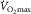Oxygen is transferred from air to mitochondria in sequential steps by means of diffusion and convection. The theoretical ceiling for whole body aerobic uptake may be determined by any factor influencing the O2 transport and utilization chain. The dispute considered here is whether peripheral O2 diffusion from microvessels, particularly capillaries, into active skeletal muscle fibres limits/regulates maximal oxygen uptake (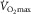 ) in healthy humans.
) in healthy humans.
Peripheral O2 diffusion does not limit 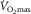
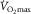 is experimentally determined by the levelling off in O2 uptake observed with increasing workload during dynamic exercise involving more than half of total muscle mass (e.g. running, cycling) (Levine, 2008). This indicates that, empirically,
is experimentally determined by the levelling off in O2 uptake observed with increasing workload during dynamic exercise involving more than half of total muscle mass (e.g. running, cycling) (Levine, 2008). This indicates that, empirically, 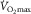 is limited, i.e. primarily restricted prior to peak muscle activation. Moreover, such limitation is attributed to a finite oxygen supply to muscle given that mitochondrial oxidative capacity exceeds that of oxygen delivery at
is limited, i.e. primarily restricted prior to peak muscle activation. Moreover, such limitation is attributed to a finite oxygen supply to muscle given that mitochondrial oxidative capacity exceeds that of oxygen delivery at 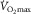 (Boushel et al. 2011). Thus, any factor related to the transport of O2 into the mitochondria might limit
(Boushel et al. 2011). Thus, any factor related to the transport of O2 into the mitochondria might limit 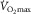 . In this regard, there is sound evidence that
. In this regard, there is sound evidence that 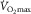 is proportionally modified in accordance with acute changes in blood O2-carrying capacity and content (Calbet et al. 2006a). In contrast, a decrease in arterial O2 partial pressure, and thereby reducing the driving force for O2 diffusion, does not affect maximal O2 uptake if O2 delivery to the exercising limbs at the same time remains preserved (Calbet et al. 2003, 2009). Importantly, these observations were not associated with any particular individual’s fitness status. Accordingly, at
is proportionally modified in accordance with acute changes in blood O2-carrying capacity and content (Calbet et al. 2006a). In contrast, a decrease in arterial O2 partial pressure, and thereby reducing the driving force for O2 diffusion, does not affect maximal O2 uptake if O2 delivery to the exercising limbs at the same time remains preserved (Calbet et al. 2003, 2009). Importantly, these observations were not associated with any particular individual’s fitness status. Accordingly, at 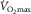 in healthy individuals there must be a physiologically relevant reserve in muscle O2 diffusing capacity, which also precludes that
in healthy individuals there must be a physiologically relevant reserve in muscle O2 diffusing capacity, which also precludes that 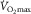 is limited by peripheral O2 diffusion from capillary into muscle.
is limited by peripheral O2 diffusion from capillary into muscle.
Does muscle O2 diffusion regulate 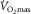 ?
?
While peripheral O2 diffusion does not limit 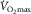 , the question arises as to whether
, the question arises as to whether 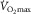 could be influenced by muscle O2 diffusing capacity in healthy individuals. This would be entirely refuted if O2 were fully extracted from capillaries supplying active muscle fibres. However, such level of resolution for O2 extraction within human muscle fibres is beyond reach with current methods (Koga et al. 2014). Instead, the large body of empirical evidence derives from O2 measures in venous blood exiting the active limb (Rud et al. 2012), in which a 100% O2 extraction seems unachievable considering temporal and spatial characteristics (Heinonen et al. 2015). For instance, the high intramuscular pressure generated during the contraction phase partly diverts blood flow toward less metabolically active tissue (Clark et al. 2000). Also, the perfusion of active muscle fibres is inherently inefficient as regards O2 delivery, since microvascular units (i.e. terminal arteriole and downstream capillaries) are not spatially coordinated with individual motor units and this may result in the overperfusion of inactive fibres (Emerson & Segal, 1997). In addition, O2 extraction may be a function of the physiological distribution of blood flow among active/inactive muscle fibres and other tissues (Kalliokoski et al. 2001, 2005; Calbet et al. 2006b). Of note, blood flow distribution is determined by the complex interplay of factors including the sympathetic drive, concentration of vasoactive substances, arterial dilator/constrictor function and microvascular structure. Therefore, a perfect matching between leg O2 delivery and metabolic demand during exercise is not expected even if neglecting the previously mentioned temporal and spatial intrinsic constraints; here, blood flow distribution is improved and O2 extraction enhanced within exercising muscles in long-term trained individuals (Kalliokoski et al. 2001). Ignoring or trivializing this fact may have distorted the contribution of muscle O2 diffusion to the limitation
could be influenced by muscle O2 diffusing capacity in healthy individuals. This would be entirely refuted if O2 were fully extracted from capillaries supplying active muscle fibres. However, such level of resolution for O2 extraction within human muscle fibres is beyond reach with current methods (Koga et al. 2014). Instead, the large body of empirical evidence derives from O2 measures in venous blood exiting the active limb (Rud et al. 2012), in which a 100% O2 extraction seems unachievable considering temporal and spatial characteristics (Heinonen et al. 2015). For instance, the high intramuscular pressure generated during the contraction phase partly diverts blood flow toward less metabolically active tissue (Clark et al. 2000). Also, the perfusion of active muscle fibres is inherently inefficient as regards O2 delivery, since microvascular units (i.e. terminal arteriole and downstream capillaries) are not spatially coordinated with individual motor units and this may result in the overperfusion of inactive fibres (Emerson & Segal, 1997). In addition, O2 extraction may be a function of the physiological distribution of blood flow among active/inactive muscle fibres and other tissues (Kalliokoski et al. 2001, 2005; Calbet et al. 2006b). Of note, blood flow distribution is determined by the complex interplay of factors including the sympathetic drive, concentration of vasoactive substances, arterial dilator/constrictor function and microvascular structure. Therefore, a perfect matching between leg O2 delivery and metabolic demand during exercise is not expected even if neglecting the previously mentioned temporal and spatial intrinsic constraints; here, blood flow distribution is improved and O2 extraction enhanced within exercising muscles in long-term trained individuals (Kalliokoski et al. 2001). Ignoring or trivializing this fact may have distorted the contribution of muscle O2 diffusion to the limitation 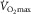 (Piiper, 2000; Koga et al. 2014). Yet O2 extraction across the leg commonly rises to 85% or more at
(Piiper, 2000; Koga et al. 2014). Yet O2 extraction across the leg commonly rises to 85% or more at 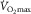 in untrained and trained individuals (Lundby et al. 2006; Rud et al. 2012), attaining an astonishing 97% in some elite athletes (Calbet et al. 2005). It follows that for muscle O2 diffusing capacity to contribute to the limitation of
in untrained and trained individuals (Lundby et al. 2006; Rud et al. 2012), attaining an astonishing 97% in some elite athletes (Calbet et al. 2005). It follows that for muscle O2 diffusing capacity to contribute to the limitation of 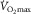 , an average of approximately 85% or more of leg blood flow should be continuously perfusing active muscle fibres.
, an average of approximately 85% or more of leg blood flow should be continuously perfusing active muscle fibres.
If leg O2 extraction, and thus 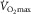 , were regulated by leg muscle O2 diffusing capacity, any increase in diffusion capacity would be reflected, at least to a degree, in an augmented leg O2 extraction. In this respect, one-leg training studies have provided compelling evidence because the impact of peripheral adaptations on
, were regulated by leg muscle O2 diffusing capacity, any increase in diffusion capacity would be reflected, at least to a degree, in an augmented leg O2 extraction. In this respect, one-leg training studies have provided compelling evidence because the impact of peripheral adaptations on 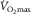 may be isolated from central haemodynamic adaptations (Gleser, 1973; Saltin et al. 1976; Klausen et al. 1982; Rud et al. 2012). All these studies have shown marked increases in one-legged cycling peak blood flow, O2 diffusing capacity, and uptake in individuals who, before training, had
may be isolated from central haemodynamic adaptations (Gleser, 1973; Saltin et al. 1976; Klausen et al. 1982; Rud et al. 2012). All these studies have shown marked increases in one-legged cycling peak blood flow, O2 diffusing capacity, and uptake in individuals who, before training, had 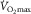 in the normal range (Gleser, 1973; Saltin et al. 1976; Klausen et al. 1982; Rud et al. 2012). However, peak O2 uptake during two-legged cycling (i.e.
in the normal range (Gleser, 1973; Saltin et al. 1976; Klausen et al. 1982; Rud et al. 2012). However, peak O2 uptake during two-legged cycling (i.e. 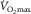 ) remained unaltered in the presence of unchanged maximal cardiac output (
) remained unaltered in the presence of unchanged maximal cardiac output (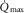 ) following one-legged training (Gleser, 1973). In line with this, the increase in
) following one-legged training (Gleser, 1973). In line with this, the increase in 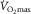 following two-legged training was reverted to the baseline level after negating the training-induced gain in
following two-legged training was reverted to the baseline level after negating the training-induced gain in 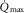 by means of phlebotomy (Bonne et al. 2014). Likewise,
by means of phlebotomy (Bonne et al. 2014). Likewise, 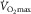 was identical prior to and after one-legged training despite the fact that blood flow, O2 extraction and uptake were enhanced during two-legged cycling in the trained versus control leg (Rud et al. 2012). This strongly suggests that two-legged O2 extraction (∼85%) was maximized relative to blood flow at
was identical prior to and after one-legged training despite the fact that blood flow, O2 extraction and uptake were enhanced during two-legged cycling in the trained versus control leg (Rud et al. 2012). This strongly suggests that two-legged O2 extraction (∼85%) was maximized relative to blood flow at 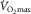 , irrespective of any training adaptation in muscle O2 diffusing capacity. Overall, these data indicate that
, irrespective of any training adaptation in muscle O2 diffusing capacity. Overall, these data indicate that 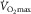 is not modulated by muscle O2 diffusing capacity. Rather,
is not modulated by muscle O2 diffusing capacity. Rather, 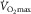 seems to be governed, in a tyrannical manner, by the amount of blood flowing into the exercising limbs.
seems to be governed, in a tyrannical manner, by the amount of blood flowing into the exercising limbs.
Several canine studies have been aimed aet examining the contribution of muscle O2 diffusing capacity to O2 extraction (Schumacker et al. 1985; Barclay, 1986; Hogan et al. 1989, 1991; Richardson et al. 1998). In these, blood flow or haemoglobin O2 affinity (P50) have been manipulated while maintaining O2 delivery constant to contracting muscle. Hence a regulatory role for O2 diffusion from capillary into muscle in O2 extraction could be pinpointed, provided that O2 delivery to active muscle fibres is not influenced by blood flow or P50 (Schumacker et al. 1987). Regardless, the majority of the evidence comparing experimental versus control conditions indicates that muscle O2 uptake is uniquely dependent on O2 delivery (Schumacker et al. 1985, 1987; Barclay, 1986; Richardson et al. 1998). Furthermore, the presence of statistical procedures raising the likelihood for type I errors to occur (i.e. the probability of making false discoveries) in divergent findings is noteworthy (Hogan et al. 1989). Taken together, the proposed regulatory role for O2 diffusion from capillary into muscle during exercise (Wagner, 1992) cannot be induced from the above animal experiments, let alone extrapolating it to humans exercising at 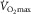 , in which, as a matter of fact, such a role is empirically absent (Calbet et al. 2003; Lundby et al. 2006).
, in which, as a matter of fact, such a role is empirically absent (Calbet et al. 2003; Lundby et al. 2006).
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘Last Word’. Please email your comment, including a title and a declaration of interest to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Biography
Carsten Lundby (left) and David Montero (right) both work at the University of Zürich in a small team focusing on Oxygen Transport and Utilization in its broadest sense. The strength of the group is its integrative approach which allows them to study O2 as it is transported all the way from inspired air and until it is oxidized in the mitochondrion. Their most recent work has focused on the relative importance of skeletal muscle and circulatory adaptations to exercise training for improvements in 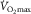 and hence is in direct line with the current cross talk.
and hence is in direct line with the current cross talk.

Additional information
Competing interests
None declared.
References
- Barclay JK. A delivery-independent blood flow effect on skeletal muscle fatigue. J Appl Physiol (1985) 1986;61:1084–1090. doi: 10.1152/jappl.1986.61.3.1084. [DOI] [PubMed] [Google Scholar]
- Bonne TC, Doucende G, Fluck D, Jacobs RA, Nordsborg NB, Robach P, Walther G. Lundby C. Phlebotomy eliminates the maximal cardiac output response to six weeks of exercise training. Am J Physiol Regul Integr Comp Physiol. 2014;306:R752–R760. doi: 10.1152/ajpregu.00028.2014. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Calbet JA, Gonzalez-Alonso J, Wright-Paradis C, Sondergaard H, Ara I, Helge JW. Saltin B. Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion. 2011;11:303–307. doi: 10.1016/j.mito.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD. Saltin B. Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M. Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol. 2005;289:R1448–R1458. doi: 10.1152/ajpregu.00824.2004. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Koskolou M. Boushel R. Importance of hemoglobin concentration to exercise: acute manipulations. Respir Physiol Neurobiol. 2006;151:132–140. doi: 10.1016/j.resp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B. Boushel R. Effects of ATP-induced leg vasodilation on VO2peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R447–R453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R. Saltin B. On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol. 2009;587:477–490. doi: 10.1113/jphysiol.2008.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MG, Rattigan S, Clerk LH, Vincent MA, Clark AD, Youd JM. Newman JM. Nutritive and non-nutritive blood flow: rest and exercise. Acta Physiol Scand. 2000;168:519–530. doi: 10.1046/j.1365-201x.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- Emerson GG. Segal SS. Alignment of microvascular units along skeletal muscle fibers of hamster retractor. J Appl Physiol (1985) 1997;82:42–48. doi: 10.1152/jappl.1997.82.1.42. [DOI] [PubMed] [Google Scholar]
- Gleser MA. Effects of hypoxia and physical training on hemodynamic adjustments to one-legged exercise. J Appl Physiol. 1973;34:655–659. doi: 10.1152/jappl.1973.34.5.655. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Koga S, Kalliokoski KK, Musch TI. Poole DC. Heterogeneity of muscle blood flow and metabolism: influence of exercise, aging and disease states. Exerc Sport Sci Rev. 2015 doi: 10.1249/JES.0000000000000044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Bebout DE. Wagner PD. Effect of increased Hb-O2 affinity on VO2max at constant O2 delivery in dog muscle in situ. J Appl Physiol (1985) 1991;70:2656–2662. doi: 10.1152/jappl.1991.70.6.2656. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Roca J, West JB. Wagner PD. Dissociation of maximal O2 uptake from O2 delivery in canine gastrocnemius in situ. J Appl Physiol (1985) 1989;66:1219–1226. doi: 10.1152/jappl.1989.66.3.1219. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Knuuti J. Nuutila P. Relationship between muscle blood flow and oxygen uptake during exercise in endurance-trained and untrained men. J Appl Physiol (1985) 2005;98:380–383. doi: 10.1152/japplphysiol.01306.2003. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J. Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001;280:E1015–E1021. doi: 10.1152/ajpendo.2001.280.6.E1015. [DOI] [PubMed] [Google Scholar]
- Klausen K, Secher NH, Clausen JP, Hartling O. Trap-Jensen J. Central and regional circulatory adaptations to one-leg training. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:976–983. doi: 10.1152/jappl.1982.52.4.976. [DOI] [PubMed] [Google Scholar]
- Koga S, Rossiter HB, Heinonen I, Musch TI. Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc. 2014;46:860–876. doi: 10.1249/MSS.0000000000000178. [DOI] [PubMed] [Google Scholar]
-
Levine BD
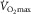 : what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar] - Lundby C, Sander M, van Hall G, Saltin B. Calbet JA. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol. 2006;573:535–547. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piiper J. Perfusion, diffusion and their heterogeneities limiting blood-tissue O2 transfer in muscle. Acta Physiol Scand. 2000;168:603–607. doi: 10.1046/j.1365-201x.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Tagore K, Haseler LJ, Jordan M. Wagner PD. Increased VO2max with right-shifted Hb-O2 dissociation curve at a constant O2 delivery in dog muscle in situ. J Appl Physiol (1985) 1998;84:995–1002. doi: 10.1152/jappl.1998.84.3.995. [DOI] [PubMed] [Google Scholar]
- Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O. Wagner PD. Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol (1985) 1989;67:291–299. doi: 10.1152/jappl.1989.67.1.291. [DOI] [PubMed] [Google Scholar]
- Rud B, Foss O, Krustrup P, Secher NH. Hallén J. One-legged endurance training: leg blood flow and oxygen extraction during cycling exercise. Acta Physiol (Oxf) 2012;205:177–185. doi: 10.1111/j.1748-1716.2011.02383.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B. Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Schumacker PT, Long GR. Wood LD. Tissue oxygen extraction during hypovolemia: role of hemoglobin P50. J Appl Physiol (1985) 1987;62:1801–1807. doi: 10.1152/jappl.1987.62.5.1801. [DOI] [PubMed] [Google Scholar]
- Schumacker PT, Suggett AJ, Wagner PD. West JB. Role of hemoglobin P50 in O2 transport during normoxic and hypoxic exercise in the dog. J Appl Physiol (1985) 1985;59:749–757. doi: 10.1152/jappl.1985.59.3.749. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc. 1992;24:54–58. [PubMed] [Google Scholar]