Abstract
Abstract
The predictive value of laboratory models for human pain processing is crucial for improving translational research. The discrepancy between peripheral and central mechanisms of pain is an important consideration for drug targets, and here we describe two models of inflammatory pain that involve ultraviolet B (UVB) irradiation, which can employ peripheral and central sensitisation to produce mechanical and thermal hyperalgesia in rats and humans. We use electrophysiology in rats to measure the mechanically- and thermally-evoked activity of rat spinal neurones and quantitative sensory testing to assess human psychophysical responses to mechanical and thermal stimulation in a model of UVB irradiation and in a model of UVB irradiation with heat rekindling. Our results demonstrate peripheral sensitisation in both species driven by UVB irradiation, with a clear mechanical and thermal hypersensitivity of rat dorsal horn neurones and enhanced perceptual responses of human subjects to both mechanical and thermal stimulation. Additional heat rekindling produces markers of central sensitisation in both species, including enhanced receptive field sizes. Importantly, we also showed a correlation in the evoked activity of rat spinal neurones to human thermal pain thresholds. The parallel results in rats and humans validate the translational use of both models and the potential for such models for preclinical assessment of prospective analgesics in inflammatory pain states.
Key points
Translational research is key to bridging the gaps between preclinical findings and the patients, and a translational model of inflammatory pain will ideally induce both peripheral and central sensitisation, more effectively mimicking clinical pathophysiology in some chronic inflammatory conditions.
We conducted a parallel investigation of two models of inflammatory pain, using ultraviolet B (UVB) irradiation alone and UVB irradiation with heat rekindling. We used rodent electrophysiology and human quantitative sensory testing to characterise nociceptive processing in the peripheral and central nervous systems in both models.
In both species, UVB irradiation produces peripheral sensitisation measured as augmented evoked activity of rat dorsal horn neurones and increased perceptual responses of human subjects to mechanical and thermal stimuli.
In both species, UVB with heat rekindling produces central sensitisation.
UVB irradiation alone and UVB with heat rekindling are translational models of inflammation that produce peripheral and central sensitisation, respectively.
Introduction
While our understanding of persistent pain mechanisms has increased considerably over the last decade, this new knowledge still needs to be translated to patients to aid the success of new analgesic drugs. A key link in this process is the predictive value of laboratory models for human pain processing. A majority of standard laboratory animal models are not or cannot be implemented in humans, even though progress in pain research is contingent with bridging the gaps between preclinical findings of nociceptive molecules and pathways to the patients. One means of strengthening the correlation of outcome measures in preclinical and clinical data is to conduct parallel investigations using both animal and human subjects (Sikandar & Dickenson, 2013). Ultraviolet B (UVB) irradiation has been successfully used across species to explore peripheral sensitisation in inflammatory pain (Rukwied et al. 2008; Bishop et al. 2009; Dawes et al. 2011). Furthermore, there are known similarities in the transcriptional profiles of UVB-irradiated rodent and human skin, including inflammatory mediators such as the chemokine CXCL5, supporting the translational value of this experimental pain model (Dawes et al. 2011).
UVB irradiation produces a local inflammatory response that entails recruitment of immune cells, as well as expression of inflammatory mediators, that sensitise or alter the function of primary afferent endings leading to a robust dose-dependent primary mechanical and thermal hypersensitivity in rodents (Saade et al. 2000, 2008; Zhang et al. 2009; Davies et al. 2011; Dawes et al. 2011) and humans (Hoffmann & Schmelz, 1999; Bishop et al. 2009; Dawes et al. 2011; Weinkauf et al. 2013) that peaks at 24–48 h. These changes correlate with enhanced responses of heat-insensitive C-fibres to suprathreshold mechanical stimuli and enhanced responses of heat-sensitive C-fibres to noxious heat in rodents (Bishop et al. 2010), as well as measures of increased axonal excitability in humans (Weinkauf et al. 2013). The lack of spontaneous activity in primary afferents, the lack of efficacy of intrathecal NMDA antagonism on behavioural hypersensitivities (Bishop et al. 2010) and the lack of induction of referred pain (Vo & Drummond, 2013) support the role of peripheral, but not central, sensitisation in UVB inflammatory pain.
However, for some purposes a translational model of inflammation will ideally induce both peripheral and central sensitisation, more effectively mimicking clinical pathophysiology in some chronic inflammatory conditions. Secondary hyperalgesia is a hallmark of central sensitisation that can be tested both preclinically and clinically, where capsaicin sensitisation with heat rekindling has been employed as a non-invasive model to produce stable primary and secondary hyperalgesia of sufficient duration to test analgesic compounds (Dirks et al. 2003). More recent rodent behavioural and motor reflex data suggest that combining UVB irradiation with heat rekindling can produce both peripheral and long-lasting central sensitisation (Davies et al. 2011; Weerasinghe et al. 2014).
Nevertheless, non-operant behavioural studies in animals usually only inform on events related to nociceptive thresholds, whereas electrophysiological measures can allow investigation of neural events evoked by suprathreshold nociceptive stimuli. Indeed, these electrophysiological measures of neuronal activity are likely to equate better to the high pain ratings in clinical pain patients, and we have previously shown a remarkable correlation between the evoked activity of spinal neurones in normal rats and perceptual outcomes in human subjects following nociceptive stimulation (Sikandar et al. 2013). Here, we have used electrophysiology in rats and quantitative sensory testing (QST) in human subjects to provide an in-depth characterisation of the effects on peripheral and central nociceptive processing in the UVB model alone and with heat rekindling. In both species, separable peripheral and central sensitisation can be observed.
Methods
Animals
All animal experiments used male Sprague–Dawley rats (210–250 g; Central Biological Services, University College London). Procedures used in this study were approved by the United Kingdom Home Office according to guidelines set by personal and project licenses and the guidelines of the Committee for Research and Ethical Issues of IASP.
Human subjects
A total of 10 healthy volunteers (age 22–32 years) were used for the study. Individuals were familiarised with the experimental protocol beforehand and gave written, informed consent. All subjects were free from pain and medical conditions that could otherwise interfere with the results of the study. All participants were asked to refrain from analgesics and anti-inflammatory or anti-histamine medication 24 h prior to the study. The study was approved by The Kings College Research Ethics Committee and conformed to the standards set by the Declaration of Helsinki.
UVB irradiation and heat rekindling
Rats
Rats were anaesthetised with isoflurane (4%; 66% N2O and 33% O2). Once under anesthesia, rats were covered with UV resistant material exposing the whole right hindpaw for UVB experiments and the upper half of the plantar surface of the right hindpaw for UVB rekindling experiments. The exposed glabrous skin was placed under a UVB lamp (Dermfix 1000MX fitted with a 9 W fluorescent UVB tube, λ max = 311 nm). The irradiance of the lamp was determined using a calibrated photometer (Solartech Inc., Glenside, PA, USA. Solarmeter 6.2 UVB Meter, Merlin Lazer), which determined the length of time required to deliver a set dose of 1000 mJ cm−2 that has previously been shown to optimally produce sensory changes without obvious signs of skin damage (Bishop et al. 2007). Following UVB irradiation the rats were placed in a temperature-controlled recovery box until the effects of the anaesthetic were completely reversed.
For the UVB rekindling study, once stable neuronal baselines were established the primary treated area of the hindpaw was subjected to heat rekindling by two exposures to a heat source kept at a constant temperature of 40°C for 5 min, with 15 min between each exposure. Evoked neuronal responses were recorded accordingly every 30 min for 180 min post rekindling.
Human subjects
An initial screening was carried out to determine the minimal erythemal dose (MED), defined as the time required to produce a uniform reddening of the area at 24 h post irradiation. UVB dosing was delivered at three times one MED to an area of 256mm2 on the volar forearm with the surrounding area covered with UV resistant material to ensure uniform burn.
Once baselines QST values for obtained in human subjects (see section headed Human quantitative sensory testing), subjects underwent UVB irradiation, followed by full QST profiling 24–30 h after irradiation. The heat rekindling procedure was carried out in a similar fashion to the rodents, but using the TSA thermal sensory testing device (TSA 2001-II; Medoc Ltd, Ramat Yishai, Israel). The thermode temperature was maintained at 40°C for 5 min, followed by a 15 min interval and a subsequent second rekindling procedure. In parallel with animal experiments, subjects were tested immediately after the rekindling procedure, and every 30 min up to 180 min post rekindling.
Rodent electrophysiology
Twenty-four to thirty hours post UVB irradiation, electrophysiology experiments were performed in rats as previously described (Dickenson & Sullivan, 1986). Rats were anaesthetised with isoflurane (1.5%; 66% N2O and 33% O2) and extracellular recordings in L4–5 segments were made from WDR neurones in the deep dorsal horn (lamina V–VI, 500–1000μm) using parylene-coated tungsten electrodes (A-M Systems, USA). Activity of neurones was visualised on an oscilloscope and discriminated on a spike amplitude and waveform basis.
For the UVB study, spinal neurones with receptive fields in the irradiated hindpaw were recorded (n = 38). Importantly, for the UVB rekindling study, spinal neurones were only recorded if they had receptive fields located in the untreated, secondary area of the hindpaw (n = 30). Control responses of WDR neurones were recorded from naive rats (n = 10).
Electrical, mechanical and thermal stimuli were applied in the peripheral receptive field of the spinal neurone on the hindpaw glabrous skin. Data were recorded and analysed by a CED 1401 interface coupled to Spike 2 software (Cambridge Electronic Design, UK). For electrical stimulation, evoked spikes to a train of 16 transcutaneous stimuli (2 ms wide pulses, 0.5 Hz, 3 × C-fibre threshold) were constructed in a post-stimulus histogram. Responses evoked by Aβ- (0–20 ms), Aδ- (20–90 ms) and C-fibres (90–350 ms) were separated and quantified on the basis of latency. Neuronal responses occurring after the C-fibre latency band of the neurone were classed as ‘post-discharge’. The input (non-potentiated C-fibre + post-discharge) and the wind-up (potentiated C-fibre + post-discharge) were calculated (Dickenson & Sullivan, 1987). For natural stimulation, brush, von Frey filaments (2 g, 8 g, 15 g, 26 g and 60 g) and water jet (35°C, 40°C, 45°C and 48°C) were applied in ascending order of intensity to the neuronal receptive field for 10 s and the total number of evoked spikes recorded. At the end of each in vivo experiment, animals were killed with an overdose of isoflurane followed by cervical dislocation.
Human quantitative sensory testing
Subjects sat comfortably in a temperature controlled, quiet room. After obtaining written informed consent, seven tests measuring 13 different parameters were undertaken as part of quantitative sensory testing (QST) according to the protocols defined by the German Research Network on Neuropathic Pain (Rolke et al. 2006). Numerical scale ratings (NRS) were given in response to stimulations on a 0–100 scale, where 0 = no pain and 100 = most pain imaginable. Measurements included mechanical detection threshold (MDT), mechanical pain threshold (MPT), mechanical pain sensitivity for pinprick stimuli (MPS), wind-up ratio (WUR), vibration detection threshold (VDT), pain pressure threshold (PPT), cold detection threshold (CDT), warm detection threshold (WDT), thermal sensory limen, paradoxical heat sensation (PHS) and heat pain threshold (HPT), dynamic mechanical allodynia (DMA).
Mapping secondary areas
Rats
Receptive fields on the plantar hindpaw were mapped for each cell with an 8 g von Frey filament (vF) applied repeatedly around the area of baseline testing until firing was depleted below 0.5 Hz. Applications were made at 30 s intervals to ensure no wind-up was elicited from the testing sequence. Receptive fields in the UVB rekindling study were mapped before and after heat rekindling. Observed receptive fields were marked onto a standard diagram of the hindpaw and digitalised using a Canon MP610 scanner. The size of each receptive field was determined using ImageJ software and calculated as a percentage of the total area of the hindpaw.
Humans
Prior to assessment of sensory changes following UVB irradiation, edges of the primary burn site were marked on the skin and an acetate template was used to mark a spider probe map at 10 mm increments along eight spokes (oriented at 45 deg intervals) radiating out from the primary area. Before and after the rekindling procedure, subjects were assessed for the development of both pinprick hyperalgesia and dynamic brush evoked allodynia in the secondary area. DMA was mapped using a paintbrush and pinprick hyperalgesia was mapped using a 256mN probe (Pinprick, MRC Systems GmbH, Heidelberg, Germany. 0.2 mm diameter) – an example stimulation was given on the contralateral arm in order for the subject to familiarise themselves with the sensation. Beginning at 8 cm from the centre of the map, the stimulation was repeated at 1 cm intervals along each spoke towards the treated area, and the subject was requested to report change of sensation. Points at which this change was reported were marked on a standard spider probe map diagram and adjacent spokes were connected to create eight triangles in order to calculate areas; the summation of these areas, minus the primary area (256 mm2), gave the total area of secondary hyperalgesia. Mapping of secondary hyperalgesia was carried out before each round of QST.
Statistical analyses
Rat behavioural data and electrophysiological data related to evoked firing of spinal neurones by mechanical and thermal stimuli were analysed using a two-way or one-way ANOVA with Bonferroni’s multiple comparisons post tests (with stimulus intensity and treatment as main factors for the two-way ANOVA). Electrically evoked measures were analysed using Student’s unpaired t test.
Psychophysical data, with the exceptions of HPT and CPT, were logged and re-tested for normality using the Kolmogorov–Smirnov test. Student’s paired t test or two-way ANOVA with stimulus intensity and treatment as main factors was performed. All data were presented as means ± SEM.
Results
UVB irradiation enhances mechanically evoked activity of rat spinal cord neurones and the perceptual responses to mechanical stimulation in human subjects
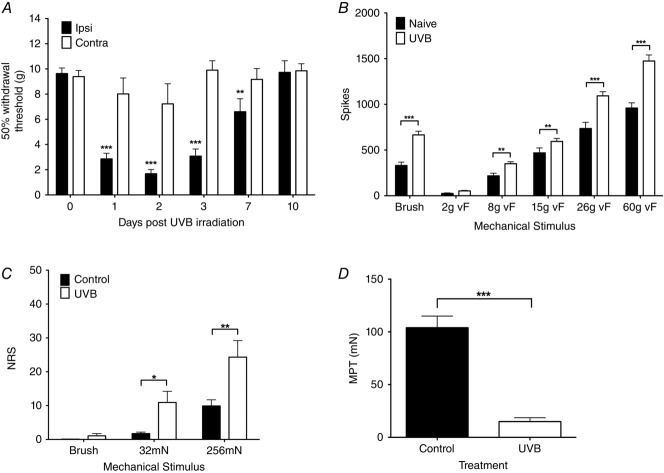
We found that UVB irradiation causes a prolonged pain-related hypersensitivity in rats exemplified by a marked reduction in the mechanical withdrawal thresholds following stimulation of the irradiated paw (Fig.1A). In line with previous reports, ipsilateral mechanical withdrawal thresholds are significantly reduced from 24 h to 7 days after UVB irradiation, with peak effect at 48 h (P < 0.001) and thresholds returning to baseline by day 10 (Bishop et al. 2007; Dawes et al. 2011).
Figure 1.
UVB irradiation enhances mechanically evoked sensory function in rats and humans
A, UVB irradiation significantly reduces mechanical withdrawal thresholds in the ipsilateral, treated paw for up to 7 days (two-way ANOVA, **P < 0.01, ***P < 0.001). B, mechanically evoked firing of rat WDR neurones in the deep dorsal horn is enhanced in UVB rats compared to naive rats (paired t test, *P < 0.05, **P < 0.01). C, human pain ratings to low-threshold dynamic mechanical and pinprick stimulation are increased following UVB irradiation (paired t test, ***P < 0.001). D, human mechanical pain thresholds are reduced following UVB irradiation (***P < 0.001)
We also used electrophysiological recordings of spinal neuronal activity in rats in order to determine changes in neural coding in the UVB inflammation state. In naive and UVB rats, we found that the evoked activity of dorsal horn WDR neurones coded for stimulus intensity of the applied von Frey mechanical stimuli in a graded fashion (Fig.1B). These neurones also showed reproducible responses to dynamic mechanical stimulation using brush stimulation. UVB inflammation did not alter the graded coding of these neurones, but markedly potentiated their evoked firing to low- and high-threshold punctate mechanical stimulation (Fig.1B; 8 g vF: 218 ± 28 to 350 ± 22 spikes, P < 0.01; 15 g vF: 469 ± 54 to 594 ± 33 spikes, P < 0.01; 26 g vF: 736 ± 68 to 1093 ± 46 spikes, P < 0.001; 60 g vF: 959 ± 57 to 1474 ± 70 spikes, P < 0.001). Importantly, there was also a significant UVB-induced increase in evoked firing to low-threshold dynamic mechanical stimulation (Fig.1B brush; 331 ± 37 to 666 ± 40 spikes, P < 0.001).
The human psychophysical responses to mechanical stimulation were also graded with stimulus intensity (Fig.1C). In parallel with the rat data, UVB irradiation produced modest pain ratings to dynamic low-threshold stimulation using brush stimulation, and also increased perceptual responses to pinprick stimulation (Fig.2C; 32 mN: 1.7 ± 0.5 to 10.9 ± 3.3, P < 0.05; 256 mN: 9.9 ± 1.8 to 24.3 ± 4.9, P < 0.05). Importantly, the mechanical pain threshold was also reduced following UVB irradiation, indicating the development of primary sensitisation (Fig.1D; 104.0 ± 11 to 15.0 ± 3.7, P < 0.001).
Figure 2.
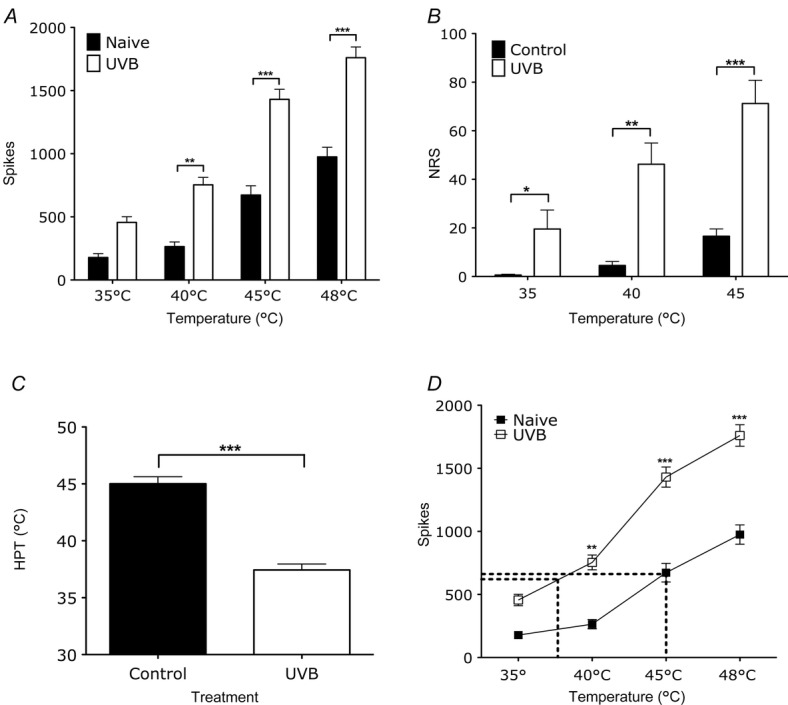
UVB irradiation enhances thermally evoked firing of rat WDR neurones that corresponds to enhanced sensory function humans
A, evoked firing of rat WDR neurones in the deep dorsal horn by low- and high-threshold thermal stimulation is enhanced in UVB rats compared to naive rats (two-way ANOVA, **P < 0.01, ***P < 0.001). B, human pain ratings to low- and high-threshold thermal stimulation is enhanced following UVB irradiation (paired t test, *P < 0.05, **P < 0.01, ***P < 0.001). C, human pain thresholds are significantly reduced following UVB irradiation (paired t test, ***P < 0.001). D, correlating thermally evoked firing of WDR neurones in naive and UVB rats with human pain thresholds in the control (37.4°C) and in the UVB condition (40°C) (two-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001).
UVB irradiation produces parallel enhancement of thermally evoked activity of rat spinal cord neurones and perceptual thermal responses of human subjects
Similar to our findings with mechanically evoked activity of dorsal horn neurones in the rat, there was a clear intensity coding of WDR neuronal firing to thermal stimuli in both naive and UVB rats (Fig.2A). After UVB irradiation, the overall discharge frequencies of these neurones to both low- and high-threshold thermal stimuli were increased (40°C: 264 + 37 to 754 + 57 spikes, P < 0.01; 45°C: 673 + 74 to 1431 ± 75 spikes, P < 0.001; 48°C 975 ± 69 to 1760 ± 81 spikes, P < 0.001). UVB irradiation in human subjects also increased perceptual responses to both low intensity and high intensity thermal stimuli (Fig.2B; 35°C: 0.6 ± 0.5 to 19.5 ± 7.8, P < 0.05; 40°C: 4.3 ± 2.4 to 46.2 ± 8.7, P < 0.01; 45°C: 16.6 ± 3.0 to 71.2 ± 9.6, P < 0.001). Human subjects also reported a reduction in heat pain threshold following UVB irradiation (Fig.2C; 45.0 ± 0.6 to 37.4 ± 0.5, P < 0.0001).
The sensitisation of dorsal horn neurones in rats following UVB irradiation was predictive of the degree of sensitisation in human subjects; the parallel rightward shift in evoked activity of WDR neurones in normal and UVB conditions correlates with the thermal pain thresholds in humans before and after UVB irradiation (Fig.2D). We analysed the stimulus–response function for these neurones and related them to human thresholds, and found that the number of action potentials produced by spinal cord neurones in response to a stimulus temperature corresponding to the mean control heat pain threshold in humans (37.4°C, 614 spikes) is remarkably similar to the evoked firing produced by the mean HPT temperature following UVB irradiation (45°C, 641 spikes). Our data therefore suggest that that the firing rate of rodent neurones correlates with human thermal thresholds, both before and after UVB treatment.
UVB irradiation alters electrically evoked responses of second order spinal neurones
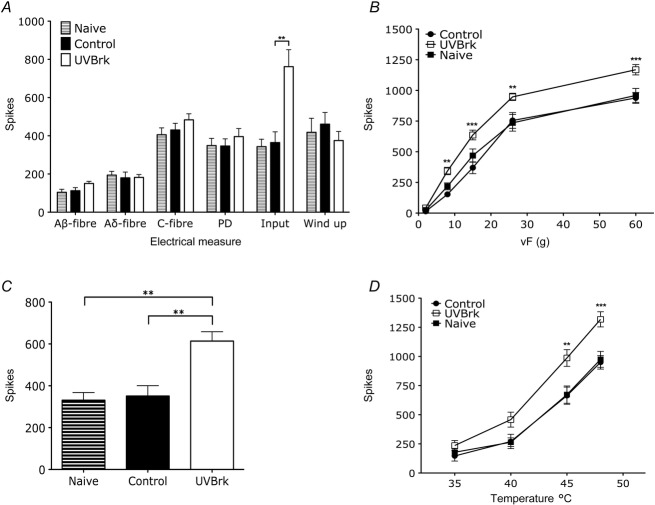
We used transcutaneous electrical stimuli in rodents to quantify UVB-induced changes of the relative primary afferent convergence onto WDR neurones and changes in neuronal excitability (Fig.3A). We found that UVB irradiation did not significantly alter A- or C-fibre input to WDR neurones, nor were measures of central sensitisation – post-discharge and wind-up – altered.
Figure 3.
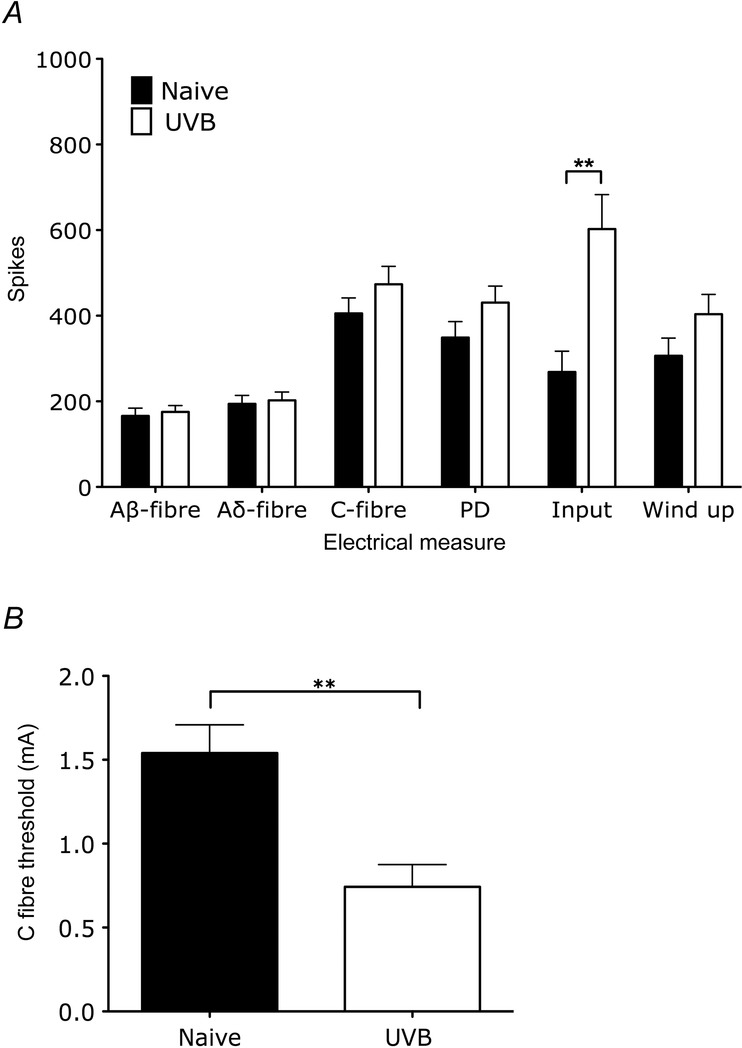
Electrically evoked changes of rat spinal neurones following UVB irradiation
A, electrically evoked responses of rat WDR neurones with electrical stimulation illustrating the convergent afferent input of recorded cells (Aβ-fibres, Aδ-fibres and C-fibres) and measures of excitability (post-discharge, input and wind-up). Only input was significantly enhanced with UVB irradiation (unpaired t test, **P < 0.01). B, C-fibre thresholds of second order neurones in UVB rats is lower compared to naive rats (unpaired t test, **P < 0.01).
Further support for peripheral sensitisation in the UVB model that compliments the observed potentiation of mechanically and thermally evoked responses in the primary area of treatment is provided by an increase in neuronal input of WDR cells in UVB rats (Fig.3A; 268 + 49 to 603 + 80 spikes, P < 0.001). This potentiated input was accompanied by a reduction in C-fibre electrical threshold following UVB irradiation (Fig.3B; 1.5 ± 0.2 to 0.7 ± 0.1 mA, P < 0.01). In rats there was no significant change in neuronal receptive field size following UVB irradiation, and in humans there was neglible area of secondary hyperalgesia, indicating a lack of central sensitisation with the UVB burn (Fig.4).
Figure 4.
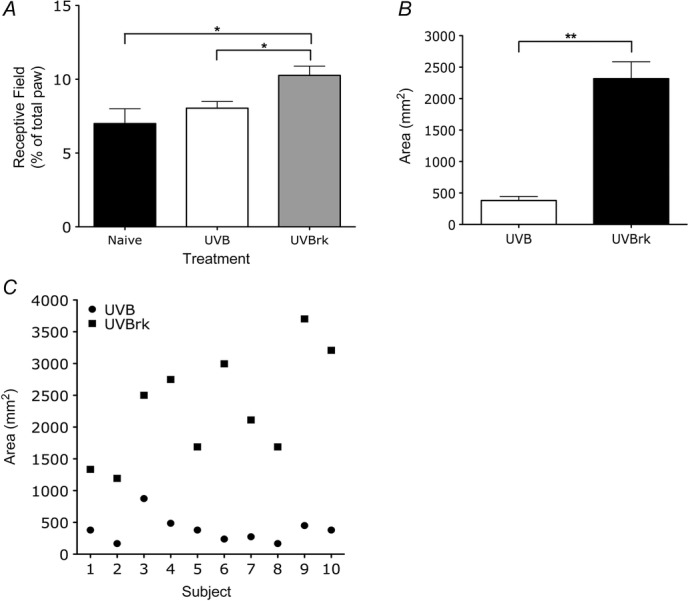
UVB irradiation with heat rekindling, but not alone, produces expansion of neuronal receptive fields in the rat spinal cord and secondary hyperalgesia in human subjects
A, neuronal receptive field size is significantly larger in rats with UVB and heat rekindling (one-way ANOVA, *P < 0.05). B and C, areas of secondary hyperalgesia in human subjects is significantly present following UVB with heat rekindling in human subjects compared to UVB alone (paired t test, **P < 0.01).
UVB heat rekindling produces central sensitisation in rodents and human subjects
Quantitative sensory profiling of human subjects using a standardised, comprehensive QST procedure confirmed sensitisation in the primary burn area following UVB irradiation for thermal and mechanical sensations (Fig.5A). All subjects reported a decrease in mechanical and thermal pain thresholds, with a pronounced gain of function for pinprick hypersensitivity. Importantly, similar gains of mechanical sensory function, and smaller gains of thermal sensory functions, were also reported in the area of secondary hyperalgesia following UVB with heat rekindling (Fig.5B). Thermal changes in sensory function before and after heat rekindling included increased sensitivity to cold stimulation, and we additionally observed increased deep pressure sensitivity.
Figure 5.
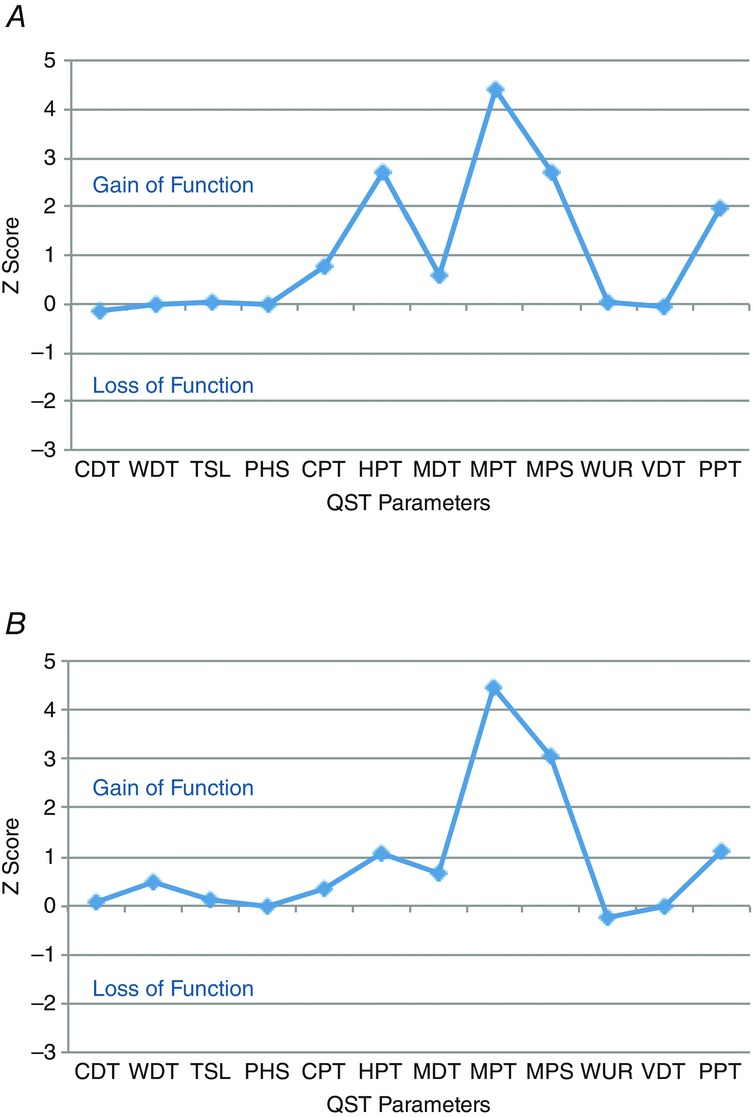
Sensory profiles of following UVB irradiation and UVB with heat rekindling are similar
Sensory profile following UVB (A) and UVB (B) with heat rekindling. The magnitude of changes are expressed as Z-scores that highlight gains or loss in somatosensory function.
Prior to the rekindling procedure, we recorded from spinal neurones with receptive fields in the area outside the UVB burn that did not extend into the primary treated area. These neurones did not have significantly different baseline responses evoked by electrical, mechanical or thermal stimulation compared to WDR neurones in naive animals, suggesting no spread of inflammatory mediators beyond the UVB treated area (Fig.6).
Figure 6.
UVB with heat rekindling enhances mechanically and thermally evoked firing of rat spinal neurones
A, electrically evoked firing of rat WDR neurones following electrical stimulation, illustrating the convergent afferent input of recorded cells in naive rats and before and after heat rekindling in UVB rats. Only input was significantly enhanced with heat rekindling (one-way ANOVA, **P < 0.01). Evoked firing of WDR neurones to graded punctate mechanical stimulation (B) and dynamic brush stimulation (C) is enhanced following heat rekindling in UVB rats (B: two-way ANOVA, **P < 0.01, ***P < 0.001; C: one-way ANOVA, **P < 0.01). D, evoked firing of WDR neurones to noxious thermal stimulation is potentiated following the heat rekindling procedure in UVB rats (two-way ANOVA, **P < 0.01, ***P < 0.001).
Following the heat rekindling procedure to the irradiated paw, WDR neurones in UVB rekindled rats showed a larger receptive field size compared to neurones in naive and UVB rats (Fig.4A; receptive field size: 7.0 ± 1.0% in naive rats to 10.3 ± 0.6% in UVB rekindled rats, P < 0.05). Similarly, in human subjects, heat rekindling produced a significant enlargement – more than 5-fold – of the secondary area of hyperalgesia, a marker of central sensitisation (Fig.4B; P < 0.01).
Additionally with UVB heat rekindling, dorsal horn neurones developed enhanced evoked activity in response to dynamic, low- and high-threshold mechanical stimulation (Fig.6B and C; brush: 331 ± 37 to 614 ± 45, P < 0.01; 8 g vF: 153 ± 9 to 343 ± 30 spikes, P < 0.01; 15 g vF: 370 ± 48 to 637 ± 40 spikes, P < 0.001; 26 g vF: 755 ± 64 to 948 ± 30 spikes, P < 0.01; 60 g vF: 939 ± 44 to 1169 ± 39 spikes, P < 0.001). Similarly, the QST procedure performed in the secondary area to the UVB burn in human subjects also produced higher psychophysical ratings to mechanical stimulation, including the development of a brush response (Fig.7A; 32 mN: 1.7 ± 0.5 to 15.9 ± 4.2, P < 0.01; 256 mN: 9.9 ± 1.8 to 28.4 ± 6.1, P < 0.01).
Figure 7.
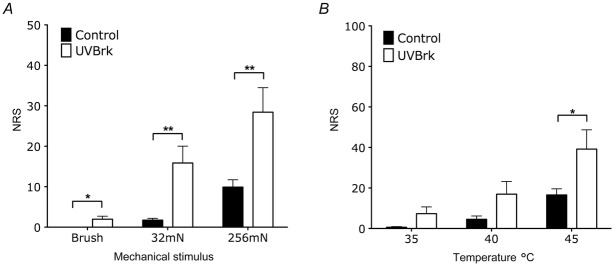
UVB with heat rekindling enhances human perceptual responses to mechanical and thermal stimulation
A, pain ratings to innocuous, dynamic brush stimulation and to pinprick stimulation are enhanced following UVB with heat rekindling compared to control conditions (paired t test, *P < 0.05, **P < 0.01). B, pain ratings to noxious thermal stimulation are enhanced following UVB with heat rekindling compared to control conditions (paired t test, *P < 0.05).
Moreover, UVB heat rekindling potentiated evoked responses to noxious thermal stimulation in both rats and human subjects (Figs6D and 7B; rat 45°C: 664 ± 74 to 987 ± 72 spikes, P < 0.01; rat 48°C: 950 ± 58 to 1318 ± 65 spikes, P < 0.001; human 45°C: 17 ± 3 to 39 ± 10, P < 0.05). Notably, UVB irradiation alone enhanced WDR firing and human perceptual responses produced by low- and high-threshold ranges of thermal stimulation (Fig.2B and D), whereas UVB heat rekindling only potentiated responses to noxious thermal stimulation.
Discussion
In this study we conducted a parallel investigation using both rats and human subjects to explore the contributions of peripheral and central sensitisation in the translational pain models of UVB inflammation and UVB with heat rekindling. Using electrophysiological recordings of rat spinal neurones and standardised QST procedures in humans, we used similar stimulation parameters in both species to characterise sensory changes in both models of inflammation and further investigated measures reflective of central sensitisation occurring after UVB irradiation alone and with heat rekindling. Our results showed parallel changes occurring in the electrophysiological measures in rodents and psychophysical measures in humans, supporting a strong translational value for the UVB model for peripheral sensitisation and the UVB heat rekindling model for central sensitisation.
We found that UVB irradiation produced primary mechanical and thermal hypersensitivity in both rats and humans, shown with enhanced evoked activity of rat WDR neurones and increased perceptual responses in human subjects. Our data also illustrate the development of hypersensitivity in the secondary area to the UVB burn in both rats and humans following UVB irradiation with heat rekindling, which was linked to neuronal receptive field expansion and development of secondary hyperalgesia – indicative measures of central sensitisation.
UVB irradiation produces primary hypersensitivity in both rats and human subjects
We observed both mechanical and thermal hypersensitivity in rats and human subjects with regard to rat behaviour and neuronal responses in the dorsal horn, as well as human psychophysical sensory evaluations (Figs 1 and 2). Evoked activity of rat spinal neurones following UVB irradiation was potentiated to both low- and high-threshold mechanical and thermal stimulation. Similarly, human mechanical and thermal pain thresholds were reduced in the primary UVB burn area and perceptual responses to pinprick and heat stimulation were enhanced.
UVB irradiation has been previously shown to sensitise rat polymodal nociceptors to both heat and mechanical stimuli across species, the former modality most likely attributed to an enhanced responsiveness of the transient receptor protein (TRP) superfamily of ion channels with lowered threshold temperatures for activation (Tominaga, 2007). Here, we show the consequential sensitisation of second order spinal neurones in rats and enhanced human perceptual response to both low- and high-intensity thermal stimulation following UVB irradiation. Additionally, secondary release of inflammatory mediators, e.g. prostaglandins, can sensitise TRPV1channels and produce axonal hyperexcitability that is correlated with heat hyperalgesia (Snyder, 1975; Wilgus et al. 2002; Kienzler et al. 2005; Sycha et al. 2005; Weinkauf et al. 2013). In fact, a selective TRVP1 antagonist has been shown to abrogate UVB-induced heat hyperalgesia (Chizh et al. 2007). Consistent with the role of inflammatory mediators in producing UVB-induced hypersensitivity, non-steroidal anti-inflammatory drugs can also alleviate UVB-induced heat hyperalgesia (Bishop et al. 2007).
In contrast to heat sensitisation, the molecular mechanisms underlying mechanical hyperalgesia are still unclear. Lowered mechanical thresholds of nociceptors and sensitisation of high-threshold mechanosensitive C-nociceptors within the primary UVB burn area have been reported, where mechanical hyperalgesia could be reduced with sequestration of nerve growth factor (Bishop et al. 2007, 2010). Recruitment of mechanically insensitive afferents may also play a role in the increased barrage of primary afferent input to the dorsal horn (Treede et al. 1998). We observed behavioural and neuronal hypersensitivity in rats, as well as increased pain ratings in humans to innocuous and noxious mechanical stimulation following UVB irradiation. Hyperalgesia to pinprick and blunt pressure, as well as heat probe, are pronounced positive sensory signs in nerve-injury-related neuropathic pain and complex regional pain syndrome in humans, validating the relevance of the UVB model to common clinical pain syndromes (Gierthmuhlen et al. 2012).
Importantly, we observed no measure of central sensitisation following UVB irradiation alone. Neuronal receptive field sizes were unaltered in rats and the secondary area of hyperalgesia was negligible in humans following UVB irradiation (Fig.4). Moreover, there was no increase in electrically induced wind-up of second order neurones following UVB irradiation, yet dorsal horn neurones in UVB rats had potentiated input (Fig.3). The latter was likely related to a decrease in C-fibre electrical thresholds. Previous studies have also reported a strong role for peripheral mechanisms in mediating UVB-induced sensitisation, given that peripherally restricted analgesics can reduce primary hyperalgesia (Bishop et al. 2007) and primary mechanical hyperalgesia is unaffected by central NMDA receptor blockade (Bishop et al. 2010).
Overall our rat and human data showed that parallel hypersensitivities develop following UVB irradiation. Furthermore, we found a strong correlation between the thermally evoked firing of rat spinal neurones and human thermal pain thresholds, such that roughly 630 action potentials of rat spinal neurones evoked by thermal stimulation corresponded to the human thermal pain threshold in control conditions or after UVB irradiation (Fig.2D). Accordingly, the evoked activity of rat spinal neurones may serve as a predictive measure of human perceptual pain thresholds to thermal stimuli.
UVB with heat rekindling produces central sensitisation in rats and human subjects
Effective translational inflammatory pain models should invoke similar mechanisms in animals and humans that adequately depict clinical symptoms in order to improve the transition of preclinical findings to pain relief in patients. Both peripheral and central sensitisation contribute to inflammatory pain in patients (Thakur et al. 2014), where secondary hyperalgesia is a prominent clinical manifestation occurring as a result of central sensitisation (Simone et al. 1991). Our data acquired from electrophysiological recordings of rat spinal neurones and human sensory testing reveal novel findings of central sensitisation in the translational inflammatory pain model of UVB with heat rekindling.
With UVB irradiation alone, we observed no measures of central sensitisation in rats or human subjects, with negligible areas of secondary hyperalgesia in human subjects and no expansion of neuronal receptive fields or wind-up in rats following UVB irradiation. However, the occurrence of secondary mechanical hyperalgesia, and thus the degree of central sensitisation in the UVB model has produced conflicting reports (Gustorff et al. 2004; Harrison et al. 2004; Bishop et al. 2009, 2010; Davies et al. 2011; Gustorff et al. 2013). Indeed, induction and maintenance of central sensitisation in nociceptive pathways requires ongoing afferent input, and the blockade of this peripheral activity should prevent the development of secondary hyperalgesia (Kinnman et al. 1997). However, previous studies have reported no change in spinal Fos expression following UVB irradiation, as well as a lack of spontaneous pain and lack of ongoing activity in peripheral afferents innervating the primary UVB burn site in rodents (Bishop et al. 2007, 2009). Other studies have reported areas of secondary pinprick hyperalgesia and dynamic mechanical allodynia, although the discrepancy with this data is likely to be related to methodological differences, such as the size of the primary burn area or differences in the von Frey filament used to assess hyperalgesia (Davies et al. 2011; Gustorff et al. 2013). It is likely that distinct afferent populations of rapidly adapting A-mechano-heat (AMH) fibres and C-mechano-heat (CMH) fibres can be activated by the differing phasic natures of von Frey filaments or automated devices; tip geometry may bend the filament to produce a sharper stimulus compared to the blunt pressure of automated devices (Schmidt et al. 2000; Bove, 2006; Davies et al. 2011).
On the other hand, we found that UVB with heat rekindling consistently led to hypersensitivity to thermal and mechanical stimuli in the secondary untreated area of both rodents and humans (Figs6 and 7). Electrophysiological recordings showed that UVB with heat rekindling potentiated evoked firing of rat spinal neurones with receptive fields outside the primary burn area to dynamic mechanical stimulation and sub- and supra-threshold punctate stimulation. Similarly, UVB irradiation with heat rekindling significantly lowered human mechanical pain thresholds and increased pain ratings to pinprick stimulation applied within the mapped area of secondary hyperalgesia. The induction of secondary mechanical hypersensitivity with heat rekindling has also been reported in recent studies measuring behavioural hyperalgesia (reduced von Frey withdrawal thresholds and dynamic brush withdrawal responses) and pain referral in secondary areas to the UVB burn (Davies et al. 2011; Weerasinghe et al. 2014). Furthermore, we also found that the sensitising effects of UVB with heat rekindling to thermal stimuli were limited to noxious stimulus intensities in both rats and human subjects.
Because the receptive fields of rat spinal neurones were distal from the treated area of the hindpaw and the stimulation of human subjects was performed in the area of mapped secondary hyperalgesia distant from the initial stimulated area, it is unlikely that the observed changes in rat neuronal and human perceptual responses can be explained by sole peripheral sensitisation that we found with UVB irradiation alone. Like the intradermal capsaicin model of central sensitisation, UVB with heat rekindling produced primary allodynia and mechanical and thermal hyperalgesia, along with secondary allodynia and mechanical hyperalgesia (Ali et al. 1996; Willis, 2001). In addition, we also observed secondary thermal hyperalgesia.
The discrepancy between tested skin sites in our rats and human subjects, as well as the lack of significant changes in UVB-induced A- and C-fibre contributions to WDR firing (Figs 3A and 6A), complicates assumptions about the distinct subsets of primary afferents mediating modality-specific pain. Nevertheless, enhanced responsiveness to punctate stimulation has been shown to be mediated primarily by capsaicin-insensitive Aδ-nociceptors, which include high-threshold mechanoreceptors and type I mechano-heat nociceptors (found in both hairy and glabrous skin) (Ziegler et al. 1999; Magerl et al. 2001). In hairy skin, large-diameter Aβ-fibres have been linked to dynamic allodynia (Koltzenburg et al. 1992; Torebjork et al. 1992), although a homologue of C tactile afferents may also contribute to allodynia in glabrous skin (Nagi & Mahns, 2013). Enhanced responses to noxious thermal stimulation observed in rats and human subjects is likely to be linked to activation of CMHs (LaMotte et al. 1982) and the observed deep pressure sensitivity may relate to sensitisation of C-mechano-insensitive afferents (Schmidt et al. 2000). Moreover, the lack of significant changes in UVB-induced A- and C-fibre contributions to WDR firing, yet an increase in the input response, is indicative of peripheral events enhancing transduction in afferents rather than excitability per se.
Conclusions
Linking preclinical and clinical findings is crucial for successful translation of pain research into improved analgesic treatments. By conducting a parallel investigation using rats and humans with similar stimulation parameters for induction of the pain model and measuring evoked responses, we explored the peripheral and central nervous system contributions to sensory changes that occur with UVB irradiation alone and with heat rekindling. Using electrophysiological recordings of rat spinal activity and quantitative sensory testing in humans, we found that UVB irradiation produces peripheral sensitisation in both species measured as behavioural hypersensitivity in rats, enhanced responses of rat spinal neurones and enhanced perceptual responses of human subjects. On the other hand, UVB with heat rekindling recruits central mechanisms of pain amplification, thus validating UVB with heat rekindling as a translational model of inflammatory pain that entails both peripheral and central sensitisation. These models may have utility in the preclinical assessment of potential analgesics alongside models of different pain states, but could also be valuable in the early Phase I human assessment of efficacy and target exposure.
Glossary
- CDT
cold detection threshold
- DMA
dynamic mechanical allodynia
- HPT
heat pain threshold
- MDT
mechanical detection threshold
- MPT
mechanical pain threshold
- MPS
mechanical pain sensitivity for pinprick stimuli
- PHS
paradoxical heat sensation
- PPT
pain pressure threshold
- QST
quantitative sensory testing
- TSL
thermal sensory limen
- UVB
ultraviolet B
- VDT
vibration detection threshold
- WDR
wide dynamic range
- WDT
warm detection threshold
- WUR
wind-up ratio
Additional information
Competing interests
None declared.
Author contributions
J.O., S.S., S.B.M. and AHD were involved in the conception and design of experiments. J.O. and S.S. collected and analysed data. S.S., AHD and S.B.M. prepared the manuscript.
Funding
This work was funded by a UCL Grand Challenges studentship, the Europain Collaboration, which has received support from the Innovative Medicines Initiative Joint Undertaking, under grant agreement no. 115007, the resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in-kind contribution (www.imi.europa.eu) and the Wellcome Trust London Pain Consortium.
References
- Ali Z, Meyer RA. Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin. Pain. 1996;68:401–411. doi: 10.1016/s0304-3959(96)03199-5. [DOI] [PubMed] [Google Scholar]
- Bishop T, Ballard A, Holmes H, Young AR. McMahon SB. Ultraviolet-B induced inflammation of human skin: characterisation and comparison with traditional models of hyperalgesia. Eur J Pain. 2009;13:524–532. doi: 10.1016/j.ejpain.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Bishop T, Hewson D, Yip P, Fahey M, Dawbarn D, Young A. McMahon S. Characterisation of ultraviolet-B-induced inflammation as a model of hyperalgesia in the rat. Pain. 2007;131:70–82. doi: 10.1016/j.pain.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Bishop T, Marchand F, Young AR, Lewin GR. McMahon SB. Ultraviolet-B-induced mechanical hyperalgesia: A role for peripheral sensitisation. Pain. 2010;150:141–152. doi: 10.1016/j.pain.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Bove G. Mechanical sensory threshold testing using nylon monofilaments: the pain field’s "tin standard". Pain. 2006;124:13–17. doi: 10.1016/j.pain.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM. Appleby JM. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Davies EK, Boyle Y, Chizh BA, Lumb BM. Murrell JC. Ultraviolet B-induced inflammation in the rat: a model of secondary hyperalgesia? Pain. 2011;152:2844–2851. doi: 10.1016/j.pain.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Dawes JM, Calvo M, Perkins JR, Paterson KJ, Kiesewetter H, Hobbs C, Kaan TK, Orengo C, Bennett DL. McMahon SB. CXCL5 mediates UVB irradiation-induced pain. Sci Transl Med. 2011;3:90ra60. doi: 10.1126/scitranslmed.3002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH. Sullivan AF. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurones in the rat dorsal horn. Pain. 1986;24:211–222. doi: 10.1016/0304-3959(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dirks J, Petersen KL. Dahl JB. The heat/capsaicin sensitization model: a methodologic study. J Pain. 2003;4:122–128. doi: 10.1054/jpai.2003.10. [DOI] [PubMed] [Google Scholar]
- Gierthmuhlen J, Maier C, Baron R, Tolle T, Treede RD, Birbaumer N, Huge V, Koroschetz J, Krumova EK, Lauchart M, et al. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain. 2012;153:765–774. doi: 10.1016/j.pain.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Gustorff B, Anzenhofer S, Sycha T, Lehr S. Kress HG. The sunburn pain model: the stability of primary and secondary hyperalgesia over 10 hours in a crossover setting. Anesth Analg. 2004;98:173–177. doi: 10.1213/01.ANE.0000093224.77281.A5. [DOI] [PubMed] [Google Scholar]
- Gustorff B, Sycha T, Lieba-Samal D, Rolke R, Treede RD. Magerl W. The pattern and time course of somatosensory changes in the human UVB sunburn model reveal the presence of peripheral and central sensitization. Pain. 2013;154:586–597. doi: 10.1016/j.pain.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Harrison GI, Young AR. McMahon SB. Ultraviolet radiation-induced inflammation as a model for cutaneous hyperalgesia. J Invest Dermatol. 2004;122:183–189. doi: 10.1046/j.0022-202X.2003.22119.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann RT. Schmelz M. Time course of UVA- and UVB-induced inflammation and hyperalgesia in human skin. Eur J Pain. 1999;3:131–139. doi: 10.1053/eujp.1998.0106. [DOI] [PubMed] [Google Scholar]
- Kienzler JL, Magnette J, Queille-Roussel C, Sanchez-Ponton A. Ortonne JP. Diclofenac-Na gel is effective in reducing the pain and inflammation associated with exposure to ultraviolet light – results of two clinical studies. Skin Pharmacol Physiol. 2005;18:144–152. doi: 10.1159/000084912. [DOI] [PubMed] [Google Scholar]
- Kinnman E, Nygards EB. Hansson P. Peripherally administrated morphine attenuates capsaicin-induced mechanical hypersensitivity in humans. Anesth Analg. 1997;84:595–599. doi: 10.1097/00000539-199703000-00024. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Lundberg LE. Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjork HE. Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2:765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA. Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–1764. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- Nagi SS. Mahns DA. Mechanical allodynia in human glabrous skin mediated by low-threshold cutaneous mechanoreceptors with unmyelinated fibres. Exp Brain Res. 2013;231:139–151. doi: 10.1007/s00221-013-3677-z. [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Dusch M, Schley M, Forsch E. Schmelz M. Nociceptor sensitization to mechanical and thermal stimuli in pig skin in vivo. Eur J Pain. 2008;12:242–250. doi: 10.1016/j.ejpain.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Saade NE, Farhat O, Rahal O, Safieh-Garabedian B, Le Bars D. Jabbur SJ. Ultra violet-induced localized inflammatory hyperalgesia in awake rats and the role of sensory and sympathetic innervation of the skin. Brain Behav Immun. 2008;22:245–256. doi: 10.1016/j.bbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Saade NE, Nasr IW, Massaad CA, Safieh-Garabedian B, Jabbur SJ. Kanaan SA. Modulation of ultraviolet-induced hyperalgesia and cytokine upregulation by interleukins 10 and 13. Br J Pharmacol. 2000;131:1317–1324. doi: 10.1038/sj.bjp.0703699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Torebjork HE. Handwerker HO. Mechano-insensitive nociceptors encode pain evoked by tonic pressure to human skin. Neuroscience. 2000;98:793–800. doi: 10.1016/s0306-4522(00)00189-5. [DOI] [PubMed] [Google Scholar]
- Sikandar S. Dickenson AH. II. No need for translation when the same language is spoken. Br J Anaesth. 2013;111:3–6. doi: 10.1093/bja/aet210. [DOI] [PubMed] [Google Scholar]
- Sikandar S, Ronga I, Iannetti GD. Dickenson A. Neural coding of nociceptive stimuli - from rat spinal neurones to human perception. Pain. 2013;154:1263–1273. doi: 10.1016/j.pain.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH. Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Snyder DS. Cutaneous effects of topical indomethacin, an inhibitor of prostaglandin synthesis, on UV-damaged skin. J Invest Dermatol. 1975;64:322–325. doi: 10.1111/1523-1747.ep12512265. [DOI] [PubMed] [Google Scholar]
- Sycha T, Anzenhofer S, Lehr S, Schmetterer L, Chizh B, Eichler HG. Gustorff B. Rofecoxib attenuates both primary and secondary inflammatory hyperalgesia: a randomized, double blinded, placebo controlled crossover trial in the UV-B pain model. Pain. 2005;113:316–322. doi: 10.1016/j.pain.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Thakur M, Dickenson AH. Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.47. [DOI] [PubMed] [Google Scholar]
- Tominaga M. Nociception and TRP channels. Handb Exp Pharmacol. 2007;179:489–505. doi: 10.1007/978-3-540-34891-7_29. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Lundberg LE. LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA. Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- Vo L. Drummond PD. High frequency electrical stimulation concurrently induces central sensitization and ipsilateral inhibitory pain modulation. Eur J Pain. 2013;17:357–368. doi: 10.1002/j.1532-2149.2012.00208.x. [DOI] [PubMed] [Google Scholar]
- Weerasinghe NS, Lumb BM, Apps R, Koutsikou S. Murrell JC. Objective validation of central sensitization in the rat UVB and heat rekindling model. Eur J Pain. 2014;18:1199–1206. doi: 10.1002/j.1532-2149.2014.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinkauf B, Main M, Schmelz M. Rukwied R. Modality-specific nociceptor sensitization following UV-B irradiation of human skin. J Pain. 2013;14:739–746. doi: 10.1016/j.jpain.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Parrett ML, Ross MS, Tober KL, Robertson FM. Oberyszyn TM. Inhibition of ultraviolet light B-induced cutaneous inflammation by a specific cyclooxygenase-2 inhibitor. Adv Exp Med Biol. 2002;507:85–92. doi: 10.1007/978-1-4615-0193-0_14. [DOI] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sitzman LA, Al-Hassani M, Cai S, Pollok KE, Travers JB. Hingtgen CM. Involvement of platelet-activating factor in ultraviolet B-induced hyperalgesia. J Invest Dermatol. 2009;129:167–174. doi: 10.1038/jid.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler EA, Magerl W, Meyer RA. Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122:2245–2257. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]