Abstract
Background
In rodent models, caloric restriction (CR) with maintenance of adequate micronutrient supply has been reported to increase lifespan and to reduce age-induced muscle loss (sarcopenia) during ageing. In the present study, we further investigated effects of CR on the onset and severity of sarcopenia in ageing male C57BL/6 J mice. The aim of this study was to investigate whether CR induces changes in behaviour of the animals that could contribute to the pronounced health-promoting effects of CR in rodents. In addition, we aimed to investigate in more detail the effects of CR on the onset and severity of sarcopenia.
Methods
The mice received either an ad libitum diet (control) or a diet matching 70 E% of the control diet (C). Daily activity, body composition (dual energy X-ray absorptiometry), grip strength, insulin sensitivity, and general agility and balance were determined at different ages. Mice were killed at 4, 12, 24, and 28 months. Skeletal muscles of the hind limb were dissected, and the muscle extensor digitorum longus muscle was used for force-frequency measurements. The musculus tibialis was used for real-time quantitative PCR analysis.
Results
From the age of 12 months, CR animals were nearly half the weight of the control animals, which was mainly related to a lower fat mass. In the control group, the hind limb muscles showed a decline in mass at 24 or 28 months of age, which was not present in the CR group. Moreover, insulin sensitivity (oral glucose tolerance test) was higher in this group and the in vivo and ex vivo grip strength did not differ between the two groups.
In the hours before food was provided, CR animals were far more active than control animals, while total daily activity was not increased. Moreover, agility test indicated that CR animals were better climbers and showed more climbing behaviours.
Conclusions
Our study confirms earlier findings that in CR animals less sarcopenia is present. The mice on the CR diet, however, showed specific behavioural changes characterized by higher bursts of activity within a short time frame before consumption of a 70 E% daily meal. We hypothesize that the positive effects of CR on muscle maintenance in rodents are not merely a direct consequence of a lower energy intake but also related to a more active behaviour in a specific time frame. The burst of activity just before immediate start of eating, might lead to a highly effective use of the restricted protein sources available.
Keywords: Caloric restriction, Sarcopenia, Skeletal muscle, Lifestyle intervention, Daily activity, Muscle function
Introduction
In rodent models, caloric restriction (CR) without malnutrition (meaning with adequate mineral and vitamin supply) has been related to longevity and a reduction in the incidence of a broad range of chronic diseases including type II diabetes, cardiovascular disease, and cancer. Moreover, it has been reported to protect against age-related loss of muscle mass and function, known as sarcopenia.1,2 Studies in humans and monkeys suggest a similar association with type II diabetes and cardiovascular diseases.3,4 However, although results are significant and pointing towards beneficial health outcomes, effects are less pronounced than in the rodent models.1,2 The differences found between rodent and primate models have fuelled the discussion whether the positive health effects of caloric restriction found in rodent models represent an artefact resulting from domestication5 or inbreeding. One of the viewpoints is that rodents in domestication are less active and consume more, while caloric restriction supposedly creates a more natural condition and hence favourable phenotype. However, a comparison of mice living in nature with laboratory animals as reported by Austad and Kristan5 and Harper et al.6 has led to the consensus that the effect of domestication only to a limited extent explains the effect of caloric restriction. Another explanation proposed for muscle preservation observed is that caloric-restricted animals are more active than ad lib fed animals. However, previous studies have shown that caloric restriction induces either no change or a reduction in total daily activity.1,2,6 At higher age, higher total daily activity has been observed. This is probably more of a consequence than a reason for the reduction in sarcopenia.6 Furthermore, no change in mass-specific metabolic rate has been reported.6 In the present study, we investigated in more detail the possible contribution of feeding-related changes in behaviour to the positive health effects of caloric restriction in mice. In addition, we aimed to investigate more specifically the effects of caloric restriction on the onset and severity of sarcopenia in ageing male C57BL/6 J mice.
The term sarcopenia describes the age-related loss of muscle mass, strength, and function.7 While muscle loss is in principle a commonly occurring phenomenon during ageing, it may become a serious problem in elderly exhibiting extensive muscle wasting.7 Sarcopenia does not only affect quality of life but is also directly linked to fall incidences, frequency of fractures, and disability.8 Moreover, it is a predictor of physical disability and is related to loss of independence. The definition and diagnostic tool of the European Working Group on Sarcopenia in Older People (representing ESPEN, IANA, and the European Geriatric Medicine Society) is currently the most used.9,10 It is based on an algorithm that includes low muscle mass with low muscle strength and/or function.
The objectives of this study were as follows: firstly, to study the effect of caloric (macronutrient) restriction with adequate micronutrient supply (CR) on development of sarcopenia with advanced age, as measured by muscle mass, strength, and function; and secondly, to provide further insights in possible underlying mechanisms (beyond the reduced food intake) related to behavioural changes, insulin sensitivity, and expression of key regulating genes in muscle metabolism.
Materials and methods
Ethics statement
The institutional and national guidelines for the care and use of animals were followed and the experiment was approved by the Local Committee for Care and Use of Laboratory Animals at Wageningen University (code number: drs-2010151b).
Animals and diets
During a 2-week run-in period, male C57BL/6 J mice [7 weeks of age; Janvier (Cedex, France)] were housed in pairs (12-h light/dark cycle and light on at 4 a.m.). The mice had free access to water and received a standard American Institute of Nutrition (AIN)-93 G diet (Research Diet Services, Wijk bij Duurstede, The Netherlands). At 9 weeks of age, the mice were individually housed and allocated to two diet intervention groups (Figure 1A). The mice received either (1) AIN-93 W diet ad libitum (n = 89) [control diet (C)] or (2) AIN-93 W-CR in portions containing 70 E% of the measured mean daily energy intake of a randomly selected subset of 20 control mice of which food intake was measured for 1 week (n = 117) [caloric-restricted diet (CR)]. Portion size of the CR groups was adjusted at the age of 6, 12, 18, and 24 months and based on measurements performed in the control group in the week prior to the switch. The portions were provided once a day at 3:30 p.m., just before the light went off. The diets of the CR mice were supplemented with vitamins, essential fatty acids, and minerals to avoid deficiencies. The composition of the diets that is listed in Table 1 and Table S1 shows the ingredient list of all diets. In Table S2, the scheme of feeding is provided. Body weight of all mice was recorded every 2 weeks. Figure 1A provides a scheme of the set-up of the study. Sixteen animals per group were killed and dissected at an age of 12 and 24 months, whereas the remaining animals were killed at 28 months. Of these animals, all 16 underwent DEXA. The other measurements are based on eight animals, because the other half of the group received an additional challenge, not reported in this paper. Figure S1 provides an overview of the context of the measured parameters.
Figure 1.
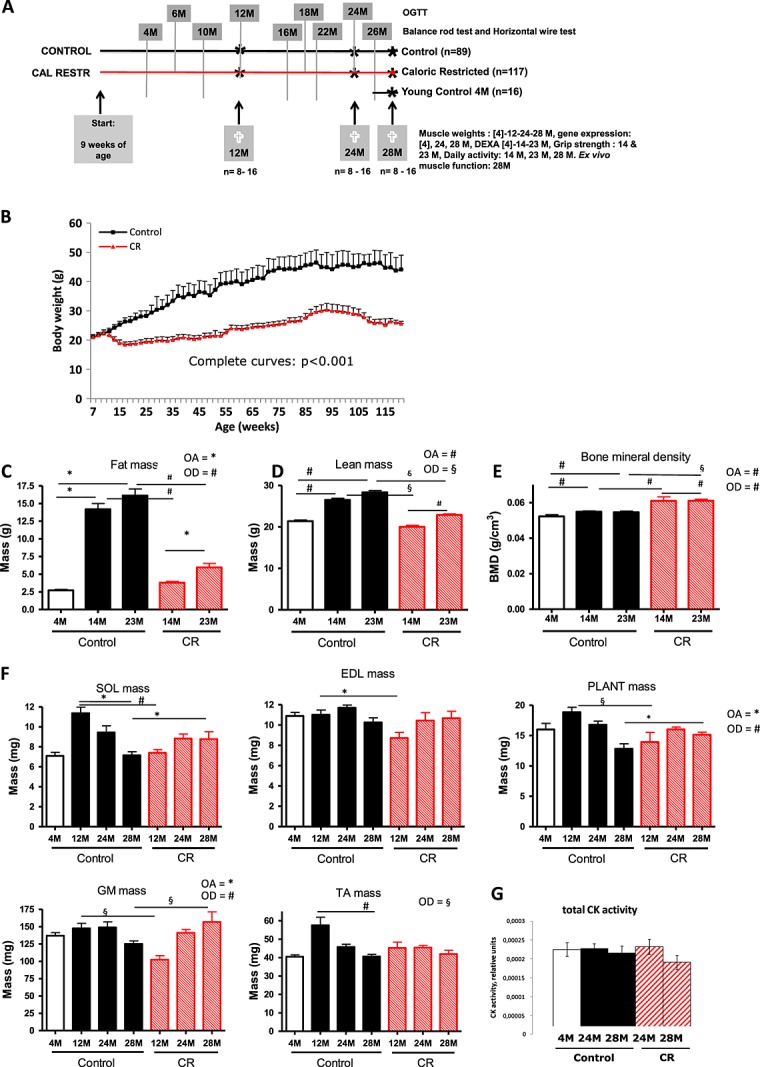
Experimental design and body composition measurements. (A) Experimental design. (B) Body weight development of the control and caloric-restricted (CR) animals. (C) Fat mass measured by dual energy X-ray absorptiometry (DEXA) scan. (D) Lean mass measured with dual energy X-ray absorptiometry scan. (E) Bone mineral density (BMD) measured by dual energy X-ray absorptiometry scan. (F) Absolute muscle mass of the mice on the different diet interventions during ageing. (G) Creatine kinase (CK) activity of whole muscle homogenates of right musculus tibialis. #P < 0.001; §P < 0.01; *P < 0.05. OA, overall age effect; OD, overall diet effect; OGTT, oral glucose tolerance test; SOL, soleus; PLANT, plantaris; GM, gastrocnemius; TA, tibialis anterior.
Table 1.
Composition of the diet
| Control |
Caloric restricted |
|||
|---|---|---|---|---|
| g% | Kcal% | g% | Kcal% | |
| Protein | 12 | 15 | 12 | 15 |
| Carbohydrate | 72 | 76 | 72 | 75 |
| Fat | 4 | 9 | 4 | 10 |
| Total | 100 | 100 | ||
| Kcal/g | 3.85 | 3.77 | ||
The diets of the control and calorie and macronutrient-restricted animals were the same, except for the fact that the diet of the restricted animals was compensated in micronutrients to such an extent that micronutrient intake was similar between all groups. The small difference in kcal/g provided between control and calorie restricted is due to the fact that the caloric-restricted diet was compensated for mineral and vitamin contents to avoid deficiencies. The complete ingredient list of the diets is provided in Table S1.
While the study was running, it became apparent that certain parameters that needed post-mortem measurements including the hind limb muscle mass measurements most likely already started to decline at a younger age. Therefore, it was decided to include an additional group (n = 16) of 4-month-old animals for comparison. In the figures, this extra control group is shown separately (open symbols). Prior to sacrifice, the mice were fasted for 10 h during the light-on phase of the experiment. Skeletal muscles (M. extensor digitorum longus (M. EDL), M. tibialis anterior, M. gastrocnemius, M. plantaris, and M. soleus) were dissected and weighed, snap frozen, and stored at −80°C until further molecular/biochemical analysis.
Functional tests
In vivo measurements
Because several of the equipment had to be borrowed, in vivo measurements could not all take place on exactly the same time. For measurement of non-invasive measurements in time, each time the same cohort of animals was used. In this way they could be their own control. Non-invasive measurements include measurements that do not influence the metabolism of the animal in time or require section (e.g. daily activity).
At 6, 12, 18, and 24 months of age (for the groups 12 and 24 months, this was 2 weeks prior to sacrifice), mice were subjected to an oral glucose tolerance test (OGTT). For this test, mice were fasted for 6 h, after which they received 1.5 mg glucose per gramme body weight via an oral gavage followed by analysis of blood glucose after 15, 30, 45, 60, 90, and 150 min via tail cut using Accu-Check blood glucose meters (Roche Diagnostics, Almere, The Netherlands).
At 14, 23, and 28 months of age, physical activity was monitored continuously (24 h) during 1 week as described previously.11–13 Activity sensors (dual technology detector DUO 240, Visonic; adapted by R. Visser, NIN, Amsterdam, The Netherlands) were mounted above the home cages, and data were analysed with MED-PC® IV software for data collection (MED associates, St Albans, VT, USA). Activity was expressed in counts per 30 min [both for the total 24-h period, the dark period (active period), and the light period (inactive period)]. Activity was calculated for each mouse separately. After an acclimatization period of 3 days, the activities of 4 days were averaged to dampen the day-to-day variability.
At approximately the same time points (12, 24, and 28 months), body composition was measured by dual energy X-ray absorptiometry (DEXA) scan, using a PIXImus imager (GE Lunar, Madison, WI, USA). The scan produced data concerning lean mass, fat mass, and bone mineral density. During the measurements, the animals were under general anaesthesia (isoflurane/N2O/O2).
At 14 and 23 months of age, forelimb grip strength measurements were performed with a calibrated grip strength apparatus from Panlab (Cornella, Spain), according to the protocol delivered with the equipment. For grip strength testing, a set included five maximum effort repetitions. From these five measurements, the mean and maximum grip strength were determined.
At 4, 10, 16, 22, and 26 months of age, agility and balance were measured with the horizontal wire test and the balance rod test.
Ex vivo measurements
At 28 months of age, ex vivo muscle performance was measured.11,12 After isolation, the EDL muscle was positioned in an organ tissue bath (Hugo Sachs Elektronik, March-Hugstetten, Germany), which was filled with Kreb's Heinselet buffer (mM: NaCl 118, KCl 4.75, MgSO4 1.18, CaCl2 2.5, KH2PO4 1.17, NaHCO3 24.9, and glucose 10). The buffer was kept at 30°C and continuously gased with 95% O2 and 5% CO2. The distal end of the muscle was connected to the base of the tissue bath, whereas the proximal end was attached to a force transducer (F30, Hugo Sachs Elektronik, March-Hugstetten, Germany). The muscle was positioned between two platinum electrodes for electrical stimulation. Isometric force signals of the force-frequency curve were analysed for maximal and total force, maximal contraction, and relaxation velocity. Furthermore, the maximal force production was followed in time during an exercise protocol.
At 4, 24, and 28 months of age, creatine kinase activity was determined ex vivo in whole muscle homogenates of right M. tibialis.
RNA isolation
Total RNA was isolated from the left M. tibialis as described previously.14 In brief, TRIzol reagent (Invitrogen, Breda, The Netherlands) was used, and the samples were treated with DNAse and purified on columns (RNAeasy micro kit, Qiagen, Venlo, The Netherlands), all according to the manufacturers' instructions. Purified RNA was immediately stored at −80°C until further use. RNA concentrations were determined using the NanoDrop ND-1000 UV-Vis spectrophotometer (Isogen, Maarsen, The Netherlands). RNA integrity was verified on an Agilent 2100 Bioanalyzer with the 6000 Nano Kit using the eukaryote total RNA Nano assay according to the manufacturer's instructions (Agilent Technologies, Amsterdam, The Netherlands). Samples were considered suitable for hybridization when they showed intact bands of 18S and 28S ribosomal RNA subunits, displayed no chromosomal peaks or RNA degradation products, and had an RNA integrity number above 8.0.
Complementary DNA synthesis and real-time quantitative PCR
Single-stranded complementary DNA (cDNA) was synthesized from 1 µg of total RNA using the reverse transcription system (iScript, Bio-Rad) following the supplier's protocol as described previously.14 cDNA was PCR amplified with Platinum Taq DNA polymerase (all reagents were from Invitrogen). Primer sequences were retrieved from the online PrimerBank database,15 or otherwise designed using the Primer3 program,16 and the sequences of the primers used are listed in Table S3 (primers). Primers were tested for specificity by basic local alignment search tool analysis. Real-time quantitative PCR (qPCR) was performed using SYBR green and a MyIQ thermal cycler (Bio-Rad laboratories BV, Veenendaal, The Netherlands). The following thermal cycling conditions were used 2 min at 94°C, followed by 40 cycles of 94°C for 15 s, and 60°C for 45 s. PCR reactions to validate ageing-induced differential gene expression were performed in duplicate, and all samples were normalized to 36B4 expression. The sequences of the primers used can be found in Table S3.
Determination of phosphorylated and unphosphorylated kinases of the mammalian target of rapamycin pathway
Ice-cold WB buffer [40 mM tris-HCl, 1 Mm ethylenediaminetetraacetic acid, 5 Mm ethylene glycol tetraacetic acid, triton X-100, PhosSTOP phosphatase inhibitor cocktail (1 tablet/10 mL), and protease inhibitor ultra tablets (a tablet/10 mL; pH 7.5)] was added to tibialis tissue that was frozen cut into pieces. Chrome steel beads, Ø2.3 mm, (BioSpec) were added and homogenization occurred shaking at 4°C using a FastPrep-24 (MP biomedical) for 3 × 30 sec on 6.5 m/s speed. Homogenates were separated with gel electrophoresis and western blotting. For detection of the proteins, the antibodies against mTOR, mTORP, Akt, Aktp, Eif4ebp1, and Eif4ebp1P derived from cell signalling were used. Detected bands were corrected for their Coomassie staining and corrected to a reference sample that occurred three times on each blot.
Creatine kinase assay
Creatine kinase activity was assessed with a coupled enzymatic reaction. The reaction buffer consisted of 100 mM imidazole, 20 mM glucose, 10 mM Mg acetate, 10 mM Adenosine diphosphate (ADP), 25 mM Adenosine monophosphate (AMP), 2 mM Nicotinamide adenine dinucleotide phosphate (NADP), 35 mM Phosphocreatine (PCr), 20 mM acetylcysteine, and 10 μM di(adenosine-5′)pentaphosphate (all obtained at Merck or Sigma). By addition of 0.5 mg/mL glucose-6-phosphate dehydrogenase and 158 U/mL hexokinase (both from Roche Diagnostics, Germany), the activity of CK leads to the formation of NADPH, which was measured colorimetric and kinetic at 340 nm (Molecular Devices, Spectra Max M2).
Statistics
For data that contained both repeated measures and multiple comparisons (different diets and different ages), mixed model statistics were used with a Bonferroni post hoc test. For repetitive measurements without multiple comparisons (body weight), the SPSS repeated measurement analysis was used with a Bonferroni post hoc test. For the bimodal data (yes or no answers like in the balance rod tests), a Pearson's X2 test was applied. All data were analysed with SPSS version 21.
Results
Body composition
Clear differences were found in body weight development between the control and the caloric-restricted groups (Figure 1B). DEXA analysis showed that most of the differences in body composition were due to a difference in fat mass (Figure 1C). Lean mass was very well conserved in the calorie and macronutrient-restricted animals (Figure 1D). Strikingly, the bone mineral density was higher in the calorie and macronutrient-restricted animals (Figure 1E). Measurement of skeletal muscle mass of individual muscles groups further support the DEXA results indicating muscle preservation or a muscle-sparing effect in the calorie and macronutrient-restricted group. Particularly at the age of 28 months, differences in muscle mass between the two intervention groups are relatively small, specifically when compared with the large difference present in body weight at all time points (Figure 1F). Relatively, therefore, the caloric-restricted animals are far more lean or less fat than their control counterparts (lean mass of total body weight C: 14 M 73%, 23 M 64% vs. CR: 14 M 85%, 23 M 77%).
In the control group, three out of the five hind limb muscles dissected showed a significant decline in muscle mass at 24 or 28 months of age. These data indicate an age-dependent decline in skeletal muscle mass of the extremities. Remarkably, this age-related decline in muscle mass of the extremities was not observed in the mice exposed to the caloric-restricted diet. For the gastrocnemius muscle (GM), there was even an increase in mass in time.
Creatine kinase activity
Total creatine kinase enzyme activity as measured in the muscle homogenate was not changed (Figure 1G) in time or between diets.
Glucose tolerance and insulin sensitivity
The OGTT tests revealed that in the caloric-restricted group, the blood glucose levels and the incremental area under the glucose curves were significantly lower at all ages (Figure 2A). The difference was most striking at the age of 12 months. As shown in Figure 2B, fasting insulin concentrations were overall significantly lower in the caloric-restricted animals when compared with the control animals. Next to that, the results presented in Figure 2B also reveal that fasting insulin levels were lower in young animals (6 months) of the control group, compared with all the other age groups. In contrast, in the caloric-restricted animals fasting, insulin levels did not increase with age.
Figure 2.
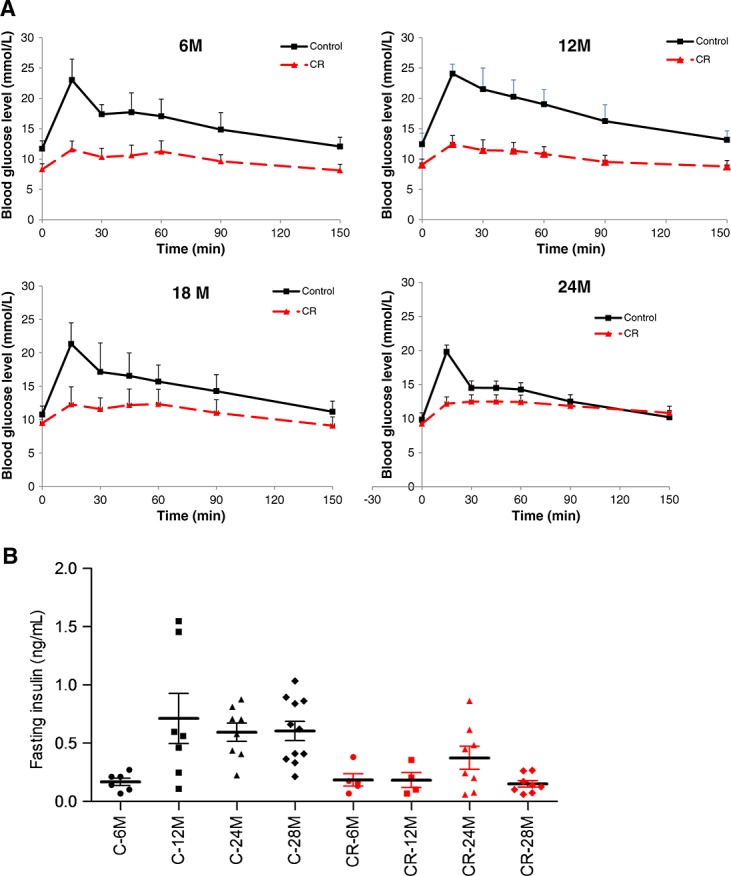
Measurements of insulin sensitivity. (A) The results of the oral glucose tolerance test of mice of different ages on caloric-restricted (CR) diet or control diet. Mice were subjected to an oral glucose tolerance test 2 weeks prior to sacrifice. Data represent mean ± standard deviation. Mixed models: difference on diet: overall and in all age groups P < 0.001. Difference on age: overall and in the control group: 12 vs. 6, 18, or 24 months P < 0.001. In control group, all age groups are compared with each other P < 0.03. (B) Insulin levels of the same mice at the different ages on caloric-restricted diet or control diet. Mixed models: difference on diet: overall and in all age groups P < 0.001. Difference on age: overall no significances. In control group: 6 vs. 12 P < 0.09, 24 P = 0.055, or 28 months P = 0.052.
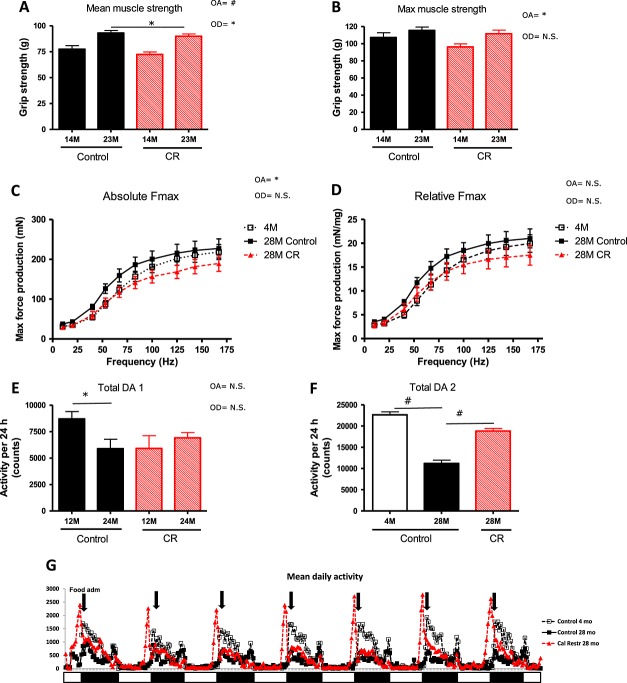
Muscle function, performance, behaviour, and overall activity
Despite the significant differences in absolute muscle mass of five measured skeletal muscle groups as well as the lean body mass between the control and the caloric-restricted groups during lifetime, the mean in vivo grip strength did not differ between the two groups (Figure 3A and B). These data suggest a higher functional capacity of the muscles and locomotor apparatus of the caloric-restricted animals. These findings are confirmed by the results obtained from the ex vivo muscle function measurements at 28 months of age (Figure 3C and D) showing a difference on age but not on diet.
Figure 3.
Locomotor function: muscle strength and daily activity of the mice on the different diet interventions during ageing. (A) Mean grip strength (mean of three median measurements out of five) and (B) maximal grip strength. Mixed model: overall and per age group no diet effect. Overall age effect: P = 0.02 but not in separate groups. (C) Maximal force production. Mixed model: difference on age (P < 0.05) but not on diet. (D) Relative force frequency. (E) Mean daily activity of the mice of 12 and 24 months of age. The data represented in this figure are from the same set of animals and can therefore also be compared towards a decrease or increase in activity in time. (F) Mean daily activity of the mice of 28 months of age when compared with the animals of 4 months of age, which were investigated at the same time. (G) An example from the mean of the raw data from 28 months. The sample is also the representative for the data found at 24 months. White and black boxes represent the light and dark periods. #P < 0.001; §P < 0.01; *P < 0.05. OA, overall age effect; OD, overall diet effect; CR, caloric restricted; n.s., no significance.
Control animals showed a significant reduction of total daily activity between the age of 12 and 24 months (Figure 3E). The calorie and macronutrient-restricted group, however, started with a lower total daily activity at 12 months, comparable with that of the control group at 24 months of age. Remarkably, in this intervention group, the activity did not decrease in time; in fact, it increased. At 28 months of age, daily activity of control animals was markedly reduced compared with the calorie-restricted mice and a group of 4 months of age (Figure 3F). We also found that for all ages, the caloric-restricted group revealed a dramatic increased level of activity concentrated in a very narrow time frame (Figure 3G). The activity counts and video behavioural observation of the animals indicated a high activity, with more frequent climbing of the caloric-restricted group compared with the control group of about 4 h before the food was provided (as a representative movie of the behaviour, the animals is added (see Supporting information, Movies S1–S2)). When the food was provided, the caloric-restricted animals started with an initial pulse feed (more than half of the food provided) and finished their meals completely within the dark period (12 h period, see Figure 7).
Figure 7.
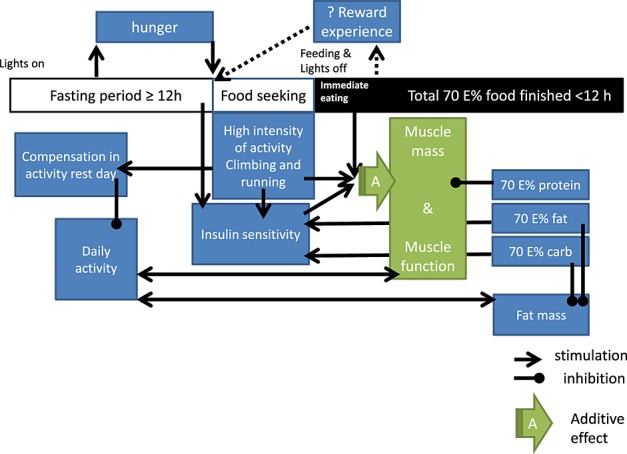
Schematic representation of the hypothesized working mechanism.
To investigate the agility and climbing capacity of the animals, a horizontal wire test was performed (Figure 4A). The ability of the animals exposed to the control diet to successfully grasp with the hind leg a horizontal wire, when being attached to the wire with its front paws, gradually diminished with increasing age. In contrast, the calorie and restricted animals did not show such a decline with advancing age. These findings suggest that caloric restriction-exposed animals maintain muscle function and coordination better over time. They might even be better climbers throughout time, which is in line with different activity patterns during the day (Figure 3G) and maintenance of muscle mass of the extremity as describe above (Figure 1F). In addition, a balance rod test was performed, a test in which both learning capacity and agility play a role (Figure 4B). Overall, mice in both intervention groups showed markedly fewer drop-offs in the second and third trials, suggesting a learning effect in this balance rod test. However, at 26 months of age, the control group showed a diminished performance on balance and a decreased learning curve. In the caloric-restricted group, however, balance was better when compared with the controls at all ages and the capacity to perform better at the second and third attempt remained high, even at old age.
Figure 4.
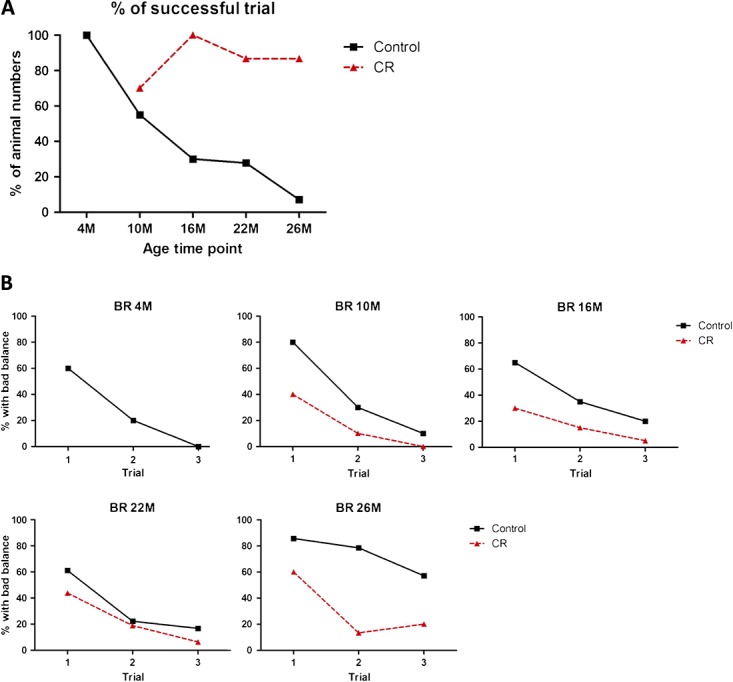
Agility and balance tests. (A) The percentage of animals that can successfully grasp with the hind leg a horizontal wire when being attached to the wire with its front paws in the horizontal wire test. Pearson's X2 analysis revealed a significant difference on age in the control group (P < 0.001) but not in the calorie-restricted (CR) group (P = 0.069). Overall control and calorie restricted were significantly different (P < 0.001). (B) The percentage of animals presenting with imbalance when performing the balance rod test at different points in time. Pearson's X2 analysis revealed a significant difference on age in the control group (P < 0.001) but not in the calorie-restricted group (P = 0.2). Overall control and calorie-restricted were significantly different (P < 0.001).
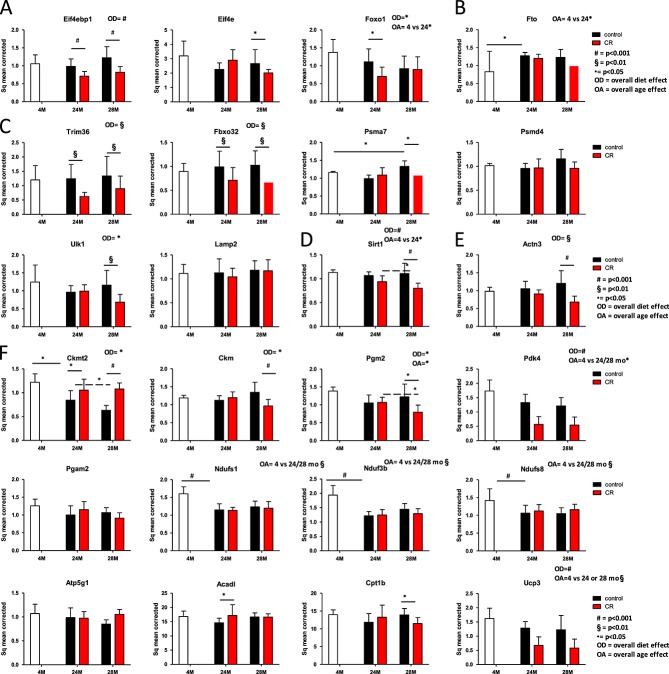
Expression of key regulating genes in muscle metabolism
Gene expression was measured to investigate the mechanisms involved in the ‘sparing or continued building’ of the muscle in caloric and macronutrient-restricted animals. Expression was measured of established representative key regulator genes of different pathways involved (see Supporting Information, Figure S1). In general, differences in gene expression found between control and caloric-restricted animals were more pronounced at a higher age. From the genes involved in protein synthesis, elongation factor 4 binding protein (Eif4ebp1) was down-regulated in caloric-restricted mice compared with mice receiving the control diet (Figure 5A, protein synthesis). Expression levels of the elongation factor itself (Elf4e) did not show an overall difference between either the diet groups or the different ages. At 24 month of age, expression of Foxo-1, a gene reducing the insulin sensitivity of the mTOR pathway, downstream of Akt, was lower in the caloric-restriction group compared with the control group. Fto (Figure 5B), a gene upstream of mTOR, involved in obesity but also in amino acid sensing was differentially expressed during growth of the animal (4 vs. 12 months), but did not change otherwise.17 At muscle protein breakdown level (Figure 5C), the myosin specific E3 ubiquitin ligases Trim63 (formerly known as Murf-1) and Fxo32 (formerly known as Atrogin-1) were overall significantly lower in CR compared with control animals. The proteasome subunit Pmsa7 was down-regulated in the CR group at 28 months, but gene expression of Psmd4 was not changed. The autophagy activating kinase Ulk-1 was down-regulated in the CR group at 28 months, but Lamp-2 was not. Sirt-1, a gene that has been linked to insulin sensitivity and longevity showed an overall diet effect, with a reduced expression in the caloric-restricted animals (Figure 5D). Actn3 (Figure 5E), a gene involved in fibre-type switch, was down-regulated at 28 months, which has been associated with a fibre-type switch towards type I fibres (aerobic profile). Next to this, there was an up-regulation of the mitochondrial creatine kinase cktm2 in the caloric-restricted mice (Figure 5F, energy metabolism). In addition, the creatine kinase present in the cytosol, Ckm was down-regulated. The genes Pgm2 (phosphoglucomutase 2) and Pdk4 (pyruvate dehydrogenase kinase, isozyme 4), both coding for enzymes of the glycolysis were also down-regulated in caloric-restricted mice compared with control. The expression of mitochondrial proteins of the oxidative phosphorylation pathway all showed an age-dependent decline in expression between 4 and 24 or 28 months of age but no diet effect. These proteins include the iron sulfur clusters from complex 1, Ndufs1 and Ndufs8, the NADH dehydrogenase (ubiquinone) 1 beta subcomplex 3 Ndufb3, and Mnf2, a mitochondrial membrane protein that participates in mitochondrial fusion and contributes to the maintenance and operation of the mitochondrial network. For the fatty acid oxidation, there was no effect of age on the gene expression profile of long-chain acyl-coenzyme A dehydrogenase (Acadl) and Cpt1b, involved in carnitine-mediated transport. For Cpt1b, only in the 28 months group there was a difference on diet. Ucp3, involved in uncoupling of complex I, showed an age-dependent decline in expression between 4 and 24 or 28 months of age. In contrast to the proteins involved in complex I, this gene was overall down-regulated in the caloric-restricted compared with the control group (Figure 5D). Figure S1 provides a detailed overview of how the gene expressions data are related to the physiological data measured.
Figure 5.
Gene expression profiles in musculus tibialis, depicted per pathway. On all data, a mixed model analysis was performed. Overall significances are depicted above the picture, within group significances are depicted with lines and symbols indicating the level of significance of the P-value. (A) Muscle protein synthesis. (B) Amino acid sensing. (C) Muscle protein breakdown. (D) Sirtuin 1. (E) Indication of fibre-type switch. (F) Energy metabolism. CR, caloric restricted.
Protein level of phosphorylated and unphosphorylated kinases of the mammalian target of rapamycin pathway
Figure 6 shows that for mTOR, in the fasted state, there was an increase in phosphorylated mTOR in the CR group (overall significant diet effect). Next to that, the total Akt protein was increased in this group. For Eif4ebp1 and Eif4ebp1P no changes occurred. The blots revered to are shown in Figure S2.
Figure 6.
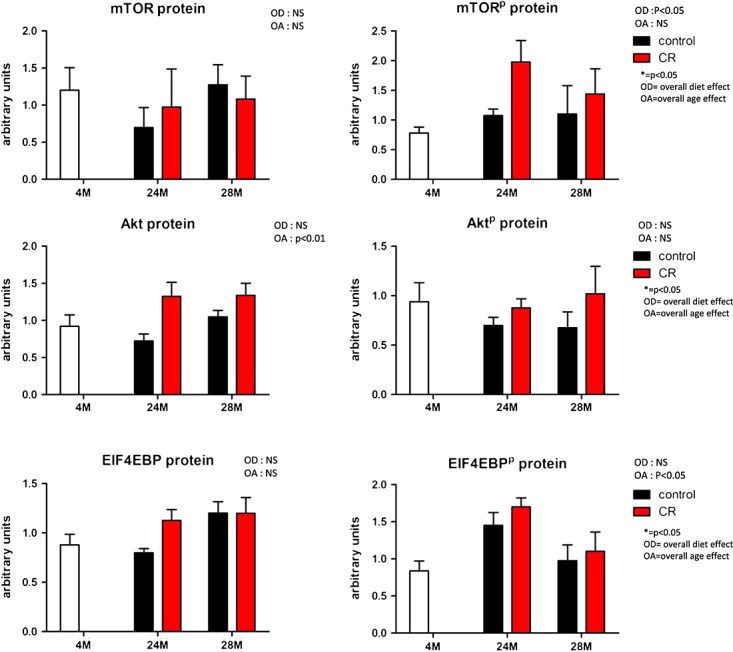
Protein levels of key regulator proteins of the mammalian target of rapamycin (mTOR) pathway both in unphosphorylated and phosphorylated forms. On all data, a mixed model analysis was performed. Overall significances are depicted above the picture, within group significances are depicted with lines and symbols indicating the level of significance of the P-value. OA, overall age effect; OD, overall diet effect; CR, caloric restricted.
Discussion
The data presented in this study indicate that caloric and macronutrient restriction with adequate micronutrient intake in rodents induces effects going beyond those directly mediated by lower energy intake. Instead, we show that this model stimulates behavioural changes which, to some extent, mimic physical exercise in humans (a combination of resistance and aerobic exercise). Increasing evidence suggests that in humans, regular physical exercise has beneficial effects on health, independently from a reduced body weight.18–20 We recorded a typical biphasic response, as has been described earlier,2,21–24 with very high activity prior to the feeding and an overall low activity during the rest of the day. This peak activity could be regarded as a so-called bout of exercise. Added to this is a clear indication of a higher agility and flexibility of the animals, and a better capacity to climb. This further suggests that not only the temporal pattern of the daily activity changed but also the type of activity performed (illustrated in Figure 7 representing an overall hypothesized working mechanism). Increased time spent on climbing might also explain the higher bone mineral density found in the caloric-restricted animals, which was rather an unexpected finding. Moreover, active climbing in the caloric-restricted group might explain why the muscle strength as measured in vivo (strength of forelimb) is similar between groups for all ages measured, despite the differences in muscle mass during younger age. It has, however, to be said that in vivo grip strength is dependent on more factors than muscle function and muscle mass alone. Differences could also be due to differences in innervation, vascularization, adenosine triphosphate supply (glycogen stores), functional bone mass, and functional ligands. Each of these functions together influences the whole locomotor unit.
These findings were confirmed by the ex vivo measurements of muscle function (strength of one of the muscles of the hind limb), of which the results were also similar between the different diets. Taken together, our observations indicate that positive effects on health and lifespan in caloric-restricted rodents are not necessarily the mere result of reduced energy intake. Instead, these effects could be considered in the light of a complete ‘lifestyle intervention’. The motivation for this changed behaviour might be getting a reward (food) after exercise.
The C57BL/6 J mouse strain used in this study is very commonly referred to in studies both on ageing and caloric restriction. It is nowadays possible to buy aged mice of this strain, which have been maintained on a high protein diet (23 E%) at the supplier, to decrease disease incidence and to reduce mortality rates. This common practice of mouse breeding till high age is in contrast with the reported results that a reduction of protein amount in the diet can, in part, mimic the effects of caloric restriction on oxidative stress and longevity.2,15,16 This is even more striking because these effects could not be reached with either a reduction of carbohydrate or a reduction in fat content of the diet,2,15,16 pointing to a special role for protein. Thus, there is a contradiction between the contribution of protein in the diet to common practice rodent breeding and the experimental findings on long-life caloric and protein restriction. In our opinion, it is hard to discriminate in rodents between effects of energy, macronutrients, and daily activity when performing diet restriction studies. We hypothesize that the effect of exercise is quite large. Otherwise, a ceiling effect for protein will occur, and the protein consumed, which is not directly used, will be degraded.25 Moreover, the timing of exercise to protein and food intake might be crucial for muscle maintenance.
An inadequate protein intake has been related to a reduction of muscle mass.26,27 In the present study, the animals were caloric restricted without malnutrition, which means that the restriction was on macronutrients, while normal levels for micronutrients were maintained. This also implicates that the total protein intake is only 70% of the normal intake. Despite this fact, the animals were very comparable in muscle strength and even muscle masses, specifically when related to body mass. There are different mechanisms that might underlie this phenomenon. First, the improved insulin sensitivity might not only affect glucose uptake but also that of amino acids and in this way stimulate the amino acid-induced protein synthesis. Our data show very clear differences in an OGTT and on fasting insulin levels that confirm several previous findings concerning the impact of caloric restriction on insulin sensitivity.28 This might be due to a lower daily intake of fat and carbohydrates in combination with the changes in time in daily activity. Our results indicate a higher content of phosphorylated mTOR in the fasted state in the CR group compared with the controls. Next to that, there was an increase in total Akt protein levels, which might suggest an increased capacity to respond to a post-prandial amino acid response. However, Miller et al. described in their recent study of caloric-restricted mice without malnutrition on a similar protocol muscle mass maintenance but no differences in the acute amino acid-induced muscle protein synthesis rate for 6, 12, or 21 months on caloric-restricted diets when compared with control-fed mice.29 Therefore, an increased synthesis rate in the fasted state seems more likely to contribute to the muscle maintenance.
A second explanation might be sought in the fact that the animals consume the diet immediately after presentation and consume it during a narrow or limited time frame. This means that the food, and in this case specifically the protein, is consumed as a boost or pulse. With respect to treatment of sarcopenia, pulse feeding is currently receiving interest as possible remedy to muscle loss. Muscle tissue continuously rebuilds, and in healthy individuals, muscle protein synthesis and breakdown are in equilibrium. Next to that, muscle tissue follows the pattern of fasting and feeding in healthy individuals. After a meal, the net protein metabolism results in muscle building, while during fasting, the net effect is muscle protein breakdown. The net muscle protein synthesis response is related to the rise in essential amino acids in the blood. A specific threshold to the anabolic action of essential amino acids has been suggested.30–32 This threshold increases with age, implicating that more protein consumption is needed to reach the threshold. In a clinical trial in elderly women, Arnal et al. showed that muscle loss during ageing could be related to a lower sensitivity of muscle protein synthesis to feeding.33 However, when the protein intake was provided as a boost at the moment that persons were most active (80% of the dietary proteins were consumed at noon), instead of a spread of the protein content during the day, this considerably improved the nitrogen balance in these elderly women. Apparently, the decreased protein sensitivity could be overcome by a feeding pattern referred to by the authors as a pulse feeding pattern. Last year, these data were confirmed in a prospective randomized study with 66 elderly malnourished patients showing a clinically relevant effect by pulse feeding on lean mass in these patients.34 Arnal et al. investigated in rats, which organs are sensitive for pulse feeding. It turned out that both skeletal muscle and liver showed an improved nitrogen balance under a pulse feeding regime, while there was no effect observed on the intestine.33 Moreover, a protein boost after resistance or mixed type of exercise have been described as most optimal for protein synthesis in humans.26,35 This optimal combination of protein supplementation combined with exercise is for example used in prevention and treatment of muscle loss in chronic obstructive pulmonary disease (COPD) patients.36–38 Also in renal patients, who often have to live with dietary protein restrictions exercise in combination with nutritional support has been shown to promote net muscle accretion and is therefore recommended in the ESPEN guidelines for renal patients.39 The results presented in this study led to the assumption that a 70 E% protein intake consumed in a boost after exercise would result in a daily net protein synthesis rate similar or nearly similar to control animals (see the overall hypothesis presented in Figure 7).
For other macronutrients, like carbohydrates, however, pulse feeding might be negative and result in high post-prandial glucose peaks, which have been described to contribute to the development of insulin resistance.40 In caloric-restricted mice, however, insulin sensitivity is higher than in control animals, despite the presence of pulse feeding. We hypothesize that this is due to different factors acting in a synergistic manner. Firstly, a fast uptake of glucose by the repeated daily exercise induced insulin sensitive muscle. Secondly, the healthy carbohydrate diet composition of AIN-93 M, being high in starch (465 g/kg) compared with dextrins (155 g/kg) and sucrose (100 g/kg), might prevent occurrence of excessive glucose peaks after pulse feeding. Finally, the reduced intake of all macronutrients (caloric restriction) has been described to be responsible for a reduction in the insulin like growth factor 1 (IGF-1) and insulin signalling contributing to the overall increased insulin sensitivity in caloric-restricted mice as also present in the presented study. This overall improved insulin sensitivity might further dampen the effect of pulse feeding on carbohydrate metabolism. Moreover, decreased fat stores in the adipose tissue might result in a fast handling of lipids into the adipose tissue and, as a consequence, less lipid-induced oxidation and inflammation. To our opinion, it is therefore important to refer to caloric restriction as a macronutrient restriction, in which also the composition of the diet should be taken into account.
In mice exposed to the control diet, a significant ageing-related reduction in muscle mass was measured in three out of the five hind limb muscles examined in suggesting that these mice suffer from sarcopenia. However, on total lean mass as measured with DEXA, no age-related reduction was found. The reason for this discrepancy is unclear. It is possible that in ageing mice a mild form of oedema is present, contributing to a less accurate measurement of lean mass by DEXA when compared with dissecting and weighing muscles. In contrast to mice that have received the control diet, in the caloric-restricted group, no age-related decline in muscle mass was observed, which could be interpreted as protecting against sarcopenia. Absolute muscle masses were approximately similar in both groups at 28 months of age as were grip strength measurements. Eif4ebp1, the binding protein and silencer of a key elongation factor in protein synthesis (Eif4), was significantly down-regulated in caloric-restricted mice compared with control mice, both overall and within groups. The elongation factor itself was, however, at 28 months, lower in expression level in the caloric-restricted group. Therefore, also on gene expression level, the total contribution of caloric restriction to muscle protein synthesis is difficult to interpret. It is, however, striking that Ulk-1 and Foxo-1, two genes downstream of the mTOR pathway and inhibited by mTOR and Akt, respectively, are down-regulated in the CR group at 28 months. The authors would like to speculate that the mTOR pathway at 28 months of age might be more active in the caloric-restricted group when compared with the control group. At muscle protein breakdown level, Murf-1 and Atrogin-1 were overall significantly lower in the caloric-restricted groups; moreover at proteasome level, Pmsa7 was significantly down-regulated at 28 months. A lower expression of genes involved in muscle protein breakdown in the caloric-restricted group might contribute to muscle maintenance, but these results need to be confirmed on protein expression level. Additionally, expression of Actn3, a gene involved in fibre-type switch, was affected in such a way that a shift towards an aerobic compared with an anaerobic profile is expected. Such a fibre-type switch has previously been reported upon duration of training and fasting.41 A fibre-type switch towards an aerobic profile is confirmed by an up-regulation of the mitochondrial creatine kinase gene in the caloric-restricted mice that coincides with down-regulated expression of the cytosolic muscle creatine kinase gene in these mice. Total creatine kinase enzyme activity as measured in the muscle homogenate was not changed, indicating that the changes induced on cytosolic and mitochondrial levels might be in equilibrium. The genes Pdk4 and Pgm2, catalysers of the glycolysis were down-regulated in caloric-restricted mice compared with control. These data further confirm a fibre-type switch towards a more aerobic profile. The expression levels of mitochondrial proteins of the oxidative phosphorylation pathway including Ndufa2, Ndufb3, Atp5g1, and Mnf2, however, revealed no difference in expression between the two diet groups. In contrast, Ucp3 expression was down-regulated in mice exposed to the CR diet, which might reflect a more efficient oxidative phosphorylation, rather than an increase in numbers of mitochondria.
Autophagy is a highly conserved process that generates nutrients during fasting and is under the control of amino acids and hormones.42 The activity of autophagy has been reported to decline with age. Caloric restriction has been described to prevent against the age-related decline of autophagic proteolysis.43 On gene expression level, for Ulk-1 such an age-dependent decline and a reduced expression in the caloric-restriction group were confirmed. However, expression of Lamp-2 was not different between the intervention groups. Ulk-1 expression is inhibited by mTOR, which, as mentioned above, might indicate that the mTOR protein synthesis pathway is active. It has been described that effects of caloric restriction on autophagy were exerted by Sirt-1. Up-regulation of the Sirt-1 has been suggested to be involved in longevity in rodents.44 Next to that, Sirt-1 knockouts have been shown to lack the initial stimulatory effect of caloric restriction on mTOR activity.45 Moreover, Chen et al.22 studied the direct effects of calorie restriction on eating behaviour. For this direct effect, it has been described that total activity increases.23,24 In the study presented, we did not examine the direct effects of calorie restriction but focused our analysis on the long-term effects of calorie restriction, which showed no increase in total daily activity up to 24 months and even a tendency to a decrease in total daily activity at 12 months. Only at 28 months, the total daily activity was higher. Interestingly, however, the study by Chen et al.22 shows that Sirt-1 KO mice do not display the initial increase in total daily activity due to the increase food anticipatory activity (FAA). Up-regulation of Sirt-1 appears, however, not to occur uniformly across tissues or across different studies.2 In this model, for example, Sirt-1 expression of the muscle was down-regulated at the age of 28 months and still there was an increased FAA in the CR group and lower total daily activity with exception of the 28 months CR group. These data indicate that more research is needed to further clarify age, strain, and tissue specific to the effects of the sirtuin and autophagy pathways during caloric restriction. Since Sirt-1 regulates the mTOR pathway, tissue-specific effects of caloric restriction might contribute to the apparent contradiction that seems to occur in caloric-restricted animals.
In general, the results have led to our following hypothesis (Figure 7): Mice on a CR diet have more hunger and therefore show an increased FAA. This behaviour stays for the rest of their life if CR maintains. This activity is different from normal daily activity in a way that it is more intense: more running and climbing. The animals compensate for the activity during the rest of the day, which might result in a decreased total daily activity. Furthermore, it is hypothesized that the increase in activity just before the meal is provided, increases the insulin sensitivity of the body and assumedly of the muscle. A lower total provided load of carbs and fat due to the CR can further contribute to an improved insulin sensitivity. Next to that triggers the bout of activity an anabolic response of the skeletal muscles involved in this activity. We moreover speculate that the increased insulin sensitivity combined with the activity-induced anabolic response lower the threshold needed for protein synthesis and therefore make maintenance of muscle protein possible.
Conclusion
Control animals showed an age-dependent sarcopenia, while caloric-restricted animals showed muscle mass and strength maintenance during lifespan. An adequate amount of protein provided as a bolus, preferably in combination with exercise, is currently recommended for sarcopenic elderly and COPD patients. These recommendations are designed to promote net muscle accretion. Our results indicate that mice on a 70 E% caloric-restriction diet show habitual changes that come close to these recommendations and therefore indicate that CR should be considered as a lifestyle and not simply a diet intervention.
Acknowledgments
The authors would like to thank Dr Giles Yeo for the help on the qPCR of Fto. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (S. von Haehling, J. E. Morley, A. J. Coats, and S. D. Anker).
Funding
Supported by the European Union's Seventh Framework Programme IDEAL (FP7/2007-2011) under grant agreement no. 259679.
Conflict of interest
None declared.
Supporting information
Supporting information may be found in the online version of this article.
Figure S1. An overview of the context of the measured parameters. For abbreviations see list of abbreviations.
Figure S2. Blots showing the proteins and phosphorylated proteins of the mTOR pathway. The 2 blots at the bottom are stained with Coomassie.
Table S1. The ingredient list of both diets.
Table S2. The provided food and measured food intake. * the portion of 58.5 kcal/week food intake resulted in a great weight loss in this young group of CR mice, which was not allowed ethically. Therefore, the food restriction was adjusted to 75-80% for the first 2 months of CR (age 2-4 month).
Table S3. List of primers used.
Movie S1 & S2. Representative movies of the behaviour of the animals.
References
- 1.Cava E, Fontana L. Will calorie restriction work in humans? Aging. 2013;5:507–514. doi: 10.18632/aging.100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speakman JR, Mitchell SE. Caloric restriction. MAM. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Bales CW, Kraus WE. Caloric restriction: implications for human cardiometabolic health. J Cardiopulm Rehabil Prev. 2013;33:201–208. doi: 10.1097/HCR.0b013e318295019e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, et al. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2011;46:23–29. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–207. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 6.Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharf G, Heineke J. Finding good biomarkers for sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:145–148. doi: 10.1007/s13539-012-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, Edwards M, Dennison E, Cooper C, Sayer Aihie. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013;42:378–384. doi: 10.1093/ageing/afs197. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Norren K, Kegler D, Argiles JM, Luiking Y, Gorselink M, Laviano A, et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br J Cancer. 2009;100:713–722. doi: 10.1038/sj.bjc.6604905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Norren K, van Helvoort A, Argiles JM, van Tuijl S, Arts K, Gorselink M, et al. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer. 2009;100:311–314. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwarkasing JT, Dijk MV, Dijk FJ, Boekschoten MV, Faber J, Argiles JM, Laviano A, et al. Hypothalamic food intake regulation in a cancer-cachectic mouse model. J Cachexia, Sarcopenia, Muscle. 2013;5:159. doi: 10.1007/s13539-013-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mischke M, Pruis MG, Boekschoten MV, Groen AK, Fitri AR, van de Heijning BJ, Verkade HJ, et al. Maternal western-style high fat diet induces sex-specific physiological and molecular changes in two-week-old mouse offspring. PLoS One. 2013;8:e78623. doi: 10.1371/journal.pone.0078623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson SJ, Raubenheimer D. Macronutrient balance and lifespan. Aging (Albany NY) 2009;1:875–880. doi: 10.18632/aging.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanz A, Gomez J, Caro P, Barja G. Carbohydrate restriction does not change mitochondrial free radical generation and oxidative DNA damage. J Bioenerg Biomembr. 2006;38:327–333. doi: 10.1007/s10863-006-9051-0. [DOI] [PubMed] [Google Scholar]
- 17.Loos RJF, Yeo GSH. The bigger picture of FTO—the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2013;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JH, Macgillivray S, McFarlane S. Interventions to promote long-term participation in physical activity after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2014;95:956–967. doi: 10.1016/j.apmr.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Santa Mina D, Clarke H, Ritvo P, Leung YW, Matthew AG, Katz J, et al. Alibhai SM. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy. 2013;100:196–207. doi: 10.1016/j.physio.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Corhay JL, Dang DN, Van Cauwenberge H, Louis R. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis. 2014;9:27–39. doi: 10.2147/COPD.S52012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Severinsen T, Fau-Munch IC, Munch IC. Body core temperature during food restriction in rats. Acta Physiol Scan. 1999;165:299–305. doi: 10.1046/j.1365-201x.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, Steele AD, Lindquist S, Guarente L. Medicine: increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 23.Gallardo CM, Hsu CT, Gunapala KM, Parfyonov M, Chang CH, Mistlberger RE, et al. Behavioral and neural correlates of acute and scheduled hunger in C57BL/6 mice. PLoS One. 2014;9:e95990. doi: 10.1371/journal.pone.0095990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luby MD, Hsu CT, Shuster SA, Gallardo CM, Mistlberger RE, King OD, et al. Food anticipatory activity behavior of mice across a wide range of circadian and non-circadian intervals. PLoS One. 2012;7:e37992. doi: 10.1371/journal.pone.0037992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemon PWR. Dietary protein requirements in athletes. J Nutr Biochem. 1997;8:52–60. [Google Scholar]
- 26.Evans WJ. Protein nutrition, exercise and aging. J Am Coll Nutr. 2004;23:601S–609S. doi: 10.1080/07315724.2004.10719430. [DOI] [PubMed] [Google Scholar]
- 27.Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26:696S–703S. doi: 10.1080/07315724.2007.10719650. [DOI] [PubMed] [Google Scholar]
- 28.Argentino DP, Dominici FP, Al-Regaiey K, Bonkowski MS, Bartke A, Turyn D. Effects of long-term caloric restriction on early steps of the insulin-signaling system in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 2005;60:28–34. doi: 10.1093/gerona/60.1.28. [DOI] [PubMed] [Google Scholar]
- 29.Miller BF, Robinson MM, Reuland DJ, Drake JC, Peelor FF, 3rd, Bruss MD, et al. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci Med Sci. 2013;68:530–538. doi: 10.1093/gerona/gls219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walrand S, Guillet C, Salles J, Cano N, Boirie Y. Physiopathological mechanism of sarcopenia. Clin Geriatr Med. 2011;27:365–385. doi: 10.1016/j.cger.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Walrand S, Zangarelli A, Guillet C, Salles J, Soulier K, Giraudet C, et al. Effect of fast dietary proteins on muscle protein synthesis rate and muscle strength in ad libitum-fed and energy-restricted old rats. Br J Nutr. 1683;106:1683–1690. doi: 10.1017/S0007114511002182. [DOI] [PubMed] [Google Scholar]
- 33.Arnal M-A, Mosoni L, Boirie Y, Houlier M-L, Morin L, Verdier E, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- 34.Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chretien P, Schauer N, et al. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin Nutr. 2013;32:186–192. doi: 10.1016/j.clnu.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, John M, Pedersen PU, Raguso C, Cicoira M, Dardai E, et al. ESPEN guidelines on enteral nutrition: cardiology and pulmonology. Clin Nutr. 2006;25:311–318. doi: 10.1016/j.clnu.2006.01.017. Epub 2006 May 2011. [DOI] [PubMed] [Google Scholar]
- 37.van de Bool C, Steiner MC, Schols AM. Nutritional targets to enhance exercise performance in chronic obstructive pulmonary disease. Curr Opin Clin Nutr Metab Care. 1097;15:553–560. doi: 10.1097/MCO.0b013e328358bdeb. [DOI] [PubMed] [Google Scholar]
- 38.Collins PF, Elia M, Stratton RJ. Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respirology. 2013;18:616–629. doi: 10.1111/resp.12070. [DOI] [PubMed] [Google Scholar]
- 39.Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, et al. ESPEN guidelines on parenteral nutrition: adult renal failure. Clin Nutr. 2009;28:401–414. doi: 10.1016/j.clnu.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care. 2013;36:4166–4171. doi: 10.2337/dc13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li P, Akimoto T, Zhang M, Williams RS, Yan Z. Resident stem cells are not required for exercise-induced fiber-type switching and angiogenesis but are necessary for activity-dependent muscle growth. Am J Physiol Cell Physiol. 2006;290:C1461–1468. doi: 10.1152/ajpcell.00532.2005. [DOI] [PubMed] [Google Scholar]
- 42.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. Epub 2005 Oct 2013. [DOI] [PubMed] [Google Scholar]
- 44.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. An overview of the context of the measured parameters. For abbreviations see list of abbreviations.
Figure S2. Blots showing the proteins and phosphorylated proteins of the mTOR pathway. The 2 blots at the bottom are stained with Coomassie.
Table S1. The ingredient list of both diets.
Table S2. The provided food and measured food intake. * the portion of 58.5 kcal/week food intake resulted in a great weight loss in this young group of CR mice, which was not allowed ethically. Therefore, the food restriction was adjusted to 75-80% for the first 2 months of CR (age 2-4 month).
Table S3. List of primers used.