Abstract
Background
In Japan, growth hormone releasing peptide-2 (GHRP-2) is clinically used as a diagnostic agent for growth hormone secretion deficiency, but the therapeutic application of GHRP-2 has not been studied in anorexia nervosa. GHRP-2 reportedly exhibits agonistic action for ghrelin receptor and increases food intake.
Methods
We administered GHRP-2 to a patient with a 20-year history of anorexia nervosa to determine whether GHRP-2 treatment increases food intake and body weight. GHRP-2 was administered before every meal by an intranasal approach for 1 year.
Results
Although the patient reported a decreased fear of eating and decreased desire to be thin by our previous treatment, she was unable to increase food intake or body weight because of digestive tract dysfunction. Vomiting after meals caused by delayed gastric emptying and incurable constipation were prolonged, and sub-ileus and hypoglycemia were observed. GHRP-2 increased the feeling of hunger and food intake, decreased early satiety and improved hypoglycemia. The patient's body weight gradually increased by 6.7 kg (from 21.1 kg to 27.8 kg) in 14 months after starting GHRP-2 administration. The fatigability and muscle strength improved, and the physical and mental activities were also increased. No obvious side effects were observed after long-term intranasal administration of GHRP-2.
Conclusions
Patients with a long-term history of eating disorder occasionally recover from the psychological problems such as fear for obesity but remain emaciated. We believe that ghrelin agonists such as GHRP-2 may be promising agents for the effective treatments of severe anorexia nervosa in a chronic condition.
Keywords: GHRP-2, Anorexia nervosa, Body weight, Ghrelin, GH, IGF-1
Introduction
Anorexia nervosa (AN), a complex illness, has the highest mortality rate among psychiatric disorders because of the severe weight loss, associated medical complications, psychological morbidity and the chronic course of the illness.1,2 It is believed that the death rates of AN could be reduced by early diagnosis and long-term specialist care. However, many studies confirm the medical seriousness of AN, and sudden death is difficult to prevent completely in severely emaciated patients despite intensive treatment and specialist intervention.
Anorexia nervosa is characterized by destructive weight loss behaviour, resulting from the interplay between debilitating cognitive, emotional, and physical processes. The gut–brain peptides have been assumed to be involved in the pathophysiology of AN.2,3 Recently, ghrelin, a 28-amino acid peptide secreted from the stomach4 was identified as the endogenous ligand of the growth hormone secretagogue (GHS) receptor. GHS includes growth hormone releasing peptide-2 (GHRP-2), a synthetic hexapeptide ( d-Ala- d-b Nal-Ala-Trp- d-Phe-Lys-NH2), discovered in the 1980s and extensively studied for their effects on growth hormone (GH) release.5 Ghrelin exhibits a potent GH-releasing effect and increases muscle mass through the GH/insulin-like growth factor-1 (IGF-1) axis.6,7 It potently stimulates food intake and promotes adiposity by a GH-independent action. Ghrelin is classified into two forms; acyl ghrelin is the active form, whereas des-acyl ghrelin is devoid of GH-releasing and orexigenic activities.
Growth hormone releasing peptide-2 is known to increase food intake in healthy men and in some animal models through GHS/ghrelin receptor.8 In Japan, GHRP-2 is used as a diagnostic agent for GH secretion deficiency, but the clinical application of GHRP-2 has not been well studied. We encountered a severely emaciated AN patient who failed to respond to medical treatment despite the vigorous efforts on nutritional rehabilitation and psychotherapy; therefore, we decided to treat her with GHRP-2 intra-nasally.
Materials and methods
Patient
Our patient was a 38-year-old Japanese woman. The patient developed AN when she entered junior college at 18 years of age. She visited many generalists and gastrointestinal specialists with the chief complaint of anorexia, edema, general fatigue, and disturbance of consciousness. Although she was diagnosed as AN and advised for follow-up with the specialists, she failed to do so because both she and her family had little insight into the disease. She consulted our department 3 years ago when she was unable to move herself because of severe emaciation, and we started medical treatment in an inpatient clinic. In the beginning, the patient was very reluctant to treatment, and anorexia continued although her nutritional status was improved by peripheral parenteral nutrition. The patient discharged temporarily after hospitalization for 3 months, but her body weight gradually decreased, and she was repeatedly hospitalized. We treated the patient with various medications including selective serotonin re-uptake inhibitors, gastro-prokinetics, and anti-constipative agents, as well as cognitive behavioural therapy over a period of 3 years. The patient's motivation for treatment improved, however, her body weight did not increase because of the gastrointestinal symptoms, and the activity gradually decreased. She suffered from vomiting after eating small amounts of food and repeated sub-ileus.
We believed that current treatment was ineffective and started treatment with GHRP-2 to improve her appetite and gastrointestinal symptoms. We monitored the patient's food intake, body weight, muscle mass, fat mass, nutritional status, hormonal changes, blood glucose levels, and subjective symptoms including appetite. We used the food diary and visual analogue scale evaluating food intake (10 stages) and appetite (three stages), respectively. Fat and muscle mass were evaluated using the bio-impedance method.
Administration of growth hormone releasing peptide-2
The composition of GHRP-2 solution is presented in Figure1. GHRP-2 was administered to the patient by nasal spray four times a day, 30 min before every meal and sleep. Approximately 100 μL of GHRP-2 solution by one push contains 100 µg of GHRP-2. After one push during the first week, we increased the dose up to 200 µg (two pushes) before every meal and 100 µg before sleep for 10 months.
Figure 1.

The composition of growth hormone releasing peptide-2 solution and the device for the nasal spray.
Laboratory measurements
Blood samples were obtained in the morning after overnight fasting and treated with hydrochloric acid for measurement of acyl-ghrelin and desacyl-ghrelin using an enzyme immunoassay kit (Mitsubishi Chemical Medicine, Tokyo, Japan) (9).
Ethics statement
In accordance with the principles of the Declaration of Helsinki, the participant gave informed, written consent to participate. The study was approved by the Institutional Committee of Kagoshima University.
Results
The patient's body temperature, blood pressure, and heart rate was 37.0°C, 100/70 mmHg, 70 beats/min, respectively. Blood examination showed no significant changes except anaemia. Severe brain atrophy by magnetic resonance imaging and low bone mineral density were observed, the latter being lower than the average of 100-year-old women (not shown).
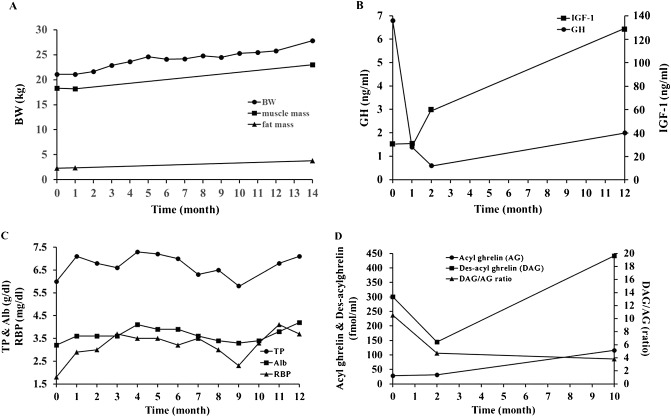
Subjective symptoms of the patient were improved dramatically by GHRP-2 treatment. The patient reported hunger and markedly decreased early satiety 30 min after treatment. Body weight, muscle mass, and fat mass were gradually increased with reduced fatigue and increased muscle strength and daily activity (Figure2A). GH and IGF-1, as well as nutritional marker retinol binding protein and albumin levels were also improved (Figure2B and C). As a result of GHRP-2 administration for 10 months, fasting des-acyl ghrelin and acyl ghrelin was increased and the des-acyl ghrelin/acyl ghrelin ratio decreased (Figure2D).
Figure 2.
(A) Changes in body weight, muscle mass, and fat mass after treatment with growth hormone releasing peptide-2 (GHRP-2). Fat and muscle mass were evaluated using the bio-impedance method. All parameters gradually increased and the increases of body weight and daily energy intake were −1.5 to 2.4 kg and 12% to 36%, respectively, after ghrelin infusion. (B) Changes in growth hormone (GH) and insulin-like growth factor 1 (IGF-1) levels after treatment with GHRP-2. GH decreased and IGF-1 increased after 12 months. (C) Changes in nutritional status. Retinol binding protein (RBP) and total protein (TP) increased rapidly, and albumin (Alb) increased after 4 months. These parameters further increased after 12 months. (D) Changes in acyl ghrelin (AG) and des-acyl ghrelin (DAG) levels after the administration of GHRP-2. Fasting DAG and AG increased and DAG/AG ratio was lowered after 10 months. BW; body weight.
The patient initially suffered from hypoglycemic symptoms with glucose level lower than 70 mg/dL. After 100 µg of GHRP-2 treatment before sleep, the patient no longer complained of hypoglycemic symptoms after meals and at midnight, but further increase of GHRP-2 dose from 100 to 200 µg appeared no additional benefits.
Discussion
Patients with AN exhibit a certain level of immune system activation that is either considered a cause of this eating disorder or an accompanying feature of the malnutrition state.3 Cytokine deregulation profiles in AN include an increase in interleukin 1-beta and tumour necrosis factor-alpha levels, which are common among anorexia–cachexia syndromes associated with many diseases including cancer.9 The clinical use of tumour necrosis factor-alpha down-regulators, such as infliximab, in the treatment of AN associated with juvenile idiopathic arthritis or Crohn's disease were successful with respect to body weight and psychopathology.10 These results indicate that despite the controversy regarding the similarities and differences between AN and cachexia, severely emaciated AN patients likely fulfil the diagnostic criteria of cachexia and could be treated similarly.
Ghrelin is the only known circulating orexigenic hormone.6,7 Ghrelin increases food intake by interacting with hypothalamic and brainstem circuits involved in energy balance, as well as reward-related brain areas. A heightened gut–brain ghrelin axis is an emerging feature of anorexia–cachexia syndromes,7 but the comparison was usually made with healthy weight subjects. We and others suggest that although acyl ghrelin tends to increase in AN patients, the increase is predominantly in the form of desacyl ghrelin2,11 that is devoid of GH-releasing and orexigenic activities. The relative abundance of desacyl form is a characteristic of both AN and cancer cachexia2,7 and agents that potentiate ghrelin signalling may exhibit a therapeutic effect for AN although most are currently in the preclinical or early clinical stage of development.
Intravenous infusion of ghrelin has been reported to stimulate appetite and food intake in patients with chronic heart failure, chronic obstructive pulmonary disease, cancer, and other diseases.7,12 In AN, only short-term treatment studies with natural ghrelin have been reported. Although single-dose continuous administration of ghrelin for 5 h failed to affect appetite in AN,13 the intravenous administration twice a day for 2 weeks in restrictive AN improved epigastric discomfort and constipation and increased hunger scores.14
Our study is the first to demonstrate a year-long therapeutic effect of the ghrelin agonist GHRP-2 by intranasal application in a very emaciated AN patient with longstanding disease history. Despite the digestive tract malfunction such as meal-induced vomiting, incurable constipation, and frequent sub-ileus, GHRP-2 enhanced feelings of hunger, improved early satiety, and increased food intake. Interestingly, GHRP-2 appears to increase sleep in the patient as reported previously.13 The treatment was well tolerated and was not associated with any adverse effects including irritation of the nasal mucosa.
In addition to nutritional status, GHRP-2 improved the patient's activities of daily living. She frequently felt general fatigue and staggered because of muscle weakness before treatment. After the start of GHRP-2, the patient could walk by herself more firmly and for a longer period of time than she had previously. The stimulation of GH and IGF-1 by GHRP-2 exhibited anabolic effects and enhanced muscle strength, particularly in the lower extremities. These effects on potentiating ghrelin signalling may be consistent with those in aged subjects in which both fat and lean body mass were increased 1 year after the oral administration of ghrelin agonists.15,16
Severe cases of AN exhibit a high prevalence of hypoglycemia. Most of the severe hypoglycemic episodes occurred in early morning, suggesting that in the majority of episodes, the liver lacked sufficient substrate in the fasting state to maintain the patient's serum glucose. Postprandial hypoglycemic episodes, occurring later in the day, would be more likely caused by a vigorous insulin response to food, which overwhelms the body's ability to maintain glucose homeostasis. In this study, GHRP-2 was effective in preventing deep hypoglycemia. When body fat is reduced by calorie restriction, ghrelin stimulates GH secretion, which allows the maintenance of glucose production, even when food intake is greatly reduced. However, in severe AN, this compensatory ghrelin and GH response will be hampered as in fat-depleted mice.17 Calorie-restricted, fat-depleted ghrelin O-acyltransferase or ghrelin knock-out mice fail to exhibit the normal increase in GH and become profoundly hypoglycemic when fasted for 18–23 h. It has been suggested that these two hormones could prolong survival in starved humans as they do in mice.17 The importance of GH and glucagon in starvation adds validity and value to the nasal administration.
By the continuous administration of GHRP-2, baseline levels of acyl ghrelin were increased, des-acyl ghrelin decreased, and the des-acyl ghrelin/acyl ghrelin ratio was decreased. This indicates the recovery of acyl ghrelin signalling. Further investigations are needed to address whether and how normalization of the ghrelin signalling and feeding–regulatory circuitry corrects metabolic, gastrointestinal, and behavioural abnormalities of the patients.
Conclusion
This is the first report of the therapeutic effects of long-term intranasal administration of ghrelin agonist GHRP-2 in a severely emaciated, long-standing AN patient. GHRP-2 increased appetite, body weight, and muscle strength, and improved fatigue, gastrointestinal functions, and hypoglycemia. The treatment was safe and tolerable without any obvious side effects. Further studies are needed for the therapeutic efficacy of GHRP-2 and other ghrelin agonists in AN.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research Project Number 24390182.
The authors of this paper certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S., Morley J. E., Coats A. J., and Anker S. D.).
Conflict of interest
None declared.
References
- 1.Suokas JT, Suvisaari JM, Gissler M, Lofman R, Linna MS, Raevuori A, et al. Mortality in eating disorders: a follow-up study of adult eating disorder patients treated in tertiary care, 1995–2010. Psychiatry Res. 2013;210:1101–1106. doi: 10.1016/j.psychres.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Ogiso K, Asakawa A, Amitani H, Inui A. Ghrelin and anorexia nervosa: a psychosomatic perspective. Nutrition. 2011;27:988–993. doi: 10.1016/j.nut.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Inui A. Eating behavior in anorexia nervosa - an excess of both orexigenic and anorexigenic signalling? Mol Psychiatry. 2001;6:620–624. doi: 10.1038/sj.mp.4000944. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 5.Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114:1537–1545. doi: 10.1210/endo-114-5-1537. [DOI] [PubMed] [Google Scholar]
- 6.Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- 7.Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61:430–481. doi: 10.1124/pr.109.001958. [DOI] [PubMed] [Google Scholar]
- 8.Laferrere B, Abraham C, Russell CD, Bowers CY. Growth hormone releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. J Clin Endocrinol Metab. 2005;90:611–614. doi: 10.1210/jc.2004-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inui A. Cancer anorexia–cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59:4493–4501. [PubMed] [Google Scholar]
- 10.Bou Khalil R, de Muylder O, Hebborn FL. Treatment of anorexia nervosa with TNF-alpha down-regulating agents. Eat Weight Disord EWD. 2011;16:300. doi: 10.1007/BF03327476. [DOI] [PubMed] [Google Scholar]
- 11.Nakahara T, Kojima S, Tanaka M, Yasuhara D, Harada T, Sagiyama K, et al. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J Psychiatr Res. 2007;41:814–820. doi: 10.1016/j.jpsychires.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Strasser F. Clinical application of ghrelin. Curr Pharm Des. 2012;18:4800–4812. doi: 10.2174/138161212803216870. [DOI] [PubMed] [Google Scholar]
- 13.Miljic D, Pekic S, Djurovic M, Doknic M, Milic N, Casanueva FF, et al. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1491–1495. doi: 10.1210/jc.2005-2304. [DOI] [PubMed] [Google Scholar]
- 14.Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocr J. 2009;56:1119–1128. doi: 10.1507/endocrj.k09e-168. [DOI] [PubMed] [Google Scholar]
- 15.Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White HK, Petrie CD, Landschulz W, MacLean D, Taylor A, Lyles K, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94:1198–1206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 17.Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012;287:17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]