Abstract
Abstract
The influence of the muscle metabolic milieu on peripheral and central fatigue is currently unclear. Moreover, the relationships between peripheral and central fatigue and the curvature constant (W ′) have not been investigated. Six men (age: 25 ± 4 years, body mass: 82 ± 10 kg, height: 179 ± 4 cm) completed four constant power handgrip tests to exhaustion under conditions of control exercise (Con), blood flow occlusion exercise (Occ), Con with 5 min post-exercise blood flow occlusion (Con + Occ), and Occ with 5 min post-exercise blood flow occlusion (Occ + Occ). Neuromuscular fatigue measurements and W ′ were obtained for each subject. Each trial resulted in significant peripheral and central fatigue. Significantly greater peripheral (79.7 ± 5.1% vs. 22.7 ± 6.0%) and central (42.6 ± 3.9% vs. 4.9 ± 2.0%) fatigue occurred for Occ than for Con. In addition, significantly greater peripheral (83.0 ± 4.2% vs. 69.0 ± 6.2%) and central (65.5 ± 14.6% vs. 18.6 ± 4.1%) fatigue occurred for Occ + Occ than for Con + Occ. W ′ was significantly related to the magnitude of global (r = 0.91) and peripheral (r = 0.83) fatigue. The current findings demonstrate that blood flow occlusion exacerbated the development of both peripheral and central fatigue and that post-exercise blood flow occlusion prevented the recovery of both peripheral and central fatigue. Moreover, the current findings suggest that W ′ may be determined by the magnitude of fatigue accrued during exercise.
Key points
Critical power represents an important threshold for neuromuscular fatigue development and may, therefore, dictate intensities for which exercise tolerance is determined by the magnitude of fatigue accrued.
Peripheral fatigue appears to be constant across O2 delivery conditions for large muscle mass exercise, but this consistency is equivocal for smaller muscle mass exercise.
We sought to determine the influence of blood flow occlusion during handgrip exercise on neuromuscular fatigue development and to examine the relationship between neuromuscular fatigue development and W ′.
Blood flow occlusion influenced the development of both peripheral and central fatigue, thus providing further evidence that the magnitude of peripheral fatigue is not constant across O2 delivery conditions for small muscle mass exercise.
W ′ appears to be related to the magnitude of fatigue accrued during exercise, which may explain the reported consistency of intramuscular metabolic perturbations and work performed for severe-intensity exercise.
Keywords: Con, control exercise; Con + Occ, control exercise with 5 min post-exercise blood flow occlusion; CP, critical power; deoxy-[Hb + Mb], deoxygenated-[haemoglobin + myoglobin]; d, displacement; EMG, electromyography; f, contraction frequency; iEMG, intergrated electromyography; LED, light-emitting diodes; MedPF, median power frequency; MVC, maximal voluntary contraction; NIRS, near infrared spectroscopy; Occ, blood flow occlusion exercise; Occ + Occ, blood flow occlusion exercise with 5 min post-exercise blood flow occlusion; oxy-[Hb + Mb], oxygenated-[haemoglobin + myoglobin]; P, power; PCr, phosphocreatine; Pi, inorganic phosphate; Ppeak, peak power; Qtw, potentiated doublet force; R, resistance; %Sat-[Hb + Mb], %Saturation-[haemoglobin + myoglobin]; Tlim, task failure; total-[Hb + Mb], total-[haemoglobin + myoglobin]; VA, voluntary activation; W ′, curvature constant
Introduction
Exercise tolerance within the severe-intensity domain is well described by a hyperbolic relationship between power output and time to exhaustion, establishing the critical power (CP) asymptote and the curvature constant (W ′) (Monod & Scherrer, 1965; Moritani et al. 1981; Poole, 2008; Vanhatalo et al. 2010; Dekerle et al. 2012; Broxterman et al. 2014). The precise mechanisms of W ′ have remained elusive, yet it appears that W ′ represents a finite capacity that when completely utilized results in similar amounts of work performed above CP and similar end-exercise intramuscular perturbations (i.e. phosphocreatine (PCr), inorganic phosphate (Pi), and hydrogen ion (H+)) (Monod & Scherrer, 1965; Moritani et al. 1981; Poole et al. 1988; Jones et al. 2008; Vanhatalo et al. 2010). The consistency of these variables suggests that the mechanisms determining W ′ must be constant within a given exercise condition. Moreover, the hyperbolic nature of the power–duration relationship implies that exercise tolerance for any power output within the severe-intensity domain is determined by the same mechanisms. Furthermore, the robust hyperbolic nature of the power–duration relationship across exercise modalities (Moritani et al. 1981; Hughson et al. 1984; Poole et al. 1988; Jones et al. 2008; Burnley, 2009; Vanhatalo et al. 2010; Cheng et al. 2012; Broxterman et al. 2014, 2015) and species (Lauderdal & Hinchcliff, 1999; Copp et al. 2010), where the determinants of exercise tolerance are likely to differ (i.e. central cardiovascular limitations, convective O2 transport limitations, diffusive O2 transport limitations, etc.), suggests the mechanisms determining exercise tolerance are common to severe-intensity exercise. Importantly, Burnley et al. (2012) demonstrated that knee-extension critical torque (the isometric exercise equivalent to CP) represents a ‘critical threshold’ for neuromuscular fatigue development and Vanhatalo et al. (2011) demonstrated that W ′ is mechanistically related to the 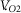 slow component and the process of muscle fatigue development. Thus, exercise tolerance within the severe-domain may be determined by the magnitude of fatigue development or degree of system impairment tolerated.
slow component and the process of muscle fatigue development. Thus, exercise tolerance within the severe-domain may be determined by the magnitude of fatigue development or degree of system impairment tolerated.
Evidence suggests that severe-intensity exercise tolerance may be limited by the attainment of specific levels of fatigue development (Saey et al. 2005; Amann et al. 2006a; Romer et al. 2007; Amann & Dempsey, 2008; Gagnon et al. 2009; Duffield et al. 2010; Rossman et al. 2012). Accordingly, Amann et al. (2013) proposed a paradigm that describes the role of group III/IV muscle afferent feedback and central motor drive in attaining a ‘sensory tolerance limit’ that leads to central motor drive becoming limited or limiting, resulting in task failure. This, however, is only one of several potential mechanisms determining exercise tolerance (Clark et al. 2000; Nybo & Nielsen, 2001; Todd et al. 2005; Meeusen et al. 2006; Amann et al. 2007). Recently, Pethick et al. (2015) demonstrated that beyond a decrease in torque-generating capacity, fatigue may also limit the ability of the neuromuscular system to adapt to external perturbation. It has been postulated that these mechanisms serve to limit the magnitude of fatigue developed during exercise, presumably as a component of homeostasis (Amann et al. 2008, 2013; Rossman et al. 2012).
The findings of Burnley et al. (2012) in combination with the proposed paradigm of Amann et al. (2013) suggest that CP may represent the exercise intensity above which exercise tolerance is limited by specific levels of fatigue development. Thus, the mechanisms determining W ′ may be related to the magnitude of fatigue developed during severe-intensity exercise. A constant level of fatigue development would constrain the amount of work that could be performed and the degree of intramuscular metabolic perturbation prior to task failure within the severe-intensity domain, which may explain the consistency in the amount of work performed and the intramuscular metabolic perturbations associated with the complete utilization of W ′ (Monod & Scherrer, 1965; Moritani et al. 1981; Poole et al. 1988; Jones et al. 2008; Vanhatalo et al. 2010). Previous research suggests that peripheral fatigue is constant for whole-body exercise (i.e. cycling) across normoxic, hypoxic and hyperoxic conditions (Amann et al. 2006a; Romer et al. 2007). However, the results are equivocal for smaller muscle mass exercise, as peripheral fatigue has been demonstrated to be similar (Millet et al. 2009) and different (Russ & Kent-Braun, 2003; Christian et al. 2014) with varying O2 delivery conditions. Thus, the magnitude of fatigue accrued during severe-intensity exercise may be different between large and small muscle mass exercise and may be a determining mechanism of W ′.
To date, we are unaware of a study that has assessed the magnitude of fatigue development using small muscle mass (handgrip) exercise with reductions in O2 delivery (via blood flow occlusion) during and post-exercise in order to determine the influence of the muscle metabolic milieu on peripheral and central fatigue. Moreover, we are aware of no study that has empirically examined the relationship between the magnitude of fatigue developed during severe intensity exercise and W ′. Therefore, the current study utilized handgrip exercise with periods of blood flow occlusion during and post-exercise to determine the influence of O2 delivery on the development of peripheral and central fatigue. Furthermore, the current study assessed the relationship between the magnitude of fatigue development and the magnitude of W ′. We tested the hypotheses that (1) peripheral and central fatigue development would be significantly exacerbated during exercise with blood flow occlusion compared to control exercise, (2) there would be no significant recovery of peripheral and central fatigue during post-exercise blood flow occlusion, and (3) a greater magnitude of peripheral and central fatigue accrued during exercise would be associated with a greater magnitude of W ′.
Methods
Ethical approval
Six recreationally active men (age: 25 ± 4 years, body mass: 82 ± 10 kg, height: 179 ± 4 cm) volunteered to participate in the study. Subjects were free of overt cardiovascular or metabolic disease, determined via medical health history evaluation. All experimental procedures were approved by the Institutional Review Board of Kansas State University and conformed to the Declaration of Helsinki. Written informed consent was attained after subjects were informed of the overall protocol and the potential risks of participation.
Experimental design
After thorough familiarization with the handgrip contraction and fatigue assessment protocol, subjects completed a total of five testing sessions. Testing sessions were separated by at least 24 h and the subjects were instructed to abstain from vigorous activity during the 24 h prior to testing. Additionally, subjects were instructed to abstain from caffeine and alcohol consumption during the 2 and 12 h, respectively, prior to testing. All testing was conducted using a custom-built two-handed handgrip ergometer (Broxterman et al. 2014), which was calibrated prior to the study. The ergometer was attached to a pneumatic cylinder by means of a cable-pulley system, which provided a fixed linear displacement of 4 cm per handgrip contraction. Resistance was controlled via pressurization of the pneumatic cylinder and work was accomplished by compressing the air within the pneumatic cylinder. Power output was calculated as P = Rdf/k, where P is power in watts (W), R is resistance in kilograms (kg), d is displacement in metres (m), f is contraction frequency, and k is the constant 6.12 for the conversion of kg m min−1 to W. Alterations in power output were accomplished via alterations in resistance (air pressure), as d and f were held constant. Subjects were seated in front of the ergometer and grasped the handrail such that both forearms were approximately at heart level. Exercise was performed using a 50% contraction duty cycle (1.5 s contraction: 1.5 s relaxation) at a rate of 20 contractions min−1. An audio recording with the specific timing was used in conjunction with feedback provided by an investigator to ensure correct timing. All testing sessions were continued until task failure (Tlim), determined as the inability to successfully complete the requisite 4 cm displacement for three consecutive contraction cycles (Fig. 1).
Figure 1.
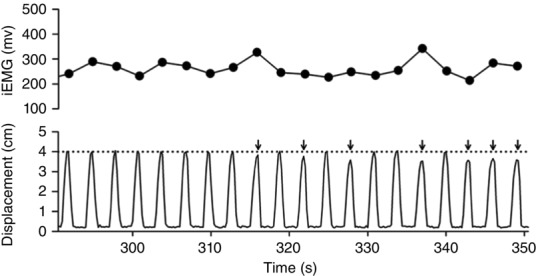
EMG and ergometer displacement at task failure in a representative subject
The integrated EMG (iEMG) values and ergometer displacement across the final 60 s of an exercise test. Task failure was determined as the inability to successfully complete the requisite 4 cm displacement for three consecutive contraction cycles. The steady iEMG despite the inability to successfully complete the contraction cycle demonstrates task failure was likely not the result of diminished effort by the subject. The arrows identify incomplete contraction cycles.
Subjects completed an incremental power output test (1.0 W + 0.5 W min−1) to determine peak power (Ppeak) during the first testing session. Ppeak was determined as the greatest power output for which at least half of the stage was completed. Subjects subsequently completed four constant-power testing sessions at 85% Ppeak (i.e. above CP). The protocols were randomly ordered conditions of control exercise (Con), blood flow occlusion exercise (Occ), control exercise with 5 min post-exercise blood flow occlusion (Con + Occ), and blood flow occlusion exercise with 5 min post-exercise blood flow occlusion (Occ + Occ) (Fig. 2). Brachial artery blood flow was occluded with a vascular cuff positioned around the brachial region of each arm, which was rapidly inflated (<0.3 s) to suprasystolic pressures (≥275 mmHg) at the onset of exercise and remained inflated until the appropriate time within the specific protocol (E20 Rapid Cuff Inflator, Hokanson, Bellevue, WA, USA). Blood flow occlusion was verified by the absence of a radial pulse and the cuff pressures were continuously monitored to ensure ≥275 mmHg. Neuromuscular function was assessed prior to and following each protocol (Fig. 2). In a recent study (Broxterman et al. 2015), the parameters of the power–duration relationship were determined for each of the current subjects for control and blood flow occlusion conditions (Con CP, Con W ′, Occ CP, Occ W ′), affording the opportunity to examine the relationships between the parameters of neuromuscular fatigue and the power–duration relationship. Importantly, Ppeak (5.8 ± 0.9 W vs. 6.1 ± 1.1 W, P = 0.1) and Tlim at similar power outputs (5.2 ± 0.9 W vs. 5.3 ± 0.9 W, P = 0.2) were not statistically different (459 ± 154 s vs. 470 ± 140 s, P = 0.8), suggesting that the physiological determinants of exercise tolerance had not changed for the subjects. In addition, all subjects reported no changes in whole-body and handgrip muscle training status.
Figure 2.
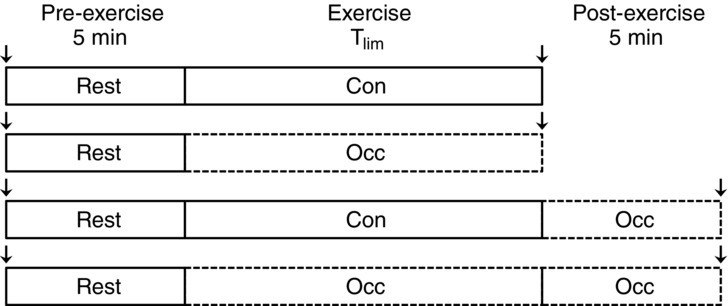
Experimental design
Control (Con) and occluded (Occ) brachial artery blood flow. The arrows signify when neuromuscular function testing was conducted.
Near-infrared spectroscopy
Oxygenation characteristics were measured during the pre-exercise and exercise portions of each protocol using a frequency-domain multi-distance NIRS system (Oxiplex TS, ISS, Champaign, IL, USA). Detailed descriptions of the principles and algorithms of the NIRS technology have previously been described (Gratton et al. 1997; Ferreira et al. 2006). Briefly, this NIRS device consists of one detector fibre bundle and eight light-emitting diodes (LED) operating at wavelengths of 690 and 830 nm (four LEDs per wavelength). The LED-detector fibre bundle separation distances are 2.0, 2.5, 3.0, and 3.5 cm. This NIRS device measures and incorporates the dynamic reduced scattering coefficients to provide absolute concentrations (μm) for deoxygenated-[haemoglobin + myoglobin] (deoxy-[Hb + Mb]), oxygenated-[Hb + Mb] (oxy-[Hb + Mb]), total-[Hb + Mb], and %Saturation-[Hb + Mb] (%Sat-[Hb + Mb]). The deoxy-[Hb + Mb] is relatively insensitive to changes in blood volume (De Blasi et al. 1993; Ferrari et al. 1997; Grassi et al. 2003) and has been used to reliably estimate the fractional oxygen extraction (De Blasi et al. 1993; Mancini et al. 1994; Ferrari et al. 1997, 2006; DeLorey et al. 2003, 2004; Grassi et al. 2003). The NIRS device was calibrated prior to each test according to the manufacturer’s recommendations. The flexor digitorum superficialis of the left arm was identified using palpation and EMG. The NIRS probe was secured along the belly of the muscle with a Velcro strap and an elastic bandage. The position of the probe was marked with indelible ink to assess movement of the probe during the testing session and for reproducible placement of the probe throughout the study. NIRS data were collected at 50 Hz and analysed using 9 s time-binned mean values.
Electromyography
Surface EMG measurements were obtained during each protocol using a commercially available system (Trigno EMG, Delsys Inc., Boston, MA, USA). The EMG sensor consists of four silver electrodes (5 × 1 mm) arranged in a 2 × 2 orientation used to make single differential EMG measurements. The flexor digitorum superficialis of the right arm was identified by palpation and strong EMG activity when the fingers were flexed, but not with ulnar or radial deviation. The sensor was secured along the belly of the muscle using adhesive surgical tape and the position marked with indelible ink. The EMG data were collected at a sampling rate of 1000 Hz and band-pass filtered (13–400 Hz) using a fifth-order Butterworth filter. The EMG signal corresponding to each muscle contraction was detected using code developed ‘in house’ (MATLAB R2011a, The Mathworks, Natick, MA, USA). The amplitude characteristics were analysed via integrated electromyography (iEMG) to provide an index of muscle activation and motorneuron firing rate. The frequency characteristics were analysed via median power frequency (MedPF) to provide an index of the muscle action potential conduction velocity. The EMG data were analysed using 9 s time-binned mean values.
Neuromuscular function
Neuromuscular function testing was conducted on the right arm with the subjects standing at the dynamometer, such that the shoulders were in-line with the dynamometer and the right arm was resting on a platform at shoulder level with the elbow fully extended. The handgrip dynamometer was attached to a calibrated force transducer (LBG1, BLH Electronics, Waltham, MA, USA) that was fixed to the platform to prevent movement. Force was sampled at 1000 Hz and displayed on a computer screen (LabVIEW, National Instruments, Austin, TX, USA). Adhesive stimulation electrodes (4 × 6 cm) were attached to the antebrachial region of the right arm for electrical stimulation of the flexor digitorum superficialis. The anode was positioned proximal to the olecranon process on the posterior brachial region of the arm and the cathode was positioned over the median nerve on the anterior antebrachial region of the arm. During the familiarization session, the cathode location that provided the greatest force development with electrical stimulation was determined. The positions of the electrodes were marked with indelible ink for reproducible placement throughout the study. The flexor digitorum superficialis was stimulated using a high-voltage constant-current electrical stimulator (DS7AH, Digitimer, Welwyn Garden City, UK). Paired stimuli (doublets) were delivered at 400 V with 100 μs square-wave pulse durations and a 10 ms pulse interval. Stimulation intensity was initiated at 50 mA and was increased in 10 mA increments until the measured force and compound muscle action potential (M-wave) no longer increased. The stimulator current was then increased by a further 19 ± 4% to ensure the stimuli were supramaximal (range 140–230 mA). Subjects subsequently performed a series of six, 3 s maximal voluntary contractions (MVCs), separated by 30 s (∼2.75 min total duration). Doublet muscle stimulations were delivered 5 s prior to each MVC, 1.5 s into the MVC, and 5 s after each MVC to obtain measurements of unpotentiated, superimposed and potentiated doublet forces, respectively. Neuromuscular assessment was completed prior to exercise and following the end of the protocol for the testing session (Fig. 1). In all cases, neuromuscular assessment was initiated < 45 s after the cessation of the protocol. MVC was measured as the greatest force attained prior to the superimposed muscle doublet stimulation. Superimposed doublet force was measured as the increment in force following the delivery of doublet stimulation during the MVC. Voluntary activation (VA) was calculated using doublet interpolation (Belanger & McComas, 1981; Behm et al. 1996; Strojnik & Komi, 1998) corrected for when the superimposed doublet stimulation did occur at MVC:
 |
Potentiated doublet force (Qtw) was measured as the greatest force produced with double stimulation 5 s after the MVC. The last four MVCs of each six MVC series were utilized for data analysis, as the degree of potentiation was lessened after the first two MVCs (Romer et al. 2006; Amann et al. 2011).
Statistical analysis
All statistical analyses were performed using a commercially available software package (SigmaStat, Systat Software Inc., Point Richmond, CA, USA). Two-way ANOVAs with repeated measures (trial × time) were used to compare main effects for all of the NIRS variables at baseline and end-exercise. One-way ANOVAs with a repeated measure were used to compare main effects for Tlim and then EMG variables at end-exercise. Tukey’s post hoc analyses were conducted when main effects were detected. Student’s paired t tests were used to compare pre- and post-exercise Qtw, MVC, %VA within each exercise test and the %change in Qtw, MVC, %VA for Con vs. Occ and Con + Occ vs. Occ + Occ. The relationships between the W ′ and MVC, Qtw, and %VA were assessed using Pearson product moment correlation analyses of the mean values between Con and Occ for each variable to avoid the influence of multiple measurements from each subject contributing to the correlation (Bland & Altman, 1995). The α-level was set at 0.05 a priori. All data are presented as means ± SD, unless otherwise noted.
Results
The Ppeak from the incremental power test was 6.1 ± 1.1 W and 85% Ppeak was 5.2 ± 0.9 W. The Tlim for the trials were: Con: 472 ± 150 s, Con + Occ: 446 ± 165 s, Occ: 131 ± 12 s, Occ + Occ: 134 ± 25 s. Tlim was significantly (P < 0.001) shorter for exercise during blood flow occlusion than for exercise during control blood flow. Occ CP (−0.7 ± 0.5 W) was significantly lower than Con CP (3.9 ± 0.8 W), while Occ W ′ (810 ± 205 J) was significantly greater than Con W ′ (550 ± 127 J).
NIRS
For all muscle oxygenation measurements at end-exercise, both Occ and Occ + Occ values were significantly different from both Con and Con + Occ, while there were no significant differences within each exercise condition (Fig. 3). Baseline muscle oxygenation values were not significantly different between trials. Deoxy-[Hb + Mb] at end-exercise was significantly greater than baseline for all trials. End-exercise total-[Hb + Mb] was significantly greater than baseline for Con and Con + Occ, while there was no significant difference between end-exercise and baseline for Occ and Occ + Occ. Oxy-[Hb + Mb] was significantly lower at end-exercise compared to baseline for Occ and Occ + Occ, but no significance differences were detected for Con or Con + Occ. %Sat-[Hb + Mb] was significantly lower at end-exercise compared to baseline for all trials (Fig. 3).
Figure 3.
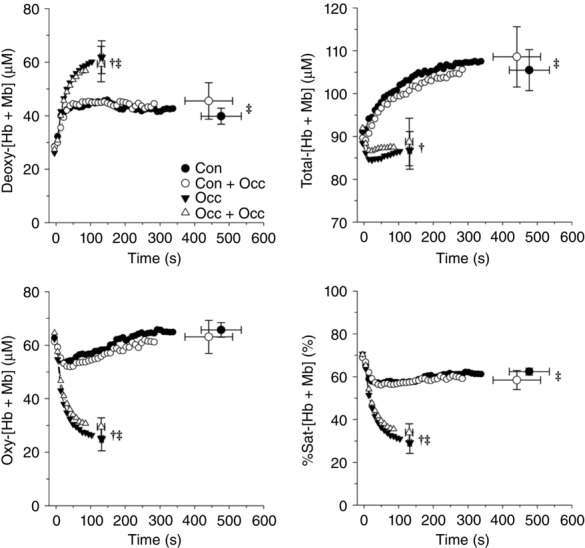
Mean NIRS muscle oxygenation data during each exercise trial
Deoxygenated-[haemoglobin + myoglobin] (deoxy-[Hb + Mb]), total-[Hb + Mb], oxygenated (oxy-[Hb + Mb]), and percent saturation (%Sat-[Hb + Mb]) data during each exercise trial. No significant differences were detected within control or occlusion exercise data. †Occ and Occ + Occ end-exercise data significantly different from Con and Con + Occ at end-exercise. ‡End-exercise significantly different from baseline within exercise condition.
EMG
EMG measurements were not significantly different between trials for the first 9 s of exercise. There were no significant differences detected for EMG measurements at end-exercise within each exercise condition (control or occlusion). MedPF was significantly lower at end-exercise for occlusion exercise than control exercise, while no significant difference was detected for iEMG (Fig. 4).
Figure 4.
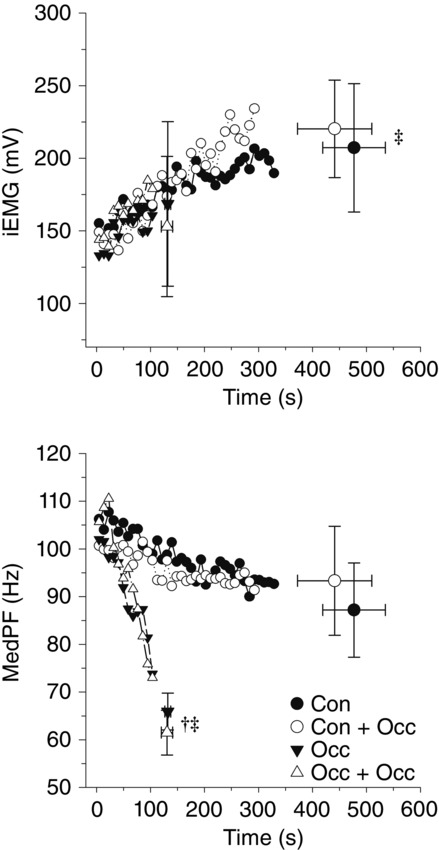
Mean EMG data for each exercise trial
Integrated EMG (iEMG) and median power frequency (MedPF) data for each exercise trial. ‡End-exercise significantly different from initial 9 s value within exercise condition. †Occ and Occ + Occ significantly different from Con and Con + Occ at end-exercise.
Neuromuscular function and W ′
For all exercise trials, post-exercise neuromuscular fatigue measurements were significantly lower than pre-exercise values. The reductions in Qtw, MVC and %VA were significantly greater for Occ than for Con (Fig. 5) and for Occ + Occ than for Con + Occ (Fig. 6). The mean W ′ between Con and Occ was significantly related to the mean pre- to post-exercise reduction in MVC (r = −0.92, P = 0.009) and Qtw (r = −0.83, P = 0.04), but not %VA (r = −0.22, P = 0.68) (Fig. 7).
Figure 5.
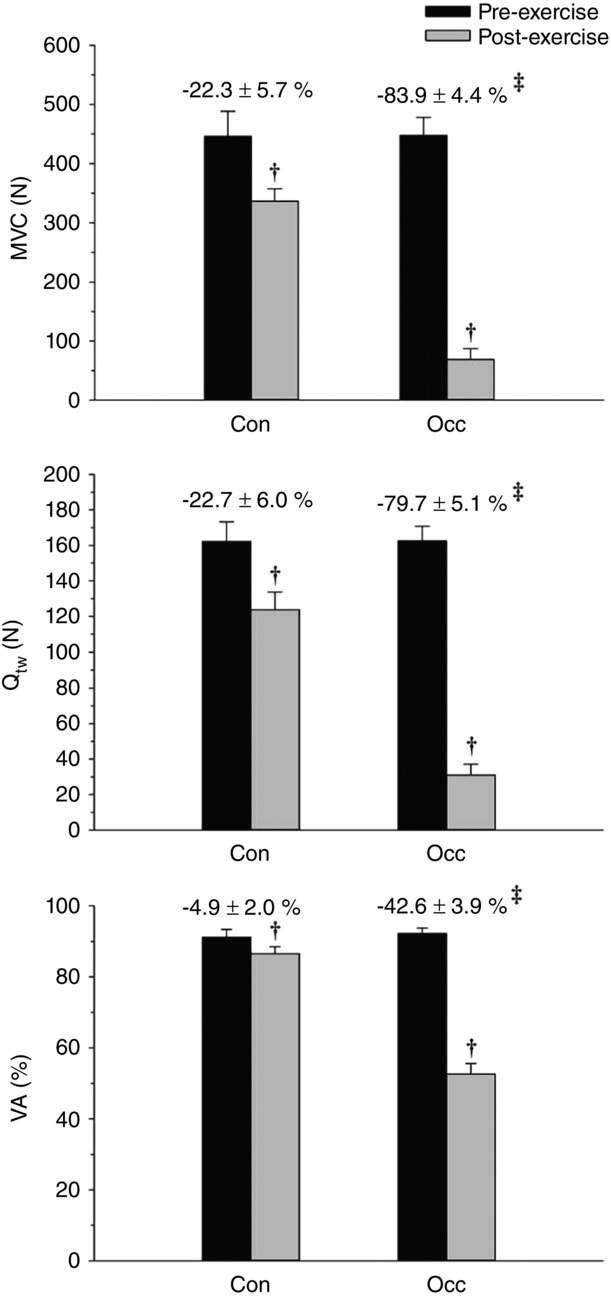
Neuromuscular function for Con and Occ exercise trials
Maximal voluntary contraction (MVC), potentiated doublet force (Qtw), and voluntary activation (%VA) determined pre- and post-exercise. The percent change from pre- to post-exercise is presented above the respective exercise trial bar graph. †Significantly different from pre-exercise. ‡Significantly different from Con percent change.
Figure 6.
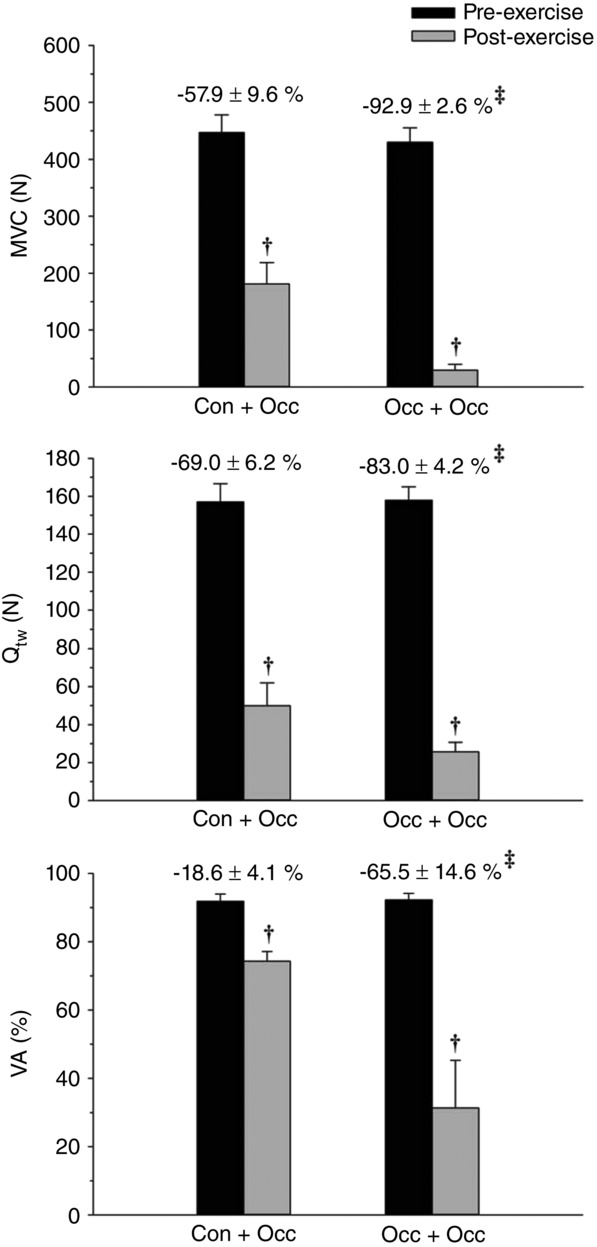
Neuromuscular function for Con + Occ and Occ + Occ exercise trials
Maximal voluntary contraction (MVC), potentiated doublet force (Qtw), and voluntary activation (%VA) determined pre- and post-exercise. The percent change from pre- to post-exercise is presented above the respective exercise trial bar graph. †Significantly different from pre-exercise. ‡Significantly different from Con + Occ percent change.
Figure 7.
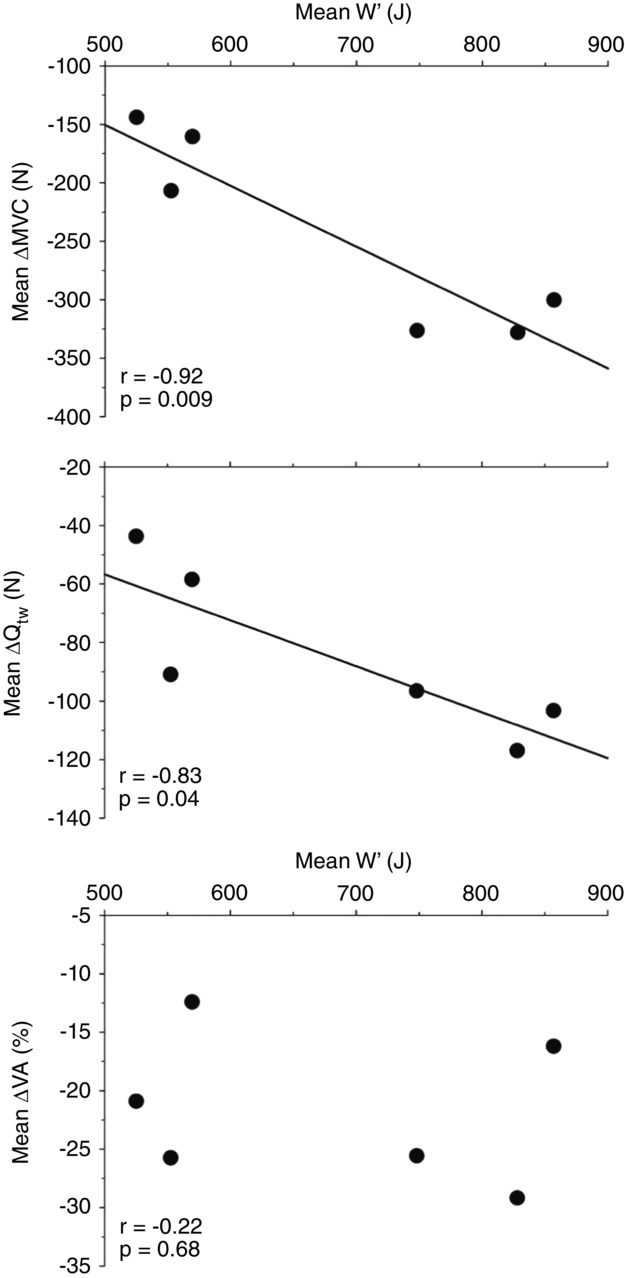
Relationship between W′ and the change in neuromuscular function variables
Maximal voluntary contraction (MVC), potentiated doublet force (Qtw) and voluntary activation (%VA) determined from neuromuscular function testing.
Discussion
The purpose of the current study was to determine the influence of reductions in O2 delivery (via blood flow occlusion) on the development of peripheral and central fatigue during handgrip exercise. It was demonstrated that blood flow occlusion during exercise exacerbated the development of both peripheral and central fatigue. Moreover, continued blood flow occlusion after the cessation of exercise prevented the recovery of (or worsened the magnitude of) peripheral and central fatigue. The current study is the first to identify a relationship between the magnitude of fatigue developed during exercise and the magnitude of W ′. This relationship suggests that the magnitude of fatigue accrued during severe-intensity exercise may be a determining mechanism of W ′.
Influence of O2 delivery on fatigue
It has been postulated that the voluntary termination of severe-intensity exercise is the result of attaining a ‘sensory tolerance limit’ (Gandevia, 2001; Amann et al. 2013). Evidence suggests that the ensemble group III/IV afferent input from the active locomotor muscles plays a vital role in determining this ‘sensory tolerance limit’ (Amann et al. 2006a,2006b, 2009; Amann & Calbet, 2008; Amann, 2011; Rossman et al. 2012; Kennedy et al. 2015) and the subsequent reduction in central motor drive (Taylor et al. 2000). This reduction in central motor drive is hypothesized to be a protective mechanism that constrains the magnitude of fatigue development within the muscle by limiting intramuscular perturbations (Amann et al. 2006a). The consistency of peripheral fatigue development (and therefore the ‘sensory tolerance limit’) during exercise is not without some degree of ambiguity. It appears that the magnitude of peripheral fatigue development for large muscle mass activity (e.g. cycling) is constant and does not vary with alterations in O2 delivery (Amann et al. 2006a; Romer et al. 2007). In contrast, a constant magnitude of peripheral fatigue development is not consistently found for small muscle mass activity (e.g. knee-extension and handgrip exercise). Christian et al. (2014) demonstrated a greater magnitude of peripheral fatigue development during knee-extension exercise in hypoxia than normoxia. Russ & Kent-Braun (2003) demonstrated that ischaemic handgrip exercise resulted in greater peripheral fatigue development than control handgrip exercise. However, Millet et al. (2009) demonstrated similar levels of peripheral fatigue with knee-extension exercise during normoxic and hypoxic exercise with and without ischaemia. The current study demonstrated that reductions in O2 delivery (via blood flow occlusion) exacerbated the magnitude of fatigue development through both peripheral and central origins for small muscle mass handgrip exercise. Moreover, the current study demonstrated a greater magnitude of peripheral fatigue was incurred during occlusion exercise. In the paradigm of Amann et al. (2013) the ‘sensory tolerance limit’ is attained via increases in afferent feedback from the active muscle and central motor drive. The current findings may be interpreted to suggest that the ‘sensory tolerance limit’ for small muscle mass exercise is sensitive to alterations in O2 delivery, as more peripheral fatigue was accrued during occlusion exercise than control exercise. These results may also be interpreted to suggest that a ‘sensory tolerance limit’ does not exist, which may provide support for other potential mechanisms determining exercise tolerance (Clark et al. 2000; Nybo & Nielsen, 2001; Todd et al. 2005; Meeusen et al. 2006; Amann et al. 2007). Consistent with this, the magnitude of the severe intensity domain has been postulated to determine exercise tolerance (Burnley & Jones, 2007; Vanhatalo et al. 2010) and it may be the breadth of this domain that constrains the magnitude of fatigue development during severe intensity exercise. Nonetheless, the consistency in the magnitude of fatigue accrued during exercise is different between large and small muscle mass exercise.
It was demonstrated in the current study that recovery of peripheral and central fatigue is prevented (and may be worsened) if blood flow occlusion is maintained post-exercise. It is well documented that the accumulation of intramuscular metabolites during exercise increases the firing frequency of group III/IV afferents (Kaufman & Rybicki, 1987; Adreani et al. 1997), which, in turn, have been implicated as determinants of the magnitude of fatigue developed during exercise (Amann, 2011; Kennedy et al. 2015). Recently, Kennedy et al. (2015) demonstrated that an ischaemic period after a fatiguing knee-extension protocol decreased VA, presumably due to activity of group III/IV muscle afferents. Consistent with this, it was demonstrated in the current study that post-exercise blood flow occlusion resulted in the persistence (or further development) of not only central fatigue, but also peripheral fatigue.
Moreover, blood flow occluded exercise resulted in greater levels of peripheral and central fatigue. Interestingly, peripheral fatigue appears to be more sensitive to the reduction in O2 delivery than central fatigue. Central fatigue has been demonstrated to be influenced by cerebral O2 delivery, as it was demonstrated that central fatigue is exacerbated during exercise with blood flow occlusion when cerebral O2 delivery is reduced via hypoxia (Millet et al. 2009, 2012). Thus, central fatigue may have been less sensitive to the effects of occlusion exercise, as cerebral oxygenation was likely not challenged during the current study. Cumulatively, these findings support the notion that the concentration of intramuscular metabolites may contribute to the magnitude of fatigue developed within the muscle, by affecting the firing frequency of group III/IV muscle afferents.
Despite an apparent difference in fatigue regulation between large and small muscle mass exercise, cycling, knee-extension and handgrip exercise have all been demonstrated to hold true to the hyperbolic power–duration relationship, even with alterations in O2 delivery (Moritani et al. 1981; Poole et al. 1988; Burnley, 2009; Vanhatalo et al. 2010; Broxterman et al. 2014, 2015). This implies that the determinants of exercise tolerance are constant within each muscle mass and O2 delivery condition, but may vary across muscle mass and O2 delivery conditions. However, some commonality in the mechanisms determining exercise tolerance must nonetheless exist, as there appears to be no deviation from the hyperbolic power–duration relationship.
Relationship between fatigue and W ′
The findings of the current study demonstrate that the magnitude of fatigue accrued during handgrip exercise is related to the magnitude of W ′. W ′ has repeatedly been associated with a finite amount of work that can be performed above CP for a given exercise condition (Monod & Scherrer, 1965; Moritani et al. 1981; Poole et al. 1988). The relationship between W ′ and fatigue suggests that the mechanisms determining when task failure occurs constrain the amount of work that can be performed above CP. Thus, the greater amount of fatigue that is accrued, the greater the amount work that can be performed above CP. For example, Broxterman et al. (2015) demonstrated a greater W ′ for exercise with blood flow occlusion than for control exercise. The findings of the current study suggest that the greater W ′ with blood flow occlusion exercise may be due to the greater magnitude of fatigue accrued. It has also been demonstrated that W ′ is associated with the attainment of consistent intramuscular metabolite concentrations at end-exercise (Poole et al. 1988; Vanhatalo et al. 2010). The magnitude of fatigue accrued during exercise would constrain the amount of intramuscular metabolic perturbation. This may explain the consistent levels measured within given exercise conditions. However, it does not appear that intramuscular metabolic perturbations at end-exercise vary with O2 delivery conditions (Hogan et al. 1999; Vanhatalo et al. 2010). Thus, it appears that different magnitudes of fatigue and amounts of work can be performed for given intramuscular metabolic perturbations. This may arise from differences in efficiency and energy yield as a result of O2 delivery to the muscle (Krustrup et al. 2003; Lanza et al. 2006; Stellingweff et al. 2006; Vanhatalo et al. 2010). Consistent with this, W ′ (determined as the amount of work performed above CP) was decreased in hyperoxia, while no change in the end-exercise intramuscular metabolic perturbations was measured (Vanhatalo et al. 2010). Importantly, the attainment of these consistent end-exercise intramuscular metabolite concentrations may not be a direct determining mechanism of W ′, as these concentrations may be attained and maintained for several minutes before the limit of exercise tolerance (see Fig. 2 in ref. (Vanhatalo et al. 2010)). Moreover, it appears that specific exercise training protocols alter peripheral and central fatigue characteristics (Zghal et al. 2015), which may potentially explain alterations in W ′ with exercise training (Jenkins & Quigley, 1991; Sawyer et al. 2014). However, as CP and W ′ were not measured in the study by Zgahal et al. (2015) it cannot be known if the reported changes in peripheral and central fatigue were related with alterations in the magnitude of W ′. The findings of the current study suggest the amount of fatigue accrued during exercise may determine W ′ and therefore exercise tolerance above CP.
Limitations
It has previously been demonstrated that compression block influences afferent activity (Garland, 1991). Thus, the vascular cuffing used in the current study potentially could have altered afferent feedback due to nerve compression. However, compression block is typically performed by occluding blood flow for ∼20 min. In the current study, blood flow occlusion was not initiated until the onset of exercise and the total occlusion duration never exceeded 20 min. There were no measurements of intramuscular metabolite concentrations or afferent firing in the current study. Therefore, inferences were made from previous studies demonstrating no effect of inspired O2 concentration on end-exercise intramuscular metabolite concentrations (Hogan et al. 1999; Vanhatalo et al. 2010), and that group III/IV afferent firing increases with metabolite accumulation (Kaufman & Rybicki, 1987; Adreani et al. 1997). Lastly, no comparisons were made between fatigue measurements from the post-exercise blood flow occlusion data and fatigue measurements obtained immediately post-exercise. This was purposeful in order to prevent attributing the difference in fatigue between these conditions to occlusion, as it is not known what the fatigue measurements would be 5 min after the cessation of exercise.
Conclusion
This study provides further evidence that the magnitude of fatigue development is not constant for small muscle mass exercise across O2 delivery conditions. Moreover, the current study demonstrated that post-exercise blood flow occlusion prevented the recovery of both peripheral and central fatigue. In combination, it appears that the consistency in the magnitude of fatigue accrued is different between large and small muscle mass exercise. The current study is the first to provide evidence of a relationship between the magnitude of fatigue development during exercise and the magnitude of W ′. This evidence suggests that W ′ may be determined by the magnitude of fatigue accrued during exercise, which may constrain the amount of work that can be performed and the intramuscular metabolic perturbations for severe-intensity exercise.
Glossary
- Con
control exercise
- Con + Occ
control exercise with 5 min post-exercise blood flow occlusion
- CP
critical power
- deoxy-[Hb + Mb]
deoxygenated-[haemoglobin + myoglobin]
- d
displacement
- EMG
electromyography
- f
contraction frequency
- iEMG
intergrated electromyography
- LED
light-emitting diodes
- MedPF
median power frequency
- MVC
maximal voluntary contraction
- NIRS
near infrared spectroscopy
- Occ
blood flow occlusion exercise
- Occ + Occ
blood flow occlusion exercise with 5 min post-exercise blood flow occlusion
- oxy-[Hb + Mb]
oxygenated-[haemoglobin + myoglobin]
- P
power
- PCr
phosphocreatine
- Pi
inorganic phosphate
- Ppeak
peak power
- Qtw
potentiated doublet force
- R
resistance; %Sat-[Hb + Mb], %Saturation-[haemoglobin + myoglobin]
- Tlim task failure; total-[Hb + Mb]
total-[haemoglobin + myoglobin]
- VA
voluntary activation
- W ′
curvature constant
Additional information
Competing interests
The authors report no competing interests for this work.
Author contributions
This work was completed at Kansas State University. R.M.B., J.C.C., S.L.W. and T.J.B. were involved in the conception and design of the study; R.M.B., J.C.C., J.R.S., S.L.W., C.J., S.W. and T.J.B. were involved in data collection; C.J. and S.W. wrote the computer code to process the data. All authors were involved in the analysis and interpretation of the data, as well as writing and revising the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Aeronautics and Space Administration (NASA) under Grant NNX10AK60G awarded to T.J.B. and by NASA Experimental Program to Stimulate Competitive Research (EPSCoR) under Grant NNX11AM05A supporting R.M.B.
References
- Adreani CM, Hill JM. Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc. 2011;43:2039–2045. doi: 10.1249/MSS.0b013e31821f59ab. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain G, Proctor LT, Sebranek JJ, Pegelow DF. Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M. Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Amann M. Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586:161–173. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF. Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF. Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influence on central neural drive during whole body endurance exercise. J Appl Physiol. 2008;105:1717–1724. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF. Dempsey JA. Opiod-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ. Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol. 2006;101:119–127. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- Amann M, Romer LM, Subudhi AW, Pegelow DF. Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol. 2007;581:389–403. doi: 10.1113/jphysiol.2007.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ. Richardson RS. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motorneuronal output. J Appl Physiol. 2013;115:355–364. doi: 10.1152/japplphysiol.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm DG, St-Pierre DMM. Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol. 1996;81:2267–2273. doi: 10.1152/jappl.1996.81.5.2267. [DOI] [PubMed] [Google Scholar]
- Belanger AY. McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bland JM. Altman DG. Calculating correlation coefficients with repeated observations: Part 2: Correlation between subjects. BMJ. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Ade CJ, Craig JC, Wilcox SL, Schlup SJ. Barstow TJ. Influence of blood flow occlusion on muscle deoxygenation characteristics and the parameters of the power–duration relationship. J Appl Physiol. 2015;118:880–889. doi: 10.1152/japplphysiol.00875.2014. [DOI] [PubMed] [Google Scholar]
- Broxterman RM, Ade CJ, Wilcox SL, Schlup SJ, Craig JC. Barstow TJ. Influence of duty cycle on the power–duration relationship: Observations and potential mechanisms. Respir Physiol Neurobiol. 2014;192:102–111. doi: 10.1016/j.resp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Burnley M. Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J Appl Physiol. 2009:975–983. doi: 10.1152/japplphysiol.91474.2008. 106, [DOI] [PubMed] [Google Scholar]
- Burnley M. Jones AM. Oxygen uptake kinetics as a determinant of sports performance. Eur J Sport Sci. 2007;7:63–79. [Google Scholar]
- Burnley M, Vanhatalo A. Jones AM. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol. 2012;113:215–223. doi: 10.1152/japplphysiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- Cheng C-F, Yang Y-S, Lin H-M, Lee C-L. Wang C-Y. Determination of critical power in trained rowers using a three-minute all-out rowing test. Eur J Appl Physiol. 2012;112:1251–1260. doi: 10.1007/s00421-011-2081-2. [DOI] [PubMed] [Google Scholar]
- Christian RJ, Bishop DJ, Billaut F. Girard O. Peripheral fatigue is not critically regulated during maximal, intermittent, dynamic leg extensions. J Appl Physiol. 2014;117:1063–1073. doi: 10.1152/japplphysiol.00988.2013. [DOI] [PubMed] [Google Scholar]
- Clark VR, Hopkins WG, Hawley JA. Burke LM. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med Sci Sports Exerc. 2000;32:1642–1647. doi: 10.1097/00005768-200009000-00019. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Musch TI. Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol. 2010;588:5077–5087. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasi RA, Cope M, Elwell C, Safoue F. Ferrari M. Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur J Appl Physiol Occup Physiol. 1993;67:20–25. doi: 10.1007/BF00377698. [DOI] [PubMed] [Google Scholar]
- Dekerle J, Mucci P. Carter H. Influence of moderate hypoxia on tolerance to high-intensity exercise. Eur J Appl Physiol. 2012;112:327–335. doi: 10.1007/s00421-011-1979-z. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM. Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate intensity exercise. J Appl Physiol. 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM. Paterson DH. Effects of prior heavy-intensity exercise on pulmonary O2 uptake and muscle deoxygenation kinetics in young and older adult humans. J Appl Physiol. 2004;97:998–1005. doi: 10.1152/japplphysiol.01280.2003. [DOI] [PubMed] [Google Scholar]
- Duffield R, Green R, Castle P. Maxwell N. Precooling can prevent the reduction in self-paced exercise intensity in the heat. Med Sci Sports Exerc. 2010;42:577–584. doi: 10.1249/MSS.0b013e3181b675da. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Binzoni T. Quaresima V. Oxidative metabolism in muscle. Philos Trans R Soc Lond B Biol Sci. 1997;352:677–683. doi: 10.1098/rstb.1997.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, Lutjemeier BJ, Townsend DK. Barstow TJ. Effects of pedal frequency on estimated muscle microvascular O2 extraction. J Appl Physiol. 2006;96:558–563. doi: 10.1007/s00421-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S. Maltais F. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol. 2009;107:832–840. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C. Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- Gratton E, Fantini S, Franceschini MA, Gratton G. Fabiani M. Measurements of scattering and absorption changes in muscle and brain. Philos Trans R Soc Lond B Biol Sci. 1997;352:727–735. doi: 10.1098/rstb.1997.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS. Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Orok CJ. Staudt LE. A high velocity treadmill running test to assess endurance running potential. Int J Sports Med. 1984;5:23–25. doi: 10.1055/s-2008-1025875. [DOI] [PubMed] [Google Scholar]
- Jenkins DG. Quigley BM. The y-intercept of the critical power function as a measure of anaerobic work capacity. Ergonomics. 1991;34:13–22. doi: 10.1080/00140139108967284. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, DiMenna FJ, Fulford J. Poole DC. Muscle metabolic responses to exercise above and below the "critical power" assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol. 2008;294:R585–593. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- Kaufman MP. Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–I65. [PubMed] [Google Scholar]
- Kennedy DS, Fitzpatrick SC, Gandevia SC. Taylor JL. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol. 2015;118:408–418. doi: 10.1152/japplphysiol.00375.2014. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RC, Kjaer M. Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE. Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdal MA. Hinchcliff KW. Hyperbolic relationship time-to-fatigue and workload. Equine Vet J Suppl. 1999;30:586–590. doi: 10.1111/j.2042-3306.1999.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Bolinger L, Li H, Kendrick K, Chance B. Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol. 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Watson P, Hasegawa H, Roelands B. Piacentini MF. Central Fatigue: the serotonin hypothesis and beyond. Sports Med. 2006;36:881–899. doi: 10.2165/00007256-200636100-00006. [DOI] [PubMed] [Google Scholar]
- Millet GY, Aubert D, Favier FB, Busso T. Benoît H. Effect of acute hypoxia on central fatigue during repeated isometric leg contractions. Scand J Med Sci Sports. 2009;19:695–702. doi: 10.1111/j.1600-0838.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- Millet GY, Mauthalib M, Jubeau M, Laursen PB. Nosaka K. Severe hypoxia affects exercise performance independently of afferent feedback and peripheral fatigue. J Appl Physiol. 2012;112:1335–1344. doi: 10.1152/japplphysiol.00804.2011. [DOI] [PubMed] [Google Scholar]
- Monod H. Scherrer J. The work capacity of a synergic muscular group. Ergonomics. 1965;8:329–338. [Google Scholar]
- Moritani T, Nagata A, DeVries HA. Muro M. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics. 1981;24:339–350. doi: 10.1080/00140138108924856. [DOI] [PubMed] [Google Scholar]
- Nybo L. Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Pethick J, Winter SL. Burnley M. Fatigue reduces the complexity of knee extensors torque fluctuations during maximal and submaximal intermittent isometric contractions in man. J Physiol. 2015;593:2085–2096. doi: 10.1113/jphysiol.2015.284380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC. Resolving the determinants of high-intensity exercise performance. Exp Physiol. 2008;94:197–198. doi: 10.1113/expphysiol.2008.045849. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ward SA, Gardner GW. Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988;31:1265–1279. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF. Dempsey JA. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R598–R606. doi: 10.1152/ajpregu.00269.2006. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF. Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Venturelli M, McDaniel J, Amann M. Richardson RS. Muscle mass and peripheral fatigue: a potential role for afferent feedback? Acta Physiol (Oxf) 2012;206:242–250. doi: 10.1111/j.1748-1716.2012.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW. Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94:2414–2422. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
- Saey D, Michaud A, Couillard A, Côté CH, Mador MJ, LeBlanc P, Jobin J. Maltais F. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1109–1115. doi: 10.1164/rccm.200408-1005OC. [DOI] [PubMed] [Google Scholar]
- Sawyer BJ, Stokes DG, Womack CJ, Morton RH, Weltman A. Gaesser GA. Strength training increases endurance time to exhaustion during high-intensity exercise despite no change in critical power. J Strength Cond Res. 2014;28:601–609. doi: 10.1519/JSC.0b013e31829e113b. [DOI] [PubMed] [Google Scholar]
- Stellingweff T, LeBlanc PJ, Hollidge MG, Heigenhauser GJF. Spriet LL. Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am J Physiol Endrocrinol Metab. 2006;290:E1180–E1190. doi: 10.1152/ajpendo.00499.2005. [DOI] [PubMed] [Google Scholar]
- Strojnik V. Komi PV. Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol. 1998;84:344–350. doi: 10.1152/jappl.1998.84.1.344. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE. Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or coriticospinal tract stimulation. J Physiol. 2000;525:793–801. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Butler JE. Taylor JL. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, DiMenna FJ. Jones AM. Influence of hyperoxia on muscle metabolic responses and the power–duration work relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol. 2010;95:528–540. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ. Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Regul Integr Comp Physiol. 2011;300:R700–R707. doi: 10.1152/ajpregu.00761.2010. [DOI] [PubMed] [Google Scholar]
- Zghal F, Cottin F, Kenoun I, Rebaϊ H, Moalla W, Dogui M, Tabka Z. Martin V. Improved tolerance of peripheral fatigue by the central nervous system after endurance training. Eur J Appl Physiol. 2015;115:1401–1415. doi: 10.1007/s00421-015-3123-y. [DOI] [PubMed] [Google Scholar]


