Abstract
The contractility of vascular smooth muscle cells within the walls of arteries is regulated by mechanical stresses and vasoactive signals. Transduction of these diverse stimuli into a cellular response occurs through many different mechanisms, one being reorganisation of the actin cytoskeleton. In addition to a structural role in maintaining cellular architecture it is now clear that the actin cytoskeleton of contractile vascular smooth muscle cells is a dynamic structure reacting to changes in the cellular environment. Equally clear is that disrupting the cytoskeleton or interfering with its rearrangement, has profound effects on artery contractility. The actin cytoskeleton associates with dense plaques, also called focal adhesions, at the plasma membrane of smooth muscle cells. Vasoconstrictors and mechanical stress induce remodelling of the focal adhesions, concomitant with cytoskeletal reorganisation. Recent work has shown that non-receptor tyrosine kinases and tyrosine phosphorylation of focal adhesion proteins such as paxillin and Hic-5 are important for actin cytoskeleton and focal adhesion remodelling and contraction.
Introduction
The maintenance of normal peripheral vascular resistance and perfusion of vital organs depends on the structure and contractile tone of small arteries within the vasculature. Changes in these properties of small blood vessels lead to cardiovascular disease. Smooth muscle cells within the artery wall respond to external stimuli such as hormones and stress (increased pressure or flow) adjusting their level of tone to maintain perfusion and resistance within normal levels. However, in response to prolonged mechanical stress, for example maintained high blood pressure, vascular smooth muscle cells (VSMCs) exhibit exaggerated contractility and remodelling of the vessel wall, as occurs in hypertension or migration and proliferation as seen in atherosclerosis (Castorena-Gonzalez et al. 2014). An important role for the actin cytoskeleton (CK) in the smooth muscle responses contraction, proliferation and migration, is now recognised (reviewed in Gerthoffer & Gunst, 2001; Gunst & Zhang, 2008; Yamin & Morgan, 2012). Cytosolic tyrosine kinases are emerging as important regulators of the actin CK, through tyrosine phosphorylation of focal adhesion proteins such as vinculin and paxillin (Gunst & Zhang, 2008). However, data describing actin CK remodelling and the signalling molecules involved are derived from multiple sources including smooth muscle cells in culture, freshly dispersed VSMCs settled on glass, or matrix and tissues which are composed of multiple cell types. The phenotypic properties and behaviour of the VSMCs in these different preparations may vary, and care should be taken when extrapolating between different models. In this review we will describe our current understanding of the regulation of actin CK dynamics in contractile VSMCs and tissue, focusing on the role of tyrosine kinases and adhesion site adaptor proteins.
Actin cytoskeleton
Until recently, the CK was perceived as a relatively fixed structure; however, this view has now been completely revised and it is recognised that the CK is a highly dynamic structure constantly assembling and disassembling in response to external stimuli. Rapid reorganisation of the CK occurs through the reversible polymerisation, of monomeric globular actin (G-actin) to the polymerised filamentous form (F-actin), markedly altering cellular properties. The organisation of the CK is regulated by signalling pathways that target proteins that influence actin assembly, in particular the stress-activated protein kinases, small heat shock proteins, cytosolic tyrosine kinases and LIM (Lin11, Isl-1 and Mec-3 domain) proteins (Gerthoffer & Gunst, 2001; Gunst & Zhang, 2008).
Actin cytoskeleton in smooth muscle
Contractile VSMCs have a highly organised CK that is required for the transmission of force. In fully differentiated smooth muscle cells in tissues the CK is attached to the cell membrane at dense plaques which are thought to act as mechanosensors transducing integrin-mediated signals to the CK (Gerthoffer & Gunst, 2001; Kim et al. 2008; Lehman & Morgan, 2012) (Fig. 1). These membrane-localised dense plaques are multiprotein complexes that cluster many of the same proteins (vinculin, α-actinin and focal adhesion kinase (FAK)) found in focal adhesions of migrating cells in culture, and are distinct from the classical dense bodies associated with the contractile machinery in the interior of the VSMCs (reviewed in Owens et al. 2004). Smooth muscle dense plaques are also called adhesion sites or focal adhesions, and will be referred to as focal adhesions (FAs) throughout this review. Recent studies in freshly dispersed differentiated VSMCs have led to the proposal that the cortical actin CK and the actin contractile filaments are formed from different actin isoforms (reviewed in Yamin & Morgan, 2012). In this model β-actin is found around intracellular dense bodies associated with the contractile apparatus (Gallant et al. 2011), whereas α-smooth muscle actin filaments interact with myosin forming the contractile actin fibres that run longitudinally and diagonally through the cell. Non-muscle γ-actin is the major component of the cortical CK at the periphery of the cell and it is the β- and γ-actin networks that alter their polymerisation state in response to stimulation (Kim et al. 2008). It is now clear that reorganisation of the actin cytoskeleton is an essential component of the contractile response of smooth muscle cells. Increases in filamentous actin have been reported in intact arteries during myogenic contraction (Cipolla et al. 2002; Moreno-Dominguez et al. 2013; Walsh & Cole, 2013) and following vasoconstrictor stimulation (Mehta & Gunst, 1999; Bárány et al. 2001; Ohanian et al. 2005; Srinivasan et al. 2008). In addition, interference with actin polymerisation blocks contraction and stabilisation of actin filaments induces relaxation in vascular tissue without affecting myosin light chain phosphorylation (Boels & Pfitzer, 1992; Gerthoffer & Gunst, 2001; Ohanian et al. 2005; Wang et al. 2014). Alterations in VSMC actin CK are also implicated in physiological (reviewed in Castorena-Gonzalez et al. 2014) and pathological remodelling of large and small arteries (Qiu et al. 2010; Saphirstein et al. 2013; Sehgel et al. 2013). However, our understanding of the mechanisms that regulate contractile VSMC actin cytoskeleton dynamics is limited.
Figure 1.
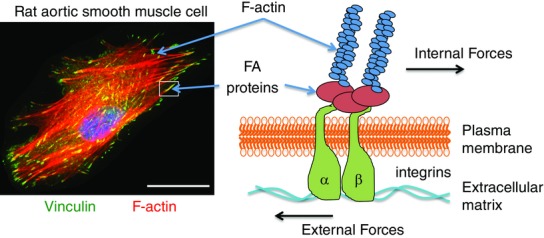
Vascular smooth muscle cell actin cytoskeleton and adhesion sites
Left-hand panel: immunofluorescence image of a rat aortic smooth muscle cell cultured on collagen-coated glass. Filamentous actin (F-actin) is stained red and vinculin, a focal adhesion (FA) marker, is shown in green. Actin filaments can be seen terminating at adhesion sites. Right-hand panel: schematic diagram of an adhesion site showing sensing of external force and transmission of internal force through actin filaments and integrin focal adhesion site. The scale bar is 0 — 25 μ.
The role of cytosolic tyrosine kinases and adhesion site remodelling in vascular smooth muscle responses
In intact small arteries we have identified two pathways activated by vasoconstrictors that are involved in regulation of the CK, p38 mitogen-activated protein kinase (p38MAPK) and cytosolic tyrosine kinase (Src and PYK2), the former through heat shock protein 27 (Hsp27) and the latter through the LIM protein paxillin and its homologue hydrogen peroxide inducible clone-5 (Hic-5) (Ohanian et al. 2001; Ward et al. 2002; Ohanian et al. 2005). Although these pathways are activated independently of each other they converge to regulate contractility through a common mechanism, promotion of F-actin formation, i.e. CK remodelling (Srinivasan et al. 2008). In agreement with our findings in small arteries, cytosolic tyrosine kinases and the FA proteins paxillin, zyxin and vinculin are involved in F-actin formation and contraction in response to acetylcholine in tracheal smooth muscle strips (reviewed in Gerthoffer & Gunst, 2001; Gunst & Zhang, 2008; Tang & Anfinogenova, 2008), emphasising the importance of this mechanism in smooth muscle tissue responses. In non-smooth-muscle cells in culture Src, PYK2, vinculin, zyxin, paxillin and Hic-5 are associated with FAs and are involved in assembly and disassembly of these structures (Zaidel-Bar et al. 2007), and activation of these proteins by vasoconstrictor hormones in smooth muscle tissues suggests that these agonists may signal to the CK through remodelling of adhesion sites. This idea is supported by two recent studies where, in A7R5 vascular smooth muscle cells, stimulation with lyso-phosphatidic acid increased cell stiffness, stress fibre formation and FA size in an Src-dependent manner (Saphirstein et al. 2013), and in freshly dispersed rat cremaster arteriole VSMCs where angiotensin II stimulation increased cell stiffness and cell adhesion to the extracellular matrix through actin CK and FA remodelling (Hong et al. 2014).
The signalling pathways and mechanisms leading to adhesion site remodelling in vascular tissues and differentiated contractile VSMCs are still unclear. Tyrosine phosphorylation is a hallmark of FA regulation and a number of cytosolic tyrosine kinases have been identified that are activated by vasoconstrictors in vascular tissue, including Src (Ohanian et al. 2001; Min et al. 2012), FAK (Min et al. 2012) and its homologue PYK2 (Ohanian et al. 2005), and Abl (Tang & Anfinogenova, 2008; Wang et al. 2013). Additionally, increased tyrosine phosphorylation of FA proteins paxillin (Ohanian et al. 2005), Hic-5 (Srinivasan et al. 2008) and p130 Crk-associated kinase (p130Cas) (Tang, 2009; Min et al. 2012) has been reported in vascular and airway smooth muscle tissue with a time course of phosphorylation consistent with the agonist-induced contractile response. Recruitment of cytosolic tyrosine kinases to FA and tyrosine phosphorylation of paxillin, Hic-5 and p130Cas regulates FA dynamics in non-smooth-muscle cells in culture (Mitra et al. 2005). Consistent with this, Src, PYK2, paxillin (Ohanian et al. 2005) and Hic-5 (Srinivasan et al. 2008) redistribute between cytosolic and cytoskeleton compartments following noradrenaline stimulation in intact small arteries. Inhibition of noradrenaline-induced redistribution of PYK2, paxillin and Hic-5 reduced the contractile response and actin CK remodelling without affecting myosin light chain phosphorylation (Ohanian et al. 2005; Srinivasan et al. 2008). Similarly in differentiated VSMCs, Src and p130Cas redistribute between soluble and insoluble fractions following phenylephrine stimulation and inhibition of their relocalisation reduces the rate of contraction of aortic tissue; again the reduction in contraction was independent of myosin light chain phosphorylation (Yamin & Morgan, 2012). Recently, it was shown in differentiated aortic VSMCs that following phenylephrine stimulation redistribution of FA proteins occurred through association with an endocytic recycling pathway (Poythress et al. 2013). Furthermore, inhibition of the endocytic pathway slowed the phenylephrine-induced rate of force development and maximum contractile response of aortic tissue (Poythress et al. 2013). Together these studies indicate that activation of cytosolic tyrosine kinases and phosphorylation of FA proteins occurs in response to vasoconstrictors in vascular smooth muscle tissues and cells leading to adhesion site and actin CK remodelling, and that this pathway is an essential component of the contractile response independent of myosin light chain phosphorylation (Fig.2).
Figure 2.
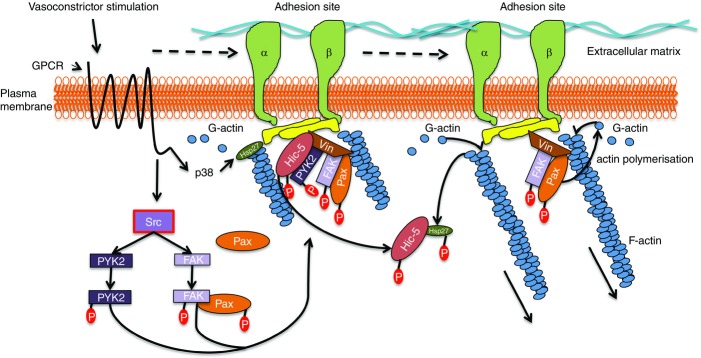
The possible role of paxillin and Hic-5 in vasoconstrictor-induced focal adhesion and actin cytoskeleton remodelling in vascular smooth muscle
Vasoconstrictor agonist acting through G protein-coupled receptor (GPCR) activates Src tyrosine kinase, leading to activation of PYK2 and FAK, and tyrosine phosphorylation of paxillin (Pax) and Hic-5. Remodelling of adhesion sites occurs such that paxillin, FAK and PYK2 relocalise to, and Hic-5 moves away from, the adhesion sites. Simultaneously vasoconstrictor activation induces p38MAPK-dependent phosphorylation of the actin-capping protein Hsp27. Phosphorylated Hsp27 and Hic-5 associate, freeing the barbed end of actin filaments promoting actin polymerisation. Phosphorylation of paxillin also promotes actin polymerisation inducing actin cytoskeleton remodelling. Abbreviation: Vin, vinculin.
In addition to transducing internal forces to the extracellular matrix during smooth muscle cell contraction, adhesion sites are important for sensing external forces such as changes in mechanical stress or strain and matrix stiffness. Signalling through adhesion sites to the actin cytoskeleton is an important mechanism for mechanosensing in VSMCs and is implicated in arterial remodelling in response to vasoconstrictors, hypertension and increased extracellular matrix stiffness (reviewed in Martinez-Lemus et al. 2009; Hill & Meininger, 2012; Castorena-Gonzalez et al. 2014; Saphirstein & Morgan, 2014). However, the signalling proteins that act as mechanosensors and mechanoresponders in vascular smooth muscle are still not fully characterised.
LIM proteins
LIM domains are versatile protein binding sites found in many adaptor proteins that facilitate the assembly of multi-protein complexes involved in cytoskeletal remodelling, cell migration and gene transcription (Kadrmas & Beckerle, 2004; Smith et al. 2014). In non-contractile cells LIM proteins are found in the nucleus where they control gene expression, and in the cytoplasm where they associate with FAs and the CK (Kadrmas & Beckerle, 2004). In expression studies in non-smooth-muscle cells, in response to force a subset of LIM proteins accumulate at actin stress fibres and are implicated in the regulation of the actin CK response to mechanical stimulation (reviewed in Smith et al. 2014). Similarly, endogenous LIM proteins Hic-5 and cysteine rich protein (CRP2) associate with stress fibres, whereas paxillin remains at FA in response to cyclical stretch in the mouse smooth muscle cell line SVS30 (Kim-Kaneyama et al. 2005). Additionally, in cultured rat VSMCs stably expressing the LIM protein zyxin–green fluorescent protein, zyxin accumulated at sites of force transmission (Sun et al. 2012). Given that zyxin, paxillin and Hic-5 are all expressed in contractile VSMCs and vascular tissues (Ward et al. 2002; Kim-Kaneyama et al. 2005; Ohanian et al. 2005; Srinivasan et al. 2008; Sun et al. 2012; Poythress et al. 2013), a role for these LIM proteins in VSMC mechanotransduction and contractility seems likely.
Hic-5
Hic-5 is a FA scaffold protein, first identified as a TGFβ and H2O2 inducible gene (Shibanuma et al. 1994). Through its scaffolding activity Hic-5 is implicated in apoptosis (Kim-Kaneyama et al. 2011; Hornigold et al. 2013; Desai et al. 2014), protein degradation (Ryan et al. 2012; Desai et al. 2014; Lei et al. 2014), fibrosis (Kim-Kaneyama et al. 2012; Hornigold et al. 2013; Desai et al. 2014), force sensing (Fujita et al. 1998; Thomas et al. 1999; Hetey et al. 2005; Kim-Kaneyama et al. 2005; Guignandon et al. 2006; Deakin et al. 2012; Smith et al. 2014), contractility (Kim-Kaneyama et al. 2005; Srinivasan et al. 2008), migration (Kim-Kaneyama et al. 2008; Deakin et al. 2012) and proliferation (Wang et al. 2011; Kim-Kaneyama et al. 2012). However, the effects of Hic-5 are cell-type specific, for instance Hic-5 is anti-apoptotic in cultured VSMCs (Kim-Kaneyama et al. 2011) and pro-apoptotic in mesangial myofibroblasts and lung fibroblasts (Hornigold et al. 2013; Desai et al. 2014), and stimulates migration in epithelial and endothelial cells, but inhibits migration in fibroblasts and smooth muscle cells (Wu et al. 2005; Avraamides et al. 2007; Dabiri et al. 2008; Kim-Kaneyama et al. 2008). Hic-5 is a homologue of paxillin (Thomas et al. 1999) and in addition to sharing a similar domain structure and localising to the same sites (FAs) Hic-5 shares many binding partners with paxillin (Fujita et al. 1998; Thomas et al. 1999). However, they must not entirely duplicate each other’s functions as paxillin knockout is embryonic lethal (Hagel et al. 2002). Furthermore, Hic-5 has unique binding partners such as Hsp27 (Jia et al. 2001; Srinivasan et al. 2008), CRP2 (Kim-Kaneyama et al. 2005) and the ubiquitin ligase Cblc (Ryan et al. 2012) and lacks the binding site for adaptor protein Crk that is present in paxillin (Thomas et al. 1999). These differences indicate that Hic-5 and paxillin will regulate different downstream pathways and so regulate different cellular functions. Indeed, forced expression of Hic-5 in fibroblasts decreases tyrosine phosphorylation of paxillin, possibly through sequestration of FAK, leading to the suggestion that Hic-5 acts as a counterbalance to paxillin opposing some of the effects of paxillin such as growth promotion and cell spreading (Fujita et al. 1998; Nishiya et al. 2001). Additionally, in the SVS30 smooth muscle cell line in response to cyclical stretch endogenous Hic-5 moved out of FAs and bound to CRP2 at actin stress fibres whereas paxillin remained within FAs, further demonstrating differences between them (Kim-Kaneyama et al. 2005). An expression study in fibroblasts has shown that the pathways regulating Hic-5 and paxillin association with vinculin at FAs also differ, with Rac1 activation regulating paxillin association with vinculin in immature FAs and RhoA activation important for Hic-5 association with vinculin in mature FAs (Deakin et al. 2012). Furthermore, when cells were placed under mechanical strain by growing in 3-D matrices Hic-5 but not paxillin interacted with vinculin at adhesion sites (Deakin et al. 2012), suggesting that in tissues that co-express Hic-5 and paxillin, Hic-5 would be the main mechanosensor through preferential interaction with vinculin at adhesion sites. Whilst paxillin is ubiquitously expressed (Brown & Turner, 2004), Hic-5 is restricted to smooth muscle, myofibroblast and epithelial cells in adults (Yuminamochi et al. 2003; Kim-Kaneyama et al. 2005) and Hic-5 is recognised as a phenotypic marker of differentiated smooth muscle cells (Wang et al. 2011). Consequently, Hic-5 would be expected to have an important role in mechanosensing and counterbalancing the effects of paxillin in smooth muscle.
Hic-5 in vascular cells and tissues
Hic-5 is abundantly expressed in differentiated contractile vascular smooth muscle cells in both large and small arteries (Yuminamochi et al. 2003; Kim-Kaneyama et al. 2005; Srinivasan et al. 2008). In femoral artery, electron microscopy studies have shown Hic-5 is localised at the cell periphery of smooth muscle cells indicative of association with adhesion sites (Kim-Kaneyama et al. 2012). Expression of Hic-5 in endothelial cells in arteries appears to be low, as it is not detectable by immunofluorescence. However, Hic-5 has been detected in endothelial cells by electron microscopy following immunogold labelling of mouse pulmonary arterioles (Kim-Kaneyama et al. 2012). The Hic-5 was present at the abluminal plasma membrane adjacent to the extracellular matrix indicating localisation to adhesion sites. In vitro studies have shown that Hic-5 regulation of FA dynamics plays a key role in endothelial cell migration (Wu et al. 2005; Avraamides et al. 2007; Komorowsky et al. 2010) stimulating interest in Hic-5 as a regulator of angiogenesis. However, the function of Hic-5 in endothelial cell responses in arteries remains unclear.
Hic-5 in vascular contractility
Two studies have implicated Hic-5 in vascular smooth muscle contractility. In a smooth muscle cell line endogenous Hic-5 translocated from FA to stress fibres in response to cyclical stretch; in mouse aorta Hic-5 and CRP2 localised to filamentous structures shown by electron microscopy. Additionally, mouse embryonic fibroblasts with forced expression of Hic-5 had a slower contraction of the 3-D gel matrix; in contrast, cells over-expressing paxillin had greater contraction. This led to the proposal that in response to cyclic stretch Hic-5 localises to stress fibres and negatively regulates contractility (Kim-Kaneyama et al. 2005). We have shown in intact small arteries that noradrenaline promoted Hic-5 association with PYK2 and increased Hic-5 tyrosine phosphorylation, which induced Hic-5 translocation from the actin cytoskeleton to the cytosol and interaction with Hsp27. This was accompanied by actin cytoskeleton remodelling and contraction, and inhibition of Hic-5 tyrosine phosphorylation-attenuated contraction suggesting Hic-5 positively regulates contractility in differentiated smooth muscle cells in the artery wall (Srinivasan et al. 2008) (Fig.2). However, a possible explanation for the apparently contradictory effects on contractility in these two studies is an effect on paxillin activity. It has been shown that over-expression of Hic-5 in fibroblasts reduces tyrosine phosphorylation of paxillin (Fujita et al. 1998; Nishiya et al. 2001) and that paxillin activation is important for tension development in fibroblasts (Deakin et al. 2012). Additionally, in airway smooth muscle tissue tyrosine phosphorylation of paxillin is important for acetylcholine-induced contraction (Gerthoffer & Gunst, 2001), and in intact small arteries noradrenaline induces paxillin tyrosine phosphorylation and association with the actin cytoskeleton (Ohanian et al. 2005). In our study in intact arteries (Srinivasan et al. 2008) we used an Src inhibitor to block Hic-5 tyrosine phosphorylation and to study contractility. Src inhibition also blocks paxillin tyrosine phosphorylation (Ohanian et al. 2005) raising the possibility that the effect on contractility was mediated through inactivation of paxillin. Obviously, further work is required to elucidate the interplay between Hic-5 and paxillin in smooth muscle contractility.
Hic-5 in vascular remodelling
Hic-5 is also implicated in vascular remodelling. Wire injury of the mouse carotid artery led to decreased Hic-5 expression in the medial smooth muscle cells and delivery of Hic-5 to the injured artery decreased neointima formation (Kim-Kaneyama et al. 2008). In vitro, over-expression of Hic-5 in a smooth muscle cell line repressed uPA expression, an activator of matrix metalloproteinase (MMP), an effect that was not duplicated by paxillin expression (Kim-Kaneyama et al. 2008). This suggests that the protective effect of Hic-5 against vascular injury is mediated through inhibition of MMP activation. However, in a mouse model of abdominal aortic aneurysm (AAA) loss of Hic-5 in aortic vascular smooth muscle cells suppressed AAA. Mechanistically, Hic-5 increased c-Jun N-terminal kinase activity by acting as a scaffold for c-Jun N-terminal kinase and its activator MKK4, leading to increased MMP activity, elastin degradation and increased susceptibility to AAA (Lei et al. 2014). These studies again highlight how the effects of Hic-5 are context specific. However, studies in non-smooth-muscle cells showing that Hic-5 regulates fibrosis (Kim-Kaneyama et al. 2012; Hornigold et al. 2013; Desai et al. 2014) support a role for Hic-5 in vascular remodelling.
Studies of Hic-5−/− mice
Given the above evidence that Hic-5 is involved in smooth muscle contractility and artery remodelling, it was surprising that genetic deletion of Hic-5 in mice produced no obvious vascular or other phenotype (Kim-Kaneyama et al. 2011). However, following wire injury of the femoral artery there was increased medial smooth muscle cell apoptosis and increased neointima formation in the Hic-5−/− mice compared to wild-type (Kim-Kaneyama et al. 2011) supporting a protective role of Hic-5 in VSMCs following vascular injury. In support of Hic-5 as a mechanosensor and regulator of the actin cytoskeleton and adhesion sites, aortic VSMCs from the Hic-5−/− mice had fewer stress fibres and in response to mechanical stimulation the FA protein vinculin relocated to the cytoplasm. Additionally, Hic-5−/− aortic VSMCs were more susceptible to stretch-induced apoptosis, suggesting that Hic-5 decreases VSMC sensitivity to stretch-induced apoptosis by stabilising stress fibres and vinculin at FAs (Kim-Kaneyama et al. 2011). Hic-5 did not protect VSMCs from cytokine- or H2O2-induced apoptosis (Kim-Kaneyama et al. 2011), further emphasising its role as a mechanoresponder in VSMCs.
Summary
It is only relatively recently that the role of the actin CK in VSMC responses has been appreciated. It is now clear that dynamic restructuring of the CK is required for contraction – independent of myosin light chain phosphorylation – and for maintenance of vascular tissue integrity. Actin CK remodelling occurs in response to both mechanical stresses and vasoactive agonist stimuli, and is implicated in increased large artery stiffness, neointima formation and small artery remodelling in cardiovascular disease. In addition to actin CK remodelling evidence is now accumulating for concomitant adhesion site remodelling in response to vasoconstrictor and mechanical stimuli. However, although it is clear that non-receptor tyrosine kinases and tyrosine phosphorylation of FA proteins is an important component of the response, the major pathways involved and their regulation and cross talk remain unclear. The goal of future research must be to unravel the complexities of VSMC actin CK and adhesion site remodelling in order to offer new areas for development of novel therapeutic intervention in cardiovascular disease.
Glossary
- CK
cytoskeleton
- FAs
focal adhesions
- FAK
focal adhesion kinase
- Hic-5
hydrogen peroxide inducible clone-5
- Hsp27
heat shock protein 27
- LIM protein
Lin11, Isl-1 and Mec-3 domain protein
- VSMCs
vascular smooth muscle cells
Biographies
Jacqueline Ohanian is currently Senior Lecturer in the Institute of Cardiovascular Sciences at the University of Manchester, UK. Her research interests focus on understanding the signalling pathways involved in the regulation of small artery contractility.

Vasken Ohanian is also currently Senior Lecturer in the Institute of Cardiovascular Sciences at the University of Manchester. His work focuses on regulation of the actin cytoskeleton by tyrosine kinases and mitogen-activated protein kinases in vascular smooth muscle cells.

Additional information
Competing interests
None declared.
Funding
None declared.
References
- Avraamides C, Bromberg ME, Gaughan JP, Thomas SM, Tsygankov AY. Panetti TS. Hic-5 promotes endothelial cell migration to lysophosphatidic acid. Am J Physiol Heart Circ Physiol. 2007;293:H193–H203. doi: 10.1152/ajpheart.00728.2006. [DOI] [PubMed] [Google Scholar]
- Bárány M, Barron JT, Gu L. Bárány K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem. 2001;276:48398–48403. doi: 10.1074/jbc.M106227200. [DOI] [PubMed] [Google Scholar]
- Boels PJ. Pfitzer G. Relaxant effect of phalloidin on Triton-skinned microvascular and other smooth muscle preparations. J Muscle Res Cell Motil. 1992;13:71–80. doi: 10.1007/BF01738430. [DOI] [PubMed] [Google Scholar]
- Brown MC. Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Castorena-Gonzalez JA, Staiculescu MC, Foote C. Martinez-Lemus LA. Mechanisms of the inward remodeling process in resistance vessels: is the actin cytoskeleton involved? Microcirculation. 2014;21:219–229. doi: 10.1111/micc.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Gokina NI. Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- Dabiri G, Tumbarello DA, Turner CE. Van De Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-β1 autocrine loop. J Invest Dermatol. 2008;128:2518–2525. doi: 10.1038/jid.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin NO, Ballestrem C. Turner CE. Paxillin and Hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA. PLoS One. 2012;7:e37990. doi: 10.1371/journal.pone.0037990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai LP, Zhou Y, Estrada AV, Ding Q, Cheng G, Collawn JF. Thannickal VJ. Negative regulation of NADPH oxidase 4 by hydrogen peroxide-inducible clone 5 (Hic-5) protein. J Biol Chem. 2014;289:18270–18278. doi: 10.1074/jbc.M114.562249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kamiguchi K, Cho D, Shibanuma M, Morimoto C. Tachibana K. Interaction of Hic-5, a senescence-related protein, with focal adhesion kinase. J Biol Chem. 1998;273:26516–26521. doi: 10.1074/jbc.273.41.26516. [DOI] [PubMed] [Google Scholar]
- Gallant C, Appel S, Graceffa P, Leavis P, Lin JJ-C, Gunning PW, Schevzov G, Chaponnier C, DeGnore J, Lehman W. Morgan KG. Tropomyosin variants describe distinct functional subcellular domains in differentiated vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;300:C1356–C1365. doi: 10.1152/ajpcell.00450.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer WT. Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Guignandon A, Boutahar N, Rattner A, Vico L. Lafage-Proust M-H. Cyclic strain promotes shuttling of PYK2/Hic-5 complex from focal contacts in osteoblast-like cells. Biochem Biophys Res Commun. 2006;343:407–414. doi: 10.1016/j.bbrc.2006.02.162. [DOI] [PubMed] [Google Scholar]
- Gunst SJ. Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A. Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetey SE, Lalonde DP. Turner CE. Tyrosine-phosphorylated Hic-5 inhibits epidermal growth factor-induced lamellipodia formation. Exp Cell Res. 2005;311:147–156. doi: 10.1016/j.yexcr.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hill MA. Meininger GA. Arteriolar vascular smooth muscle cells: Mechanotransducers in a complex environment. Int J Biochem Cell Biol. 2012;44:1505–1510. doi: 10.1016/j.biocel.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Sun Z, Li M, Li Z, Bunyak F, Ersoy I, Trzeciakowski JP, Staiculescu MC, Jin M, Martinez-Lemus L, Hill MA, Palaniappan K. Meininger GA. Vasoactive agonists exert dynamic and coordinated effects on vascular smooth muscle cell elasticity, cytoskeletal remodelling and adhesion. J Physiol. 2014;592:1249–1246. doi: 10.1113/jphysiol.2013.264929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornigold N, Johnson TS, Huang L, Haylor JL, Griffin M. Mooney A. Inhibition of collagen I accumulation reduces glomerulosclerosis by a Hic-5-dependent mechanism in experimental diabetic nephropathy. Lab Invest. 2013;93:553–565. doi: 10.1038/labinvest.2013.42. [DOI] [PubMed] [Google Scholar]
- Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ. Smoyer WE. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem. 2001;276:39911–39918. doi: 10.1074/jbc.M103510200. [DOI] [PubMed] [Google Scholar]
- Kadrmas JL. Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- Kim HR, Gallant C, Leavis PC, Gunst SJ. Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Kaneyama J-r, Lei X-F, Arita S, Miyauchi A, Miyazaki T. Miyazaki A. Hydrogen peroxide-inducible clone 5 (Hic-5) as a potential therapeutic target for vascular and other disorders. J Atheroscler Thromb. 2012;19:601–607. doi: 10.5551/jat.10736. [DOI] [PubMed] [Google Scholar]
- Kim-Kaneyama J-r, Suzuki W, Ichikawa K, Ohki T, Kohno Y, Sata M, Nose K. Shibanuma M. Uni-axial stretching regulates intracellular localization of Hic-5 expressed in smooth-muscle cells in vivo. J Cell Sci. 2005;118:937–949. doi: 10.1242/jcs.01683. [DOI] [PubMed] [Google Scholar]
- Kim-Kaneyama J-r, Takeda N, Sasai A, Miyazaki A, Sata M, Hirabayashi T, Shibanuma M, Yamada G. Nose K. Hic-5 deficiency enhances mechanosensitive apoptosis and modulates vascular remodeling. J Mol Cell Cardiol. 2011;50:77–86. doi: 10.1016/j.yjmcc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Kim-Kaneyama J-r, Wachi N, Sata M, Enomoto S, Fukabori K, Koh K, Shibanuma M. Nose K. Hic-5, an adaptor protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Biochem Biophys Res Commun. 2008;376:682–687. doi: 10.1016/j.bbrc.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Komorowsky C, Samarin J, Rehm M, Guidolin D. Goppelt-Struebe M. Hic-5 as a regulator of endothelial cell morphology and connective tissue growth factor gene expression. J Mol Med. 2010;88:623–631. doi: 10.1007/s00109-010-0608-3. [DOI] [PubMed] [Google Scholar]
- Lehman W. Morgan KG. Structure and dynamics of the actin-based smooth muscle contractile and cytoskeletal apparatus. J Muscle Res Cell Motil. 2012;33:461–469. doi: 10.1007/s10974-012-9283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X-F, Kim-Kaneyama J-r, Arita-Okubo S, Offermanns S, Itabe H, Miyazaki T. Miyazaki A. Identification of Hic-5 as a novel scaffold for the MKK4/p54 JNK pathway in the development of abdominal aortic aneurysms. J Am Heart Assoc. 2014;3:e000747. doi: 10.1161/JAHA.113.000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Hill MA. Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- Mehta D. Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol. 1999;519:829–840. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Reznichenko M, Poythress RH, Gallant CM, Vetterkind S, Li Y. Morgan KG. Src modulates contractile vascular smooth muscle function via regulation of focal adhesions. J Cell Physiol. 2012;227:3585–3592. doi: 10.1002/jcp.24062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA. Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Moreno-Dominguez A, Colinas O, El-Yazbi A, Walsh E, Hill MA, Walsh MP. Cole WC. Calcium sensitization due to myosin light chain phosphatase inhibition and cytoskeletal reorganization in the myogenic response of skeletal muscle resistance arteries. J Physiol. 2013;591:1235–1250. doi: 10.1113/jphysiol.2012.243576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya N, Tachibana K, Shibanuma M, Mashimo JI. Nose K. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001;21:5332–5345. doi: 10.1128/MCB.21.16.5332-5345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian J, Cunliffe P, Ceppi E, Alder A, Heerkens E. Ohanian V. Activation of p38 mitogen-activated protein kinases by endothelin and noradrenaline in small arteries, regulation by calcium influx and tyrosine kinases, and their role in contraction. Arterioscler Thromb Vasc Biol. 2001;21:1921–1927. doi: 10.1161/hq1201.100264. [DOI] [PubMed] [Google Scholar]
- Ohanian V, Gatfield K. Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension. 2005;46:93–99. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS. Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Poythress RH, Gallant C, Vetterkind S. Morgan KG. Vasoconstrictor-induced endocytic recycling regulates focal adhesion protein localization and function in vascular smooth muscle. Am J Physiol Cell Physiol. 2013;305:C215–C227. doi: 10.1152/ajpcell.00103.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RRG, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA. Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PE, Kales SC, Yadavalli R, Nau MM, Zhang H. Lipkowitz S. Cbl-c ubiquitin ligase activity is increased via the interaction of its RING finger domain with a LIM domain of the paxillin homolog, Hic 5. PLoS One. 2012;7:e49428. doi: 10.1371/journal.pone.0049428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphirstein RJ, Gao YZ, Jensen MH, Gallant CM, Vetterkind S, Moore JR. Morgan KG. The focal adhesion: a regulated component of aortic stiffness. PLoS One. 2013;8:e62461. doi: 10.1371/journal.pone.0062461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphirstein RJ. Morgan KG. The contribution of vascular smooth muscle to aortic stiffness across length scales. Microcirculation. 2014;21:201–207. doi: 10.1111/micc.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA. Vatner SF. Increased vascular smooth muscle cell stiffness; a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1281–H1287. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibanuma M, Mashimo J, Kuroki T. Nose K. Characterization of the TGFβ1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem. 1994;269:26767–26774. [PubMed] [Google Scholar]
- Smith MA, Hoffman LM. Beckerle MC. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014;10:575–583. doi: 10.1016/j.tcb.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Forman S, Quinlan RA, Ohanian J. Ohanian V. Regulation of contractility by Hsp27 and Hic-5 in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2008;294:H961–H969. doi: 10.1152/ajpheart.00939.2007. [DOI] [PubMed] [Google Scholar]
- Sun Z, Huang S, Li Z. Meininger GA. Zyxin is involved in regulation of mechanotransduction in arteriole smooth muscle cells. Front Physiol. 2012;3:472. doi: 10.3389/fphys.2012.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD. p130 Crk-associated substrate (CAS) in vascular smooth muscle. J Cardiovasc Pharmacol Ther. 2009;14:89–98. doi: 10.1177/1074248409333490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD. Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther. 2008;13:130–140. doi: 10.1177/1074248407313737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Hagel M. Turner CE. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J Cell Sci. 1999;112:181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- Walsh MP. Cole WC. The role of actin filament dynamics in the myogenic response of cerebral resistance arteries. J Cereb Blood Flow Metab. 2013;33:1–12. doi: 10.1038/jcbfm.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Cleary RA, Wang T, Li J. Tang DD. The association of cortactin with profilin-1 is critical for smooth muscle contraction. J Biol Chem. 2014;289:14157–14169. doi: 10.1074/jbc.M114.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Cleary RA, Wang R. Tang DD. Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J Biol Chem. 2013;288:20713–20722. doi: 10.1074/jbc.M112.439877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu G, Betts C, Harmon EY, Keller RS, Van De Water L. Zhou J. Transforming growth factor-β1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J Biol Chem. 2011;286:41589–41599. doi: 10.1074/jbc.M111.250878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DT, Alder AC, Ohanian J. Ohanian V. Noradrenaline-induced paxillin phosphorylation, ERK activation and MEK-regulated contraction in intact rat mesenteric arteries. J Vasc Res. 2002;39:1–11. doi: 10.1159/000048988. [DOI] [PubMed] [Google Scholar]
- Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA. Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin R. Morgan KG. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J Physiol. 2012;590:4145–4154. doi: 10.1113/jphysiol.2012.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuminamochi T, Yatomi Y, Osada M, Ohmori T, Ishii Y, Nakazawa K, Hosogaya S. Ozaki Y. Expression of the LIM proteins paxillin and Hic-5 in human tissues. J Histochem Cytochem. 2003;51:513–521. doi: 10.1177/002215540305100413. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R. Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]


