Abstract
Purpose: Increased expression of transforming growth factor-β2 (TGF-β2) is reported in the conjunctiva of dry eye patients with no increase of anti-inflammatory activity of TGF-β2. Our aim was to compare the expression of molecules involved in TGF-β2 activation, thrombospondin-1 (TSP-1) and CD36, during murine and human conjunctival inflammation.
Methods: Human conjunctival tissue from cadaveric donors, human conjunctival epithelial primary cells and fibroblasts, and murine conjunctivas were immunostained for TSP-1, CD36, or TGF-β2. Inflamed conjunctival tissues were obtained from C57BL/6 wild-type (WT) mice induced to develop experimental dry eye (EDE) with 10 days of desiccating conditions and scopolamine injections and TSP-1-deficient (TSP1−/−) mice, which spontaneously develop Sjögren's syndrome-associated conjunctival inflammation with age. Immunostaining intensities were compared using ImageJ software. Cultures of human conjunctival fibroblasts were stimulated with IL-1β and both secreted protein and message levels of TSP-1, CD36, and TGF-β2 were analyzed.
Results: TSP-1 and CD36 were detectable in human and murine conjunctival tissues as well as primary conjunctival epithelial cells and fibroblasts. Increased conjunctival immunostaining of TGF-β2 and reduced CD36 were detected in EDE mice compared with WT mice. Interestingly, increased TGF-β2 and CD36 conjunctival immunostaining was detected in TSP1−/− mice. The expression of TSP-1 and CD36 was downregulated in IL-1β-stimulated conjunctival fibroblasts at both the protein and message level, while active TGF-β2 was undetected.
Conclusions: The absence or reduced expression of either of the molecules involved in TGF-β2 activation supports proinflammatory conditions in the conjunctiva. Changes in TSP-1 and CD36 may serve as potential biomarkers of conjunctival inflammation.
Introduction
Conjunctiva is the mucosal tissue of the ocular surface and, like other mucosa in the body, contains an associated lymphoid tissue, namely the conjunctival-associated lymphoid tissue (CALT). CALT contains all of the components necessary for a complete immune response.1 In many cases, diseases of the ocular surface are associated with inflammation and the ocular mucosa plays an imperative role in modulating and resolving inflammation.2,3
In the mucosal surfaces of the intestine and lung, transforming growth factor-β2 (TGF-β2) is the predominant isoform of TGF-β and plays an important role during inflammatory diseases.4–6 Recently, TGF-β2 has also been described as the predominant isoform in mouse conjunctiva.7 Moreover, increased levels of TGF-β2 in the conjunctiva in inflammatory diseases such as dry eye (DE) or vernal keratoconjunctivitis have been reported,8–10 suggesting ineffectiveness of its anti-inflammatory activity. TGF-β2 is secreted in a complex with its propeptide, latency-associated protein 2 (LAP2). To become biologically active, this complex, LAP2/TGF-β2, must be dissociated during physiologic or pathologic events in a process termed latent TGF-β activation. Appropriate levels of active TGF-β2 are essential to avoid immunological and inflammatory disturbances due to its importance in mediating immune privilege, creating an immunomodulatory environment when activated.11,12 Integrins, which are upregulated during conjunctival inflammation,13 cannot activate TGF-β2 due to the absence of the integrin-binding arginine-glycine-aspartic acid (RGD) sequence in the LAP isoform associated with TGF-β2.14,15
Thrombospondin-1 (TSP-1) is a matricellular protein reported to efficiently activate TGF-β216 through ligation of its receptor CD36.17 The role of TSP-1 in the ocular surface as a modulator in avascular repair and immunoregulation has been reviewed.18,19 On the other hand, CD36 is involved in diverse processes in the ocular surface, including angiogenesis, inflammation, and oxidative stress scavenging, depending on the ligands with which CD36 can interact.20,21
The notion that both TSP-1 and CD36 are critical for ocular surface homeostasis has recently arisen as TSP-1 and CD36-deficient goblet cells have been reported as incapable of activating their endogenous TGF-β2.7 Moreover, TSP-1-deficient mice spontaneously develop conjunctival inflammation with age.22 Regarding TSP-1 expression in human conjunctiva, some studies reported a mild and focal TSP-1 staining in normal human conjunctival epithelium,23 while others reported detection of the protein in corneal, but not in conjunctival epithelia,24,25 suggesting a pivotal role of TSP-1 in preventing corneal angiogenesis. A reported correlation between a polymorphism in the TSP-1 gene (THBS-1) and chronic ocular surface inflammation in humans further supports the importance of TSP-1 in immunomodulation.26 In addition, TSP-1 has been colocalized with CD36 in murine conjunctival goblet cells and simultaneous TGF-β2 activation was detected, which points to a relevant role of TSP-1-dependent TGF-β2 activation in regulating mucosal immunity.7
Based on these findings, we hypothesize that TSP-1 and CD36, both TGF-β2-activating factors, are affected by the surrounding inflammatory microenvironment, which would explain the lack of TGF-β2 activation in an inflamed conjunctiva. Thus, our aim was to study changes in TSP-1, CD36, and TGF-β2 in different models of conjunctival inflammation. Murine models of ocular surface inflammatory diseases and in vitro stimulation of conjunctival fibroblasts with inflammatory cytokines were used. We conclude that the absence or reduced expression of either of the molecules involved in TGF-β2 activation supports proinflammatory conditions in the conjunctiva, suggesting the use of these molecules as potential inflammatory biomarkers.
Methods
Materials
All materials used were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Dulbecco's modified Eagle's medium (DMEM)/F12 and some of its supplements, such as fetal bovine serum (FBS) and penicillin/streptomycin, were from Invitrogen-GIBCO (Inchinnan, United Kingdom). Human serum (HS) was from Lonza Group Ltd., (Basel, Switzerland) and human epidermal growth factor (EGF) and bovine insulin were from Invitrogen (Eugene, OR). Cell culture plates and multichamber Permanox® or glass slides were from Nunc (Roskilde, Denmark).
Primary antibodies (Abs) used for immunofluorescence were goat anti-TSP-1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-CD36 (Abcam, Cambridge, United Kingdom), and rat anti-TGF-β2 (R&D systems, Abingdon, United Kingdom). Secondary Abs were Alexa Fluor-conjungated Abs from Invitrogen. Fluoromount-G™ mounting media were from SouthernBiotech (Birmingham, AL). To display fluorescence micrographs, a Leica DMI 6000B microscope and the LAS AF Lite software from Leica Microsystems (Wetzlar, Germany) were used.
Enzyme-linked immunosorbent assay (ELISA) kits for human TSP-1 and TGF-β2 were from R&D systems and for human CD36 from Biorbyt (Cambridge, United Kingdom). AlamarBlue® colorimetric indicator assay used to calculate numbers of cells was from AbD Serotec (Oxford, United Kingdom). The SpectraMAX® M5 multidetection microplate reader and the SoftMax Pro 4.8 software used to analyze ELISA results were from Molecular Devices (Sunnyvale, CA).
The kit for RNA isolation, RNeasy® Mini Kit, was from Qiagen (Valencia, CA), and kits for RNA quantification (Quant-iT™ RNA assay) and cDNA synthesis (SuperScript® VILO™ cDNA kit) were from Invitrogen. Reagents for reverse transcription–polymerase chain reaction (RT-PCR) were from Biotools B&M Labs S.A. (Madrid, Spain), except Blue Juice™ gel-loading buffer 10× (Invitrogen) and TSP-1 and CD36 primers (OriGene Technologies, Rockville, MD). SYBR Green PCR Master Mix was from Applied Biosystems (Carlsbad, CA). To visualize agarose gels, the ChemiDoc® gel documentation system and Quantity One software from Bio-Rad Laboratories (Hercules, CA) were used. The conventional RT-PCR was done in a MyGene™ L Series Peltier Thermal Cycler from LongGene® Scientific Instruments Co., Ltd. (Tuen Mun, Hong Kong), and real-time RT-PCR or quantitative RT-PCR (qPCR) was done in a 7500 Real-Time PCR System from Applied Biosystems.
For statistical analyses, Statistical Package for the Social Sciences software (SPSS 20.0; SPSS Inc., Chicago, IL) was used.
Mice and murine experimental dry eye model
Wild-type C57BL/6 (H-2b) mice (WT) between 6 and 12 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME) and Charles River Laboratories (Wilmington, MA). TSP-1-deficient mice of C57BL/6 background (TSP1−/−) were purchased from The Jackson Laboratory. These mice were subsequently bred in-house in a pathogen-free facility at Boston University, Boston, MA, and were used at 10 weeks of age. C57BL/6 and TSP-1-deficient mice were euthanized by CO2 inhalation and their eyeballs with attached eyelids were excised. All animal experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee (IACUC) at Boston University School of Medicine.
Tissue sections of eyeballs with attached eyelids from C57BL/6 mice, to which experimental dry eye (EDE) had been induced as previously described,27 were kindly provided by Dr. Michael E. Stern from Allergan, Inc., (Irvine, CA). Briefly, EDE was induced by injecting scopolamine (0.5 mg in 0.2 mL) subcutaneously thrice a day in alternate flanks for 10 days. Mouse cages were placed in front of a fan directed to blow air through the wired screen side of the cage 24 h per day with controlled humidity (less than 40%). After 10 days under desiccating conditions and scopolamine injections, eye tissues were excised.
Human tissues
Healthy human conjunctival tissues were obtained from corneoscleral buttons from cadaveric donors (n=8; mean age±standard error of the mean: 77.57±4.02 years). Corneoscleral buttons were obtained with informed research consent from the Barraquer Eye Bank (Barcelona, Spain). This study was in strict accordance with the tenets of the Declaration of Helsinki and Spanish Regulations concerning the use of human tissues for biomedical research and had the approval of the Ethics Committee of the University of Valladolid.
Isolation and culture of epithelial and stromal primary cells from human conjunctival tissues
Human bulbar conjunctiva was carefully isolated, and epithelial and stromal (fibroblasts) cells were obtained using the explant technique as previously described28 and optimized.29 In brief, conjunctiva was carefully cut into small pieces and plated in 12-well plates using the epithelial culture media described below. Explants were fed by superficial tension until cell growth was observed. After that, more culture medium was added. The epithelial culture medium comprised DMEM/F12 supplemented with 2.5 μg/mL fungizone, 5,000 units/mL penicillin/streptomycin, 1 μg/mL insulin, 0.5 μg/mL hydrocortisone, 2 ng/mL EGF, and 10% HS.
Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C and the medium was changed every other day. To ensure the purity of the cultures, differential trypsinization with 0.25% trypsin/EDTA was used. Different trypsinization times were used to obtain fibroblasts (2 min) or epithelial cells (5 min). Cell morphology was evaluated by phase-contrast microscopy, showing the common polygonal or spindle-shaped cells for epithelial or fibroblast cell types, respectively. Polygonal cells were fed with the epithelial culture medium described above, and fibroblasts with culture medium comprising DMEM/F12 supplemented with 2.5 μg/mL fungizone, 5,000 units/mL penicillin/streptomycin, and 10% FBS.
Epithelial cells from passages 0 to 1 or conjunctival fibroblasts from passages 2 to 4 were used. Previously, the purity of epithelial and fibroblast cultures had been assessed by specific lineage markers using previously tested Abs.29
In vitro induction of inflammation to human conjunctival primary cells
Conjunctival fibroblasts were cultured for 48 h in a 24-well plate. Before treatment, the cells were maintained in serum-free medium for 1 h. Then, the cells were stimulated with 10 ng/mL IL-1β in 10% serum-supplemented or serum-free culture medium for 24 h. Untreated cells were used as controls. After treatment, the supernatants were collected and RNA extracted. The AlamarBlue assay was performed after supernatant collection to calculate the number of cells. At least 3 independent experiments were performed.
TSP-1, CD36, and TGF-β2 detection by immunofluorescence
TSP-1, CD36, and TGF-β2 were immunodetected in murine and human conjunctival tissues and in cultured conjunctival cells. Murine and human ocular tissues were rinsed with 15% sucrose in phosphate-buffered saline (PBS), and then placed in 30% sucrose at 4°C overnight. Tissues were then embedded in optimal cutting temperature compound and frozen. Cryostat sections (7 μm) were collected on poly-l-lysine-treated slides, fixed with acetone for 10 min, and kept at −80°C during shipment and until use.
Epithelial cells and fibroblasts were seeded onto 8-well multichamber Permanox® or glass slides, respectively, and grown for 48 h. Then, the cells were fixed in ice-cold methanol and kept at −20°C until use.
Tissue cryosections and fixed cells were washed in PBS and incubated at room temperature (RT) for 2 h with blocking buffer comprising 2% bovine serum albumin (BSA) and 0.3% Triton X-100 in PBS to block nonspecific binding and allow permeability of the cell membrane. Afterward, primary Abs against TSP-1 and CD36 (5 μg/mL) and TGF-β2 (10 μg/mL) were incubated overnight at 4°C in blocking buffer. Alexa Fluor-conjugated secondary Abs (10 μg/mL) were incubated for 1 h at RT. Nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI) or Hoechst. Negative controls included omission of primary Abs. Cells were observed under a dry objective and same exposure time, gain, and intensity were used for all the pictures taken. To quantify TSP-1, CD36, or TGF-β2 immunofluorescence in murine conjunctivas, the two-channel micrographs were analyzed using ImageJ software v. 1.49 (http://imagej.nih.gov/ij/; National Institutes of Health, Bethesda, MD). Then, micrographs were split into different channels and each channel thresholded. To subtract the background in the red channel (corresponding to the protein of interest), negative controls were used to set the threshold and the same value was used in all micrographs being analyzed from the same experiment at the same time. The threshold for the blue channel, corresponding to the nuclei area, was set according to the area stained. Mean gray value was measured, redirecting the measurement to the corresponding channel. The results shown are the ratio of the mean gray value for the red channel to that obtained from the blue channel from 2 independent animals in triplicate.
TSP-1, CD36, and TGF-β2 measurement by ELISA
An instant ELISA was used to determine the levels of TSP-1, CD36, and TGF-β2 in cell culture supernatants of conjunctival primary cells. The assays were performed according to the manufacturers' protocols. For TGF-β2 measurements, nonacidified (active TGF-β2) was assayed. The minimum detectable doses for the human TSP-1, CD36, and TGF-β2 ELISAs were 355, 10, and 7 pg/mL, respectively, according to each ELISA kit's instructions.
The obtained concentration values were normalized against cell numbers obtained using the AlamarBlue assay. A standard curve representing the relationship between fluorescence intensity (560 nm excitation/590 nm emission) and number of cells seeded had been done previously for each cell type to normalize quantified concentrations. The results shown are the mean of concentration/number of cells calculated.
Quantification of TSP-1 and CD36 mRNA expression levels by reverse transcription–polymerase chain reaction and SYBR Green qPCR
Cell lysates were prepared from human conjunctival tissues, conjunctival epithelial cells, and fibroblasts. Isolation and purification of total RNA were done using the RNeasy Mini Kit following the manufacturer's instructions. Samples were then incubated with DNase for 10 min at RT to remove any contaminating genomic DNA. Total RNA was quantified using the Quant-it RNA Assay Kit with the Qubit fluorometer. Then, cDNA was synthesized using the SuperScript VILO cDNA synthesis Kit with 1 μg of RNA. For RT-PCR, 1 μL of primers, 50 ng of cDNA, 5 μL of PCR buffer 10×, 1 μL of dNTPs, and 1 μL of Taq polymerase were mixed in a final volume of 50 μL. The thermal profile used was as follows: 95°C for 120 s, 39 cycles at 95°C for 20 s, 60°C for 30 s, 72°C for 40 s, and 72°C for 600 s. RT-PCR products were resolved on 4% agarose gels.
For qPCR, 1 μL of primers, 10 ng of cDNA, and 10 μL of SYBR Green PCR Master Mix were mixed in a final volume of 20 μL using a thermal profile of 50°C for 120 s, 95°C for 600 s, followed by 40 cycles at 95°C for 15 s and 60°C for 60 s, and a final cycle at 95°C for 90 s. All reactions were performed in duplicate. Relative mRNA expression levels were determined using the 2−ΔΔCt method30 using the endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) signal for normalization. A negative no-template control was included. In addition, to ensure the specificity of the PCR products, a melting curve analysis was performed. At least three independent experiments were performed.
The primer pairs used for both RT-PCR and qPCR were GAPDH (sense: 5′-GAACGTGAAGGTCGGAGTCAAC-3′; antisense: 5′CGTGAAGATGGTGATGGGATTTC-3′), TSP-1 (Catalog No. HP206797), and CD36 (Catalog No. HP200058).
Statistical analyses
Data are expressed as the mean±standard error of the mean (SEM). A Student's t-test was used to compare groups. If groups had variances significantly different (F test), Welch's correction was done. Statistical significance of p≤0.05 was considered.
Results
Altered conjunctival expression of TGF-β2 and CD36 in experimental mouse models with conjunctivitis
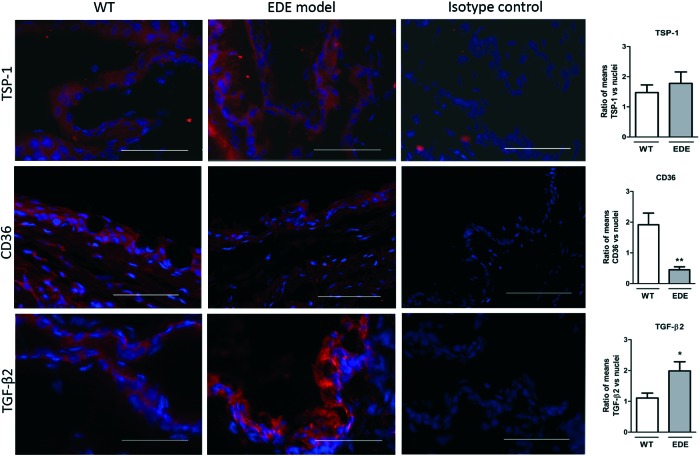
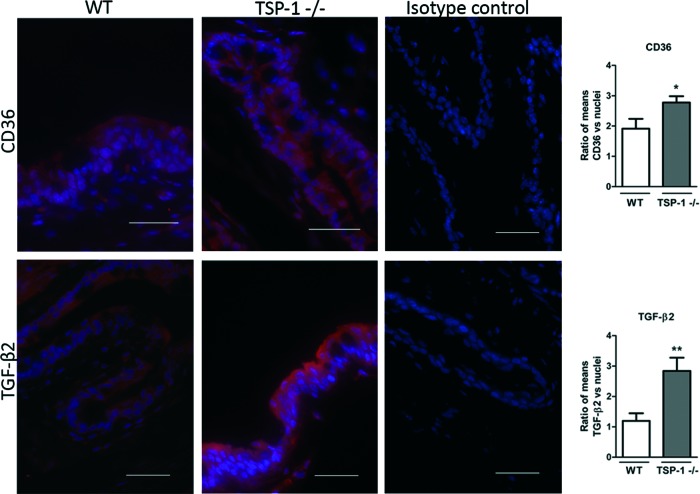
To determine the expression of TSP-1, CD36, and TGF-β2 in conjunctival inflammation, mouse models with reported conjunctivitis were used. In the EDE model, exposure to a desiccating stress environment is known to induce conjunctival inflammation, whereas it is reported to develop spontaneously in TSP-1-deficient mice. Frozen sections of conjunctiva tissues harvested from WT, EDE, and TSP-1-deficient mice were immunostained for CD36 and TGF-β2, while WT and EDE tissues were also immunostained for TSP-1. The staining pattern for all the 3 molecules was evenly distributed predominantly in the epithelial layer of the mouse conjunctiva. The signal quantification revealed significant differences between mice with normal and inflamed conjunctiva. Significantly increased TGF-β2 conjunctival staining was detected in both EDE and TSP-1-deficient mice compared with normal WT mice (Figs. 1 and 2). No difference was detectable in TSP-1 immunofluorescence, while staining for CD36 was significantly reduced in EDE conjunctival tissues compared with control conjunctival tissues (Fig. 1). However, immunofluorescence of CD36 was significantly increased in TSP-1-deficient conjunctiva compared with normal control conjunctival tissue (Fig. 2).
FIG. 1.
Changes in transforming growth factor-β2 (TGF-β2) and CD36 conjunctival immunostaining in experimental dry eye (EDE) mice compared with wild-type (WT) mice. Representative micrographs of thrombospondin-1 (TSP-1), CD36, and TGF-β2 immunostaining in WT and EDE model conjunctivas. Nuclei were stained in blue with Hoechst dye. Isotype controls include omission of primary antibodies. Scale bar: 50 μm. Immunofluorescence intensity analysis of images is presented in bar graphs as the mean ratio between protein of interest/nuclei±SEM. Statistically significant differences between samples are indicated with asterisks (t-test; *p≤0.05, **p≤0.01). SEM, standard error of the mean. Color images available online at www.liebertpub.com/jop
FIG. 2.
Changes in TGF-β2 and CD36 conjunctival immunostaining in TSP-1-deficient (TSP1−/−) mice compared with WT mice. Representative micrographs of CD36 and TGF-β2 immunostaining in conjunctivas of WT and TSP-1-deficient mice. Nuclei were stained in blue with DAPI. Isotype controls include omission of primary antibodies. Scale bar: 50 μm. Immunofluorescence intensity analysis of images is presented in bar graphs as the mean ratio between protein of interest/nuclei±SEM. Statistically significant differences between samples are indicated with asterisks (t-test; *p≤0.05, **p≤0.01). Color images available online at www.liebertpub.com/jop
Expression of TSP-1 and CD36 in human conjunctival tissue and primary cells
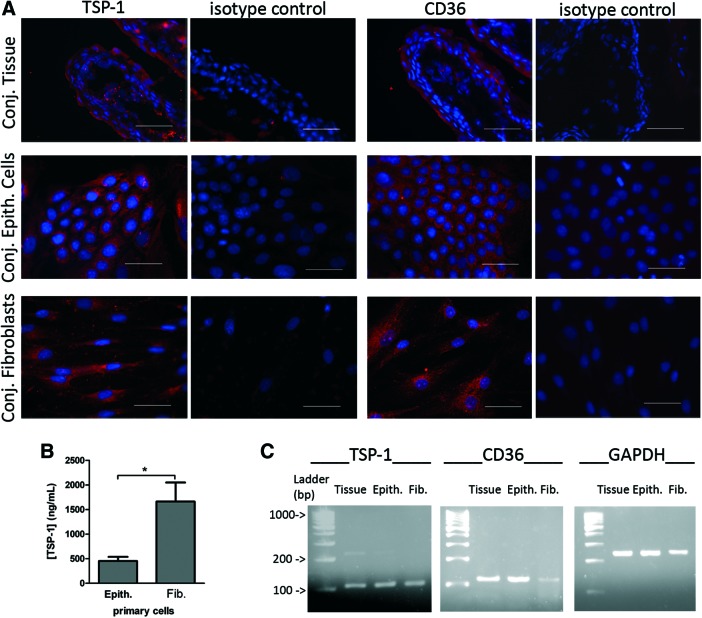
To determine if TSP-1 and CD36 are detectable in the human conjunctiva, their expression was analyzed in human conjunctival tissues and primary cultures of conjunctival epithelial cells and fibroblasts. Both TSP-1 and CD36 were immunolocalized in human bulbar conjunctival tissue as well as in primary epithelial cells and fibroblasts (Fig. 3A). In conjunctival tissue, TSP-1 and CD36 were mainly localized in conjunctival epithelium, although some staining was detectable in the stroma. In epithelial cells and fibroblasts, TSP-1 appeared distributed in the cultured cell, showing higher intensity in the perinuclear area. CD36, an integral membrane receptor, was evenly distributed in the epithelial and fibroblast cell membrane. The levels of TSP-1 were assessed in culture supernatants collected from confluent monolayers of primary conjunctival cells cultured for 3 days in the presence of 10% serum. Significantly increased basal levels of TSP-1 were detected in supernatants derived from conjunctival fibroblast cell cultures compared with those derived from conjunctival epithelial cell cultures (Fig. 3B). CD36 was undetectable in conjunctival cell supernatants. Message for TSP-1 and CD36 was detected in conjunctival tissue as well as primary conjunctival cells (Fig. 3C).
FIG. 3.
The expression of TSP-1 and CD36 in human conjunctival tissue and primary cultures. (A) Immunofluorescence micrographs of TSP-1 and CD36 (in red) of conjunctival tissue (Conj. Tissue), conjunctival epithelial cells (Conj. Epith. Cells), and fibroblasts (Conj. Fibroblasts). Nuclei of cells were stained in blue with Hoechst dye. Isotype controls include omission of primary antibodies. Scale bar: 50 μm. (B) Detection of TSP-1 in culture supernatants of confluent epithelial cells (Epith.) and fibroblasts (Fib.) after 3 days in serum-supplemented culture by enzyme-linked immunosorbent assay (ELISA). Bars represent the mean TSP-1 concentration (ng/mL)±SEM. Statistically significant differences between samples are indicated with asterisks (t-test; *p≤0.05). (C) Agarose gels showing mRNA expression of TSP-1, CD36, and GAPDH, determined by RT-PCR of conjunctival tissue (tissue), conjunctival epithelial cells (Epith.), and fibroblasts (Fib.). GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Color images available online at www.liebertpub.com/jop
Changes in TSP-1, CD36, and TGF-β2 expression in IL-1β-stimulated conjunctival fibroblasts
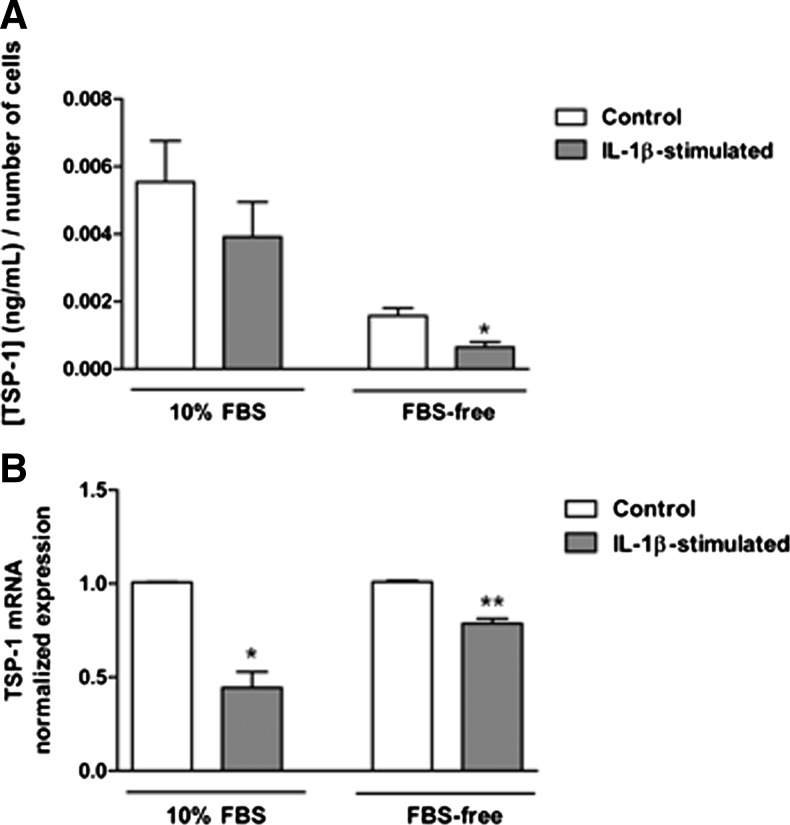
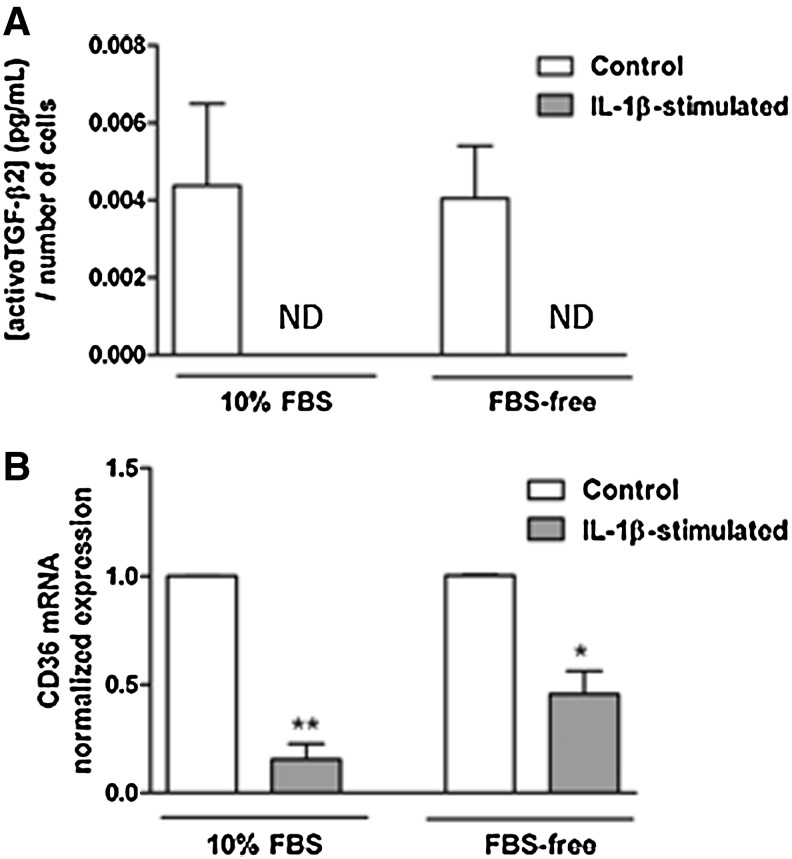
The proinflammatory cytokine, IL-1β, is detectable in human ocular surface tissue during DE and is considered a consistent marker of this condition.31–33 Therefore, primary cultures of conjunctival stromal cells, fibroblasts, were stimulated with IL-1β and changes in their expression of TSP-1 and CD36 were assessed in the context of their ability to secrete active TGF-β. First, the toxicity of IL-1β was determined by assessing cell numbers using the AlamarBlue assay. Both serum-containing (10% FBS) and serum-free culture conditions were tested. No significant cell loss was detected in either culture condition in IL-1β-stimulated cells compared with untreated control cells (data not shown). Levels of TSP-1 and active TGF-β2 protein secreted in culture supernatants were determined by ELISA. While no significant change in TSP-1 levels was detected in serum-containing cultures after IL-1β stimulation, in serum-free conditions, TSP-1 levels were significantly reduced after IL-1β stimulation compared with untreated control cultures (Fig. 4A). However, qPCR analysis indicated significant reduction in TSP-1 mRNA in IL-1β-stimulated cells in both culture conditions (Fig. 4B). Active TGF-β2 levels were undetectable in culture supernatants collected from IL-1β-stimulated cells compared with untreated cells (Fig. 5A). These results are consistent with significantly reduced CD36 mRNA levels in IL-1β-stimulated conjunctival fibroblasts cultured under both serum-containing and serum-free conditions (Fig. 5B).
FIG. 4.
Changes in TSP-1 expression in conjunctival fibroblasts mediated by IL-1β stimulation. (A) Detection of TSP-1 in culture supernatants as determined by ELISA. (B) TSP-1 mRNA levels normalized to GAPDH levels and referenced to control conditions, determined by quantitative polymerase chain reaction (qPCR). Ten percent serum-supplemented conditions are indicated by 10% fetal bovine serum (FBS) and serum-free conditions by FBS-free. Values are expressed as mean±SEM from 3 independent experiments. Statistically significant differences, when compared with untreated control cells, are indicated with asterisks (t-test; *p≤0.05, **p≤0.01).
FIG. 5.
Changes in active TGF-β2 levels and CD36 mRNA levels in conjunctival fibroblasts stimulated with IL-1β. (A) Levels of active TGF-β2 secreted by conjunctival fibroblasts in serum-free conditions determined by ELISA. Concentration values were normalized to cell numbers determined by the AlamarBlue® assay. (B) Levels of CD36 mRNA normalized to GAPDH levels and referenced to control conditions, determined by qPCR. Ten percent serum-supplemented conditions are indicated by 10% FBS and serum-free conditions by FBS-free. Values are expressed as mean±SEM from at least 3 independent experiments. Statistically significant differences, when compared with untreated control cells, are indicated with asterisks (t-test; *p≤0.05, **p≤0.01). ND, not detected.
Discussion
Inflammatory diseases of the ocular surface cause pain and affect the quality of life in patients for whom no immunomodulatory therapies have proven to be completely efficient so far. The presence of conjunctival inflammation together with increased levels of TGF-β2 in DE patients suggests a lack of activation of the LAP2/TGF-β2 complex in conjunctival inflammation. While integrins cannot activate the TGF-β2 isoform, TSP-1, through its ligation of CD36, is known to efficiently activate it. Therefore, understanding how an inflammatory environment regulates TSP-1 and CD36 expression in conjunctival cells may be of great value to enhance the knowledge of DE pathophysiology and address potential novel therapies.
In this study, we used not only inflammatory murine models but also cytokine-induced inflammation in human conjunctival fibroblasts to analyze changes in TSP-1, CD36, and/or TGF-β2. Murine models included an EDE model34 induced in C57BL/6 mice and a spontaneous model in TSP-1-deficient mice that develop a well-characterized chronic DE.22,35 The induced EDE model has been characterized showing a reduction in goblet cell number and increased inflammatory cytokines in the conjunctiva.34 In addition, in TSP-1-deficient mice, a damaged and inflamed conjunctival epithelial barrier develops spontaneously with age accompanied by increased apoptosis in the lacrimal gland and reduced goblet cell density and tear mucin secretion. Proinflammatory cytokines, such as IL-6 and TNF-α, among others, are increased in the conjunctiva of 6-week-old TSP-1-deficient mice.22 Our results showed higher expression of TGF-β2 in conjunctival tissues of EDE and TSP-1-deficient mice compared with those tissues of WT mice, as determined by immunofluorescence detection. It has been previously reported that no TGF-β2 increase is detectable at message or protein levels in mice exposed to desiccating stress for 5 or 10 days.8,36 In those studies, mice were exposed to the airflow acutely during a period of 16 or 18 h, while our model included 24 h of airflow exposure. This fact, together with different techniques used to compare TGF-β2 expression, may explain the different results obtained. Our results obtained in murine models are in agreement with previously reported results in human conjunctival cells harvested from DE patients with respect to increased levels of TGF-β2.9
In addition, our results indicate unaltered TSP-1 expression in EDE conjunctiva with significantly reduced CD36 expression, in contrast to the increased CD36 expression in TSP-1-deficient conjunctiva. Several in vitro studies have reported expression of both proinflammatory37,38 and anti-inflammatory39–41 cytokines in macrophages resulting from the ligation of CD36 by ligands, such as apoptotic cells, oxidized low-density lipoprotein, and TSP-1, but not on epithelial cells. However, in vivo studies appear to predominantly support the anti-inflammatory role of CD36.41–43 In particular, reduced CD36 expression in respiratory as well as intestinal mucosa was associated with the development of inflammation.42,43 In conjunctival epithelial cells, TSP-1:CD36 interaction is reported to activate the immunoregulatory cytokine, TGF-β.7 Therefore, it is conceivable that in conjunctival mucosa, CD36 downmodulates inflammatory immune responses through binding TSP-1 and activating TGF-β. On the one hand, such a function of CD36 may explain its reduced expression in EDE, while on the other hand, the absence of TSP-1 may allow increased detection of unoccupied CD36 as noted in TSP-1-deficient mice. Thus, together, our findings corroborate other studies supporting the anti-inflammatory role of CD36 and suggest that the absence or reduced expression of either CD36 or TSP-1, molecules involved in TGF-β2 activation, may result in inflammatory conditions in the conjunctiva.
We evaluated TSP-1 and CD36 expression in human conjunctival tissue from cadaveric donors and in human conjunctival epithelial cells and fibroblasts. To our knowledge, characterization of TSP-1 and CD36 had not been previously reported in human conjunctival primary cells. Expression of TSP-1 and CD36 in murine goblet cell primary cultures has been recently described.7 We detected the expression of both TSP-1 and CD36 at the protein and message levels in conjunctival epithelial cells and fibroblasts. Therefore, both conjunctival epithelial cells and fibroblasts can activate TGF-β2 locally in the conjunctiva. Since both showed a similar expression pattern, but fibroblasts expressed higher levels of TSP-1 than epithelial cells, this cell type was chosen to study further the role of TSP-1 in an inflammatory environment. The activation of fibroblasts during keratoconjunctivitis further supports the use of this cell type.44
In addition, in inflammation-based diseases such as DE, loss of TGF-β2-expressing goblet cells in the ocular surface45 suggests an involvement of other cell types in the immunomodulatory process. Recent research indicates that fibroblasts can contribute to the inflammatory reaction by releasing inflammatory modulators in response to different cytokines. In particular, IL-1β has been previously identified in the tears and conjunctival epithelium of patients with ocular inflammatory disease.31–33 Moreover, IL-1β has been previously used to study the conjunctival inflammatory processes as increased release of inflammatory cytokines and chemokines, such as IL-6 or IL-8, has been observed in IL-1β-stimulated conjunctival cells.46,47 Therefore, we chose to activate our conjunctival fibroblast cultures with the inflammatory cytokine, IL-1β, in 10% serum-supplemented or serum-free culture medium, and changes in TSP-1, CD36, and TGF-β2 were analyzed. It is also important to take into account IL-1β-mediated regulation of the fibroblast cell proliferation rate as reported previously.48,49 For this reason, the cell number was measured after stimulation and used to normalize secreted protein levels. Consistent with previous reports, we detected increased proliferation after IL-1β stimulation of conjunctival fibroblasts in serum-free conditions (data not shown).
We observed that conjunctival fibroblasts respond to an in vitro IL-1β-induced inflammatory stimulation by altering their TSP-1, CD36, and TGF-β2 expression. Reduced expression of TSP-1 was detected at both the protein and message levels in IL-1β-stimulated conjunctival fibroblasts. This result agreed with a previously reported association between reduced levels of TSP-1 with increased IL-1β in ocular surface epithelial cells derived from individuals with chronic DE.26 Although consistent with previously reported increased TGF-β2 message in human conjunctiva,9 we detected increased TGF-β2 protein in mouse conjunctiva during DE; interestingly, biologically active TGF-β2 was undetectable in IL-1β-stimulated cells. This result was consistent with their downregulated CD36 and TSP-1 message. These findings further support the importance of TSP-1 and CD36 in TGF-β2 activation.
Detection of changes in TSP-1 and CD36 expression by conjunctival fibroblasts in serum-free culture condition, but not in serum-supplemented cultures, can be explained by the high TSP-1 content of the serum. Clearly, the changes induced by IL-1β stimulation in cells were masked in the presence of serum-derived exogenous TSP-1. The clear differences noted in our results in the presence and absence of serum may also help explain the differences in observations often encountered with the use of cell lines and primary cells that differ in their serum supplementation needs for growth in culture.
To conclude, in this study, we show that TSP-1 and CD36 are affected by the inflammatory environment both in vitro and in vivo. Conjunctival fibroblasts are able to respond to inflammatory mediators such as IL-1β in the tissue environment by downregulating TSP-1 and CD36 expression. This suggests a lower availability of these molecules for TGF-β2 activation and would consequently allow progression of the inflammation. How this scenario would affect epithelial cell response remains to be elucidated and warrants further studies. Our results suggest that TSP-1 or CD36 may serve as a potential biomarker and conjunctival fibroblasts a potential screening tool to detect ocular surface inflammation.
Acknowledgments
The authors thank Michael Stern, PhD (Allergan, Inc., CA), for kindly allowing preparation and shipment of eyeball tissue sections from EDE mice, and Jorge Montesinos-Selfa, MSc, and Bruce Turpie, A.B., for technical support. This work was supported by an FEDER-CICYT Grant MAT2013-47501-C02-1-R (Spanish Ministry of Economy and Competitiveness, Y.D.) and NEI Grant No. EY015472 (S.M.). Other authors were supported by the Regional JCyL Scholarship/European Social Fund Program VA098-12 and University of Valladolid Mobility Program 2014 (L.S.-R.) and by FPI Scholarship Program BES-2011-048381 (Spanish Ministry of Science and Innovation, L.G.-P.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Knop N., and Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest. Ophthalmol. Vis. Sci. 41:1270–1279, 2000 [PubMed] [Google Scholar]
- 2.Dartt D.A., Hodges R.R., Li D., Shatos M.A., Lashkari K., and Serhan C.N. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J. Immunol. 186:4455–4466, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dartt D.A., and Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 14:464–470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzar S., Chu H.W., Silkoff P., et al. . Increased TGF-beta2 in severe asthma with eosinophilia. J. Allergy Clin. Immunol. 115:110–117, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chu H.W., Balzar S., Seedorf G.J., et al. . Transforming growth factor-beta2 induces bronchial epithelial mucin expression in asthma. Am. J. Pathol. 165:1097–1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maheshwari A., Kelly D.R., Nicola T., et al. . TGF-beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140:242–253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras-Ruiz L., and Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 10:e0120284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Paiva C.S., Chotikavanich S., Pangelinan S.B., et al. . IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2:243–253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benito M.J., Calder V., Corrales R.M., et al. . Effect of TGF-beta on ocular surface epithelial cells. Exp. Eye Res. 107:88–100, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Leonardi A., Di Stefano A., Motterle L., Zavan B., Abatangelo G., and Brun P. Transforming growth factor-beta/Smad-signalling pathway and conjunctival remodelling in vernal keratoconjunctivitis. Clin. Exp. Allergy 41:52–60, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Cousins S.W., Trattler W.B., and Streilein J.W. Immune privilege and suppression of immunogenic inflammation in the anterior chamber of the eye. Curr. Eye Res. 10:287–297, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Hori J., Vega J.L., and Masli S. Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye. Ocul. Immunol. Inflamm. 18:325–333, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Abu El-Asrar A.M., Al-Mansouri S., Tabbara K.F., Missotten L., and Geboes K. Immunopathogenesis of conjunctival remodelling in vernal keratoconjunctivitis. Eye (Lond) 20:71–79, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Munger J.S., Huang X., Kawakatsu H., et al. . The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319–328, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Annes J.P., Rifkin D.B., and Munger J.S. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 511:65–68, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro S.M., Poczatek M., Schultz-Cherry S., Villain M., and Murphy-Ullrich J.E. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 274:13586–13593, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Yehualaeshet T., O'Connor R., Green-Johnson J., et al. . Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am. J. Pathol. 155:841–851, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiscott P., Paraoan L., Choudhary A., Ordonez J.L., Al-Khaier A., and Armstrong D.J. Thrombospondin 1, thrombospondin 2 and the eye. Prog. Retin. Eye Res. 25:1–18, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Masli S., Sheibani N., Cursiefen C., and Zieske J. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr. Eye Res. 39:759–774, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mwaikambo B.R., Sennlaub F., Ong H., Chemtob S., and Hardy P. Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 47:4356–4364, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Cursiefen C., Maruyama K., Bock F., et al. . Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 208:1083–1092, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contreras-Ruiz L., Regenfuss B., Mir F.A., Kearns J., and Masli S. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjogren's syndrome. PLoS One 8:e75937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aspiotis M., Tsanou E., Gorezis S., et al. . Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond) 21:1095–1101, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Sekiyama E., Nakamura T., Cooper L.J., et al. . Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest. Ophthalmol. Vis. Sci. 47:1352–1358, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Chen H.C., Yeh L.K., Tsai Y.J., et al. . Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest. Ophthalmol. Vis. Sci. 53:5615–5623, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Contreras-Ruiz L., Ryan D.S., Sia R.K., Bower K.S., Dartt D.A., and Masli S. Polymorphism in THBS1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Ophthalmology 121:1389–1397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras-Ruiz L., Zorzi G.K., Hileeto D., et al. . A nanomedicine to treat ocular surface inflammation: performance on an experimental dry eye murine model. Gene Ther. 20:467–477, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Diebold Y., Calonge M., Fernandez N., et al. . Characterization of epithelial primary cultures from human conjunctiva. Graefes Arch. Clin. Exp. Ophthalmol. 235:268–276, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Posadas L., Arranz-Valsero I., Lopez-Garcia A., Soriano-Romani L., and Diebold Y. A new human primary epithelial cell culture model to study conjunctival inflammation. Invest. Ophthalmol. Vis. Sci. 54:7143–7152, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Livak K.J., and Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Boehm N., Riechardt A.I., Wiegand M., Pfeiffer N., and Grus F.H. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest. Ophthalmol. Vis. Sci. 52:7725–7730, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Massingale M.L., Li X., Vallabhajosyula M., Chen D., Wei Y., and Asbell P.A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 28:1023–1027, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Na K.S., Mok J.W., Kim J.Y., Rho C.R., and Joo C.K. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Invest. Ophthalmol. Vis. Sci. 53:5443–5450, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Dursun D., Wang M., Monroy D., et al. . Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv. Exp. Med. Biol. 506:647–655, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Turpie B., Yoshimura T., Gulati A., Rios J.D., Dartt D.A., and Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 175:1136–1147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X., de Paiva C.S., Li D.Q., Farley W.J., and Pflugfelder S.C. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest. Ophthalmol. Vis. Sci. 51:3083–3091, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collot-Teixeira S., Martin J., McDermott-Roe C., Poston R., and McGregor J.L. CD36 and macrophages in atherosclerosis. Cardiovasc. Res. 75:468–477, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Janabi M., Yamashita S., Hirano K., et al. . Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler. Thromb. Vasc. Biol. 20:1953–1960, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Ferracini M., Rios F.J., Pecenin M., and Jancar S. Clearance of apoptotic cells by macrophages induces regulatory phenotype and involves stimulation of CD36 and platelet-activating factor receptor. Mediators Inflamm. 2013:950273, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rios F.J., Koga M.M., Pecenin M., Ferracini M., Gidlund M., and Jancar S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm. 2013:198193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., Xiong Z., Lechner E.J., et al. . Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 7:440–448, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharif O., Matt U., Saluzzo S., et al. . The scavenger receptor CD36 downmodulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. J. Immunol. 190:5640–5648, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Cupi M.L., Sarra M., De Nitto D., et al. . Defective expression of scavenger receptors in celiac disease mucosa. PLoS One 9:e100980, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumagai N., Fukuda K., Fujitsu Y., Yamamoto K., and Nishida T. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog. Retin. Eye Res. 25:165–187, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Carracedo G., Recchioni A., Alejandre-Alba N., et al. . Signs and symptoms of dry eye in keratoconus patients: a pilot study. Curr. Eye Res. 1–7, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Stahl J.L., Cook E.B., Graziano F.M., and Barney N.P. Differential and cooperative effects of TNFalpha, IL-1beta, and IFNgamma on human conjunctival epithelial cell receptor expression and chemokine release. Invest. Ophthalmol. Vis. Sci. 44:2010–2015, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Zhang J.Z., Cavet M.E., VanderMeid K.R., Salvador-Silva M., Lopez F.J., and Ward K.W. BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells. Mol. Vis. 15:2606–2616, 2009 [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt J.A., Mizel S.B., Cohen D., and Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J. Immunol. 128:2177–2182, 1982 [PubMed] [Google Scholar]
- 49.Bigildeev A.E., Zezina E.A., Shipounova I.N., and Drize N.J. Interleukin-1 beta enhances human multipotent mesenchymal stromal cell proliferative potential and their ability to maintain hematopoietic precursor cells. Cytokine 71:246–254, 2015 [DOI] [PubMed] [Google Scholar]