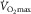At maximal oxygen uptake (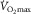 ) we know that (1) muscle O2 extraction is not 100%, yet (2) hyperoxia increases
) we know that (1) muscle O2 extraction is not 100%, yet (2) hyperoxia increases 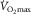 . The reason for (1) is diffusion limitation of O2 from the muscle microvessels to the mitochondria. This does not exclude ‘central’ factors from also affecting
. The reason for (1) is diffusion limitation of O2 from the muscle microvessels to the mitochondria. This does not exclude ‘central’ factors from also affecting 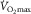 , as will be explained.
, as will be explained.
Two simple, direct, published, and undisputed observations document the first point. They are that, when the inspired O2 fraction (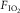 ) is acutely altered (in random order within a single day):
) is acutely altered (in random order within a single day):
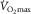 is higher in hyperoxia and lower in hypoxia, compared to room air (Welch, 1982, 1987; Knight et al. 1993).
is higher in hyperoxia and lower in hypoxia, compared to room air (Welch, 1982, 1987; Knight et al. 1993).
Muscle venous blood still contains significant amounts of O2 at
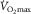 , in hypoxia, normoxia and hyperoxia (Roca et al. 1989; Knight et al. 1993).
, in hypoxia, normoxia and hyperoxia (Roca et al. 1989; Knight et al. 1993).
This shows unequivocally that the muscles are capable of using more O2 in normoxia (or hypoxia) than they can extract, proving the existence of an extraction limit, contributing to 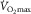 limitation.
limitation.
The extraction limit could result from any of three possibilities: (a) shunting of arterial blood around exercising muscle; (b) heterogeneity in the distribution of blood flow with respect to metabolic demand; (c) diffusion limitation of O2 transport from microvessels to mitochondria.
While there may be minor contributions from the first two, diffusion limitation appears to be the major basis of limited extraction. The most compelling evidence comes from studies of isolated in situ, canine gastrocnemius muscle in which maximal contractions (and 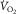 ) were produced by nerve stimulation, holding O2 delivery into the muscle constant while one factor was varied – haemoglobin O2 affinity defined by (P50) (Hogan et al. 1991; Richardson et al. 1998). Arterial [O2] and blood flow were kept constant (animals breathed 100% O2; muscle blood flow was pump-controlled). Hogan’s study reduced P50 (to impair diffusive extraction by reducing microvascular
) were produced by nerve stimulation, holding O2 delivery into the muscle constant while one factor was varied – haemoglobin O2 affinity defined by (P50) (Hogan et al. 1991; Richardson et al. 1998). Arterial [O2] and blood flow were kept constant (animals breathed 100% O2; muscle blood flow was pump-controlled). Hogan’s study reduced P50 (to impair diffusive extraction by reducing microvascular 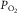 ); Richardson’s study increased P50 (to enhance diffusive extraction by increasing microvascular
); Richardson’s study increased P50 (to enhance diffusive extraction by increasing microvascular 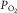 ). Importantly, neither shunting nor heterogeneity would alter extraction at constant O2 delivery and blood flow, as only P50 is varied. As predicted,
). Importantly, neither shunting nor heterogeneity would alter extraction at constant O2 delivery and blood flow, as only P50 is varied. As predicted, 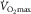 increased as P50 was raised, and fell when P50 was reduced. Moreover, the amount by which
increased as P50 was raised, and fell when P50 was reduced. Moreover, the amount by which 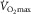 changed was predicted by the laws of diffusion from the concomitant changes in mean microvascular
changed was predicted by the laws of diffusion from the concomitant changes in mean microvascular 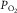 :
: 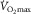 was proportional to mean microvascular
was proportional to mean microvascular 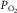 , a finding also noted in humans (Roca et al. 1989; Knight et al. 1993).
, a finding also noted in humans (Roca et al. 1989; Knight et al. 1993).
Additional evidence for diffusion limitation of O2 between muscle microvessels and mitochondria comes from com-putational modelling (Groebe & Thews, 1990), frozen myoglobin spectroscopy (Gayeski & Honig, 1988) and magnetic resonance spectroscopy (Richardson et al. 1995).
Scientific progress calls for not only supporting one’s views with data, but also reconciling them with other views. The major difference with others’ opinions is in understanding the role of cardiac output in limiting 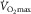 . That cardiac output contributes to
. That cardiac output contributes to 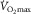 limitation is not in dispute. Pericardiectomy in dogs increases maximal cardiac output and
limitation is not in dispute. Pericardiectomy in dogs increases maximal cardiac output and 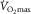 (Stray-Gundersen et al. 1986). The Saltin group, comparing two-legged and one-legged cycling (Rowell et al. 1986), showed that specific
(Stray-Gundersen et al. 1986). The Saltin group, comparing two-legged and one-legged cycling (Rowell et al. 1986), showed that specific 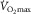 is higher in one-legged than two-legged cycling, associated with higher specific muscle blood flow. Additionally, Powers et al. (1989) showed that pulmonary gas exchange inefficiency affected
is higher in one-legged than two-legged cycling, associated with higher specific muscle blood flow. Additionally, Powers et al. (1989) showed that pulmonary gas exchange inefficiency affected 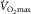 ; severe anaemia is also well known to reduce exercise capacity. That is exactly what would be expected of an in-series O2 transport system – every step must play a role in affecting overall outcome (
; severe anaemia is also well known to reduce exercise capacity. That is exactly what would be expected of an in-series O2 transport system – every step must play a role in affecting overall outcome (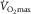 ).
).
Pro-cardiac-output-is-the-limiting-factor-advocates (PCOITLFA) cite the Fick principle (O2 uptake = blood flow × arteriovenous [O2] difference) applied to elite athletes versus the rest of us. The main, undisputed, difference is in cardiac output (blood flow) and not in arteriovenous [O2] difference. Ergo, the PCOITLFA conclude that cardiac output, not extraction, explains the differences in 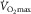 . What the PCOITLFA forget is that if all else were similar between us, the elite athlete’s higher cardiac output would shorten red cell transit time for O2 unloading in the muscle microvessels. This would offset much of the benefit of higher blood flow by reducing diffusive O2 unloading (Wagner, 1996). However, despite higher blood flow, athletes are able to extract higher amounts of O2: femoral venous
. What the PCOITLFA forget is that if all else were similar between us, the elite athlete’s higher cardiac output would shorten red cell transit time for O2 unloading in the muscle microvessels. This would offset much of the benefit of higher blood flow by reducing diffusive O2 unloading (Wagner, 1996). However, despite higher blood flow, athletes are able to extract higher amounts of O2: femoral venous 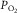 is usually lower than in the rest of us. This means that the athlete’s diffusive conductance supporting O2 movement from microvessels to mitochondria is greater than in the rest of us, allowing the maintenance of a large arteriovenous [O2] difference in the face of higher blood flow. But even so, elite athletes increase
is usually lower than in the rest of us. This means that the athlete’s diffusive conductance supporting O2 movement from microvessels to mitochondria is greater than in the rest of us, allowing the maintenance of a large arteriovenous [O2] difference in the face of higher blood flow. But even so, elite athletes increase 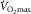 with added O2, which takes us back to the initial arguments of this article.
with added O2, which takes us back to the initial arguments of this article.
The simplest way to understand how the O2 transport system works, with every step contributing to limiting 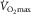 , is graphically, using a diagram relating
, is graphically, using a diagram relating 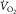 to muscle venous
to muscle venous 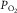 on the basis of the two main transport equations involved. One underlies the previously mentioned Fick principle (O2 uptake = blood flow × arteriovenous [O2] difference), i.e.
on the basis of the two main transport equations involved. One underlies the previously mentioned Fick principle (O2 uptake = blood flow × arteriovenous [O2] difference), i.e.
| 1 |
And the second is the equation underlying the Fick law of diffusion:
| 2 |
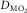 is muscle O2 diffusional conduc-tance,
is muscle O2 diffusional conduc-tance, 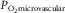 is mean microvascular
is mean microvascular 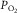 within muscle, and
within muscle, and  is mitochondrial
is mitochondrial 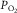 , which appears to be so low compared to
, which appears to be so low compared to 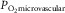 (Richardson et al. 1995) that it can here be neglected. Because
(Richardson et al. 1995) that it can here be neglected. Because 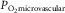 and muscle venous
and muscle venous 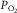 rise and fall in proportion to one another (Roca et al. 1989), we can replace
rise and fall in proportion to one another (Roca et al. 1989), we can replace 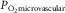 by
by 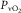 , the venous
, the venous 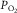 , times a constant, say, k. With these approximations, eqn 2 may be re-written:
, times a constant, say, k. With these approximations, eqn 2 may be re-written:
| 3 |
Equations 1 and 3 embody the same undisputed law: conservation of O2 mass during its transport. As a consequence they apply simultaneously: at their solution, both 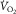 and
and 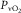 must be the same in the two equations. Because they both relate
must be the same in the two equations. Because they both relate 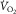 to muscle venous O2 levels, they can be plotted on one diagram with
to muscle venous O2 levels, they can be plotted on one diagram with 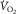 on the ordinate and
on the ordinate and 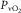 on the abscissa (Fig.1, modified from Wagner, 1996). Their intersection point is the only point where conservation of mass exists – the same
on the abscissa (Fig.1, modified from Wagner, 1996). Their intersection point is the only point where conservation of mass exists – the same 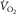 at the same
at the same 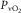 – indicating the value of
– indicating the value of 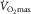 for the given values of
for the given values of 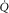 ,
, 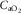 and
and 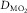 . Change any one of these three, and the lines will shift, yielding a different intersection point (i.e. different
. Change any one of these three, and the lines will shift, yielding a different intersection point (i.e. different 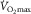 ). Since
). Since 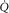 represents cardiac function,
represents cardiac function, 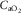 represents pulmonary gas exchange and blood [Hb], and
represents pulmonary gas exchange and blood [Hb], and 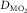 represents muscle O2 diffusional properties, it is evident that all steps of the O2 pathway significantly impact
represents muscle O2 diffusional properties, it is evident that all steps of the O2 pathway significantly impact 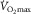 . That is how an in-series system must work, and why muscle O2 diffusion limitation does contribute to limitation of
. That is how an in-series system must work, and why muscle O2 diffusion limitation does contribute to limitation of 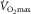 .
.
Figure 1.
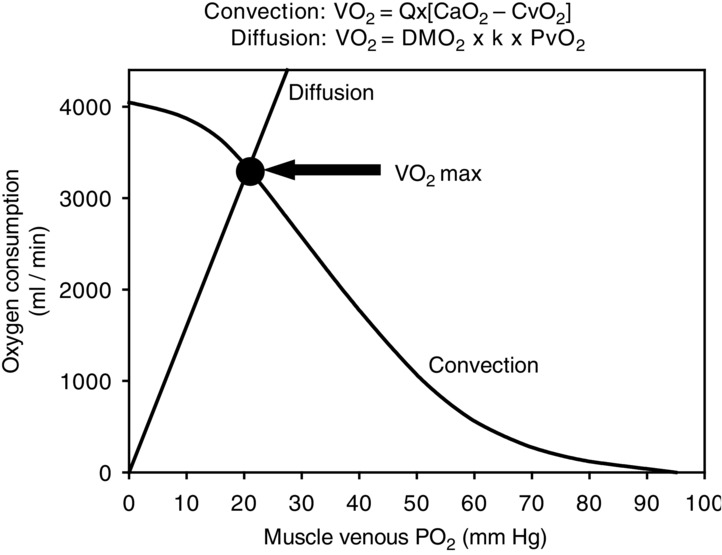
Determinants of 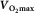
Plot of O2 consumption (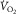 ) against muscle venous
) against muscle venous 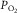 showing the two conservation of mass equations describing convective flow of O2 into the muscle microcirculation (Fick principle), and subsequent diffusive flow of O2 from the microcirculation to the mitochondria (Fick law of diffusion). Conservation of mass occurs only at their point of intersection, indicating the value of
showing the two conservation of mass equations describing convective flow of O2 into the muscle microcirculation (Fick principle), and subsequent diffusive flow of O2 from the microcirculation to the mitochondria (Fick law of diffusion). Conservation of mass occurs only at their point of intersection, indicating the value of 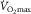 when the independent variables
when the independent variables 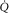 ,
, 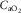 and
and 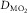 are those at
are those at 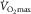 (modified from Wagner, 1996).
(modified from Wagner, 1996).
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘Last Word’. Please email your comment, including a title and a declaration of interest to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Biography
Peter Wagner is Distinguished Professor of Medicine and Bioengineering at the University of California, San Diego. His research addresses the theoretical and experimental basis of oxygen transport and its limitations in the lungs and skeletal muscles in health and disease. A particular focus ismuscle capillary growth regulation usingmolecular biological approaches in integrated systems: the role of O2,microvascular haemodynamics, physical factors, nitric oxide and inflammatory mediators in transcriptional regulation of angiogenic growth factors. Of particular interest is the role of VEGF in both pulmonary and skeletalmuscle structure and function.

Additional information
Competing interests
The author has no conflicts of interest associated with this manuscript.
Funding
Funding was provided by NIH HL091830.
References
- Gayeski TE. Honig CR. Intracellular PO2 in long axis of individual fibers in working dog gracilis muscle. Am J Physiol. 1988;254:H1179–H1186. doi: 10.1152/ajpheart.1988.254.6.H1179. [DOI] [PubMed] [Google Scholar]
- Groebe K. Thews G. Calculated intra- and extracellular gradients in heavily working red muscle. Am J Physiol. 1990;259:H84–H92. doi: 10.1152/ajpheart.1990.259.1.H84. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Bebout DE. Wagner PD. Effect of increased Hb-O2 affinity on VO2max at constant O2 delivery in dog muscle in situ. J Appl Physiol (1985) 1991;70:2656–2662. doi: 10.1152/jappl.1991.70.6.2656. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE. Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol (1985) 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Powers SK, Lawler J, Dempsey J, Dodd JA. Landry G. Effects of incomplete pulmonary gas exchange on VO2 max. J Appl Physiol (1985) 1989;66:2491–2495. doi: 10.1152/jappl.1989.66.6.2491. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS. Wagner PD. Myoglobin O2 desaturation during exercise: evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Tagore K, Haseler L, Jordan M. Wagner PD. Increased VO2max with a right shifted Hb-O2 dissociation curve at a constant O2 delivery in dog muscle in situ. J Appl Physiol (1985) 1998;84:995–1002. doi: 10.1152/jappl.1998.84.3.995. [DOI] [PubMed] [Google Scholar]
- Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O. Wagner PD. Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol (1985) 1989;67:291–299. doi: 10.1152/jappl.1989.67.1.291. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Saltin B, Kiens B. Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Stray-Gundersen J, Musch TI, Haidet GC, Swain DP, Ordway GA. Mitchell JH. The effect of pericardiectomy on maximal oxygen consumption and maximal cardiac output in untrained dogs. Circ Res. 1986;58:523–530. doi: 10.1161/01.res.58.4.523. [DOI] [PubMed] [Google Scholar]
- Wagner PD. A theoretical analysis of factors determining VO2max at sea level and altitude. Respir Physiol. 1996;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. [DOI] [PubMed] [Google Scholar]
- Welch HG. Hyperoxia and human performance: a brief review. Med Sci Sports Exerc. 1982;14:253–262. doi: 10.1249/00005768-198204000-00001. [DOI] [PubMed] [Google Scholar]
- Welch HG. Effects of hypoxia and hyperoxia on human performance. Exerc Sports Sci Rev. 1987;15:191–221. [PubMed] [Google Scholar]