Abstract
Background
Pancreatic cancer is often accompanied by cachexia, a syndrome of severe weight loss and muscle wasting. A suboptimal response to nutritional support may further aggravate cachexia, yet the influence of nutrition on protein kinetics in cachectic patients is poorly understood.
Methods
Eight cachectic pancreatic cancer patients and seven control patients received a primed continuous intravenous infusion of l-[ring-2H5]phenylalanine and l-[3,3-2H2]tyrosine for 8 h and ingested sips of water with l-[1-13C]phenylalanine every 30 min. After 4 h, oral feeding was started. Whole body protein breakdown, protein synthesis, and net protein balance were calculated. Results are given as median with interquartile range.
Results
Baseline protein breakdown and protein synthesis were higher in cachectic patients compared with the controls (breakdown: 67.1 (48.1–79.6) vs. 45.8 (42.6–46.3) µmol/kg lean body mass/h, P = 0.049; and synthesis: 63.0 (44.3–75.6) vs. 41.8 (37.6–42.5) µmol/kg lean body mass/h, P = 0.021). During feeding, protein breakdown decreased significantly to 45.5 (26.9–51.1) µmol/kg lean body mass/h (P = 0.012) in the cachexia group and to 33.7 (17.4–37.1) µmol/kg lean body mass/h (P = 0.018) in the control group. Protein synthesis was not affected by feeding in cachectic patients: 58.4 (46.5–76.1) µmol/kg lean body mass/h, but was stimulated in controls: 47.9 (41.8–56.7) µmol/kg lean body mass/h (P = 0.018). Both groups showed a comparable positive net protein balance during feeding: cachexia: 19.7 (13.1–23.7) and control: 16.3 (13.6–25.4) µmol/kg lean body mass/h (P = 0.908).
Conclusion
Cachectic pancreatic cancer patients have a higher basal protein turnover. Both cachectic patients and controls show a comparable protein anabolism during feeding, albeit through a different pattern of protein kinetics. In cachectic patients, this is primarily related to reduced protein breakdown, whereas in controls, both protein breakdown and protein synthesis alterations are involved.
Keywords: Cancer cachexia, Pancreatic cancer, Nutrition, Protein synthesis, Protein breakdown, Anabolic resistance
Background
Pancreatic cancer is the eighth leading cause of cancer deaths in men and the ninth in women with 138 100 and 127 900 annual deaths worldwide, respectively.1 Cancer cachexia, a complex syndrome characterized by weight loss and muscle wasting, is a major problem of pancreatic cancer and greatly decreases survival and quality of life.2,3 It is responsible for more than 80% of pancreatic cancer related deaths.4 The exact pathogenesis of cancer cachexia is not fully understood, but systemic inflammation, a negative energy balance, malabsorption, and anorexia play an important role.5 Metabolic changes are most profound in protein metabolism, peripheral tissues furnishing nitrogenous and carbon substrate to sustain the acute phase response, and tumour growth. This leads to net negative peripheral protein metabolism, presenting as muscle wasting.
A key feature of cancer cachexia is that it cannot be fully reversed by regularly used nutritional support.3,5 This lack of response indicates that nutrient handling and especially protein metabolism are altered in cachectic patients. This might be due to a failing anabolic response (anabolic resistance), increased breakdown, or a reprioritisation of nitrogen economy away from peripheral tissues (muscle) towards increased hepatic production of acute phase proteins.6–9 The latter theory was studied previously by our group in cachectic pancreatic cancer patients with an acute phase response.2,10 We found normal albumin11 but increased fibrinogen synthesis rates12 in fasted cachectic pancreatic cancer patients. Moreover, feeding in such patients was associated with a marked increase in fibrinogen synthesis,13 supporting the theory of reprioritisation. However, the mechanisms involved remain unclear, and studies providing information on whole body protein synthesis and breakdown and their response on feeding in cachectic cancer patients are lacking.
This study aimed to investigate the effect of protein meal feeding on whole body protein turnover, protein synthesis, and protein breakdown in cachectic pancreatic cancer patients compared with non-oncologic surgical control patients by using established methods of primed continuous infusions of stable isotope-labelled amino acids.
Materials and methods
Subjects
To investigate the effect of enteral feeding on protein metabolism in cancer cachexia, cachectic patients with pancreatic head cancer were studied. Inclusion criteria were unresectable biopsy-proven, primary or recurrent pancreatic cancer, and cachexia. Cancer cachexia was defined as weight loss of >5% in 6 months, according to international consensus.5 Age-matched and sex-matched weight-stable patients admitted for surgery for benign disease (cholecystectomy or inguinal hernia repair) were included as a control group. Exclusion criteria for both groups were radiotherapy or chemotherapy, surgery in the month preceding the study, endocrine or metabolic disorders, fever, anaemia, and steroid use. Height, bodyweight, bodyweight loss, triceps skinfold thickness, mid-arm muscle circumference, and handgrip strength were measured on admission. Body weight loss was estimated by subtracting current measured body weight from reported normal body weight 6 months ago. For handgrip strength, the highest grip strength was recorded after measuring three times for both hands. Laboratory measurements that were taken at admission as part of standard care were recorded. Body composition was assessed by multiple frequency bioelectrical impedance measurements at 5 and 200 kHz with either a four terminal RJL BIA 101 (RJL Systems, Detroit, USA) or the Bodystat Dualscan 2005 (Bodystat Ltd., UK).14 Since the study protocol is demanding, especially for severely ill patients, a sample size of eight cachectic patients and seven control patients was chosen. This sample size was proven by our research group to provide adequate (patho)physiological data in tracer studies in the past.13,15
Study protocol
Patients and healthy subjects were studied after an overnight fast. Starting at 8 a.m., two cannulas were inserted: one into the antecubital vein for stable isotope infusion and one into the dorsal hand vein in the contralateral arm for arterialized blood sampling using a custom made heated box (50°C, J. Cambell and Dr. H. Brash, Department of Medical Physics, Royal Infirmary of Edinburgh). After a baseline blood sample had been taken for measurement of background isotope enrichment and C-Reactive-Protein (CRP), patients received a priming dose of l-[1-13C]phenylalanine (2.3 µmol/kg), l-[ring-2H5]phenylalanine (2.2 µmol/kg), l-[3,3-2H2]tyrosine (0.9 µmol/kg), and l-[ring-2H4]tyrosine (0.3 µmol/kg) followed by a continuous intravenous infusion of l-[ring-2H5]phenylalanine (3.5 µmol/kg/h) and l-[3,3-2H2]tyrosine (1.3 µmol/kg/h). Patients also received a primed continuous infusion of l-[2H8]phenylalanine for a separate research question not discussed in this paper. Subsequent arterialized blood samples were drawn hourly. For the first 4 h of the study, subjects drank 60 ml of water mixed with l-[1-13C]phenylalanine every 30 min to obtain a steady ‘infusion rate’ of 3.5 µmol/kg/h. Thereafter, subjects started drinking a commercially available sip feeding (Fortisip, Nutricia Ltd, Wiltshire, UK) mixed with l-[1-13C]phenylalanine at a rate of 60 ml per 30 min for another 4 h. Macronutrient composition of the sip feed was protein 50 g/L (casein), carbohydrate 180g/L, and fat 65g/L. The amino acid composition was alanine 1.7 g/L, arginine 2.0 g/L, aspartic acid 3.9 g/L, cysteine 0.15 g/L, glutamine 12.5 g/L, glycine 1.0 g/L, histidine 1.6 g/L, isoleucine 2.85 g/L, leucine 5.25 g/L, lysine 5.1 g/L, methionine 1.65 g/L, phenylalanine 2.8 g/L, proline 5.0 g/L, serine 3.3 g/L, threonine 2.45 g/L, tryptophan 0.7 g/L, tyrosine 3.05 g/L, and valine 3.6 g/L. The protocol was approved by the Lothian Research Ethical Committee of the Local Health Board in Edinburgh. Written informed consent was obtained from all patients.
Plasma analyses
For serum CRP, blood was collected in serum-gel clotting tubes and centrifuged for 5 min at 2500 rpm at 5°C. Aliquots of supernatant were frozen in liquid nitrogen and stored at −70°C until analysis. CRP was measured by fluorescent polarization immunoassay using an Abbott TDX analyzer and Abbott reagents (Abbott Laboratories, Maidenhead, UK) with a limit of detection of 1 mg/L. Samples for plasma amino acid measurements were collected in pre-chilled lithium-heparin tubes, transported to the laboratory on ice, and centrifuged for 5 min at 2500 rpm at 5°C. Plasma proteins were precipitated using 5% sulfosalicylic acid. The samples were then frozen in liquid nitrogen and stored at −70°C until analysis. Plasma amino acids were measured by routine high-performance liquid chromatography techniques as described previously.16 Plasma enrichments of l-[1-13C]phenylalanine, l-[ring-2H5]phenylalanine, l-[3,3-2H2]tyrosine, and l-[ring-2H4]-tyrosine were determined by liquid chromatography–mass spectrometry (LC–MS).17 During transamination in vivo, l-[2H8]phenylalanine exchanges the hydrogen atom at C2 position with body water, forming l-[2H7]phenylalanine.18 During ionization in the LC–MS, l-[2H8]phenylalanine exchanges with unlabelled phenylalanine, giving rise to some L-[2H1]phenylalanine. This interfered with the analysis of l-[1-13C]phenylalanine, causing overestimation of the M + 1/M ratio, and the used LC–MS method was not able to distinguish the contribution of the 1-13C and the 2H1. However, because of the isotopic distribution of both tracers, l-[1-13C]phenylalanine will also generate a sufficient increase of the M + 2 mass, while the 2H1 will only generate a very small amount. We therefore used the M + 2/M ratio to back calculate the M + 1/M ratio, caused by l-[1-13C]phenylalanine. Alanine transaminase, gamma-glutamyl transpeptidase, alkaline phosphatase, and bilirubin levels were analysed as part of standard hospital care in the Department of Clinical Chemistry of the Royal Infirmary of Edinburgh, Scotland, according to hospital protocols.
Calculations
All data concerning amino acid metabolism were corrected for lean body mass. In the post-absorptive state, protein breakdown is the only source of essential amino acids. The plasma rate of appearance of essential amino acids, such as phenylalanine, can therefore be used as an index of protein breakdown. Phenylalanine and tyrosine kinetics were calculated according to the approach of Tessari et al.19 The rate of appearance of phenylalanine (RaPHE) was calculated from the infusion rate of l-[ring-2H5]phenylalanine (IR2H5-PHE) and l-[ring-2H5]phenylalanine tracer–tracee ratio (TTR) in plasma (TTR2H5-PHE) according to the following formula:
| (1) |
Phenylalanine hydroxylation (PH) was calculated from the tyrosine plasma rate of appearance calculated from l-[3,3-2H2]-tyrosine TTR (TTR2H2-TYR) and l-[3,3-2H2]-tyrosine infusion rate (IR2H2-TYR) according to Equation 1 and from the ratio between TTR2H5-PHE and the plasma TTR of its derivative l-[ring-2H4]tyrosine (TTR2H4-TYR):
| (2) |
The rate of non-hydroxylative phenylalanine disposal (NHPD) is an index of incorporation of phenylalanine in protein and, therefore, of protein synthesis. In steady state conditions, the sum of NHPD and PH equals RaPHE. NHPD could thus be calculated from the results of Equations 1 and 2:
| (3) |
Splanchnic extraction (SPEPHE), the fraction of phenylalanine that is taken up by the gut and/or liver during its first-pass, was calculated as the ratio of RaPHE calculated with the intravenous (RaPHE-IV) and enteral phenylalanine tracer (RaPHE-ENT):
| (4) |
Endogenous RaPHE was used as a measure of endogenous protein breakdown (endoPB). This was calculated as the difference between the total rate of appearance of phenylalanine and the exogenous, enteral phenylalanine ingestion rate (EIRPHE), corrected for SPEPHE:
| (5) |
The phenylalanine net balance, an index of protein balance, could be calculated from protein synthesis (NHPD, Equation 3) and protein breakdown (endogenous RaPHE, Equation 5):
| (6) |
Statistics
All data are presented as median and interquartile range (IQR). Tracer fluxes are expressed in µmol/kg lean body mass/h. Data were analysed using IBM SPSS 20 for Microsoft Windows®. Due to the sample size and high likelihood of a non-normal distribution, non-parametric tests were used. Differences between groups were analysed using the Mann–Whitney U test or Fisher's exact test, where appropriate. To analyse differences within groups at different time points, the Wilcoxon signed-rank test and Friedman test were used. For correlations, Spearman's ranked correlation coefficient (rs) was used. A P-value of <0.05 was considered significant.
Results
Patient characteristics
Eight cachectic pancreatic cancer patients and seven control patients (six with gallstones and one with bilateral inguinal hernia) were included in this study. Patient characteristics are displayed in Table 1. Two pancreatic cancer patients had proven liver metastasis. Groups were comparable with respect to age, sex, height, lean body mass, and male/female ratio. Weight, BMI, triceps skinfold thickness, and mid-arm muscle circumference were significantly lower in the cachexia group. Patients did not show signs of ascites or oedema. The percentage weight loss in the cachexia group was very high with almost all (n = 7) cachectic patients having more than 10% weight loss. All pancreatic cancer patients had some degree of functional status loss but were able to come to the hospital themselves using public transportation or a taxi. Patients were mildly to moderately anorexic but tolerated the protocol well. During the last 2 h of the protocol, some cancer patients consumed less liquid feed due to anorexia, resulting in a small non-significant difference in sip feed intake between the cachexia group and control group: 8.1 (IQR: 5.6–10.0) vs. 10.3 (IQR: 8.5–11.6) ml/kg lean body mass protein drink corresponding with 34.2 (IQR: 23.7–42.6) vs. 43.7 (IQR: 36.1–49.3) µmol/kg lean body mass/h phenylalanine (P = 0.105).
Table 1.
Patient characteristics
| Cachectic patients (n = 8) |
Control patients (n = 7) |
||||
|---|---|---|---|---|---|
| Sex(n) | Female(5) | Male(3) | Female(4) | Male(3) | P-valueb 1.000 |
| Age (yrs) | 71 (20) | 67 (16) | 66 (12) | 77 (17) | 0.451 |
| Body weight (kg) | 47.6 (25.3) | 53.7 (16.6) | 72.3 (22.4) | 77.6 (28.0) | 0.003 |
| Height (cm) | 153 (13) | 175 (16) | 158 (5) | 170 (7) | 0.684 |
| Body mass index (kg/m2) | 20.3 (7.7) | 19.4 (3.75) | 29.0 (7.2) | 26.9 (7.6) | 0.005 |
| Weight lossa (kg) | 7.7 (13.4) | 19.2 (19.8) | — | — | 0.001 |
| Weight lossa (%) | 16.6 (28.3) | 37.2 (40.9) | — | — | 0.001 |
| Lean body mass (kg) | 33.7 (23.9) | 46.6 (9.6) | 42.8 (8.8) | 56.3 (12.2) | 0.132 |
| Triceps skin fold (mm) | 14.0 (3.0) | 11.0 (7.0) | 27.5 (11.0) | 22.0 (21.0) | 0.018 |
| Mid-arm circumference (cm) | 23.3 (7.0) | 22.5 (3.0) | 31.8 (4.0) | 31.0 (7.0) | 0.003 |
| Handgrip strength (kg) | 6.0 (13.0) | 24.0 (1.0) | 17.0 (6.0) | 32.0 (13.0) | 0.566 |
Data represent median and range
In 6 months
Cachexia group compared with control group
Inflammation and clinical chemistry
Serum CRP levels were significantly higher in the cachectic group compared with the controls (Table 2). Liver enzymes (alanine transaminase, gamma-glutamyl transferase and alkaline phosphatase) were significantly elevated in cachectic patients compared with controls, whereas bilirubin levels were similarly low in both groups.
Table 2.
Laboratory results and amino acid concentrations
| Cachectic patients (n = 8) | Control Patients (n = 7) | P-value | |
|---|---|---|---|
| CRP (mg/L) | 8.3 (4.2–31.3) | 0 (0–1.8) | 0.002 |
| WBC (*109/L) | 8.4 (4.6–10.5) | 7.1 (4.9–8.3) | 0.431 |
| Bilirubin (µmol/L) | 10.0 (8.0–18.0) | 10.5 (7.8–18.5) | 0.943 |
| ALT (IU/L) | 36.0 (34.0–67.0) | 20.5 (14.5–30.8) | 0.015 |
| GGT (IU/L) | 93.0 (47.0–168.0) | 21.0 (15.8–30.5) | 0.010 |
| ALP (IU/L) | 214.0 (149.0–231.0) | 67.5 (60.3–101.5) | 0.007 |
| Urea (mmol/L) | 4.9 (3.5–6.3) | 5.2 (5.0–7.7) | 0.391 |
| Creatinine (µmol/L) | 81.0 (74.0–101.0) | 92.0 (82.5–106.3) | 0.199 |
| Phenylalanine (fasted, µmol/L) | 44.5 (41.3–60.5) | 67.0 (59.0–71.0) | 0.024 |
| Phenylalanine (fed, µmol/L) | 62.5 (56.3–69.5) | 84.0 (70.0–96.0) | 0.009 |
| Tyrosine (fasted, µmol/L) | 50.0 (41.8–65.0) | 53.0 (45.0–59.0) | 0.817 |
| Tyrosine (fed, µmol/L) | 64.0 (45.0–75.0) | 74.0 (55.0–90.0) | 0.246 |
| Valine (fasted, µmol/L) | 124.5 (109.3–154.5) | 204.0 (179.0–220.0) | 0.005 |
| Valine (fed, µmol/L) | 162.0 (143.3–176.0) | 252.0 (223.0–281.0) | 0.001 |
Laboratory results and amino acid concentrations are presented as median and interquartile range. Amino acid concentrations are given as average concentrations in the fasted (1–4 h) and fed state (5–8 h). All amino acid concentrations increased significantly (P < 0.05) during feeding except for Tyrosine in the cachexia group (P = 0.062).
ALP, alkaline phosphatase; ALT, alanine transaminase; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; WBC, white blood cells
Isotopic steady state
Plasma amino acid concentrations are given in Table 2. Isotopic steady state was achieved for all administered tracers within 1 h. A new steady state equilibrium was formed again within 1 h after the start of the oral ingestion of the sip feed (Figure 1).
Figure 1.
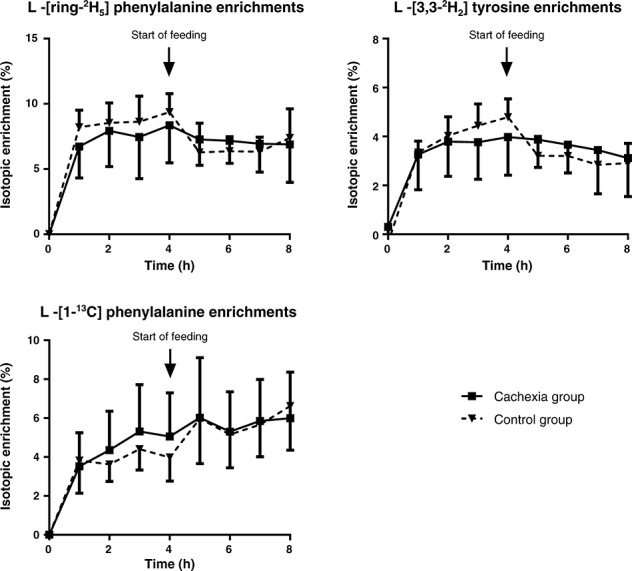
Mean isotope enrichments and standard deviations of phenylalanine and tyrosine over time. Feeding was started after 4 h (arrow). Steady states of all tracers were significantly different during the fasted state compared with the fed state (cachexia: P = 0.019; control: P < 0.001; Friedman test). Steady states of all tracers did not differ significantly between groups in both fasted (1–4 h) and fed (5–8 h) states (Mann–Whitney U test).
Protein breakdown (phenylalanine flux) and splanchnic extraction
At baseline, protein breakdown (RaPHE) was significantly higher in cachectic cancer patients compared with control patients (67.1 IQR: 48.1–79.6 vs. 45.8 IQR: 42.6–46.3 µmol/kg lean body mass/h; P = 0.049, Figure 2A). This was positively correlated with serum CRP levels (rs = 0.658, P = 0.008). During ingestion of the study feed, endo PB decreased significantly to 45.5 (IQR: 26.9–51.1) µmol/kg lean body mass/h (P = 0.012) in cancer patients and 33.7 (IQR: 17.4–37.1) µmol/kg lean body mass/h (P = 0.018) in control subjects (Figure 2B). The magnitude of this decrease was not significantly different between both groups (P = 0.132). Splanchnic extraction was similar between both groups during feeding (P = 0.418, Figure 2C).
Figure 2.
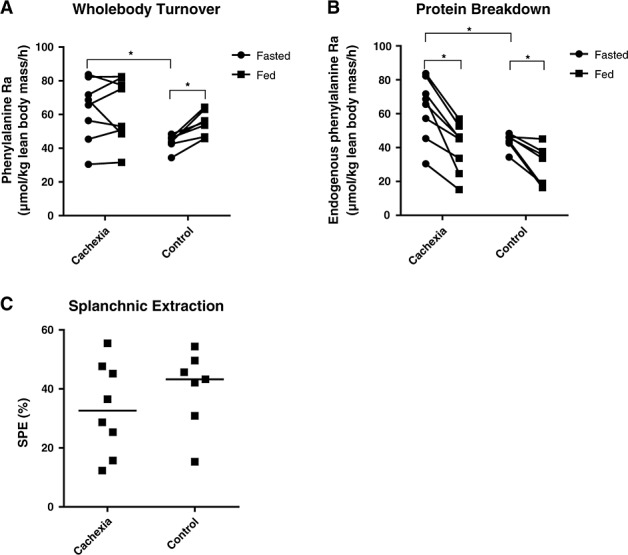
Whole body protein turnover, protein breakdown, and splanchnic extraction. Dots represent individual patients. (A) Whole body protein turnover expressed as total phenylalanine rate of appearance. (B) Protein breakdown expressed as endogenous phenylalanine rate of appearance. (C) Splanchnic extraction during feeding. Bars represent the median. There was no significant difference in splanchnic extraction between groups. *P < 0.05, SPE, splanchnic extraction.
Protein synthesis (non-hydroxylative phenylalanine disposal)
Protein synthesis was higher in weight-losing cancer patients than in controls at baseline (63.0 IQR: 44.3–75.6 vs. 41.8 IQR: 37.6–42.5 µmol/kg lean body mass/h (P = 0.021, Figure 3A). In cachectic patients, protein synthesis did not respond to sip feeding (58.4 IQR: 46.5–76.1 µmol/kg lean body mass/h; P = 1.000), whereas protein synthesis in control patients increased significantly (47.9 IQR: 41.8–56.7 µmol/kg lean body mass/h; P = 0.018). Accordingly, the response of protein synthesis on feeding (ΔNHPD) was significantly lower in cachectic patients (−0.6 IQR: −3.8 to 6.5 µmol/kg lean body mass/h) compared with control patients (9.9 IQR: 5.3–16.0 µmol/kg lean body mass/h; P = 0.021). Phenylalanine hydroxylation rates increased during feeding in the control group (4.5 IQR: 3.1–5.8 to 6.3 IQR: 4.2–7.4 µmol/kg lean body mass/h; P = 0.018) but not in the cachexia group (4.1 IQR: 2.6–5.0 to 3.6 IQR: 2.8–5.7 µmol/kg lean body mass/h; P = 0.779, Figure 3B).
Figure 3.
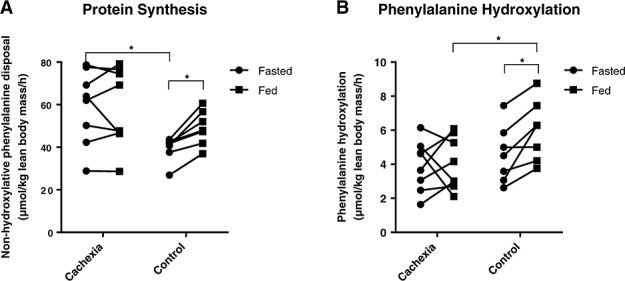
Protein synthesis and phenylalanine hydroxylation. Dots represent individual patients. (A) Protein synthesis expressed as non-hydroxylative phenylalanine disposal. (B) Phenylalanine hydroxylation rates. *P < 0.05.
Protein balance
Both cachectic patients and control patients were able to achieve a positive and comparable net protein balance during feeding as shown in Figure 4. Net protein balance in the cachexia group increased from −4.1 (IQR: −5.0 to −2.6) to 19.7 (IQR: 13.1–23.7) µmol/kg lean body mass/h (P = 0.012), while in the control group, it increased from −4.5 (IQR: −5.8 to −3.1) to 16.3 (IQR: 13.6–25.4) µmol/kg lean body mass/h (P = 0.018).
Figure 4.
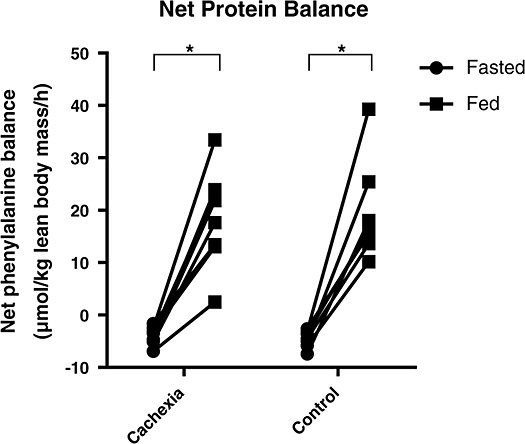
Net protein balance expressed as net phenylalanine balance. Dots represent individual patients *P < 0.05.
Discussion
This study has analysed the effect of sip feeding on whole body protein turnover in cachectic pancreatic cancer patients. It demonstrated that whole body protein turnover is higher in cancer patients compared with that in control patients, possibly due to inflammation. Although both patient groups were able to generate a similar anabolic response to feeding, cachectic patients were only able to achieve this by reducing protein breakdown, whereas controls were also able to increase protein synthesis.
Previous studies investigating protein metabolism in cancer cachexia (gastrointestinal20 and hepatocellular carcinoma21) found a higher whole body protein turnover compared with healthy controls, in line with our study. In cachexia associated with benign disease, the results are variable: whole body protein turnover was increased in patients with heart failure22 but not in tuberculosis (TBC).23 Cachectic patients with chronic obstructive pulmonary disease (COPD) may have an increased whole body protein turnover,24 but this is not always the case.25 These mixed results may indicate a different pathogenesis for non-cancer cachexia compared with cancer cachexia. Federica et al.26 did not find an increased whole body protein turnover in cachectic gastric cancer patients. This could be due to the fact that they included a small group (n = 4 cancer patients) and did not correct for lean body mass. This is important since body composition of cachectic patients differs greatly from that of healthy individuals.3 Enteral feeding is preferred to parenteral feeding, since this mimics the normal situation better and generates a higher anabolic response. Previous studies have mainly investigated the effects of total parenteral nutrition (TPN) or parenteral amino acids and found mixed results. Winter et al.27 found a normal anabolic response of whole body protein turnover following hyperaminoacidemia using intravenous amino acid infusions in moderately cachectic non-small cell lung carcinoma patients. Bozzetti et al.28 studied the effect of TPN on protein synthesis and breakdown in cachectic gastric cancer patients. Unlike our study, they found an increase in protein synthesis in response to TPN and no change in breakdown. However, the number of patients was very low (n = 3) and did not include a control group.
The study by Williams et al.,29 which analysed the fractional synthetic rate (FSR, protein synthesis) in the vastus lateralis muscle of colorectal cancer patients, found that FSR did not increase in cancer patients after the administration of a mix of parenteral amino acids, whereas FSR increased in matched healthy controls. Although FSR and whole body protein synthesis are difficult to compare, their general message was in line with our results. They also found a trend towards increased leg muscle protein breakdown in cachectic patients that did not change after feeding. Interestingly, after surgical removal of the tumour, the FSR increased as a result of feeding in these patients. Only two studies have assessed the effect of enteral feeding on protein kinetics in cachectic patients with benign disease, but apart from the present study, no data on the response of protein kinetics on enteral feeding in cancer-cachexia are at hand. First, Macallan et al. (1998)23 studied the effect of hourly feeding in cachectic TBC patients compared with that in non-cachectic malnourished and healthy controls. They found that whole body protein synthesis did not increase during feeding in cachectic TBC patients, whereas both malnourished and healthy controls showed a significant increase, which is in line with our results. Second, studies of Engelen et al.24 and Jonker et al.30 studied the effect of respectively sip feeding and bolus feeding on protein kinetics in cachectic COPD patients. In contrast with our results, both studies found a significant increase in protein synthesis in COPD patients, which was similar to the response in young healthy individuals in the study of Engelen et al. There are several reasons which may explain this difference in anabolic response. Firstly, our study population suffered from very severe weight loss (>10%), which could mean that their cachectic state was more advanced than in the COPD patients resulting in a worse anabolic response. Secondly, the COPD patients consumed a drink with higher whey protein content than our study drink. Whey protein is known to stimulate muscle protein synthesis more than other milk proteins (e.g. casein) in elderly patients.31,32 Finally, as mentioned earlier, the more chronic pathogenesis of COPD-related cachexia might be different from the relatively acute pathogenesis of pancreatic cancer cachexia, which could influence the way patients respond on feeding.6,33
Another reason for the different protein kinetics found in the studies referred to above is that of all solid cancers, pancreatic cancers are strongly related to a pro-inflammatory state.6,7 In recent years, it has become evident that any rapidly proliferating tumour is associated with severe inflammatory activity in the tumour itself, in surrounding tissues, and systemically, which stimulates cancer cell proliferation. This leads to acute phase protein synthesis, proliferation of leukocytes, cancer cells, and synthesis of extracellular matrix which all contribute to increasing protein and DNA synthesis.9,11–13 This may partly explain the increase in basal protein synthesis in the present study.
The fact that net protein balance during feeding was similar in both groups despite the lack of increase in protein synthesis in cachectic patients indicates that protein conservation is regulated differently in cachexia. While control patients respond with both a decrease in protein breakdown and an increase in protein synthesis, cachectic patients seem only to decrease protein breakdown to preserve a positive net balance. An explanation for the lack of protein synthesis is that cachectic patients might have a higher anabolic threshold. This is also seen in the healthy elderly. Ingestion of a large bolus (35 g) of whey protein generates a higher whole body anabolic response and muscle protein synthesis than a smaller bolus (10 or 20 g).34 Thus, a single protein bolus might be more effective than sip feeding (which is comparable to continuous feeding). Moreover, studies using TPN indicate that a high infusion rate or a high content of branched chain amino acids stimulates whole body protein synthesis in cachectic cancer patients while standard TPN infusion does not.35,36 Clinical application will be difficult, however. Satiety effects of protein might be different in cachectic cancer patients, most patients suffer from anorexia,5,7,37 and dietary patterns and food preference vary greatly among cachectic cancer patients.38 Therefore, individualized dietary care might be needed for adequate nutritional support in cancer cachexia.
The current study has some limitations. We used bioelectrical impedance measurements for assessment of body composition. This method can underestimate the fat free mass compared with computed tomography or dual-energy X-ray absorptiometry analyses in surgical and oncologic patients because of fluid shifts.39,40 However, since our patients did not show any signs of oedema, ascites, or dehydration (see urea levels in Table 2), underestimation is likely to be a minor issue. Another issue of the current study is that some patients in the cachexia groups consumed less sip feed than the control patients during the last 2 h of the study protocol. This might result in an underestimation of the phenylalanine rate of appearance and protein breakdown measurements. However, the difference was small (and non-significant) and did not influence the amino acid enrichments (see Figure 1). We are therefore confident that even though there may be some underestimation, the effect of the lower sip feed intake is too small to change the conclusions of this study.
In conclusion, this study shows that cachectic pancreatic cancer patients have a higher basal protein turnover, which is positively correlated with CRP levels. Both cachectic patients and controls are able to achieve a comparable positive net balance during feeding, suggesting that anabolic resistance may be less of an issue in cachectic patients than previously thought. However, in cachectic patients, this is only achieved by reduced protein breakdown, whereas in controls, both protein breakdown and protein synthesis are modulated. Impaired nutritional stimulation of protein synthesis in pancreatic cancer cachexia should be a topic in future studies to develop more effective nutritional interventions.
Acknowledgments
The help of Dr Nabil Dowidar in some of the studies is greatly appreciated. We are indebted to Dr A. Ajami from Mass Trace (USA) for providing us with some of the 2H3-Valine isotopolog. The technical assistance of Mrs G. A. M. ten Have, BSc, Mr H. M. H. van Eijk, PhD, and Mr J. L. J. M. Scheyen, BSc, is greatly appreciated. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S., Morley J. E., Coats A. J. S., Anker S. D. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.).
Funding
David van Dijk is supported as a PhD candidate by the Netherlands Organization for Scientific Research (NWO grant 022.003.011). Cornelis Dejong was a recipient of a grant from the Niels Stensen Stichting, Amsterdam, The Netherlands, and was supported as a clinical research fellow by the Netherlands Organization for Scientific Research (Zon-MW/NWO grant 907-00-033). This research was funded by a grant from the Sir Stanley and Lady Davidson Endowment Fund (E08473), University of Edinburgh, and grant CZG/4/1/5 from the Chief Scientist Office, Edinburgh, Scotland.
Conflict of Interest
None declared.
References
- 1.Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wigmore S, Plester C, Richardson R, Fearon K. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75:106–9. doi: 10.1038/bjc.1997.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 4.Dewys W, Begg C, Lavin P, Band P, Bennett J, Bertino J, Cohen M, Douglass H, Engstrom P, Ezdinli E, Horton J, Johnson G, Moertel C, Oken M, Perlia C, Rosenbaum C, Silverstein M, Skeel R, Sponzo R, Tormey D. Prognostic effect of weight loss prior to chemotherapy in cancer patients. ECOG Am. J. Med. 1980;69:491–7. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 5.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 6.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Tisdale M. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 8.Hasselgren P, Pedersen P, Sax H, Warner B, Fischer J. Current concepts of protein turnover and amino acid transport in liver and skeletal muscle during sepsis. Arch Surg. 1988;123:992–9. doi: 10.1001/archsurg.1988.01400320078016. (Chicago, Ill: 1960) [DOI] [PubMed] [Google Scholar]
- 9.Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. 1999;23:584–8. doi: 10.1007/pl00012351. [DOI] [PubMed] [Google Scholar]
- 10.Wigmore S, Plester C, Ross J, Fearon K. Contribution of anorexia and hypermetabolism to weight loss in anicteric patients with pancreatic cancer. Br J Surg. 1997;84:196–7. [PubMed] [Google Scholar]
- 11.Fearon K, Falconer J, Slater C, McMillan D, Ross J, Preston T. Albumin synthesis rates are not decreased in hypoalbuminemic cachectic cancer patients with an ongoing acute-phase protein response. Ann Surg. 1998;227:249–54. doi: 10.1097/00000658-199802000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston T, Slater C, McMillan D, Falconer J, Shenkin A, Fearon K. Fibrinogen synthesis is elevated in fasting cancer patients with an acute phase response. J Nutr. 1998;128:1355–60. doi: 10.1093/jn/128.8.1355. [DOI] [PubMed] [Google Scholar]
- 13.Barber M, Fearon K, McMillan D, Slater C, Ross J, Preston T. Liver export protein synthetic rates are increased by oral meal feeding in weight-losing cancer patients. Am J Physiol Endocrinol Metab. 2000;279:E707–14. doi: 10.1152/ajpendo.2000.279.3.E707. [DOI] [PubMed] [Google Scholar]
- 14.Hannan W, Cowen S, Plester C, Fearon K, deBeau A. Comparison of bio-impedance spectroscopy and multi-frequency bio-impedance analysis for the assessment of extracellular and total body water in surgical patients. Clin Sci. 1995;89:651–8. doi: 10.1042/cs0890651. (London, England: 1979) [DOI] [PubMed] [Google Scholar]
- 15.van de Poll MC, Wigmore SJ, Redhead DN, Beets-Tan RG, Garden OJ, Greve JW, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Effect of major liver resection on hepatic ureagenesis in humans. Am J Physiol Gastrointest Liver Physiol. 2007;293:62. doi: 10.1152/ajpgi.00366.2006. [DOI] [PubMed] [Google Scholar]
- 16.Vissers Y, Dejong C, Luiking Y, Fearon K, von Meyenfeldt M, Deutz N. Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am J Clin Nutr. 2005;81:1142–6. doi: 10.1093/ajcn/81.5.1142. [DOI] [PubMed] [Google Scholar]
- 17.van Eijk H, Rooyakkers D, Soeters P, Deutz N. Determination of amino acid isotope enrichment using liquid chromatography-mass spectrometry. Anal Biochem. 1999;271:8–17. doi: 10.1006/abio.1999.4112. [DOI] [PubMed] [Google Scholar]
- 18.Preston T, Small AC. Improved measurement of protein synthesis in human subjects using 2H-phenylalanine isotopomers and gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:549–453. doi: 10.1002/rcm.4402. [DOI] [PubMed] [Google Scholar]
- 19.Tessari P, Kiwanuka E, Vettore M, Barazzoni R, Zanetti M, Cecchet D, Orlando R. Phenylalanine and tyrosine kinetics in compensated liver cirrhosis: effects of meal ingestion. Am J Physiol Gastrointest Liver Physiol. 2008;295:604. doi: 10.1152/ajpgi.00355.2007. [DOI] [PubMed] [Google Scholar]
- 20.Borzotta A, Clague M, Johnston I. The effects of gastrointestinal malignancy on whole body protein metabolism. J Surg Res. 1987;43:505–12. doi: 10.1016/0022-4804(87)90123-5. [DOI] [PubMed] [Google Scholar]
- 21.O'Keefe S, Ogden J, Ramjee G, Rund J. Contribution of elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. Cancer Res. 1990;50:1226–30. [PubMed] [Google Scholar]
- 22.Nørrelund H, Wiggers H, Halbirk M, Frystyk J, Flyvbjerg A, Bøtker H, Schmitz O, Jørgensen JO, Christiansen JS, Møller N. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Intern Med. 2006;260:11–21. doi: 10.1111/j.1365-2796.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 23.Macallan D, McNurlan M, Kurpad A, de Souza G, Shetty P, Calder A, Griffin GE. Whole body protein metabolism in human pulmonary tuberculosis and undernutrition: evidence for anabolic block in tuberculosis. Clin Sci. 1998;94:321–31. doi: 10.1042/cs0940321. (London, England: 1979) [DOI] [PubMed] [Google Scholar]
- 24.Engelen M, De Castro C, Rutten E, Wouters E, Schols A, Deutz N. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clin Nutr. 2012;31:616–24. doi: 10.1016/j.clnu.2012.04.006. (Edinburgh, Scotland) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutten E, Franssen F, Engelen M, Wouters E, Deutz N, Schols A. Greater whole-body myofibrillar protein breakdown in cachectic patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2006;83:829–34. doi: 10.1093/ajcn/83.4.829. [DOI] [PubMed] [Google Scholar]
- 26.Federica D, Paola F, Cecilia G, Carmen M, Federico B. Effects of cachexia due to cancer on whole body and skeletal muscle protein turnover. Cancer. 1998;82:42–8. [PubMed] [Google Scholar]
- 27.Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012;31:765–73. doi: 10.1016/j.clnu.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Bozzetti F, Gavazzi C, Ferrari P, Federica D. Effect of total parenteral nutrition on the protein kinetics of patients with cancer cachexia. Tumori. 2000;86:408–11. doi: 10.1177/030089160008600508. [DOI] [PubMed] [Google Scholar]
- 29.Williams J, Phillips B, Smith K, Atherton P, Rankin D, Selby A, Liptrot S, Lund J, Larvin M, Rennie M. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012;96:1064–70. doi: 10.3945/ajcn.112.045708. [DOI] [PubMed] [Google Scholar]
- 30.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr. 2014;33:211–20. doi: 10.1016/j.clnu.2013.06.014. (Edinburgh, Scotland) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennings B, Boirie Y, Senden J, Gijsen A, Kuipers H, van Loon L. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 32.Luiking YC, Deutz NE, Memelink RG, Verlaan S, Wolfe RR. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: a randomized controlled trial. Nutr J. 2014;13:9. doi: 10.1186/1475-2891-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schols A, Gosker H. The pathophysiology of cachexia in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3:282–7. doi: 10.1097/SPC.0b013e328331e91c. [DOI] [PubMed] [Google Scholar]
- 34.Pennings B, Groen B, de Lange A, Gijsen A, Zorenc A, Senden J, van Loon L. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:9. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 35.Hyltander A, Warnold I, Edén E, Lundholm K. Effect on whole-body protein synthesis after institution of intravenous nutrition in cancer and non-cancer patients who lose weight. Eur J Cancer. 1991;27:16–21. doi: 10.1016/0277-5379(91)90051-e. [DOI] [PubMed] [Google Scholar]
- 36.John AT, Bruce RB, Dermot JH, Ramon M, Lyle LM, George LB. Improved protein kinetics and albumin synthesis by branched chain amino acid-enriched total parenteral nutrition in cancer cachexia: a prospective randomized crossover trial. Cancer. 1986;58:147–57. doi: 10.1002/1097-0142(19860701)58:1<147::aid-cncr2820580126>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Op den Kamp C, Langen R, Haegens A, Schols A. Muscle atrophy in cachexia: can dietary protein tip the balance? Curr Opin Clin Nutr Metab Care. 2009;12:611–6. doi: 10.1097/MCO.0b013e3283319399. [DOI] [PubMed] [Google Scholar]
- 38.Hutton JL, Martin L, Field CJ, Wismer WV, Bruera ED, Watanabe SM, Baracos VE. Dietary patterns in patients with advanced cancer: implications for anorexia-cachexia therapy. Am J Clin Nutr. 2006;84:1163–70. doi: 10.1093/ajcn/84.5.1163. [DOI] [PubMed] [Google Scholar]
- 39.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied physiology, nutrition, and metabolism = Physiologie appliquée, nutrition et métabolisme. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 40.Haverkort EB, Reijven PL, Binnekade JM, de van der Schueren MA, Earthman CP, Gouma DJ, de Haan RJ. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: a systematic review. Eur J Clin Nutr. 2015;69:3–13. doi: 10.1038/ejcn.2014.203. [DOI] [PubMed] [Google Scholar]


