Abstract
Background
Although commonly observed, malnutrition is poorly characterized and frequently underdiagnosed in patients with metastatic renal cell carcinoma (RCC). The ability of nutritional screening tools to predict overall survival (OS) in patients with RCC has not been adequately validated. The objective of this study was to investigate the performance of nutritional screening tools and their additional prognostic value in patients with metastatic RCC treated with targeted therapies.
Methods
Patients were prospectively recruited from three tertiary hospitals between 2009 and 2013. Nutritional status was evaluated using the Geriatric Nutritional Risk Index (GNRI) and the Mini Nutritional Assessment–Short Form (MNA–SF). Their OS and early grade 3/4 adverse events were recorded as outcomes of interest, and their associations with nutritional status were assessed using Cox regression and logistic regression, respectively. The incremental value in prognostication was evaluated using concordance index and decision curve analyses.
Results
Of the 300 enrolled patients, 95 (31.7%) and 64 (21.3%) were classified as being at risk of malnutrition according to the GNRI and MNA–SF, respectively. Both GNRI and MNA–SF were independent predictors of OS in multivariate analyses and provided significant added benefit to Heng risk classification. Compared with the MNA–SF, the GNRI contributed a higher increment to the concordance index (0.041 vs. 0.016). Nutritional screening, however, was not associated with early grade 3/4 adverse events in multivariate analyses. Further investigations are needed using more comprehensive and accurate assessment tools.
Conclusions
This prospective study confirmed the importance of nutritional screening tools in survival prognostication in patients with metastatic RCC. The standardized and objective measurements would allow clinicians to identify metastatic RCC patients at risk of poor survival outcomes. Individualized nutritional assessment and intervention strategies may be included in the multidisciplinary treatment.
Keywords: Geriatric Nutritional Risk Index (GNRI), Mini Nutritional Assessment–Short Form (MNA–SF), Nutritional screen, Overall survival, Renal cell carcinoma, Targeted therapy
Introduction
The management of metastatic renal cell carcinoma (RCC) has changed dramatically over the last decade. The introduction of novel targeted therapies has significantly improved median overall survival (OS) to almost double that of immunotherapy.1,2 Nevertheless, treatment outcomes vary widely, emphasizing the need for prompt and accurate prognostic stratification. An integrated prognostic model based on 645 patients with metastatic RCC treated with VEGF-targeted therapy from North America,3 known as the Heng risk model, has been externally validated in an international setting and was shown to significantly stratify risk.4 This model uses six independent predictors of poor survival, including Karnofsky performance status <80%, <1 year from diagnosis to treatment, anemia (hemoglobin concentration < normal range [NR]), hypercalcemia (corrected calcium concentration > NR), neutrophilia (neutrophil count > NR), and thrombocytosis (platelet count > NR); with patients stratified into favorable (no factors), intermediate (1–2 factors), and poor (>2 factors) risk groups.3 Based on this benchmark model, many studies have sought to identify additional clinically applicable prognostic markers.
These factors and models, however, are limited in predicting outcomes in patients with metastatic RCC. Few have been prospectively validated in a multicenter setting. Some involve only patients in clinical trials, limiting their applicability to patients outside clinical trials. Furthermore, prognostic studies of RCC have mainly focused on tumor-related factors, such as pathological features, disease burden, and serum markers secreted by the tumor. Few attempts have been made to evaluate patient characteristics other than performance status and symptoms. Because the validated Heng risk model showed only moderate discriminatory performance (Harrell's concordance index: 0.66), new factors should be evaluated to determine whether they show better prognosis.
The process of nutritional decline is commonly seen in patients with metastatic RCC. Cachexia, a manifestation of malnutrition, is found in up to 50% of these patients.5 As one factor in paraneoplastic syndrome, cachexia may be relieved by removal of the primary tumor,6 which indicates the importance of RCC in altering host catabolism. Other causes of malnutrition include host demographic factors, treatment-related toxicity, comorbidities, and socioeconomic status. In other cancer types, malnutrition is associated with higher mortality, dysfunctional and impaired quality of life.7 Despite the importance of early detection and prevention of malnutrition, it is frequently underdiagnosed and difficult to recognize during anti-cancer treatment, making it necessary to systematically screen cancer patients for malnutrition. Although several instruments are currently used in the nutritional screening of the geriatric population,8 the ability of these tools to predict survival in cancer patients has not been adequately validated.
This study therefore tested the hypothesis that assessment of nutritional status may provide additional prognostic value in patients with metastatic RCC. Using a prospective multicenter design, we were able to demonstrate the applicability of nutritional status by overcoming methodological drawbacks.
Materials and methods
Study population
The study population was recruited from three tertiary medical centers in Eastern China, Fudan University Shanghai Cancer Center, Qingdao University Affiliated Hospital, and Ningbo University Affiliated First Hospital. The study protocol was approved by the institutional review board of each hospital, and informed consent was obtained from each patient prior to participation.
Patients aged 18 years or older with confirmed metastatic RCC and referred to begin targeted therapy were eligible for the study. Other inclusion criteria included Eastern Cooperative Oncology Group performance status < 2; stable condition without severe comorbidities, ensuring that patients would survive after targeted therapy, and no limitations to food access or intake. Of the 319 consecutive patients recruited during the study period, five had short-expected survival (<6 months) and were excluded, as were 14 patients with missing medical records. Finally, a total of 300 patients were prospectively enrolled from 2009 to 2013.
Assessment of nutritional status and covariates
Nutritional status was assessed in each patient within 2 weeks prior to starting targeted therapy. Nutritional parameters were assessed by trained nurses, who were blinded to risk stratification and treatment information. The parameters included weight, height, body mass index (BMI), and two nutritional screen scores, the Mini Nutritional Assessment–Short Form (MNA–SF) and the Geriatric Nutritional Risk Index (GNRI). Serum total albumin concentration, white blood cell count, differential count, platelet count and hemoglobin concentration were measured using standardized protocols.
The guidelines of the European Society of Parenteral and Enteral Nutrition have recommended using the MNA–SF to detect malnutrition among the elderly.9 MNA–SF scores ranged from 0 to 14, with ≥12 points indicating normal nutrition, 8–11 points indicating a low risk of malnutrition and ≤7 points indicating a high risk of malnutrition.
The GNRI was first developed to assess at-risk elderly patients.10 This index is calculated using the equation: GNRI = [1.489 × albumin (g/L)] + [41.7 × (weight/ideal body weight)]. Ideal body weight (in kg) was calculated (using height in cm) from the Lorentz equations as (height−100−[(height−150)/4]) for men; and (height−100−[(height−150)/2.5]) for women. In line with a previous study, we set weight/ideal body weight = 1 when weight exceeded ideal body weight.10 GNRIs of <82, 82–92, 92–98, and >98 were defined as patients at high, moderate, low, and no risk of malnutrition, respectively.10 Because few patients in this study were categorized as being at high risk, patients at high and moderate risk were combined. This modified three-level categorization was also more comparable to the MNA–SF score.11,12
Demographic data, disease characteristics, and geographic location were recorded as covariates. Specifically, Heng risk classification was used as a benchmark model.
Outcomes of interest
The primary endpoint of this study was overall survival (OS), defined as the time from targeted agent administration to the date of death or last contact. The secondary outcome was significant (grade 3/4) adverse events that occurred within 30 days after patients' nutritional status evaluations, as assessed by clinical staff according to Common Terminology Criteria for Adverse Events 3.0. (Table S1).
Sample size calculation
To properly estimate regression coefficients in new data, the number of considered covariables should be in reasonable balance with the number of events. Harrell proposed that at least 10 events are required per variable.13 For a 10-variable Cox regression model, we calculated a priori sample size of 100 events for death. Patients with metastatic RCC have an estimated 2-year OS rate of nearly 60%.3 Therefore, the projected sample size of the prospective cohort was at least 250 patients. Because of possible missing values or early drop outs, we enrolled a total of 300 patients according to the sample size estimation.
Statistical analyses
Categorical data were presented as frequencies and percentages, and continuous data as means and standard deviations. Categorical variables were compared using Pearson's χ2 test or Fisher's exact test, and continuous variables by one-way analysis of variance and test for trend. Cohen's kappa test was used to evaluate the agreement between the two nutrition scores (i.e., the MNA–SF and GNRI scores).
The OS was estimated using the Kaplan–Meier method and compared using the log-rank test. Cox regression analyses were performed to calculate adjusted hazard ratios (HRs) of covariates. Other than nutritional screening scores, the multivariate analyses included age, sex, Heng risk stratification, Charlson Comorbidity Index, prior nephrectomy, systemic inflammation score (neutrophil lymphocyte ratio, [NLR]), and geographic location. Harrell's concordance index (C index) was calculated to evaluate the discriminatory power of Cox models.14 We used the likelihood ratio (LR) χ2 test for the nested models to assess whether new variables added predictive value to the baseline models. To compare competing nutrition scores, we calculated the adequacy index; that is, the fraction of total LR χ2 explained by a set of variables.15 Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were derived from Cox regression models based on the Heng risk model, with and without nutritional scores. Additional benefit was also evaluated using decision curve analysis (DCA). Briefly, DCA is used to calculate the net benefit of new markers across various risk thresholds by taking account of weighted risks and benefits.16 The associations between nutritional screening scores and significant early adverse events were evaluated by logistic regression analysis. All statistical analyses were performed using R software. The level of statistical significance was set at P < 0.05. All P-values are two-sided.
Results
Table 1 shows baseline characteristics of the 300 enrolled patients, which included 203 (67.7%) men and 97 (32.3%) women. Mean patient age was 56.21 (27–81) years; mean BMI was 22.86 kg/m2, which was close to the average in China (22.9 kg/m2 in 201117). Most subjects received tyrosine kinase inhibitors, including sunitinib (N = 122), sorafenib (N = 142), axitinib (N = 14), pazopanib (N = 8), and famitimib (N = 10), although four were treated with mammalian target of rapamycin inhibitor everolimus. Of the 300 patients, 216 (72%) received targeted therapies as first-line treatment. The Heng risk classification classified 63% of these patients as being at intermediate risk.
Table 1.
Correlations of GNRI and MNA–SF scores with categories of patient characteristics, anthropometric results and laboratory measures
| Variables | Overall Population | GNRI <92 | GNRI 92–98 | GNRI >98 | ANOVA P-value | Trend P-value | MNA–SF <8 | MNA–SF 8–11 | MNA–SF >11 | ANOVA P-value | Trend P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 300 | 47 | 48 | 205 | 12 | 52 | 236 | ||||
| Age (years) | 56.02 (12.32) | 56.67 (10.49) | 56.31 (13.14) | 55.80 (12.58) | 0.539 | 0.315 | 50.75 (14.20) | 54.10 (15.29) | 56.71(111.43) | 0.124 | 0.125 |
| Sex (%) | 0.437 | 0.028 | |||||||||
| Male | 202 (67.33) | 33 (70.21) | 28 (58.33) | 141 (68.78) | 11 (91.67) | 29 (55.77) | 163 (69.07) | ||||
| Female | 98 (32.67) | 14 (29.79) | 20 (41.67) | 64 (31.22) | 1 (8.33) | 23 (44.23) | 73 (30.93) | ||||
| Weight (kg) | 63.99 (11.50) | 54.35 (8.25) | 60.19 (8.19) | 67.12 (11.31) | <0.001 | <0.001 | 51.88 (3.30) | 51.23 (6.72) | 67.35 (10.17) | <0.001 | <0.001 |
| BMI (kg/m2) | 22.85 (3.47) | 19.54 (2.36) | 21.89 (2.34) | 23.85 (3.36) | <0.001 | <0.001 | 17.98 (1.04) | 18.88 (1.55) | 23.95 (2.98) | <0.001 | <0.001 |
| Heng risk (%) | <0.001 | 0.038 | |||||||||
| High | 36 (12.00) | 18 (38.30) | 8 (16.67) | 9 (4.39) | 2 (16.67) | 10 (19.23) | 24 (10.17) | ||||
| Intermediate | 201 (67.00) | 28 (59.57) | 38 (79.17) | 135 (65.85) | 10 (83.33) | 37 (71.15) | 154 (65.25) | ||||
| Low | 63 (21.00) | 1 (2.13) | 2 (4.16) | 61 (29.76) | 0 (0.00) | 5 (9.62) | 58 (24.58) | ||||
| Laboratory investigation | |||||||||||
| WBC × 109/L | 6.29 (2.06) | 6.45 (2.37) | 6.73 (2.01) | 6.15 (1.98) | 0.185 | 0.362 | 5.61 (1.14) | 6.27 (2.91) | 6.33 (1.86) | 0.500 | 0.240 |
| Lymphocytes × 109/L | 2.69 (1.85) | 2.56 (1.99) | 2.59 (2.03) | 2.74 (1.78) | 0.763 | 0.539 | 2.85 (1.88) | 2.80 (2.48) | 2.66 (1.69) | 0.848 | 0.723 |
| Neutrophil × 109/L | 2.96 (1.82) | 3.22 (2.32) | 3.38 (2.03) | 2.80 (1.61) | 0.084 | 0.155 | 2.24 (1.21) | 2.81 (1.99) | 3.03 (1.80) | 0.274 | 0.139 |
| NLR (%) | 0.001 | ||||||||||
| >5 | 17 (5.67) | 5 (10.64) | 7 (14.58) | 5 (2.44) | 0 (0.00) | 4 (7.69) | 13 (5.51) | 0.689 | |||
| ≤5 | 283 (94.33) | 42 (89.36) | 41 (85.42) | 200 (97.56) | 12 (100.00) | 48 (92.31) | 223 (94.49) | ||||
| Hemoglobin g/L | 123.00 (21.15) | 103.85 (23.19) | 112.74 (17.94) | 129.74 (17.41) | <0.001 | <0.001 | 120.83 (22.49) | 115.71 (21.05) | 124.68 (20.80) | 0.021 | 0.514 |
| Platelet × 109/L | 231.19 (103.96) | 296.89 (160.91) | 252.04 (81.73) | 211.25 (82.83) | <0.001 | <0.001 | 229.42 (112.61) | 259.56 (104.94) | 225.46 (102.82) | 0.117 | 0.754 |
| Albumin g/L | 39.52 (5.24) | 32.09 (3.96) | 35.70 (3.25) | 42.14 (3.33) | <0.001 | <0.001 | 36.41 (6.46) | 28.51 (5.49) | 39.90 (5.06) | 0.025 | 0.021 |
| Creatinine clearance rate | 80.54 (1.57) | 76.30 (3.45) | 77.02 (3.86) | 82.71 (25.78) | 0.205 | 0.149 | 76.41 (7.98) | 75.14 (19.56) | 81.84 (1.82) | ||
| Comorbidities | |||||||||||
| CCI | 6.11 (0.37) | 6.09 (0.41) | 6.04 (0.30) | 6.13 (0.29) | 0.295 | 0.440 | 6.12 (0.07) | 6.14 (0.41) | 6.11 (0.02) | 0.850 | 0.583 |
| Diabetes mellitus (%) | 16 (5.33) | 1 (2.12) | 1 (2.08) | 14 (6.83) | 0.245 | 1 (8.33) | 2 (3.84) | 13 (5.50) | 0.807 | ||
| Myocardial infarction (%) | 2 (0.67) | 0 (0.00) | 0 (0.00) | 2 (0.98) | 0.630 | 0 (0.00) | 1 (1.92) | 1 (0.42) | 0.455 | ||
| Congestive heart failure (%) | 3 (1.00) | 0 (0.00) | 0 (0.00) | 3 (1.46) | 0.499 | 0 (0.00) | 0 (0.00) | 3 (1.27) | 0.667 | ||
| Liver disease (%) | 4 (1.33) | 0 (0.00) | 1 (2.08) | 3 (1.46) | 0.643 | 0 (0.00) | 0 (0.00) | 4 (1.69) | 0.582 | ||
| Peptic ulcer disease (%) | 8 (2.67) | 3 (6.38) | 0 (0.00) | 5 (2.43) | 0.148 | 1 (8.33) | 3 (5.76) | 4 (1.69) | 0.113 | ||
Abbreviations: GNRI, Geriatric Nutritional Risk Index; MNA–SF, Mini Nutritional Assessment–Short Form; BMI, body mass index; WBC, white blood cells; NLR, neutrophil lymphocyte ratio; CCI, Charison Comorbidity Index; ANOVA, analysis of variance.
Based on the GNRI and MNA–SF scores, 95 (31.7%) and 64 (21.3%) patients, respectively, were classified as being at risk of malnutrition. Both low BMI and poor Heng risk classification disease were significantly associated with impaired nutritional status (Table 1). In addition, GNRI score was strongly associated with NLR, an indicator of systemic inflammation. Interestingly, MNA–SF score and inflammatory markers were only weakly associated.
The GNRI took less time to implement (average: 2 min vs. 10 min) and showed higher reproducibility (0.98 vs. 0.91) than the MNA–SF. GNRI and MNA–SF scores were weakly correlated (κ = 0.212, P < 0.001), with GNRI scores tending to be more sensitive to malnutrition than MNA–SF scores (Table 2). For example, of 48 patients classified by GNRI score as being at severe risk for malnutrition, 20 (41.6%) patients were ranked as normal using the MNA–SF score.
Table 2.
Weighted Cohen's kappa test for agreement between GNRI and MNA–SF scores
| MNA–SF <8 | MNA–SF 8–11 | MNA–SF >12 | |
|---|---|---|---|
| N = 12 | N = 52 | N = 236 | |
| GNRI <92 N = 47 | 8 | 20 | 19 |
| GNRI 92–98 N = 48 | 1 | 11 | 36 |
| GNRI >98 N = 205 | 3 | 21 | 181 |
| Kappa (95% CI) | 0.212 (0.122–0.302) | ||
| P-value | <0.0001 |
Abbreviations: GNRI, Geriatric Nutritional Risk Index; MNA–SF, Mini Nutritional Assessment–Short Form
Over a median follow-up period of 30.8 months, 185 deaths were observed. The 2-year OS rate for all patients was 38%. Survival curves were significantly stratified by GNRI score. The median survival of the no-risk group was three times longer than that of the high-risk group (25.9 months vs 8.6 months). Similar results were seen for patients stratified by MNA–SF scores (21.3 months vs 8.9 months). The predictive value of nutrition scores were also seen in patients with intermediate Heng risk (Figure1C and D). In this subgroup, patients with impaired nutritional status had significantly poorer outcomes, comparable with those of patients with the most adverse Heng risk scores. We also analyzed whether the prognostic significance of nutritional score was caused by other underlying factors, including age, sex, comorbidities, disease risk, systemic inflammation, geographic location, and prior treatment. We tested for interaction between Heng score and nutritional score but found no significant relationship (data not shown). Multivariate analyses, performed to calculate the adjusted HR for malnutrition, showed that both GNRI and MNA–SF scores were independent predictors of OS (Table 3).
Figure 1.
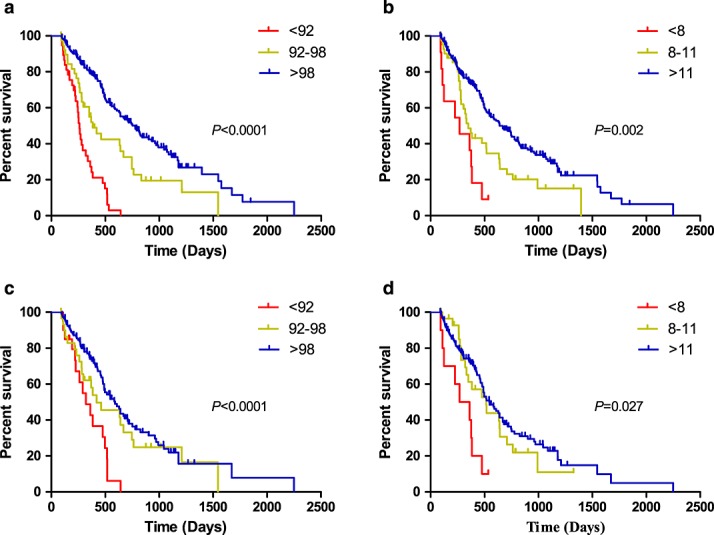
Kaplan–Meier survival curves showing patient overall survival stratified by different nutrition scores, including (A) GNRI score, (B) MNA–SF score, (C) GNRI score in patients with intermediate Heng risk score, and (D) MNA–SF score in patients with intermediate Heng risk score.
Table 3.
Multivariate Cox regression models analyzing the associations between nutritional status, as assessed by the GNRI and MNA–SF scores, and overall survival
| GNRI model | MNA–SF model | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex | ||||
| Male | Ref. | Ref. | ||
| Female | 0.883 (0.638–1.224) | 0.637 | 1.070 (0.771–1.486) | 0.684 |
| Age | 1.004 (0.971–1.038) | 0.836 | 1.016 (0.982–1.051) | 0.366 |
| Charlson Comorbidity Index | 0.807 (0.544–1.195) | 0.248 | 0.789 (0.529–1.176) | 0.244 |
| Heng risk stratification | ||||
| Low | Ref. | Ref. | ||
| Intermediate | 2.601 (1.630–4.148) | <0.001 | 2.741 (1.724–4.359) | <0.001 |
| High | 3.929 (2.155–7.163) | <0.001 | 5.696 (3.185–10.19) | <0.001 |
| Nephrectomy | ||||
| No | Ref. | Ref. | ||
| Yes | 0.560 (0.228–1.378) | 0.207 | 0.492 (0.198–1.222) | 0.126 |
| NLR | ||||
| ≤5 | Ref. | Ref. | ||
| >5 | 1.541 (0.728–3.264) | 0.259 | 1.266 (0.208–2.638) | 0.528 |
| Geographic location | ||||
| Urban | Ref. | Ref. | ||
| Rural | 1.506 (1.085–2.091) | 0.014 | 1.696 (1.226–2.346) | 0.001 |
| Nutrition risk | ||||
| Normal | Ref | Ref. | ||
| Low | 1.502 (1.069–2.498) | 0.023 | 1.320 (0.886–1.967) | 0.172 |
| High | 3.157 (2.273–5.353) | <0.001 | 2.784 (1.506–6.065) | 0.002 |
| Incremental performance (using Heng model as benchmark) | ||||
| △ C index | 0.041 | 0.016 | ||
| IDI at 2 years (95%CI) | 0.066 (0.024–0.113) | P = 0.001 | 0.013 (-0.005-0.050) | P = 0.239 |
| NRI at 2 years (95%CI) | 0.177 (0.057–0.363) | P = 0.013 | 0.123 (-0.134–0.245) | P = 0.286 |
| △ Net benefit at threshold of 50% at 2 years | 1.2 per 100 patients | 0.9 per 100 patients | ||
| △ Net benefit at threshold of 62%* at 2 years | 4.5 per 100 patients | 2.8 per 100 patients |
Abbreviations: GNRI, Geriatric Nutritional Risk Index; MNA–SF, Mini Nutritional Assessment–Short Form; HR, hazard ratio; NLR, neutrophil lymphocyte ratio; C index, Harrell's concordance index; IDI, integrated discrimination improvement index; NRI, net reclassification index
For the entire group, 2-year survival rate was 38%.
After we performed a later sensitivity analysis that included adjustments for the hospitals where the patients received their care, we found similar results: GNRI high risk HR: 3.293, 95% CI: 2.166–5.007, P < 0.001; low risk HR: 1.660, 95% CI: 1.112–2.478 P = 0.013; and MNA–SF high risk: HR: 2.704 95% CI: 1.296–5.641, P = 0.008; low risk: HR: 1.335, 95% CI: 0.906–1.966, P = 0.144. When interactions were tested between Heng scores and nutritional scores, we found no significant interaction in predicting OS (data not shown).
In assessing the added benefit of nutritional score in prognostication, we found that adding nutritional scores to baseline models significantly improved the discriminative ability of the latter. Compared with MNA–SF score, GNRI score contributed a larger increment to the C index (Table 3). The likelihood ratio test showed that the GNRI score, with a higher adequacy index, provided better estimates, as the inclusion of GNRI score in a model containing the MNA–SF score resulted in greater statistical improvement (P < 0.001), whereas the inclusion of MNA–SF score in a model containing the GNRI score had no effect (P = 0.254; Figure S1). When GNRI was entered into the Heng model, IDI, and NRI at 2 years were estimated as 0.066 (95% CI: 0.024–0.113; P = 0.001) and 0.177 (95% CI: 0.057–0.363; P = 0.013). MNA–SF, however, showed no significant incremental value (Table 3). DCA showed that adding nutritional scores would lead to better prediction of outcomes if the threshold of 2-year mortality probability were above 30%. For example, 4.5 per 100 patients would have better risk stratification from adding GNRI assessment to Heng models at a threshold of 62%, with MNA–SF showing similar results (Table 3). Similar results were observed when the Memorial Sloan-Kettering Cancer Center risk stratification model was used in place of the Heng risk model (data not shown).
Finally, we evaluated whether nutritional scores were associated with an increased risk of adverse events. Of our 300 patients, 32.7% had an early grade 3/4 adverse event. In unadjusted analyses, impaired nutritional status was associated with a higher probability of a significant adverse event, although multivariate analysis showed that nutritional status was not an independent predictor of early grade 3/4 adverse events (Table 4).
Table 4.
Multivariate logistic regression models analyzing the association between nutritional status, as assessed by the GNRI and MNA–SF, and grade 3/4 adverse events
| GNRI model | MNA–SF model | |||
|---|---|---|---|---|
| Parameters | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Sex | ||||
| Male | Ref. | Ref. | ||
| Female | 2.755 (1.605–4.734) | <0.001 | 2.920 (1.689–5.050) | <0.001 |
| Age | 1.018 (0.997–1.039) | 0.097 | 1.016 (0.995–1.037) | 0.142 |
| Charlson comorbidity index | 1.169 (0.589–2.324) | 0.655 | 1.191 (0.600–2.365) | 0.618 |
| Heng risk stratification | ||||
| Low | Ref. | Ref. | ||
| Intermediate | 0.884 (0.324–2.451) | 0.810 | 1.111 (0.441–2.798) | 0.823 |
| High | 0.574 (0.244–1.349) | 0.203 | 0.654 (0.294–1.453) | 0.297 |
| Nephrectomy | ||||
| No | Ref. | Ref. | ||
| Yes | 0.962 (0.250–3.694) | 0.954 | 1.525 (0.449–5.184) | 0.937 |
| NLR | ||||
| ≤5 | Ref. | Ref. | ||
| >5 | 1.113 (0.452–2.742) | 0.815 | 0.873 (0.369–2.065) | 0.757 |
| Geographic location | ||||
| Urban | Ref. | Ref. | ||
| Rural | 1.873 (1.093–3.212) | 0.023 | 2.035 (1.211–3.298) | 0.009 |
| Nutrition risk | ||||
| Normal | Ref. | Ref. | ||
| Low | 0.889 (0.324–2.330) | 0.812 | 1.616 (0.485–5.701) | 0.456 |
| High | 1.550 (0.686–3.506) | 0.292 | 0.635 (0.315–1.279) | 0.345 |
Abbreviations: GNRI, Geriatric Nutritional Risk Index; MNA–SF, Mini Nutritional Assessment–Short Form; HR, hazard ratio; NLR, neutrophil lymphocyte ratio
Discussion
To our knowledge, this is the first prospective, multicenter study to evaluate the impact of nutritional parameters on the prognosis of patients with metastatic RCC. Using nutritional screening tools, we presented a more accurate and complete description of nutritional status than previously reported.18,19 Using a standardized approach, we found that patients with malnutrition before targeted therapies were at significantly greater risk of mortality. Moreover, GNRI score was superior to MNA–SF score for risk discrimination. These results suggest that early nutritional assessment using GNRI will not only aid in accurately predicting mortality in patients with metastatic RCC but may serve as an important basis for implementation of individualized nutritional care plans, which may be beneficial in treating these patients.
Nutritional assessment was found to be an important predictor of outcomes in a study of 369 patients with localized RCC.18 In that study, however, the criteria for classifying malnutrition were prompted by the investigators. Our results extend these findings to patients with advanced disease, which showed that up to 15.7% of our patients were severely malnourished according to GNRI score. Although these results were not surprising in patients with advanced disease, they highlight the need for standardized and objective assessment methods. For example, using the average Chinese BMI as a cutoff (22.8 kg/m2), we found 14.3% of our overweight patients to have nutritional risks. Similar results were seen using albumin concentration and weight loss alone as criteria.
Nutritional status in cancer patients is influenced by many factors, which makes it more complex and difficult to study.20 Therefore, whether the association between nutrition and outcome is simply a reflection of underlying predictors is unclear. For example, elderly patients frequently have compromised nutritional status and are also vulnerable to cancer-related deaths. After adjusting for potential covariates in multivariate analyses, we found that malnutrition remained an independent risk factor for death. The HR of nutritional status was independent and as high as 3.349 for those with severe malnutrition. Because of the close relationship between malnutrition and systemic inflammation, the impact of nutritional screening was examined in the context of well-established inflammatory markers.21 Our results showed that the prognostic value of nutritional status was not improved by inclusion of NLR, indicating that inflammation cannot fully explain the association between nutrition and survival.
Based on the recommended framework for prognostic studies, we examined statistics that seemed clinically relevant. DCA analysis showed a moderate net benefit in risk attribution based on a mortality threshold >30% at 2 years.
In addition to its significant statistical performance, nutritional screening has several other advantages. The tools were easy to use, less time consuming and cost-free. Our study and other reports jointly showed that the established cutoffs from geriatric studies were satisfied in the oncology setting.22 In particular, these scores were able to classify patients at intermediate Heng risk, thereby providing additional information on ‘gray-zone’ RCC patients. Because GNRI score had a superior C statistic, was less time consuming, and had less observer variability that MNA–SF score, GNRI score may be a good screening tool for routine practice. Nevertheless, patients found to be at risk during screening should undergo meticulous subsequent nutritional evaluation. An important aspect of our findings is the identification of a risk factor that is potentially amenable to intervention. In contrast with most prognostic factors in metastatic RCC, which are innate to the disease and therefore fixed, nutritional status represents a promising modifiable host risk factor. Although there are no solid supportive data that nutritional intervention can reduce mortality risk, the next step is to enhance at-risk patients' nutritional statuses and investigate how this affects outcomes. The mechanism that underlies the effect of nutrition on OS in patients with metastatic RCC is unclear, although malnutrition may be associated with vulnerability to treatment toxicity.23 However, we failed to find a positive correlation between malnutrition and early grade 3/4 adverse events. Alternatively, malnutrition may be associated with the secretion of pro-angiogenic factors24 or a compromised immune response. Further investigations are needed to explore mechanisms and develop interventions to improve outcomes.
This study had several limitations. Firstly, our study represented only the first step toward nutritional evaluation in patients with metastatic RCC. Additional investigations, using more comprehensive and accurate assessment tools, are needed. Moreover, associations between nutrition and other outcomes, including quality of life, are also of interest. Secondly, the general applicability of the GNRI to various populations requires further evaluation, although reports in patients on dialysis and those with other chronic diseases have confirmed the general validity of the GNRI in western countries.25,26
The MNA was widely validated to detect malnutrition among patients aged 70 years and older, and reportedly has predictive value in metastatic lung and upper gastrointestinal cancers.27 It is an accurate assessment tool for nutritional status but is too long for routine screening. The MNA–SF was created to shorten the MNA while preserving accuracy.9 The MNA uses a questionnaire with no biological indicators and is more suited to older patients in nursing home settings because of the mature biases of the questionnaire.10
The GNRI scores are based only on albumin assessment and comparison of patients' weight with desirable weight. Albumin alone can indicate both disease severity and chronic malnutrition or poor dietary habits28; whereas, with regard to weight loss, GNRI seems to indicate both systemic disease severity and protein–calorie stores that a patient needs to cope with acute stress.22 GNRI can be used to classify patients by risk of complications in relation to illnesses often associated with malnutrition.
In conclusion, using standardized and objective measures, malnutrition was characterized as a common condition in patients with metastatic RCC. Our results suggested that the addition of nutritional screen tools could better identify patients at risk for poorer survival outcomes. Individualized nutritional assessment and possible intervention strategies may be included in a multidisciplinary approach to treat patients with metastatic RCC.
Acknowledgments
This work was supported by the Shanghai Leaders' Foundation and the Project of the National Natural Science Foundation of China [grants number 81001131, 81370073, 81202004].
The authors of this paper certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD).
Conflict of interest
None declared.
Supporting information
Supporting Information is available at Journal of Cachexia, Sarcopenia and Muscle online.
Table S1. Summary of common adverse events collected during 30-days for nutritional status evaluation.
Figure S1. Cox regression models showing the predictive performance of Heng score combined with GNRI and/or MNA-SF scores.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New Eng J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, Group TS. Sorafenib in advanced clear-cell renal-cell carcinoma. New Eng J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol: Offic J Am Soc Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, Rha SY, Agarwal N, Kollmannsberger C, Rini BI, Choueiri TK. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14:141–8. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.August D, Huhmann M. American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. ASPEN clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33:472–500. doi: 10.1177/0148607109341804. [DOI] [PubMed] [Google Scholar]
- 6.Gold PJ, Fefer A, Thompson JA. Paraneoplastic manifestations of renal cell carcinoma. Seminars in urologic oncology. 1996;14:216–22. [PubMed] [Google Scholar]
- 7.Hebuterne X, Lemarie E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38:196–204. doi: 10.1177/0148607113502674. [DOI] [PubMed] [Google Scholar]
- 8.Cereda E, Pedrolli C, Zagami A, Vanotti A, Piffer S, Opizzi A, Rondanelli M, Caccialanza R. Nutritional screening and mortality in newly institutionalised elderly: a comparison between the geriatric nutritional risk index and the mini nutritional assessment. Clinical nutrition. 2011;30:793–8. doi: 10.1016/j.clnu.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56:M366–72. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 10.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–83. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 11.Cereda E, Pusani C, Limonta D, Vanotti A. The ability of the Geriatric Nutritional Risk Index to assess the nutritional status and predict the outcome of home-care resident elderly: a comparison with the Mini Nutritional Assessment. Br J Nutr. 2009;102:563–70. doi: 10.1017/S0007114509222677. [DOI] [PubMed] [Google Scholar]
- 12.Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO, Amin GE. The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr. 2014;33:1108–16. doi: 10.1016/j.clnu.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 15.Tan MH, Li H, Choong CV, Chia KS, Toh CK, Tang T, Tan PH, Wong CF, Lau W, Cheng C. The Karakiewicz nomogram is the most useful clinical predictor for survival outcomes in patients with localized renal cell carcinoma. Cancer. 2011;117:5314–24. doi: 10.1002/cncr.26193. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making: Int J Soc Med Decis Making. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon-Larsen P, Wang H, Popkin BM. Overweight dynamics in Chinese children and adults. Obes Rev: Offic J Int Assoc Stud Obes. 2014;15:37–48. doi: 10.1111/obr.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith JA, Jr, Clark PE. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59:923–8. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko K, Park YH, Lee JW, Ku JH, Kwak C, Kim HH. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC) BJU Int. 2013;112:775–80. doi: 10.1111/bju.12275. [DOI] [PubMed] [Google Scholar]
- 20.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 21.Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P, Lee CK. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013;109:147–53. doi: 10.1038/bjc.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cereda E, Pedrolli C. The Geriatric Nutritional Risk Index. Curr Opin Clin Nutr Metab Care. 2009;12:1–7. doi: 10.1097/MCO.0b013e3283186f59. [DOI] [PubMed] [Google Scholar]
- 23.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–9. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Shibata M, Nishiyama H, Soeda S, Furukawa S, Gonda K, Takenoshita S, Fujimori K. Elevated serum levels of vascular endothelial growth factor is effective as a marker for malnutrition and inflammation in patients with ovarian cancer. Biomed Rep. 2013;1:197–201. doi: 10.3892/br.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87:106–13. doi: 10.1093/ajcn/87.1.106. [DOI] [PubMed] [Google Scholar]
- 26.Narumi T, Arimoto T, Funayama A, Kadowaki S, Otaki Y, Nishiyama S, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Watanabe T, Kubota I. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol. 2013;62:307–13. doi: 10.1016/j.jjcc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Gioulbasanis I, Martin L, Baracos VE, Thezenas S, Koinis F, Senesse P. Nutritional assessment in overweight and obese patients with metastatic cancer: does it make sense? Anna oncol: Offic J Eur Soc Med Oncol / ESMO. 2014 doi: 10.1093/annonc/mdu501. [DOI] [PubMed] [Google Scholar]
- 28.Cereda E, Vanotti A. Short dietary assessment improves muscle dysfunction identification by Geriatric Nutritional Risk Index in uncomplicated institutionalised patients over 70 years old. Clin Nutr. 2008;27:126–32. doi: 10.1016/j.clnu.2007.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of common adverse events collected during 30-days for nutritional status evaluation.
Figure S1. Cox regression models showing the predictive performance of Heng score combined with GNRI and/or MNA-SF scores.


