Abstract
We evaluated safety and feasibility of high-pressure transvenous limb perfusion in an upper extremity of adult patients with muscular dystrophy, after completing a similar study in a lower extremity. A dose escalation study of single-limb perfusion with 0.9% saline was carried out in nine adults with muscular dystrophies under intravenous analgesia. Our study demonstrates that it is feasible and definitely safe to perform high-pressure transvenous perfusion with 0.9% saline up to 35% of limb volume in the upper extremities of young adults with muscular dystrophy. Perfusion at 40% limb volume is associated with short-lived physiological changes in peripheral nerves without clinical correlates in one subject. This study provides the basis for a phase 1/2 clinical trial using pressurized transvenous delivery into upper limbs of nonambulatory patients with Duchenne muscular dystrophy. Furthermore, our results are applicable to other conditions such as limb girdle muscular dystrophy as a method for delivering regional macromolecular therapeutics in high dose to skeletal muscles of the upper extremity.
Introduction
In human studies of muscular dystrophy, delivery of therapeutic macromolecules including gene therapy to skeletal muscles has been limited to direct intramuscular injection.1–4 Single-limb perfusion has been used in delivering gene therapy to multiple muscles in limbs in preclinical studies.5–8 We have published a dose escalation study of high-pressure transvenous perfusion with 0.9% saline in the lower extremities of adult patients with muscular dystrophy to address the safety and feasibility of this approach in the leg.9 However, for single-limb gene-based therapy studies of muscular dystrophy, the nondominant arm offers advantages over a leg. Current single-limb delivery methods do not encompass the proximal muscles of the hip and shoulder. Walking is the primary function of the legs and it requires bilateral proximal strength for meaningful improvement in function. In contrast, preservation of isolated distal strength and function sufficient to operate a computer touchpad or an electric wheelchair in even one hand can have a major impact on quality of life and can be tested easily. The smaller muscle mass of the arm also means that a smaller overall dose will be needed, reducing both expense and potentially dose-related systemic toxicity since regional deliveries of viral vectors also inevitably deliver a systemic dose.8 Finally, an adverse outcome in which the muscles in the distal nondominant arm were damaged would leave the dominant arm functional and not, as in the legs, possibly lead to loss of ambulation.
Our results obtained in the lower extremities cannot be assumed to be valid in the upper extremities since the venous system and muscle compartments are different. In the upper extremities the superficial venous system, rather that the deep system, is dominant, and there are fewer perforating veins communicating between the two systems.10,11 The anatomy of the muscle compartments and their relation to nerves and blood vessels in the forearm are different from the calf.12 Therefore, we undertook a dose escalation safety study of high-pressure transvenous perfusion with 0.9% saline in the upper extremities of adult patients with muscular dystrophy.
Materials and Methods
Study subjects
Study inclusion criteria were biopsy-confirmed or mutation-proven Becker muscular dystrophy (BMD) or other clinically diagnosed muscular dystrophies, including limb girdle muscular dystrophy (LGMD), and ability to give informed consent. Exclusion criteria included positive pregnancy test; evidence of cardiomyopathy, pulmonary insufficiency, or renal insufficiency; history of local injury to upper extremities such as neuropathy, vascular injury (including arterial or venous thrombosis), surgery, or trauma; or compartment syndrome. The study was approved by the Office of Research Ethics of the University of North Carolina at Chapel Hill (IRB). Supplementary Material (available online at www.liebertpub.com/hum) includes the potential benefits and risks from consent form. Written informed consent was obtained from each subject. This study is registered on ClinicalTrials.gov with an identifier of NCT00873782.
Pre- and postperfusion studies
Outpatient pre- and postperfusion visits within 1 week of the perfusion included the following: Doppler ultrasound to assess venous and arterial damage,13 electrodiagnostic testing using standard neurographic techniques of four nerves (distal ulnar, anterior interosseus, posterior interosseus, and superficial radial nerves) in upper extremities performed in triplicate by the same examiner, quantitative muscle testing strength assessments with a handheld dynamometer (JTechAA104; JTech Medical, Salt Lake City, UT), and the action research arm test (ARAT). The ARAT assesses upper extremity function with four ordinal subscales: grasp, grip, pinch, and gross movement.14 Preperfusion studies were done bilaterally in the upper extremities. Postperfusion neurography and Doppler studies were only performed in the perfused arm. Also obtained at these visits were detailed history, neurological examination, basic metabolic panel (Na+, K+, Cl−, CO2, BUN, and creatinine), serum creatine kinase [CK], plasma and urine myoglobin, pregnancy test for women, and limb photos. Limb segmental volume (below shoulder) was measured by water immersion.
Perfusion procedure
The subjects were instructed to take no solid food after midnight and no liquids after 6 am. They were admitted to the pediatric intensive care unit the morning of the perfusion study. An intravenous catheter was placed in the peripheral vein of the control arm for blood draws and intravenous anesthesia (IVA). IVA was administered by a board-certified anesthesiologist with a combination of opioids, benzodiazepines, and propofol with monitoring following the American Society of Anesthesiology guidelines. Blood pressure, heart rate, EKG, respiratory rate, and pulse oximetry were continuously monitored. A Somanetics INVOS near-infrared oxygen monitor sensor (Somanetics Corporation, Troy, MI) was placed on each forearm to monitor distal limb tissue oxygenation,15 while a second pulse oximetry was placed onto the finger of the perfused limb.
Basic metabolic panel, CK, urine, and serum myoglobin were checked at baseline (immediately preperfusion) and hourly postperfusion until the patient was deemed ready for discharge. A portable transthoracic echocardiograph (Sonos 5500; Philips Healthcare, Andover, MA) was used to assess cardiac systolic function and measure systolic and diastolic dimensions. In addition, we continuously observed for microcavitation in the right atrium during infusion that would indicate saline leak because of loss of cuff occlusion. An upper extremity was chosen for the study for each subject (the nondominant hand unless the subject requested otherwise). An intravenous catheter (1.5 inches long and 18 or 20 gauge) was placed percutaneously in a peripheral vein in dorsum of the hand. Using the segmental limb volume premeasured by water displacement and the protocol-specified perfusion dose, the volume of normal 0.9% saline to be infused as a percent of the segmental limb volume was determined. A pneumatic tourniquet (ATS 2000; Zimmer, Warsaw, IN) was placed below the shoulder at the line marked during limb volume measurements at the preperfusion visit. The inflation pressure was set to 310–325 mm Hg and tested briefly while the subject was awake. This is the pressure recommended for intravenous regional anesthesia.16
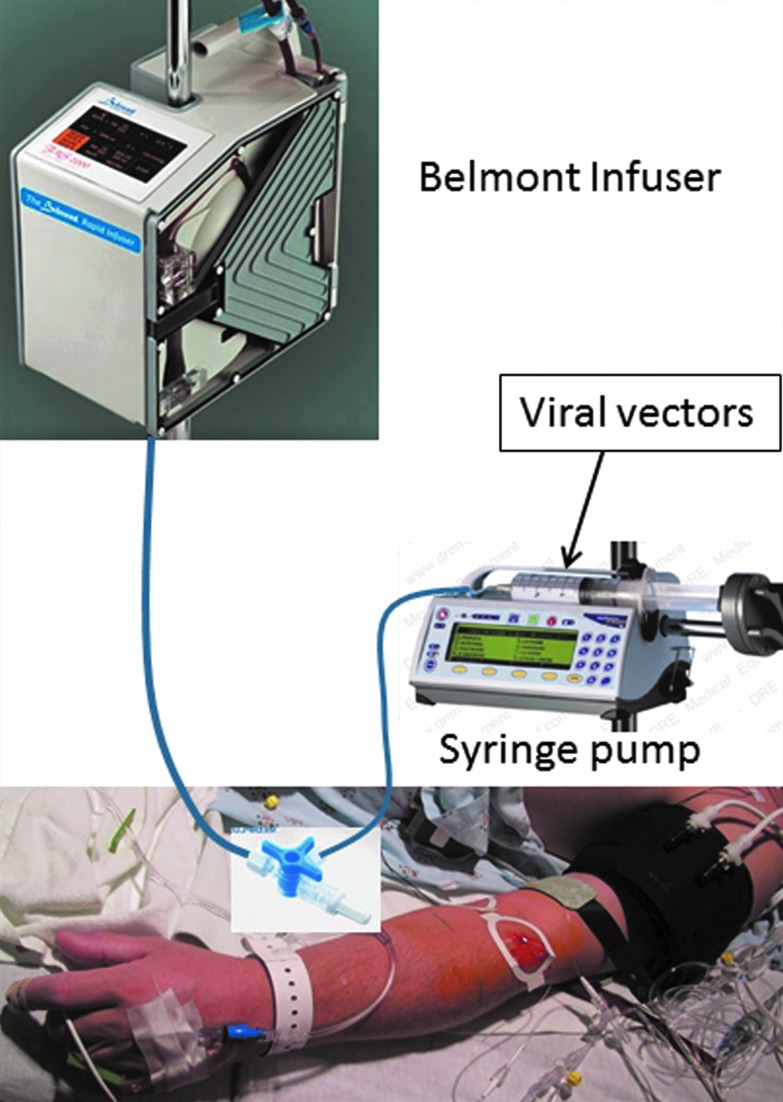
Saline was infused with a dual-pump system that consisted of an FMS 2000 Rapid Infuser (Belmont Instrument Corporation, Billerica, MA) and a Medfusion 3500 syringe pump, both of which are approved for clinical use (Medex Inc., Carlsbad, CA) (Fig. 1).17 We modified our lower extremity perfusion protocol to add the syringe pump downstream from the Belmont pump. We did so after infusion of adeno-associated virus vector serotype 8 (AAV8)-CMV-GFP vector at the standard dose of 1×1013 v.g./kg body weight in a canine limb perfusion experiment using the Belmont system alone failed to produce detectable green fluorescent protein expression. We suspected that the internal aluminum heating plates, which have very large surface areas, were adsorbing and/or inactivating the AAV viral vectors. This new dual-pump system has successfully been tested in the canine DMD model with reproducible efficacy of gene transfer identical to the original animal studies using the Harvard pump, which is not approved for human subject.18 The infusion rate was set at 80 ml/min for the Belmont pump. The Belmont FMS 2000 Rapid Infuser will infuse to a maximum line pressure of 300 mm Hg. When a line pressure of 300 mm Hg is reached, the infusion rate is automatically decreased. The syringe pump was set to infuse 20 ml of saline at a rate of 5 ml/min delivered in the initial 4–5 min of the perfusion simultaneously with Belmont pump. Following completion of the infusion, the tourniquet remained inflated for 1 min and was then deflated. Photos of the limb were taken before, during, and after perfusion.
Figure 1.
Dual-pump infusion system. Color images available online at www.liebertpub.com/hum
We measured compartmental pressures in the dorsal and volar compartments in the forearm of the perfused limb in all subjects during the perfusion and immediately after the tourniquet was released. We employed continuous monitoring by indwelling catheters (Indwelling Slit Catheter; Stryker Surgical, Kalamazoo, MI) before, during, and after the perfusion until the pressures returned to less than 35 mm Hg.
Magnetic resonance imaging acquisition and data analysis
Seven subjects (10–16) had magnetic resonance imaging (MRI) studies preperfusion at baseline and immediately postperfusion. It was technically challenging to position both arms in the scanner in a position that was comfortable for the subjects. The arms were either positioned straight on the abdomen over a plastic frame or crossed over on the chest/abdomen on a plastic frame, in order for both forearms to be imaged. T2 fat-saturated (FS) images were obtained using the same protocols as our prior study,9 and then manually segmented into several groups of muscles using open source software ITK-SNAP 2.0.19,20 The pre- and postperfusion images were aligned using the ulna as a landmark. Volumetric data and T2FS intensity data were obtained after the segmentation was finalized.
Dose escalation
The initial study was performed with an infusion volume of 5% limb volume. Infusion volume was then increased in a stepwise fashion with approval of the independent safety monitor (Table 1).
Table 1.
Infusion parameters
| Subject | Volume (% limb) | Segmental limb volume (liter) | Volume infused Belmont (ml) | Volume by syringe pump (ml) | Infusion catheter gauge | Infusion time (min) | Average Belmont flow rate (ml/min) | Peak compartment pressure (dorsal/volar) (mm Hg) | Circumference immediately postperfusion (cm) |
|---|---|---|---|---|---|---|---|---|---|
| 08 | 5 | 2.7 | 136 | 0 | 18 | 2 | 80 | 10/23 | 0 |
| 09 | 10 | 2.2 | 200 | 20 | 18 | 3 | 80 | 98/34 | 0 |
| 10 | 20 | 2.2 | 420 | 20 | 20 | 10 | 42 | 15/12 | +2.5 |
| 11 | 20 | 3.1 | 601 | 20 | 18 | 8 | 75 | 59/112 | +2.0 |
| 12 | 25 | 2.3 | 555 | 20 | 18 | 8 | 69 | —/71 | +1.7 |
| 13 | 30 | 2.7 | 798 | 20 | 18 | 11 | 73 | 82/104 | +3.0 |
| 14 | 43a | 2.4 | 1024 | 20 | 20 | 18 | 57 | 43/13 | +3.0 |
| 15 | 40 | 2.6 | 921 | 20 | 20 | 16 | 58 | 142/30 | +2.5 |
| 16 | 35 | 1.7 | 575 | 20 | 20 | 16 | 36 | 108/134 | +4.5 |
This subject was intended to have 35% perfusion volume.
Safety boundaries
Safety boundaries were set conservatively in anticipation that these changes may not be clinically significant but may predict problems at the next higher dose. The safety boundaries for limb tissue oximetry (<31%) and compartment pressures (>35 mm Hg) were based on a study of chronic exertional compartment syndrome with transient pain during exercise without muscle damage.15 For electrodiagnostic nerve testing, the postperfusion versus preperfusion changes were set to >1 msec for the distal motor latency, <75% of compound muscle action potential (CMAP) or sensory nerve action potential (SNAP) amplitude and velocity. Cardiac function and dimensions were to remain within 90% of baseline. Quantitative muscle testing and timed walking were set to be within 85% baseline. Venous Doppler was used to evaluate for venous stenosis/thrombosis and arterial Doppler to detect stenosis. For laboratory values, we set criteria for serum creatinine of >0.5 mg/dl over baseline, and for serum potassium of >0.5 mM over baseline or >5.0 mM. We did not specify the safety boundary for CK, urine, and serum myoglobin, as ascribing clinical significance to specific values with high baseline values and underlying muscle disease is problematic because of high variability.21
Safety monitoring
A local independent safety monitor reviewed the safety report prepared after the completion of each subject's study and approved the infusion parameters for the next subject.
An external independent safety officer was appointed by National Institute of Arthritis and Musculoskeletal and Skin Diseases. Biannual safety reports were submitted via KAI Research, Inc. (KAI Research, Inc., Rockville, MD).
Results
Subjects
Nine subjects, aged 23–50 years, were studied. Seven were male and two were female. One had BMD, one had limb LGMD 2A, three had LGMD 2B, two had LGMD 2E, and two had LGMD of unknown subtype. One subject (08) was studied before our implementation of the dual-pump system (see Materials and Methods section). All other subjects were studied with the dual-pump system.
The perfusion procedure
Perfusion
The initial study was performed with an infusion volume of 5% limb volume. Infusion volume was then increased in a stepwise fashion per protocol. Because of abnormalities in nerve conduction studies (NCS) observed at 40% limb volume infusion in subject 15, infusion volume was decreased to 35% for subject 16. Table 1 details the specific perfusion parameters. Average infusion rate varied with the size of the perfusion catheter placed. The average rate was 75 ml/min in 5 subjects with 18-gauge catheters and 48 ml/min in 4 subjects with 20-gauge catheters. All subjects had visible limb volume expansion, firmness to touch, and enlargement of forearm circumference. Four (12, 13, 15, and 16) developed painless petechiae under the tourniquet. There was no cardiac dysfunction or signs of systemic fluid overload as measured by echocardiogram, EKG, heart rate, respiratory rate, or cutaneous oxygen saturation (Supplementary Table S1). None of the nine reported discomfort or remembered the perfusion procedure after recovering from anesthesia (∼10–15 min postperfusion). Some subjects commented that they felt that the perfused arm was “tight” up to 1–2 hr postperfusion.
Subject 10 was a 27-year-old woman who was the only participant who had been treated with long-term corticosteroids (∼17 years). She had noticeably small veins and thin skin on her hand/forearm by visual inspection. The line pressure of 300 mm Hg (which causes the Belmont infuser to automatically adjust the perfusion rate down) was reached often during her study, and as a consequence she had one of the lowest average perfusion rates (42 ml/min). Other parameters of her study were not different from the remaining subjects. Subject 14, who received 43% rather than 35% rather limb perfusion because of calculation error, had more prolonged recovery of his limb size to baseline, but no other issues.
Compartment pressure and tissue oxygen saturation monitoring
In all subjects, postperfusion compartment pressure returned to below the safety cutoff of 35 mm Hg within 15 min and postperfusion tissue oxygen saturation returned to baseline or above baseline within 5 min. The highest peak compartment pressure was 142 mm Hg in subject 15 with 40% perfusion volume (Table 1).
Pre- and postperfusion studies
Clinical assessment
None of the postperfusion assessments showed any evidence of injury related to perfusion except for the NCS (detailed results below). All subjects had returned to subjective baseline except for healing needle puncture sites at the postperfusion visit. All subjects had a telephone follow-up within 2 weeks of postperfusion and none reported problems in the perfused arm. Petechiae typically resolved completely by 2–3 weeks per phone follow-up. No serious adverse events occurred during this study.
Laboratory studies
None had abnormal levels of renal function (creatinine) or potassium. The range of fluctuation in CK was consistent with normal variation described in muscular dystrophies attributed to normal activity (Supplementary Table S1).21,22
Venous and arterial Doppler study
All subjects had normal venous and arterial studies pre- and postperfusion (Supplementary Table S1).
Quantitative muscle strength test and functional arm test: action research arm test
Table 2 summarizes the quantitative muscle strength test data postperfusion compared with preperfusion. Large variations, similar in perfused and control arms, were observed as have been previously reported.23 There were no decreases in postperfusion arm/hand function measured by ARAT (Supplementary Table S1).
Table 2.
Changes in quantitative muscle strength testing (postperfusion compared with baseline)
| Subject | Perfused arm | Control arm |
|---|---|---|
| 08 | −9% to +25% | −3% to +53% |
| 09 | −20% to +50% | −14.7% to +32% |
| 10 | 0 to +90% | −14.7% to +33% |
| 11 | −43% to +54% | −29.8% to +50% |
| 12 | −11% to +64% | −27% to +35% |
| 13 | −24% to +10% | −12.5% to +6.5% |
| 14 | 0 to +42% | −20% to +6% |
| 15 | −75% to +94% | −75% to +39% |
| 16 | −2% to +8% | −32% to +28% |
The symbol “−” denotes strength reduction and “+” denotes strength increase in postperfusion test compared with preperfusion baseline. Quantitative muscle strength was measured in pounds (lb).
Electrophysiology studies
All except subject 15 had unchanged postperfusion NCS. Subject 15 had abnormal postperfusion studies at 3 days postperfusion in which the amplitude of the distal CMAP in all three nerves tested exceeded the prespecified safety boundary of more than a 25% reduction (Table 3), while all other parameters, including distal motor latencies and conduction velocities, remained unchanged compared with baseline (Supplementary Table S1). The postperfusion NCS were repeated twice. At 25 days postperfusion, only the amplitude of the distal CMAP of the anterior interosseous nerve exceeded the 25% reduction boundary and this returned to baseline at 5 weeks postperfusion (Table 3). At no time were there symptoms or clinical neurological deficits associated with the abnormal electrophysiological findings.
Table 3.
Amplitude of distal compound motor action potential of three nerves in subject 15
| Baseline (mV) | Poststudy 1a(mV) | Change post-vs. preperfusion (%) | Poststudy 2a(mV) | Change post- vs. preperfusion (%) | Poststudy 3a(mV) | Change post vs. preperfusion (%) | ||
|---|---|---|---|---|---|---|---|---|
| Anterior interosseous nerve | Trial 1 | 1.50 | 1.20 | — | 0.90 | — | 1.60 | — |
| Trial 2 | 1.90 | 1.20 | — | 1.00 | — | 1.60 | — | |
| Trial 3 | 1.60 | 1.20 | — | 1.20 | — | 1.60 | — | |
| Mean | 1.67 | 1.20 | −28% | 1.03 | −38% | 1.60 | −4.2% | |
| Posterior interosseous nerve | Trial 1 | 1.5 | 1.3 | — | 1.3 | — | — | — |
| Trial 2 | 2.1 | 1.3 | — | 2.3 | — | — | — | |
| Trial 3 | 2.5 | 1.2 | — | 3.2 | — | — | — | |
| Mean | 2.0 | 1.3 | −35% | 2.3 | +15% | |||
| Ulnar nerve | Trial 1 | 13.7 | 9.0 | — | 13.2 | — | — | — |
| Trial 2 | 13.9 | 9.6 | — | 12.5 | — | — | — | |
| Trial 3 | 12.4 | 9.5 | — | 12.7 | — | — | — | |
| Mean | 13.3 | 9.4 | −29% | 12.8 | −4% | — | — |
Poststudy 1 was performed 3 days postperfusion, poststudy 2 was performed 25 days postperfusion, and poststudy 3 was performed 5 weeks postperfusion. Values in bold indicate values that exceeded the prespecified safety boundary of more than a 25% reduction.
MRI studies
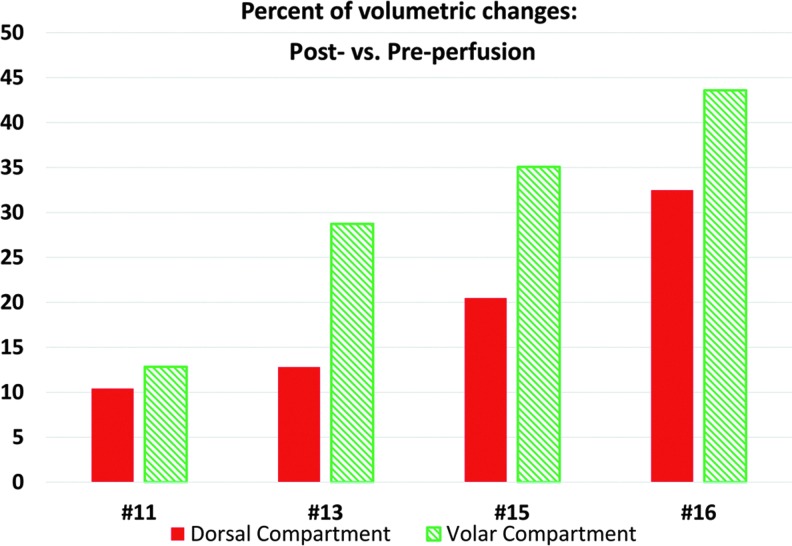
Seven subjects (10–16) had MRI studies. Three studies (10, 12, and 13) were excluded from analysis because of marked artifacts and low image quality. For subjects 11, 13, 15, and 16, the T2 fat saturation (T2FS) sequences were started at 55, 41, 63, and 43 min postperfusion. Postperfusion MRI showed increases in volume of the volar and dorsal compartments (Fig. 2). Compartmental volume increases ranged from 13% to 43% for the 30–40% limb volume perfusion studies in subjects (patients 13, 15, and 16) and were larger in the volar compartment (Fig. 3).
Figure 2.
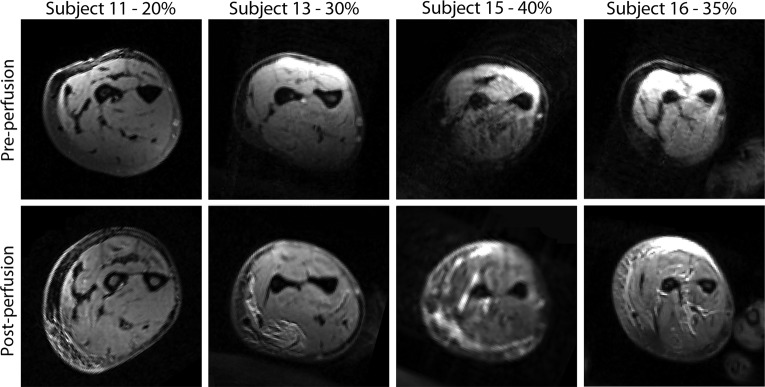
Axial view of preperfusion and postperfusion at the level of proximal two-thirds in the forearm. Increased intensity areas in these T2 fat saturation images indicate increased fluid.
Figure 3.
Postperfusion versus preperfusion muscle volume changes in percent by compartments. Color images available online at www.liebertpub.com/hum
Discussion
Our study demonstrates that it is feasible and definitely safe to perform high-pressure transvenous perfusion with 0.9% saline up to 35% of limb volume in the upper extremities of young adults with muscular dystrophy. Perfusion at 40% limb volume was associated with short-lived physiological changes in peripheral nerves without clinical correlates in one subject. With the infusion parameters used, there was no detectable regional injury to the arteries, veins, or muscles in the limb, nor was there systemic cardiac or renal toxicity observed in any subject. Subjects recovered within 4 hr and were discharged home. Furthermore, a phone follow-up in 2 weeks revealed no self-reported concerns of the subjects. In contrast to arterial limb perfusion methods, no surgical procedure such as femoral artery catheterization or cut-down was necessary.24
The clinical significance of the transiently reduced amplitudes of 28–38% in CMAP is unclear. It is unlikely due to an acute nerve injury such as neurapraxia, as conduction velocities were normal rather than slowed as they would be with this condition. Axonal injury was considered but the relatively rapid resolution of the reduced CMAP amplitudes and the absence of significant denervation on needle EMG refutes this. One possibility is that the reduction of amplitude was because of fluid entering the muscle and dampening the evoked response similar to electroporation reported by Collins et al.25 The time course of abnormality is not typical of that seen in ischemic injury to the nerve.26 Given that the conduction velocities and distal latencies of the same nerves in this subject were unchanged postperfusion, and that there was no evidence for nerve/muscle impairment by history or clinical evaluation, we concluded that these electrophysiologic abnormalities were not of clinical significance.
This study used lower cuff pressures than in nonhuman primate studies (310–325 mm Hg vs. 450–700 mm Hg in nonhuman primates).5,27 The maximum perfusion volumes of 30–40% in this upper extremity study were higher than the maximum of 20% in our lower extremity study.9 This 30–40% volume was similar to that used for whole pelvic limb perfusion in dogs with the AAV-mini-dystrophin construct that has produced robust mini-dystrophin expression.28 Peak tissue/compartment pressures of 142 mm Hg during the perfusion were comparable to the peak pressures of 155 mm Hg in our lower extremity study9 and 175 mm Hg in studies of nonhuman primates.5 The largest volume infused (∼1 liter) was delivered in less than 18 min, a time frame that is well within the established safety time limit of 1.5–2 hr of tourniquet time for regional intravenous limb anesthesia.29
The rate of infusion used in this study was up to 80 ml/min, which is comparable to that used in some large animal studies of 60–120 ml/min.28,30,31 Based on experience from preclinical canine therapeutic trials, we adopted a dual-pump system.18 With this system, the syringe pump can be used to deliver the therapeutic agent (such as a viral vector) bypassing the Belmont infuser, while the needed volume and pressure are delivered by the Belmont infuser (Fig. 1). The patients in this study consisted of LGMD and BMD. The only patient in this study who had been on long-term corticosteroid use (∼17 years) was subject 10 with LGMD 2E. She was Cushingoid with a BMI of 27. She also had the lowest average perfusion rate of 42 ml/min. Long-term use of steroids may cause smaller and more fragile vasculatures, including superficial venous structures, and we did not observe any adverse effects with the high pressures that we employed. The vasculature of DMD patients with long-term use of corticosteroid may be different and perfusion rates lower than in this study may be effective. Perfusion rates of 10 or 35 ml/min have been used in dogs to successfully deliver gene therapy.7 However, lower perfusion rates mean longer perfusion and anesthesia time. Additional studies are needed to address these questions.
A recent safety, dose escalation, and efficacy study in golden retriever muscular dystrophy used high-pressure transvenous perfusion of the forelimb to deliver recombinant AAV8 carrying a modified U7snRNA sequence promoting exon skipping to restore a functional in-frame dystrophin transcript. Improvement in muscle strength was most consistently achieved with a dose of 5×1013 vg/kg infused at 7–20 mL/min with peak infusion pressures of 150 and 380 mm Hg at 20% and 40% of the forelimb volume, respectively.7 Our study has demonstrated that these same infusion parameters can be easily implemented and safely employed in patients with muscular dystrophy. These two studies provide the basis for a phase 1/2 clinical trial using high-pressure transvenous delivery into upper limbs of patients with Duchenne muscular dystrophy. Furthermore, our results are applicable to conditions other than muscular dystrophy as a method for delivering regional macromolecular therapeutics in high dose to skeletal muscles of the upper extremity.
Supplementary Material
Acknowledgments
This study was supported by USPHS Grant 1U54AR056953-01DE and the University of North Carolina at Chapel Hill.
We are indebted to our study subjects and their families for their dedication to making a difference in the battle against muscular dystrophy. We thank the following people for their assistance in carrying out the trial: Joe Kornegay, DVM, PhD; Xiao Xiao, PhD; Mitchel Cockman, CNCT; Doreen Marlowe, RN; Sabrina Thompson, RN; Spencer Weig, MD; Jesse Thomas, RVT; Niki Baker, RVT; Diane Meyer, RPT; Thomas Delviscio, RPT; Susan Gisler, RPT; Tom Beckman, RT(R); Lewis Daughtry, RT(R); Kathy Wilber; Jon Wolff, MD, PhD; Julia Hegge, PhD; Richard Jude Samulski, PhD; and Robert Leshner, MD.
Author Disclosure
No authors have any financial conflict of interest related to the submitted article.
References
- 1.Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010;363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. Limb-girdle muscular dystrophy type 2d gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009;66:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendell JR, Sahenk Z, Malik V, et al. A phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol Ther 2015;23:192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stedman H, Wilson JM, Finke R, et al. Phase I clinical trial utilizing gene therapy for limb girdle muscular dystrophy: Alpha-, beta-, gamma-, or delta-sarcoglycan gene delivered with intramuscular instillations of adeno-associated vectors. Hum Gene Ther 2000;11:777–790 [DOI] [PubMed] [Google Scholar]
- 5.Hagstrom JE, Hegge J, Zhang G, et al. A facile nonviral method for delivering genes and sirnas to skeletal muscle of mammalian limbs. Mol Ther 2004;10:386–398 [DOI] [PubMed] [Google Scholar]
- 6.Kamimura K, Zhang G, Liu D. Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther 2010;18:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Guiner C, Montus M, Servais L, et al. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther 2014;22:1923–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toromanoff A, Cherel Y, Guilbaud M, et al. Safety and efficacy of regional intravenous (R.I.) versus intramuscular (I.M.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther 2008;16:1291–1299 [DOI] [PubMed] [Google Scholar]
- 9.Fan Z, Kocis K, Valley R, et al. Safety and feasibility of high-pressure transvenous limb perfusion with 0.9% saline in human muscular dystrophy. Mol Ther 2012;20:456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browse N, Burnand K, Irvine A, Wilson N. Disease of the Veins. (Arnold, London: ), 1999 [Google Scholar]
- 11.Glovicki P. Venous embryology and anatomy. In: The Vein. Bergan J, Bunke-Paquette N, eds. (Oxford University Press, New York, NY: ). 2014; pp. 17–26 [Google Scholar]
- 12.Konstantakos EK, Dalstrom DJ, Nelles ME, et al. Diagnosis and management of extremity compartment syndromes: An orthopaedic perspective. Am Surg 2007;73:1199–1209 [PubMed] [Google Scholar]
- 13.Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg 2003;38:793–798 [DOI] [PubMed] [Google Scholar]
- 14.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair 2008;22:78–90 [DOI] [PubMed] [Google Scholar]
- 15.Van Den Brand JG, Nelson T, Verleisdonk EJ, Van Der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: A prospective study in 50 patients. Am J Sports Med 2005;33:699–704 [DOI] [PubMed] [Google Scholar]
- 16.Marsch SC, Sluga M, Studer W, et al. 0.5% versus 1.0% 2-chloroprocaine for intravenous regional anesthesia: A prospective, randomized, double-blind trial. Anesth Analg 2004;98:1789–1793, table of contents [DOI] [PubMed] [Google Scholar]
- 17.Comunale ME. A laboratory evaluation of the level 1 rapid infuser (h1025) and the Belmont Instrument Fluid Management System (FMS 2000) for rapid transfusion. Anesth Analg 2003;97:1064–1069, table of contents [DOI] [PubMed] [Google Scholar]
- 18.Fan Z, Powers W, Kornegay J, Xiao X. Pressurized transvenous-retrograde extremity perfusion. In: Gene and Cell Therapy: Therapeutic Mechanisms and Strategies. Templeton N, ed. (CRC Press, New York, NY: ). 2015; pp. 439–449 [Google Scholar]
- 19.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006;31:1116–1128 [DOI] [PubMed] [Google Scholar]
- 20.Yushkevich PA, Gerig G, Soldea O, Gao Y. (2014). www.itksnap.org (last accessed June22, 2015)
- 21.Florence JM, Fox PT, Planer GJ, Brooke MH. Activity, creatine kinase, and myoglobin in Duchenne muscular dystrophy: A clue to etiology? Neurology 1985;35:758–761 [DOI] [PubMed] [Google Scholar]
- 22.Jackson MJ, Round JM, Newham DJ, Edwards RH. An examination of some factors influencing creatine kinase in the blood of patients with muscular dystrophy. Muscle Nerve 1987;10:15–21 [DOI] [PubMed] [Google Scholar]
- 23.Escolar DM, Henricson EK, Mayhew J, et al. Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. Muscle Nerve 2001;24:787–793 [DOI] [PubMed] [Google Scholar]
- 24.Rodino-Klapac LR, Montgomery CL, Mendell JR, Chicoine LG. AAV-mediated gene therapy to the isolated limb in rhesus macaques. Methods Mol Biol 2011;709:287–298 [DOI] [PubMed] [Google Scholar]
- 25.Collins JM, Despa F, Lee RC. Structural and functional recovery of electropermeabilized skeletal muscle in-vivo after treatment with surfactant poloxamer 188. Biochim Biophys Acta 2007;1768:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelzer JD, Zochodne DW, Low PA. Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci USA 1989;86:1639–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegge JO, Wooddell CI, Zhang G, et al. Evaluation of hydrodynamic limb vein injections in nonhuman primates. Hum Gene Ther 2010;21:829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao C, Li J, Zheng H, et al. Hydrodynamic limb vein injection of adeno-associated virus serotype 8 vector carrying canine myostatin propeptide gene into normal dogs enhances muscle growth. Hum Gene Ther 2009;20:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barry L, Balliana S, Galeppi A. Intravenous regional anesthesia (Bier block). Techn Reg Anesth Pain Manag 2006;10:123–131 [Google Scholar]
- 30.Vigen KK, Hegge JO, Zhang G, et al. Magnetic resonance imaging-monitored plasmid DNA delivery in primate limb muscle. Hum Gene Ther 2007;18:257–268 [DOI] [PubMed] [Google Scholar]
- 31.Kornegay JN, Li J, Bogan JR, et al. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther 2010;18:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.