Abstract
Abstract
The present study aimed to test whether submitting the healthy heart to intermittent and tolerable amounts of workload, independently of its nature, could result in an adaptive cardiac phenotype. Male Wistar rats were subjected to treadmill running (Ex) (n = 20), intermittent cardiac overload with dobutamine (ITO) (2 mg kg–1, s.c.; n = 20) or placebo administration (Cont) (n = 20) for 5 days week–1 for 8 weeks. Animals were then killed for histological and biochemical analysis or subjected to left ventricular haemodynamic evaluation under baseline conditions, in response to isovolumetric contractions and to sustained LV acute pressure overload (35% increase in peak systolic pressure maintained for 2 h). Baseline cardiac function was enhanced only in Ex, whereas the response to isovolumetric heartbeats was improved in both ITO and Ex. By contrast to the Cont group, in which rats developed diastolic dysfunction with sustained acute pressure overload, ITO and Ex showed increased tolerance to this stress test. Both ITO and Ex developed cardiomyocyte hypertrophy without fibrosis, no overexpression of osteopontin-1 or β-myosin heavy chain, and increased expression of sarcoplasmic reticulum Ca2+ protein. Regarding hypertrophic pathways, ITO and Ex showed activation of the protein kinase B/mammalian target of rapamycin pathway but not calcineurin. Mitochondrial complex IV and V activities were also increased in ITO and Ex. Chronic submission to controlled intermittent cardiac overload, independently of its nature, results in an adaptive cardiac phenotype. Features of the cardiac overload, such as the duration and magnitude of the stimuli, may play a role in the development of an adaptive or maladaptive phenotype.
Key points
The present study aimed to test whether a chronic intermittent workload could induce an adaptive cardiac phenotype
Chronic intermittent workload induced features of adaptive hypertrophy
This was paralleled by protection against acute pressure overload insult
The heart may adapt favourably to balanced demands, regardless of the nature of the stimuli.
Introduction
Cardiac hypertrophy is a universal response of the heart to chronic workload elevations but, depending on the underlying cause, different clinical outcomes are attained. Under chronic loading conditions induced by disease states such as hypertension or aortic stenosis, the heart undergoes hypertrophic growth to normalize wall stress and maintain or increase cardiac output (Kehat & Molkentin, 2010). Despite having a compensatory effect in the short-term, cardiac hypertrophy is commonly recognized as a major independent risk factor for morbidity and mortality (Meijs et al. 2010), and is associated with increased interstitial fibrosis, cell death and cardiac dysfunction (Ooi et al. 2014). By contrast, chronic workload elevations elicited, for example, by exercise training or pregnancy, induce reversible cardiac hypertrophic growth, which is characterized by normal cardiac morphology (i.e. no fibrosis or apoptosis) and normal or enhanced cardiac function (Ooi et al. 2014). Hypertrophic growth can also be distinguished at the molecular level. Exercise-induced cardiac hypertrophy is mainly mediated by signalling through insulin-like growth factor 1 and growth hormone and is transduced by the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (Akt/mTOR) pathway, whereas the calcineurin/nuclear factor of activated T-cells and Ca2+/calmodulin-dependent kinase II pathways appear to be centrally involved in overloading diseases (Kehat & Molkentin, 2010; Mann & Rosenzweig, 2012; Ooi et al. 2014).
The reason for such a divergent response is usually attributed to the nature of the overloading stimuli. However, features of the cardiac overload, namely its duration and intensity, may also play a role (Kultz, 2003, 2005; Fulda et al. 2010). When cells are subjected to severe or prolonged stress and do not have sufficient time to recover, their integrity can be compromised and cellular death pathways are favoured (Chrousos, 2009). In turn, when the stress demands are matched by the cellular response, pro-survival pathways are activated and an improved homeostatic capacity is attained (increased tolerance) (Chrousos, 2009). In this sense, even the exercise benefits may be dose-dependent. Extreme exercise has been associated with biochemical and functional evidences of acute damage (Dawson et al. 2008; Trivax et al. 2010) and, over time, the cumulative effects of repetitive injury can possibly cause adverse structural and electrical cardiac remodelling (Anversa et al. 1985; Benito et al. 2011; La Gerche, 2013). Therefore, it can be hypothesized that, independently of the nature of the stimuli, the balance between the functional demands, recovery periods, and compensatory and regenerative mechanisms of the heart may dictate whether an adaptive or a maladaptive cardiac phenotype is developed.
The present study aimed to determine whether submitting the heart to intermittent and tolerable amounts of stress, independently of the inducing nature, could induce a cardiac phenotype that would fit in the ‘physiological’ spectrum. To test this hypothesis, rats were subjected to controlled chronic intermittent cardiac overload induced by β-adrenergic stimulation. This strategy allowed the imposition of a cardiac overload that shares some similarities to the one imposed by exercise training, namely its duration and magnitude. We then analysed the resultant functional, structural and molecular adaptations and compared them with the normal sedentary and exercised heart.
Methods
Ethical approval
Animal experiments were performed in accordance with the Portuguese law on animal welfare and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, Revised 2011). The ethical committee of the University of Porto, Portugal, approved all of the studies.
Preliminary haemodynamic experiments
Preliminary haemodynamic experiments were performed to determine the dose of dobutamine that could reproduce some aspects of our exercise training protocol. Specifically, we were aiming to identify a dosage that could induce a similar haemodynamic demand (∼40% increase heart rate and ∼15% increase in peak systolic pressure) (Miki et al. 2002), be maintained for the same period (90 min) and be applied daily for several weeks (5 days week–1 for 8 weeks). To perform this first task, male Wistar rats (n = 10; aged 5–6 weeks; Charles River Laboratories, Barcelona, Spain) were anaesthetized by inhalation of a mixture of sevoflurane (4%) and oxygen, intubated for mechanical ventilation (60 cpm, tidal volume set at 1 ml 100 g–1; TOPO; Kent Scientific, Torrington, CT, USA) and placed over a heating pad (body temperature is maintained at 37°C). One pressure–volume catheter (model-FTM-1912B-8018, 1.9F; Scisense, London, ON, Canada) was introduced in the left ventricle (LV) through the right carotid artery, as described in detail previously (Pacher et al. 2008). After stabilization, dobutamine (Mayne Pharma, Cascais, Portugal) was administered s.c. and haemodynamic parameters were recorded every 10 min for at least 100 min. Considering the previous data from literature, different doses of this drug were tested to define which was the most suitable (Liang et al. 1979; Davidson et al. 1986; Buttrick et al. 1988; Buser et al. 1989; Tipton & Sebastian, 1997), namely 4, 2 and 1 mg kg−1. Data were stored and analysed with PVAN, version 3.5 (Millar Instruments, Houston, TX, USA). The results that best fit our criteria were obtained with the administration of 2 mg kg−1 of dobutamine. After the experiments, all animals were killed by exsanguination when under anaesthesia with sevoflurane. Results from three independent experiments with acute dobutamine were averaged and are shown in Fig.1. Dobutamine induced an increase of ∼15% in peak systolic pressure, ∼30% in heart rate (HR) and ∼190% in peak rate of pressure rise (dP/dtmax) (Fig.1A–C).
Figure 1.
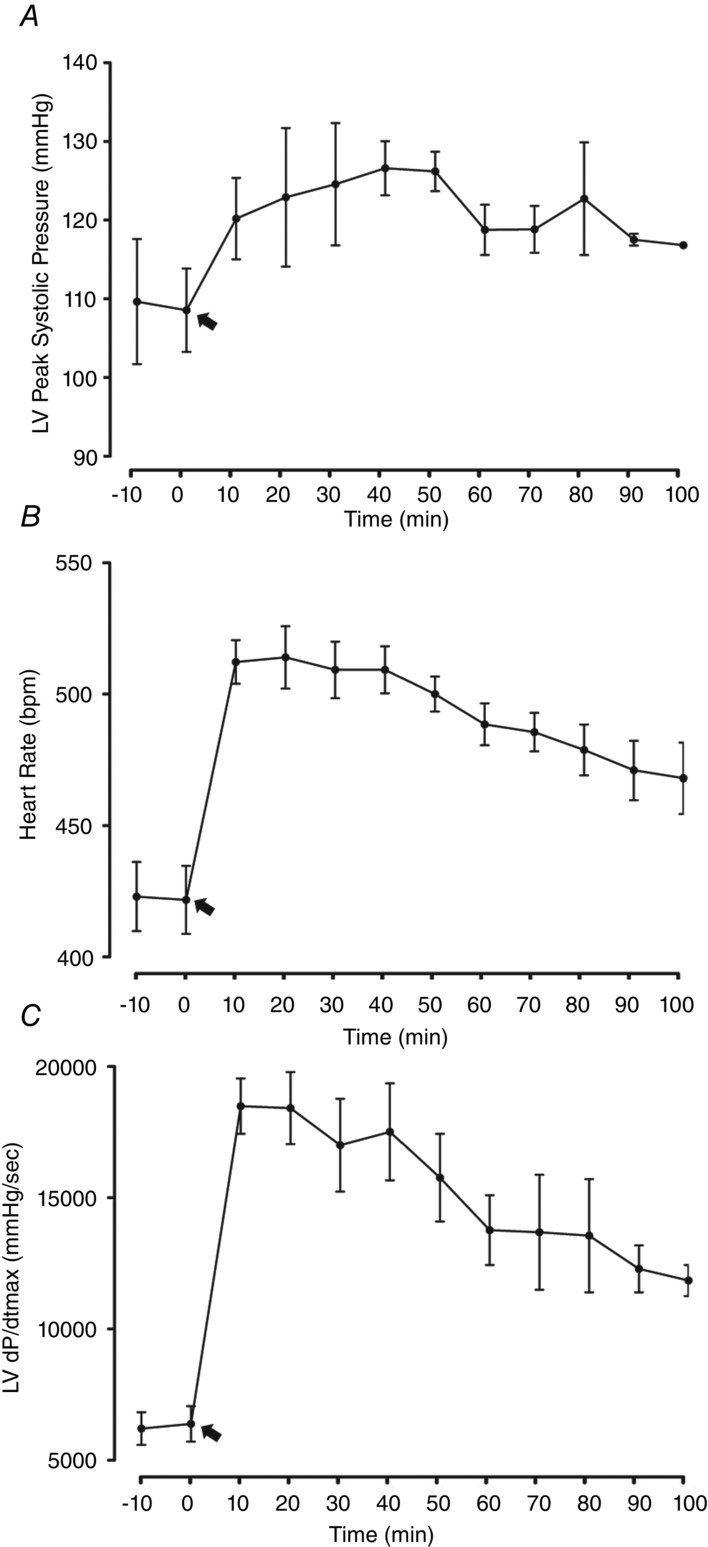
Acute effects of s.c. administration of 2 mg kg–1 of dobutamine on LV haemodynamics
A, LV Peak Systolic Pressure. B, Heart Rate. C, LV dP/dtmax. Black arrows indicate the time when the drug was administrated. Three animals per group were considered for analysis. Error bars indicate the mean ± SD.
Study design
Male Wistar rats (n = 60; aged 5 weeks; Charles River Laboratories) were housed in groups of five rats per cage, in a controlled environment at a room temperature of 22°C, with an inverted 12:12 h light/dark cycle, to match both animal handling and training with their most active period. All animals had free access to food and water. After 1 week of quarantine, they were randomly attributed to one of three protocols: (1) treadmill exercise training (Ex) (n = 20); (2) intermittent overload by dobutamine administration (ITO) (n = 20); and (3) placebo administration (Cont) (n = 20). Animals assigned to the Ex group trained for 8 weeks, 5 days week−1. Exercise duration and treadmill speed were gradually increased over the course of the first 3 weeks of training until animals achieved 90 min day−1 at 25 m min−1 (estimated work rate of 60% maximum oxygen consumption) (Lawler et al. 1993). Subsequently, both intensity and exercise duration were maintained constant. Animals from ITO group were injected (s.c.) with 2 mg kg−1 of dobutamine (Mayne Pharma) for 8 weeks, once a day, 5 days week−1. Animals from the Cont group and the Ex group received an equal volume of sodium chloride (NaCl) 0.9% (s.c.). Dosages were adjusted weekly according to the body weight (BW) and dilutions were performed with 0.9% NaCl.
Haemodynamic evaluation
Twenty-four hours after ending the protocols, half of the animals from each group were anaesthetized by inhalation of a mixture of sevoflurane (4%) and oxygen, and were killed by exsanguination. Tissue samples were collected and prepared for histological analysis and biochemical studies, as outlined below. The remaining animals were also anaesthetized by inhalation of a mixture of sevoflurane (4%) and oxygen, intubated for mechanical ventilation (60 cpm, tidal volume set at 1 ml 100 g–1; TOPO) and placed over a heating pad (body temperature maintained at 37°C). Under binocular surgical microscopy (Wild M651.MS-D; Leica, Herbrugg, Switzerland), the right jugular vein was cannulated for fluid administration (pre-warmed lactated Ringer solution) to compensate for perioperative fluid losses. The heart was exposed by a median sternotomy and the pericardium was widely opened. Descending thoracic aorta was dissected and a silk suture 2/0 was placed around it and passed through a plastic tube to allow aortic constriction during the experimental protocol. LV haemodynamic function was measured with conductance catheters (model-FTM-1912B-8018, 1.9F; Scisense), connected to a MVP-300 conductance system (Millar Instruments) through an interface cable (PCU-2000 MPVS, FC-MR-4; Scisense), coupled to a PowerLab16/30 converter (ADInstruments, Dunedin, New Zealand) and a personal computer for data acquisition. Parameters from the conductance catheter were recorded at a sampling rate of 1000 Hz to accurately capture all of the features of the pressure–volume waveforms produced by the fast beating hearts of rats.
Experimental protocol
After complete instrumentation, the animal preparation was allowed to stabilize for 15 min. Haemodynamic recordings were performed under baseline conditions and under inferior vena cava or ascending aortic occlusions, with the latter producing isovolumetric heartbeats. Sustained and selective acute pressure overload to the LV was obtained by controlled banding of the thoracic descending aorta, just above the diaphragm, for 120 min (min). Briefly, this was performed by gently pulling a silk suture, previously placed around the descending thoracic aorta, against a plastic tube, until an elevation of ∼35% of left ventricular peak systolic pressure (LVPmax) was obtained. At that time, the constriction was fixed with the help of a clamp and the imposed overload was monitored continuously. Adjustment of the constriction was provided to maintain the same cardiac overload during the entire protocol. Haemodynamic measurements were made under baseline steady-state conditions (immediately before banding) and 60 and 120 min after banding. All recordings were obtained with the ventilation suspended. Data were stored and analysed with PVAN, version 3.5. After the experiments, all animals were killed by exsanguination when under anaesthesia with sevoflurane.
Measured parameters
HR, peak systolic pressure (Pmax), end-systolic pressure (ESP), end-diastolic pressure (EDP), dP/dtmax, peak rate of pressure fall (dP/dtmin), time constant of isovolumetric pressure decay (Tau), maximum volume (Vmax), minimum volume (Vmin), end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF), cardiac output (CO), stroke work (SW) and maximal elastance (Emax) were obtained using PVAN, version 3.5. To assess intrinsic load independent myocardial function, end-systolic pressure–volume relationship (ESPVR), preload-recrutable stroke work (PRSW), end-diastolic pressure–volume relationship (EDPVR) and arterial elastance (Ea) were determined from pressure–volume loops recorded during transient preload reduction by gently pulling the inferior vena cava with a silk suture previously placed around it.
Conductance calibration
Parallel conductance values were obtained by the injection of approximately 100 μl of 10% NaCl into the right atrium. Calibration from the relative volume units conductance signal to absolute volumes (μl) was undertaken using a previously validated method of comparison with known volumes in Perspex wells (Pacher et al. 2008).
Tissue preparation
The heart and right gastrocnemius (Gast) muscle from animals killed at the end of the chronic protocols (not subjected to haemodynamic evaluation) were excised and weighed. Under binocular magnification (3.5×), the LV+septum (LV+S) was dissected from the right ventricle and weighed separately. Heart weight, LV and Gast were normalized to BW. Samples from the LV were fixed and prepared for light microscopy in accordance with routine procedures, or frozen with liquid nitrogen for protein and enzymatic studies.
Microscopic evaluation
Cubic pieces originating from the basal, intermediate and apical cardiac regions of each LV were fixed [4% (v/v) buffered paraformaldehyde] by diffusion for 24 h and subsequently dehydrated with graded ethanol and included in paraffin blocks. Xylene was used in the transition between dehydration and impregnation. LV blocks were embedded in the upright position to distinguish the endocardium, mid-wall and the epicardium of the LV free wall in cross-sections. Serial sections (5 μm thickness) of paraffin blocks were cut by a microtome and mounted on silane-coated slides. The slides were dewaxed in xylene and hydrated through graded alcohol, finishing in PBS prepared by dissolving Na2HPO4 (1.44 g), KH2PO4 (0.24 g), NaCl (8 g), KCl (0.2 g) and adjusting the pH to 7.2. Deparaffinized sections from the LV were stained for haematoxylin and eosin, performed by immersing slides in Mayer’s haematoxylin solution for 3–4 min, followed by immersion in 1% eosin solution for 7 min and dehydration with graded alcohol through xylene, and were mounted with DPX. The cardiomyocyte surface area was measured and only round to ovoid nucleated myocyte were considered for analysis. An average of 1000 cardiomyocytes per group was analysed. To determine the amount of cardiac fibrosis, LV sections were stained with Picrosirius red and quantified as described previously (Falcão-Pires et al. 2011). For quantitative comparisons, four random microscopic fields (magnification of 400×) from five animals were considered.
Left ventricular mitochondrial isolation
LV mitochondria isolation was performed using the conventional methods of differential centrifugation, as described in detail previously (Ascensao et al. 2005). All procedures were performed at 0–4°C. Briefly, after being excised, samples from the LV (pools of two animals) were immediately minced in an ice-cold isolation medium containing 250 mm sucrose, 0.5 mm EGTA, 10 mm Hepes-KOH (pH 7.4) and 0.1% defatted BSA (catalogue number A6003; Sigma, St Louis, MO, USA). The minced blood-free tissue was resuspended in isolation medium containing protease subtilopeptidase A type VIII (catalogue number P5380; Sigma; 1 mg g−1 tissue) and homogenized with a tightly fitted Potter-Elvehjen homogenizer and Teflon pestle. The suspension was incubated for 1 min (4°C) and rehomogenized. An aliquot of 0.5 ml of cardiac muscle homogenate was reserved for western blotting analysis of specific protein targets and the remaining homogenate was centrifuged at 14,500 g for 10 min. The supernatant fluid was decanted, and the pellet, essentially devoid of protease, was gently resuspended in isolation medium. The suspension was centrifuged at 750 g for 10 min, and the resulting supernatant was centrifuged at 12,000 g for 10 min. The pellet was resuspended and repelleted at 12,000 g for 10 min. The final pellet, containing the mitochondrial fraction, was gently resuspended in a washing medium containing 250 mm sucrose and 10 mm Hepes-KOH (pH 7.4). The mitochondrial protein concentration was estimated spectrophotometrically using a colorimetric method comprising the RC DC protein assay (Bio-Rad, Hercules, CA, USA) with BSA as standard.
Blue-native (BN)-PAGE separation of mitochondrial membrane complexes and in-gel activity of respiratory chain complexes IV and V
BN-PAGE was performed as described by Schagger & von Jagow (1991). Briefly, mitochondrial fractions (200 μg of protein) from each experimental group were pelleted by centrifugation at 20,000 g for 10 min and then resuspended in solubilization buffer (50 mm NaCl, 50 mm imidazole, 2 mm ɛ-amino n-caproic acid, 1 mm EDTA, pH 7.0) with 1% (w/v) digitonin. After 10 min on ice, insoluble material was removed by centrifugation at 20,000 g for 30 min at 4ºC. Soluble components were combined with 0.5% (w/v) Coomassie blue G250, 50 mm ɛ-amino n-caproic acid, 4% (w/v) glycerol and separated on a 4–13% gradient acrylamide gradient gel with 3.5% sample gel on top. Anode buffer contained 25 mm imidazole (pH 7.0). Cathode buffer (50 mm tricine and 7.5 mm imidazole, pH 7.0) containing 0.02% (w/v) Coomassie blue G250 was used for 1 h at 70 V, which is the time needed for the dye front to reach approximately one-third of the gel. Cathode buffer was then replaced by buffer containing only 0.002% (w/v) Coomassie blue G250 and the native complexes were separated at 200 V for 4 h at 4ºC. A native protein standard HMW-native marker (GE Healthcare, Little Chalfont, UK) was used. The gels were stained with colloidal Coomassie for protein visualization or incubated at 37ºC with 35 mm Tris and 270 mm glycine buffer (pH 8.3), supplemented with 14 mm MgSO4, 0.2% (w/v) Pb(NO3)2 and 8 mm ATP for evaluation of the ATP hydrolysis activity of complex V. Lead phosphate precipitation, which is proportional to the enzymatic ATP hydrolysis activity, was stopped by 50% (v/v) methanol (30 min), and the gels were then transferred to water. Gels were scanned using the Molecular Imager Gel Doc XR+ System (Bio-Rad). Band detection and analysis were performed using QuantityOne Imaging, version 4.6.3 (Bio-Rad).
Spectrophotometric evaluation of respiratory chain complex V was also measured as described previously (Zerbetto et al. 1997). The phosphate produced by the hydrolysis of ATP reacts with ammonium molybdate in the presence of reducing agents to form a blue-colour complex, for which the intensity is proportional to the concentration of phosphate in solution. Oligomycin was used as an inhibitor of mitochondrial ATPase activity.
Western blotting analysis
Equivalent amounts of total protein from each group were electrophoresed on a 12.5% SDS-PAGE as described previously (Laemmli, 1970). One sample from each of the groups studied was applied in the same gel. Gels containing total proteins or mitochondrial proteins (separated by two-dimensional BN-PAGE) were blotted onto a nitrocellulose membrane (Protan®; Schleicher & Schuell, Dassel, Germany) and non-specific binding was blocked with 5% (w/v) dry non-fat milk in TBS-T (100 mm Tris, 1.5 mm NaCl, pH 8.0, and 0.5% Tween 20). Membranes were then incubated with primary antibody solution (1:1000 dilution; mouse anti-ATP synthase subunit β, ab14730: Abcam, Cambridge, MA, USA; mouse anti-SERCA2 ATPase, ab2861: Abcam; rabbit anti-calcineurin A, ab52761; mouse anti-3-nitrotyrosine, clone 2A8.2: Chemicon International Inc., Temecula, CA, USA; rabbit anti-osteopontin, ab8448; rabbit anti-Akt, #9272: Cell Signaling Technology, Beverly, MA, USA; rabbit anti-phospho-Akt, #4058: Cell Signaling; rabbit anti-mTOR, #2983: Cell Signaling; rabbit anti-phospho-mTOR, #2971: Cell Signaling; rabbit anti-atrogin-1, #AP2041: ECM Bioscience, Versailles, KY, USA). After 2 h of incubation, the membrane was washed with TBS-T and incubated with anti-mouse or anti-rabbit IgG peroxidase secondary antibody (1:1000 dilution; Amersham Pharmacia Biotech, Piscataway, NJ, USA). Immunoreactive bands were detected with enhanced chemiluminescence reagents (ECL, Amersham Pharmacia Biotech) in accordance with the manufacturer’s instructions and images were recorded using X-ray films (Kodak Biomax light Film; Sigma). The films and the gels were scanned using the Molecular Imager Gel Doc XR+ System (Bio-Rad) and were analysed with QuantityOne, version 4.6.3. Four independent experiments were considered for the analysis. Equal loading was confirmed by staining the membrane with Ponceau S.
Myosin heavy chain (MHC) isoform determination
LVs were weighed and transferred to a glass homogenizer. A 1:19 ratio of 100 mm phosphate buffer (pH 7.4) containing 0.02% BSA was added. Tissue sections were thoroughly homogenized with a tightly fitted Potter-Elvehjen homogenizer and Teflon pestle. Total protein concentration was assayed spectrophotometrically using a colorimetric method comprising the RC DC protein assay (Bio-Rad) with BSA as standard. α- and β-isoforms of cardiac myosin heavy chain were separated by gel electrophoresis in accordance with the procedure described by Talmadge & Roy (1993). The amount of protein run on the gel was 1 mg per lane. To avoid intergel variation, one sample from each of the groups studied was applied in the same gel. The stacking gel consisted of 30% glycerol and 4% acrylamide:N,N’-methylene-bis-acrylamide at the ratio of 50:1, 70 mm Tris (pH 6.7), 4 mm EDTA and 0.4% SDS. The separating gels were composed of 30% glycerol, 8% acrylamide-bis (50:1), 0.2 m Tris (pH 8.8), 0.1 m glycine and 0.4% SDS. Polymerization was initiated with 0.05% N,N,N’,N’-tetramethylethylenediamine and 0.1% ammonium persulphate. The gels were run in a Mini-Protean system (Bio-Rad) at 4°C. The running conditions were 70 V (constant voltage) for 30 h. The gels were stained with colloidal Coomassie, scanned in a Molecular Imager Gel Doc XR+ System (Bio-Rad, Hercules, CA, USA) and optical density analysis of MHC bands was performed using QuantityOne Imaging, version 4.6.3. Five independent experiments assayed in duplicate were considered for the analysis.
Statistical analysis
A Kolmogorov–Smirnov test was performed to check the normality of the data. A Kruskal–Wallis test followed by Dunn’s test was used for non-normal data (cross-sectional analysis of cardiomyocytes). Between group’s comparisons of baseline haemodynamics, morphometric, fibrosis, western blotting, MHC, BN-PAGE and enzymatic activity data were performed by one-way ANOVA. For comparisons of haemodynamic data during pressure overload, a repeated measures two-way ANOVA test was performed. Significant differences were evaluated with Tukey’s post hoc analysis. All statistical analysis was performed with Prism, version 5.0 (GraphPad Software Inc., San Diego, CA, USA). Data are expressed as the mean ± SD. P < 0.05 was considered statistically significant.
Results
General morphometric features of animals subjected to the chronic protocols
Table1 summarizes the morphometric parameters analysed. Both ITO and Ex groups presented a lower BW (P < 0.001 vs. Cont). GAST was reduced in the Ex group (P < 0.05 vs. Cont) but not when normalized to BW. Only exercise training resulted in a significant increase of heart weight (HW)/BW (P < 0.001 vs. Cont). LV mass evaluated by the LV/BW ratio was significantly increased in ITO (P < 0.05 vs. Cont) and Ex (P < 0.01 vs. Cont).
Table 1.
General morphometric characterization
| Cont | ITO | Ex | |
|---|---|---|---|
| BW (g) | 437 ± 34 | 376 ± 49* | 365 ± 37* |
| Gast (g) | 2.54 ± 0.3 | 2.33 ± 0.2 | 2.26 ± 0.3* |
| Gast/BW (g kg–1) | 5.8 ± 0.9 | 6.4 ± 0.5 | 6.3 ± 0.7 |
| HW (g) | 1.10 ± 0.1 | 1.05 ± 0.1 | 1.08 ± 0.2 |
| HW/BW (g kg–1) | 2.5 ± 0.2 | 2.8 ± 0.2 | 3.0 ± 0.1* |
| LV+S (g) | 0.74 ± 0.1 | 0.71 ± 0.1 | 0.71 ± 0.1 |
| LV+S/BW (g kg–1) | 1.69 ± 0.2 | 1.91 ± 0.2 * | 1.96 ± 0.2 * |
Ten animals per group were considered for analysis. Data are expressed as the mean ± SD.
P < 0.05 vs. Cont;
Characterization of cardiac function under baseline steady-state conditions
Full haemodynamic data are summarized in Table2. HR was significantly increased in Ex compared to all other groups (P < 0.05). No differences were noted on Pmax, ESP, EDP or dP/dtmin. dP/dtmax was reduced in ITO (P < 0.05 vs. Cont). Relaxation, as evaluated by the constant time Tau, was improved only in Ex (P < 0.05 vs. all other groups). Considering volume-derived parameters, and despite ITO and Ex having a higher CO and EF, no differences were detected.
Table 2.
Baseline haemodynamic characterization
| Cont | ITO | Ex | |
|---|---|---|---|
| HR (bpm) | 392.1 ± 38.2 | 399 ± 37 | 422 ± 24*† |
| Pmax (mmHg) | 121.8 ± 14.5 | 115.4 ± 8.9 | 120.1 ± 12.4 |
| ESP (mmHg) | 113.2 ± 16.5 | 106.0 ± 8.9 | 111.2 ± 12.5 |
| EDP (mmHg) | 5.8 ± 2.5 | 4.6 ± 2.1 | 3.9 ± 0.9 |
| dP/dtmax (mmHg s–1) | 8868.4 ± 1708.3 | 7154.7 ± 1192.4* | 8788.5 ± 2177.3 |
| dP/dtmin (mmHg s–1) | –9221.3 ± 1919.1 | –8109.6 ± 1780.2 | –9607.5 ± 2182.3 |
| Tau W | 9.0 ± 1.0 | 9.0 ± 1.0 | 7.7 ± 0.9*† |
| ESV (μl) | 56.1 ± 21.5 | 46.1 ± 25.7 | 42.4 ± 14.9 |
| EDV (μl) | 146.8 ± 34.3 | 151.8 ± 51.2 | 142.8 ± 25.5 |
| SV (μl) | 101.1 ± 24.1 | 117.8 ± 50.1 | 106.6 ± 18.6 |
| EF (%) | 67.8 ± 9.1 | 74.7 ± 11.9 | 74.3 ± 7.3 |
| CO (μl min–1) | 39696.5 ± 10350.0 | 46740.5 ± 19162.1 | 44814.8 ± 7362.7 |
| SW (mmHg × μL) | 9978.3 ± 2320.4 | 11072.9 ± 5052.2 | 10305.2 ± 2634.2 |
| Ea (mmHg μl–1) | 1.2 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.2 |
| ESPVR (mmHg μl–1) | 2.4 ± 1.1 | 2.0 ± 1.0 | 3.8 ± 1.5*† |
| PRSW | 95.3 ± 25.7 | 119.9 ± 55.8 | 169.9 ± 32.9*† |
| Emax | 5.8 ± 2.4 | 6.4 ± 2.7 | 10.5 ± 4.6*† |
| EDPVR (mmHg μl–1) | 0.06 ± 0.0 | 0.05 ± 0.0 | 0.06 ± 0.0 |
Data are presented as the Mean ± SD.
P < 0.05 vs. Cont
P < 0.05 vs. ITO.
Pressure–volume derived parameters obtained from inferior vena cava occlusion, namely ESPVR, PRSW and Emax, were increased only in Ex (P < 0.05 vs. all other groups). No alterations were observed in EDPVR (P > 0.05).
Characterization of cardiac function in response to beat-to-beat isovolumetric contractions and to sustained acute pressure elevations
As shown in Fig.2, isovolumetric heartbeats presented similar peak systolic pressure and dP/dtmax in all groups but a shorter time constant Tau (faster relaxation) in Ex and ITO (P < 0.05 vs. Cont).
Figure 2.
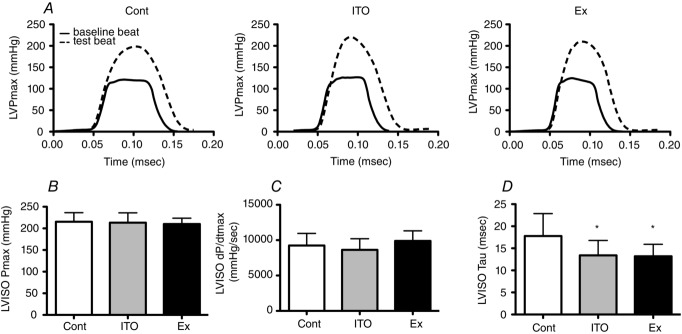
Effects of total occlusion of the ascending aorta (isovolumetric heartbeats)
A, typical baseline and test beats. B, LV Pmax. C, dP/dtmax. D, Tau. Ten animals per group were considered for analysis. Error bars indicate the mean ± SD. *P < 0.05 vs. Cont.
Pressure overload by descending thoracic aortic banding (Fig.3A) induced a 35% increase in systolic pressure in all groups, as shown by the rise in LV Pmax (Fig.3B). All groups were able to maintain the imposed overload for the entire duration of the banding, and no differences in LV Pmax were observed between groups at any time. dP/dtmax (Fig.3C) showed a compensatory increase at 60 min of banding in Ex and ITO, although this was only significant in the latter (P < 0.05 vs. baseline). At 120 min of pressure overload, dP/dtmax increased further in Ex (P < 0.05 vs. Cont and ITO) (Fig.3C). Regarding the impact of acute pressure overload on diastolic function, in opposition to Cont, ITO and Ex exhibited preserved EDP. Both ITO and Ex groups had an increase in dP/dtmin at 60 min (P < 0.05 vs. respective baseline values) but were normalized at 120 min (Fig.3E). Finally, a slower relaxation (prolonged time constant Tau) (Fig.3F), was observed in the Cont group after 60 min (P < 0.05 vs. baseline, ITO and Ex) and 120 min (P < 0.05 vs. baseline and Ex) of banding.
Figure 3.

Effects of 120 min of acute pressure overload on LV systolic and diastolic function
A, procedure of descending aortic pressure overload. B, LVPmax. C, LV dP/dtmax. D, LV EDP. E, LV dP/dtmin. F, LV Tau. Baseline values were considered as 100% and are indicated by the dashed line. Values at 60 and 120 min are indicated as a percentage of variation relative to baseline. Ten animals per group were considered for analysis. Error bars indicate the mean ± SD. #P < 0.05 vs. baseline *P < 0.05 vs. Cont; †P < 0.05 vs. ITO, ‡P < 0.05 vs. Ex.
Characterization of the hypertrophic phenotype
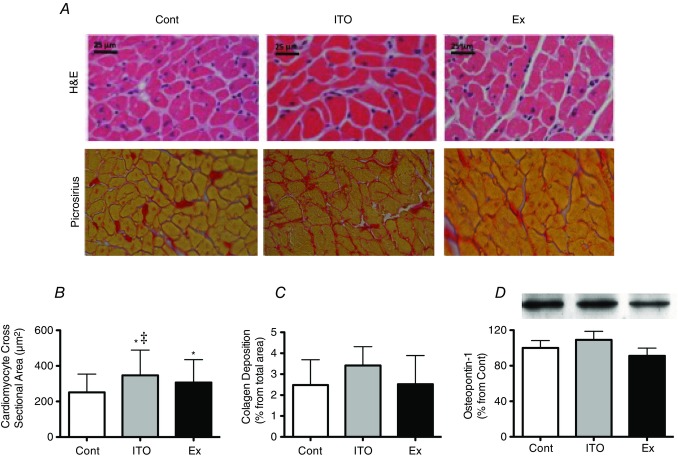
As shown in Fig.4A and B, cardiomyocyte hypertrophy was found in ITO (38%) (P < 0.001 vs. Cont) and Ex (22%), although it was more marked in ITO (P < 0.001 vs. Ex). No alterations were detected in terms of collagen deposition or osteopontin-1 protein expression (Fig.4C and D).
Figure 4.
Effects of the different chronic protocols on hypertrophy and fibrosis:
A (upper) and B, cardiomyocyte hypertrophy. A (lower) and C, fibrosis. D, osteopontin-1 protein levels. The Four random microscopic fields of LV from five animals per group were considered for histological analysis. Four independent experiments containing two different samples from each group were considered for protein analysis. Error bars indicate the mean ± SD. *P < 0.05 vs. Cont; ‡P < 0.05 vs. Ex.
Sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) (Fig.5A) was significantly increased in ITO and Ex (P < 0.05 vs. Cont). A modest increase in the β/α-MHC ratio was present in all groups (Fig.5B), although this was not significant (P > 0.05). Regarding calcineurin protein expression (Fig.5C), similar values were observed in all groups. The Akt/mTOR pathway was also assessed. No differences were noted in the expression of total Akt. However, both ITO and Ex groups exhibited a significant increase of Ser473 phosphorylation of Akt (P < 0.05 vs. Cont) (Fig.5D). Regarding mTOR, a significant increase was found for the expression of its total levels and for its phosphorylation at Ser2448 in the ITO and Ex groups (P < 0.05 vs. Cont).
Figure 5.
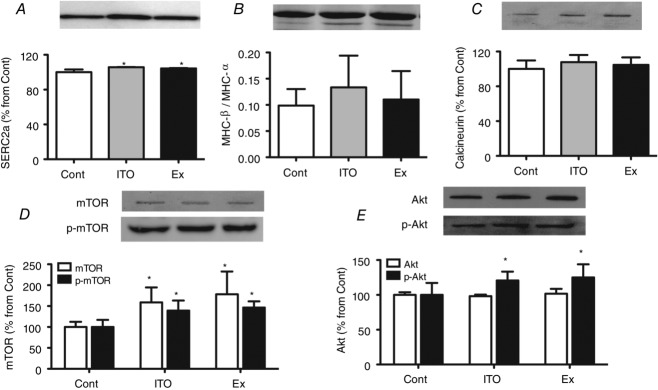
Effects of the different chronic protocols on molecular markers of cardiac remodelling:
A, SERCA2a protein expression. B, MHC isoforms shift. C, calcineurin protein expression A. D, total and phosphorated mTOR protein expression. E, total and phosphorated Akt protein expression. Four independent experiments containing two different sample from each group were considered for protein analysis. Equal loading was confirmed by staining the membrane with Ponceau S. Error bars indicate the mean ± SD. *P < 0.05 vs. Cont.
BN-PAGE densitometric analysis did not reveal differences in the protein complexes organization, as can be seen from the representative density traces for the complex bands (Fig.6A). In-gel activity showed an elevated complex IV in both ITO and EX groups (P < 0.05 vs. Cont), although this was greater in ITO (P < 0.05 vs. EX) (Fig.6B), whereas complex V activity was similarly increased in ITO and EX (P < 0.05 vs Cont). Spectrophotometric quantification of respiratory chain complex V corroborated the in-gel activity of complex V (P < 0.05 vs. Control) (Fig.6D).
Figure 6.
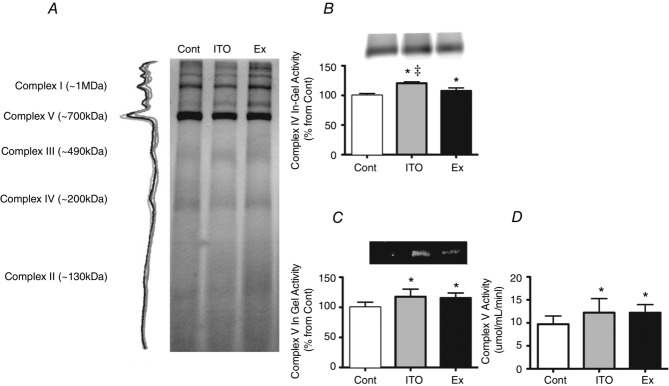
Effects of the different chronic protocols on oxidative phosphorylation
A, LV mitochondrial BN-PAGE profile of the experimental groups. B, representative images of histochemical staining, with semi-quantitative analysis of in-gel activity of complex IV. C, representative images of histochemical staining, with semi-quantitative analysis of in-gel activity of complex V. D, activity of complex V assayed by spectrophotometry. Five independent experiments assayed in duplicate were considered for analysis. Error bars indicate the mean ± SD. *P < 0.05 vs. Cont; ‡P < 0.05 vs. Ex.
Discussion
The present study investigated whether submitting the normal healthy heart to intermittent and tolerable amounts of stress may result in an adaptive cardiac phenotype. Our data suggest that the intermittence and magnitude of cardiac workload elevations may indeed play a role in cardiac adaptation, independently of the nature of the stimuli. Animals from the ITO group presented normal cardiac function and showed improved tolerance to acute stress tests. Similar to exercise-trained animals, ITO developed cardiomyocyte hypertrophy without fibrosis, no overexpression of osteopontin-1 or β-MHC, and increased expression of SERCA2a protein. Regarding hypertrophic pathways, similar to Ex, ITO showed activation of Akt/mTOR pathway but not calcineurin. Mitochondrial complex IV and V activities were also improved in ITO.
The reason why some cardiac overloading stimuli are beneficial, whereas others are deleterious, remains intriguing. It has been suggested that the nature of the stimulus, rather than the duration of stress or the hypertrophic growth per se, determines the molecular trigger for cardiac dysfunction (Perrino et al. 2006). However, even exercise, under certain conditions (i.e. strenuous and prolonged), can possibly result in adverse structural and electrical cardiac remodelling (Anversa et al. 1985; Benito et al. 2011; La Gerche, 2013). Thus, the features of the overload may indeed be important, independently of the nature of the underlying stimuli. To test this, we induced controlled intermittent cardiac overloads with dobutamine. This strategy allowed us to control the duration and magnitude of the haemodynamic overload, reasonably mimicking the overload imposed by the exercise training protocol used (Fig.1) (Miki et al. 2002).
To evaluate the cardiac adaptations obtained with a controlled chronic intermittent overload, we compared the haemodynamic, structural and molecular features of ITO with the normal sedentary and exercised heart. The ITO group presented normal cardiac function under resting conditions, whereas the EX group showed visible improvements. Under stress conditions, the ITO and EX groups both exhibited increased tolerance to a sudden and sustained pressure overload stress test. These data suggest that an intermittent controlled cardiac overload does not lead to cardiac dysfunction. Evaluation of the hypertrophic phenotype revealed that cardiomyocyte hypertrophy in the ITO and Ex groups occurred without fibrosis. Consistently, we found no changes of osteopontin-1 expression, a matricellular protein that is increased during stress-induced cardiac remodelling and mediates cardiac fibrosis and diastolic dysfunction (Yu et al. 2009). These data, in addition to the unchanged diastolic stiffness (normal EDPVR and EDP at baseline), suggest normal intrinsic myocardial function.
To further explore the hypertrophic phenotype developed by each of the interventions, we assessed Akt/mTOR and calcineurin protein expression, comprising two pathways with distinct roles in the promotion of adaptive or maladaptive hypertrophy, respectively (Kehat & Molkentin, 2010; Mann & Rosenzweig, 2012; Ooi et al. 2014). Our data show that, although the former was activated in the ITO and Ex groups, the latter was not. Activation of the Akt/mTOR signal cascade is associated with improved contractile function, cytoprotection, and an increased synthesis of normal contractile proteins and metabolic enzymes (Kemi et al. 2008), which could explain the increased performance against the acute pressure overload insult.
SERCA2a protein levels are typically increased in adaptive cardiac hypertrophy. We found increased total protein levels of SERCA2a in Ex and ITO which, together with the unaffected dP/dtmin and time constant Tau after acute pressure overload, suggest preserved/improved activity of SERCA2a (Demirel et al. 2001) and a more efficient transport of calcium to the sarcoplasmic reticulum (Miyamoto et al. 2000). This is important because the accumulation of calcium in the cytosol is implicated in cardiac dysfunction by favouring mitochondrial swelling (Starnes et al. 2007; Moreira-Gonçalves et al. 2011), apoptosis and proteolysis (French et al. 2006; French et al. 2008), and activation of the calcineurin/nuclear factor of activated T-cells and Ca2+/calmodulin-dependent kinase II pathway (Kehat & Molkentin, 2010; Mann & Rosenzweig, 2012; Ooi et al. 2014).
A modest increase in the β- to α-MHC ratio was observed in the ITO and Ex groups, without compromising the ability to tolerate an increased overload. Our data are corroborated by Hwang et al. (2005) who reported increased β-MHC in the LV of trained rats also exhibiting an improved cardiac response to a brief period of ischaemia and reperfusion (Hwang et al. 2005). The shift from the α- to β-MHC isoform is considered a classic marker of heart failure, although different studies provide contrasting results. For example, although transgenic mice with cardiac-specific expression of β-MHC developed cardiac dysfunction and failure under chronic isoproterenol challenge or in a post-infarction failure model, no signs of cardiac failure were observed in response to exercise training (Krenz & Robbins, 2004). Moreover, transgenic mice overexpressing the α-MHC isoform showed only subtle benefits in response to myocardial infarction or LV pressure overload (James et al. 2010). Therefore, a shift from the α- to β- MHC isoform might not represent a maladaptive response of the heart but rather an adaptation towards greater energetic efficiency. The link to cardiac dysfunction might occur when concurrent downstream alterations such as disturbed thick–thin filament interactions or calcium kinetics are present (Rice et al. 2010). In addition, the greater activity of mitochondrial complexes IV and V found in ITO and Ex suggests that these groups were more prepared to support the energetic cost of an elevated cardiac overload, without compromising the ATP that is needed to maintain intracellular homeostasis.
Overall, the findings of the present study suggest that, in the presence of balanced demands, and independently of the inciting haemodynamic stimuli, the heart may adapt favourably and exhibit features of adaptive hypertrophy. We suggest that intermittent and balanced workloads may induce a cardiac phenotype that fits inside the range of the ‘physiological’ spectrum.
Acknowledgments
We are very thankful to Miss Celeste Resende for her technical support regarding animal care and the training protocol. DM-G takes responsibility for the reliability and freedom from bias of the reported data, as well as their interpretation.
Glossary
- Akt/mTOR
protein kinase B/mammalian target of rapamycin
- BN
blue native
- BW
body weight
- CO
cardiac output
- dP/dtmax
peak rate of pressure rise
- dP/dtmin
peak rate of pressure fall
- ESP
end-systolic pressure
- EDP
end-diastolic pressure
- ESV
end-systolic volume
- EDV
end-diastolic volume
- Ea
arterial elastance
- EF
ejection fraction
- ESPVR
end-systolic pressure volume relation
- Emax
maximal elastance
- EDPVR
end-diastolic pressure volume relation
- Gast/BW
gastrocnemius weight/body weight
- Gast
gastrocnemius weight
- HR
heart rate
- HW
heart weight
- HW/BW
heart weight/body weight
- LV
left ventricle
- LV+S
left ventricle+septum
- LV+S/BW
left ventricle+septum/body weight
- LVPmax
left ventricular peak systolic pressure
- MHC
myosin heavy chain
- PRSW
preload-recrutable stroke work
- Pmax
peak systolic pressure
- SERCA2a
sarcoplasmic reticulum Ca2+ ATPase
- SV
stroke volume
- SW
stroke work
- Tau
constant time
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
DMG, THC, ALM and JAD participated in the conception and design of the experiments, the analysis and interpretation of data, and the drafting of the manuscript. DMG, HF, SV and AFS were responsible for the implementation of protocols, as well as haemodynamic and histological assessments. RF, AIP, CS and FA performed all of the biochemical assessments, and were also involved in the analyses and interpretation of data.
Funding
This work was supported by Fundação Para a Ciência e Tecnologia PTDC/DES/104567/2008 and EXCL/BIM-MEC/0055/2012 (partially funded by FEDER through COMPETE). The Research Centre on Physical Activity Health and Leisure (CIAFEL) is supported by Pest-OE/SAU/UI0617/2011. The Cardiovascular Research Centre is supported by PEst-C/SAU/UI0051/2014. DMG and HF are supported by an individual grant from Fundação Para a Ciência e Tecnologia through QREN – POPH (SFRH/BPD/90010/2012 and SFRH/BPD/78259/2011, respectively).
Translational perspective.
The heart adapts favourably to certain overloading stimuli, such as exercise training or pregnancy, but not to others, such as aortic stenosis or hypertension. This divergent response is usually attributed to the nature of the overloading stimuli. We hypothesized that features of the cardiac overload, namely its duration and intensity, may also play a role. Our results show that chronic controlled intermittent overload induced by β-adrenergic stimulation with dobutamine resulted in beneficial adaptations. Our observations suggest that, when subjected to balanced haemodynamic demands, and independently of the inducing stimuli, the heart develops a cardiac phenotype that fits inside the range of the ‘physiological’ spectrum.
References
- Anversa P, Beghi C, Levicky V, McDonald SL, Kikkawa Y. Olivetti G. Effects of strenuous exercise on the quantitative morphology of left ventricular myocardium in the rat. J Mol Cell Cardiol. 1985;17:587–595. doi: 10.1016/s0022-2828(85)80027-4. [DOI] [PubMed] [Google Scholar]
- Ascensao A, Magalhaes J, Soares JMC, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ. Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol. 2005;289:H722–H731. doi: 10.1152/ajpheart.01249.2004. [DOI] [PubMed] [Google Scholar]
- Benito BA, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif J-C, Brugada J, Nattel S. Mont L. cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training/clinical perspective. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- Buser PT, Camacho SA, Wu ST, Higgins CB, Jasmin G, Parmley WW. Wikman-Coffelt J. The effect of dobutamine on myocardial performance and high-energy phosphate metabolism at different stages of heart failure in cardiomyopathic hamsters: a 31P MRS study. Am Heart J. 1989;118:86–91. doi: 10.1016/0002-8703(89)90076-8. [DOI] [PubMed] [Google Scholar]
- Buttrick P, Malhotra A, Factor S, Geenen D. Scheuer J. Effects of chronic dobutamine administration on hearts of normal and hypertensive rats. Circ Res. 1988;63:173–181. doi: 10.1161/01.res.63.1.173. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Davidson WR, Jr, Banerjee SP. Liang CS. Dobutamine-induced cardiac adaptations: comparison with exercise-trained and sedentary rats. Am J Physiol Heart Circ Physiol. 1986;250:H725–H730. doi: 10.1152/ajpheart.1986.250.5.H725. [DOI] [PubMed] [Google Scholar]
- Dawson EA, Whyte GP, Black MA, Jones H, Hopkins N, Oxborough D, Gaze D, Shave RE, Wilson M, George KP. Green DJ. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol (1985) 2008;105:1562–1568. doi: 10.1152/japplphysiol.90837.2008. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J. Naito H. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–2212. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- Falcão-Pires I, Palladini G, Gonçalves N, van der Velden J, Moreira-Gonçalves D, Miranda-Silva D, Salinaro F, Paulus W, Niessen H, Perlini S. Leite-Moreira A. Distinct mechanisms for diastolic dysfunction in diabetes mellitus and chronic pressure-overload. Basic Res Cardiol. 2011;106:801–14. doi: 10.1007/s00395-011-0184-x. [DOI] [PubMed] [Google Scholar]
- French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA. Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J. 2008;22:2862–2871. doi: 10.1096/fj.07-102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KKW. Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H128–H136. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- Fulda S, Gorman AM, Hori O. Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H, Reiser PJ. Billman GE. Effects of exercise training on contractile function in myocardial trabeculae after ischemia-reperfusion. J Appl Physiol. 2005;99:230–236. doi: 10.1152/japplphysiol.00850.2004. [DOI] [PubMed] [Google Scholar]
- James J, Hor K, Moga MA, Martin LA, Robbins J. Effects of myosin heavy chain manipulation in experimental heart failure. J Mol Cell Cardiol. 2010;48:999–1006. doi: 10.1016/j.yjmcc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I. Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G. Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- Krenz M. Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004;44:2390–2397. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Kultz D. Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J Exp Biol. 2003;206:3119–3124. doi: 10.1242/jeb.00549. [DOI] [PubMed] [Google Scholar]
- Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- La Gerche A. Can intense endurance exercise cause myocardial damage and fibrosis? Curr Sports Med Rep. 2013;12:63–69. doi: 10.1249/JSR.0b013e318287488a. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc. 1993:251259–64. [PubMed] [Google Scholar]
- Liang C, Tuttle RR, Hood WB., Jr Gavras H. Conditioning effects of chronic infusions of dobutamine. Comparison with exercise training. J Clin Invest. 1979;64:613–619. doi: 10.1172/JCI109501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann N. Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126:2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijs MFL, Vergouwe Y, Cramer MJM, Vonken E-JA, Velthuis BK, Verton DJ, Graaf YVD, Visseren FL, Mali WP, Doevendans PA, Bots ML. Group SS. A prediction model for left ventricular mass in patients at high cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17:621–627. doi: 10.1097/HJR.0b013e328332d4bc. [DOI] [PubMed] [Google Scholar]
- Miki K, Kosho A. Hayashida Y. Method for continuous measurements of renal sympathetic nerve activity and cardiovascular function during exercise in rats. Exp Physiol. 2002;87:33–39. doi: 10.1113/eph8702281. [DOI] [PubMed] [Google Scholar]
- Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A. Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Gonçalves D, Henriques-Coelho T, Fonseca H, Ferreira RM, Amado F, Leite-Moreira A. Duarte JA. Moderate exercise training provides left ventricular tolerance to acute pressure overload. Am J Physiol Heart Circ Physiol. 2011;300:H1044–H1052. doi: 10.1152/ajpheart.01008.2010. [DOI] [PubMed] [Google Scholar]
- Ooi JYY, Bernardo BC. McMullen JR. The therapeutic potential of miRNAs regulated in settings of physiological cardiac hypertrophy. Future Med Chem. 2014;6:205–222. doi: 10.4155/fmc.13.196. [DOI] [PubMed] [Google Scholar]
- Pacher P, Nagayama T, Mukhopadhyay P, Batkai S. Kass DA. Measurement of cardiac function using pressure–volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C, Prasad SVN, Mao L, Noma T, Yan Z, Kim H-S, Smithies O. Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Guinto P, Dowell-Martino C, He H, Hoyer K, Krenz M, Robbins J, Ingwall JS, Tardiff JC. Cardiac myosin heavy chain isoform exchange alters the phenotype of cTnT-related cardiomyopathies in mouse hearts. J Mol Cell Cardiol. 2010;48:979–88. doi: 10.1016/j.yjmcc.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H. von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- Starnes JW, Barnes BD. Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol. 2007;102:1793–1798. doi: 10.1152/japplphysiol.00849.2006. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ. Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Tipton CM. Sebastian LA. Dobutamine as a countermeasure for reduced exercise performance of rats exposed to simulated microgravity. J Appl Physiol. 1997;82:1607–1615. doi: 10.1152/jappl.1997.82.5.1607. [DOI] [PubMed] [Google Scholar]
- Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, Colar JM, Haines DE. McCullough PA. Acute cardiac effects of marathon running. J Appl Physiol. 2010;108:1148–1153. doi: 10.1152/japplphysiol.01151.2009. [DOI] [PubMed] [Google Scholar]
- Yu Q, Vazquez R, Khojeini EV, Patel C, Venkataramani R. Larson DF. IL-18 induction of osteopontin mediates cardiac fibrosis and diastolic dysfunction in mice. Am J Physiol Heart Circ Physiol. 2009;297:H76–H85. doi: 10.1152/ajpheart.01285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbetto E, Vergani L. Dabbeni-Sala F. Quantification of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis. 1997;18:2059–2064. doi: 10.1002/elps.1150181131. [DOI] [PubMed] [Google Scholar]