Abstract
Abstract
Hypoadiponectinaemia is closely associated with endothelial dysfunction and insulin resistance, and microvasculature plays a critical role in the regulation of insulin action in muscle. Here we tested whether adiponectin replenishment could improve metabolic insulin sensitivity in male rats fed a high-fat diet (HFD) via the modulation of microvascular insulin responses. Male Sprague–Dawley rats were fed either a HFD or low-fat diet (LFD) for 4 weeks. Small resistance artery myograph changes in tension, muscle microvascular recruitment and metabolic response to insulin were determined. Compared with rats fed a LFD, HFD feeding abolished the vasodilatory actions of globular adiponectin (gAd) and insulin on pre-constricted distal saphenous arteries. Pretreatment with gAd improved insulin responses in arterioles isolated from HFD rats, which was blocked by AMP-activated protein kinase (AMPK) inhibition. Similarly, HFD abolished microvascular responses to either gAd or insulin and decreased insulin-stimulated glucose disposal by ∼60%. However, supplementing gAd fully rescued insulin’s microvascular action and significantly improved the metabolic responses to insulin in HFD male rats and these actions were abolished by inhibition of either AMPK or nitric oxide production. We conclude that HFD induces vascular adiponectin and insulin resistance but gAd administration can restore vascular insulin responses and improve insulin’s metabolic action via an AMPK- and nitric oxide-dependent mechanism in male rats.
Key points
Adiponectin is an adipokine with anti-inflammatory and anti-diabetic properties. Hypoadiponectinaemia is closely associated with endothelial dysfunction and insulin resistance in obesity and diabetes.
Insulin resistance is present in muscle microvasculature and this may contribute to decreased insulin delivery to, and action in, muscle.
In this study we examined whether adiponectin ameliorates metabolic insulin resistance by affecting muscle microvascular recruitment.
We demonstrated that a high-fat diet induces vascular adiponectin and insulin resistance but globular adiponectin administration can restore vascular insulin responses and improve insulin’s metabolic action via an AMPK- and nitric oxide-dependent mechanism.
This suggests that globular adiponectin might have a therapeutic potential for improving insulin resistance and preventing cardiovascular complications in patients with diabetes via modulation of microvascular insulin responses.
Introduction
Adiponectin is a well-known insulin sensitizer and patients with obesity and diabetes exhibit hypoadiponectinaemia (Arita et al. 1999; Hotta et al. 2000; Weyer et al. 2001). Similarly, obese and diabetic animals have low plasma adiponectin levels and replenishment of adiponectin ameliorates insulin resistance in liver, adipose tissue and skeletal muscle (Berg et al. 2001; Combs et al. 2001; Fruebis et al. 2001; Yamauchi et al. 2001). In normotensive young spontaneously hypertensive rats hypoadiponectinaemia plays a causal role in the development of vascular insulin resistance (Xing et al. 2013). Adenovirus-mediated adiponectin overexpression improves muscle insulin sensitivity in Wistar rats (Satoh et al. 2005). Hypoadiponectinaemia is also associated with endothelial dysfunction in obese KKAy (Ohashi et al. 2006) and diabetic (db/db) mice (Lee et al. 2012) while adiponectin repletion significantly improves endothelial function.
Adiponectin binds to adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) to exert its biological effects, including vasodilatation and insulin sensitization (Kadowaki & Yamauchi, 2005). Multiple mechanisms may contribute to the insulin-sensitizing action of adiponectin, including the activation of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α, and stimulation of fatty acid oxidation (Nedvídková et al. 2005). We have recently demonstrated that globular adiponectin (gAd) can enhance muscle insulin action by recruiting capillaries to increase microvascular perfusion and muscle insulin delivery and action in healthy, insulin sensitive rats, which links the adiponectin’s vascular effects to its metabolic effects and provides a new mechanistic insight into the insulin-sensitizing effect of adiponectin in vivo (Zhao et al. 2013).
Muscle microvasculature provides an endothelial surface area for substrate exchange between plasma and muscle interstitium and plays a pivotal role in the regulation of insulin delivery to, and action in, muscle (Clerk et al. 2004; Barrett et al. 2009). We and others have shown that factors that increase muscle microvascular perfusion, such as exercise, insulin, glucagon-like peptide1, ranolazine and angiotensin II type 1 receptor blockade are all capable of increasing muscle insulin delivery and action (Vincent et al. 2004; Inyard et al. 2007; Chai et al. 2011, 2012; Fu et al. 2013). For insulin, blocking nitric oxide (NO) synthase inhibits its action to recruit microvasculature and this reduces insulin-stimulated glucose disposal by up to 40% (Vincent et al. 2003). Insulin’s microvascular effect is blunted or abolished in the insulin-resistant states in both animal models and humans (de Jongh et al. 2004; Clerk et al. 2006; Liu et al. 2009). Mounting evidence from both clinical and animal studies suggests that microvascular dysfunction is present in obesity which may play a pathogenesis role in the development of metabolic insulin resistance in obesity and type 2 diabetes (Wallis et al. 2002; de Jongh et al. 2004; Clerk et al. 2006; Jonk et al. 2007). We considered that enhancing microvascular perfusion in insulin-resistant states could improve insulin’s metabolic actions by augmenting insulin delivery to muscle. Indeed, we have recently reported that systemic administration of losartan was able to recruit muscle microvasculature and restore fully muscle insulin sensitivity in rats receiving systemic lipid infusion (Wang et al. 2013).
In the present study we examined the acute microvascular and metabolic insulin responses in the presence or absence of gAd administration in a rodent model of chronic insulin resistance induced by 4 weeks of high-fat diet (HFD). Isolated distal saphenous arteries were used to assess the vasoactive actions of gAd and insulin ex vivo and contrast-enhanced ultrasound (CEU) technology was used to assess microvascular perfusion in vivo. Our results suggest that HFD rats exhibit both gAd and insulin resistance in small resistance arteries and microcirculation, and treatment with gAd restores vascular insulin responses and improves the metabolic action of insulin in muscle of HFD rats.
Methods
Ethical approval
The study conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication No. 85-23, revised 1996). The study protocols were approved by the Animal Care and Use Committee of the University of Virginia, which conforms to the principles of UK regulations, as described in Drummond (2009).
Animal preparations and experimental protocols
Adult male Sprague–Dawley rats (200–250 g, Charles River Laboratories, Wilmington, MA, USA) were placed on either a high-fat diet (HFD, 60% calories from saturated fat) or a low-fat diet (LFD, 10% calories from saturated fat; Research Diets, New Brunswick, NJ, USA) for 4 weeks. Rats were housed at 22 ± 2°C on a 12 h light–dark cycle and given water ad libitum. Rats were then fasted overnight and anaesthetized with pentobarbital sodium (50 mg kg−1i.p., Abbott Laboratories, North Chicago, IL, USA). They were placed in a supine position on a heating pad to ensure euthermia and intubated to maintain a patent airway. The carotid artery and the jugular vein were cannulated with PE50 polyethylene tubing (Fisher Scientific, Newark, DE, USA) for blood pressure monitoring, arterial blood sampling and various infusions. After a 30–45 min baseline period to ensure haemodynamic stability and stable anaesthesia, rats were studied under the following two protocols. At the end of each study, rats were killed via a pentobarbital sodium overdose.
In protocol 1, HFD rats (5 rats in this group) received an i.p. injection of rat gAd (0.4 μg (g body wt)−1; Biovision Inc., Mountain View, CA, USA). Hindlimb muscle microvascular blood volume (MBV) and microvascular blood flow velocity (MFV) were measured using CEU before, and 30, 60 and 120 min after, gAd injection as previously described (Vincent et al. 2004). Microvascular blood flow (MBF) was calculated as the product of MBV and MFV.
In protocol 2 seven groups of rats were studied (5–6 rats in each group). Rats received a 2 h euglycaemic hyperinsulinaemic clamp (3 mU kg−1 min−1) after an i.p. injection of either rat gAd (0.4 μg (g body wt)−1) or saline in the presence or absence of an i.p. injection of Compound C (10 μg (g body wt)−1; Calbiochem, Billerica, MA, USA) (McCullough et al. 2005) or l-NG-nitroarginine methyl ester (l-NAME) co-infusion which started 30 min prior to the initiation of insulin clamp. Arterial blood glucose was determined every 10 min using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN, USA) and 30% dextrose was infused at a variable rate to maintain blood glucose within 10% of basal. Steady-state whole body glucose infusion rates were measured. Hindlimb muscle MBV, MFV and MBF were determined at time 0, 30, 60 and 120 min. Rats were then killed and gastrocnemius muscle and aorta removed and freeze-clamped for later measurement of adiponectin and insulin signalling molecules using Western blot analysis as described below.
Throughout the study, mean arterial blood pressure (MAP) was monitored via a sensor connected to the carotid arterial catheter (Harvard Apparatus, Holliston, MA, USA and ADInstruments, Inc., Colorado Springs, CO, USA). Pentobarbital sodium was infused throughout the study at a variable rate to maintain steady levels of anaesthesia and blood pressure. gAd was given as a single intraperitoneal dose due to its long plasma half-life of ∼3–6 h (Pajvani et al. 2003; Hoffstedt et al. 2004).
Measurement of plasma adiponectin, insulin levels and NO concentration
Plasma adiponectin was measured using an Adiponectin Rat ELISA kit (Abcam, San Francisco, CA, USA), which recognizes both full-length and globular adiponectin. Plasma insulin concentrations were determined using a Rat Insulin ELISA kit (Mercodia AB, Uppsala, Sweden). Plasma NO levels were measured using a 280i Nitric Oxide Analyser (GE Analytical Instruments, Boulder, CO, USA), according to the manufacturer’s instructions. In brief, ice-cold ethanol was mixed with plasma samples at a ratio of 2:1, kept at 0°C for 30 min, and then centrifuged at ∼18,000 g for 5 min. The supernatant was then used for NO analysis based on a gas-phase chemiluminescent reaction between NO and ozone.
Myograph studies
The distal saphenous arteries were dissected from additional HFD and LFD rats (4–6 rats in each group) immediately after the rats were anaesthetized. After removing the adhering connective tissue, each artery was cut into segments of ∼2 mm in length and vessel functions were measured as previously described (Dong et al. 2013). Briefly, each segment was mounted in a Multi Myograph System (Danish Myo Technology, Aarhus, Denmark). The organ chamber was filled with 6 ml of physiological salt solution buffer (130 mm NaCl, 4.7 mm KCl, 1.6 mm CaCl2, 1.17 mm MgSO4, 1.18 mm KH2PO4, 14.9 mm NaHCO3, 0.026 mm EDTA and 5.5 mm glucose, pH 7.4), which was constantly bubbled with 95% O2–5% CO2 and maintained at 37°C. Each ring was stretched initially to an optimal tension (5 mN) and then allowed to stabilize at baseline tone. After pre-constriction with phenylephrine, arterial responses to rat gAd and/or insulin were recorded. Additional experiments were done in the presence of Compound C (an AMPK inhibitor, 5 μm) (Meijer et al. 2013) or l-NAME (10 μm) pretreatment for 30 min.
Determination of protein expression and phosphorylation in muscle and aorta
Protein expression of AdipoR1, AdipoR2, adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1 (APPL1), total AMPK, endothelial nitric oxide synthase (eNOS), protein kinase B (Akt) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), and the phosphorylation of AMPK, eNOS, Akt and ERK1/2 in muscle or aorta were determined by Western blot analysis, as previously described (Vincent et al. 2004; Chai et al. 2008). Primary antibodies against AdipoR1 (cat. no. ab70362) and AdipoR2 (ab77613) were obtained from Abcam (Cambridge, MA, USA) and those against APPL1 (no. 3276), phospho-AMPKα (Thr172) (no. 2531), total AMPK (no. 5831), phospho-eNOS (Ser1177) (no. 9571), total eNOS (no. 9572), phospho-Akt (Ser473) (no. 9271), total Akt (no. 9272), phospho-ERK (Thr202/Tyr204) (no. 4370) and total ERK1/2 (no. 4695) were purchased from Cell Signaling Technology (Beverly, MA, USA). Molecular markers were used to verify the molecular weights of the respective proteins. All blots were developed using enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). The images were captured by the UVP imaging system (UVP LLC, Upland, CA, USA) and quantified using ImageJ software (National Institutes of Health, Besthesda, MD, USA). For protein phosphorylation analyses, both the total and phospho-specific densities were quantified and the ratios of phospho-specific to total density calculated.
Statistical analysis
All data are presented as means ± SEM. Statistical analyses were performed with SigmaStat 11.0 software (Systat Software, Inc., San Jose, CA, USA), using either Student’s t test or ANOVA with post hoc analysis as appropriate. A P value < 0.05 was considered statistically significant.
Results
gAd restores endothelial function despite its blunted vasodilatory action in small resistance arteries from HFD fed rats
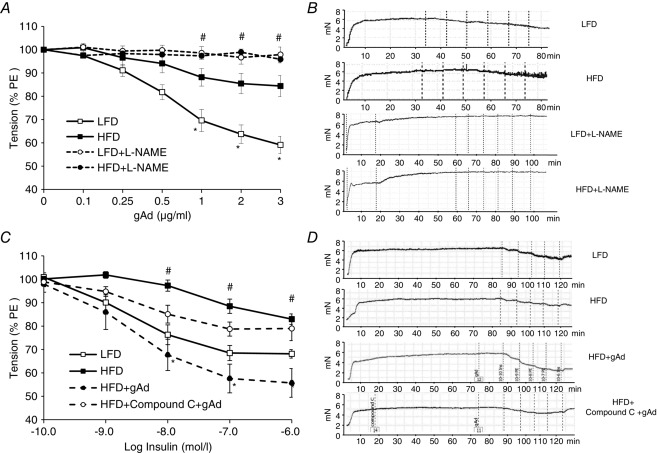
We first examined ex vivo the direct effects of gAd on the isolated distal saphenous artery (<250 μm) as it descends on the medial surface of the leg and at the distal part of the medial thigh muscles, where muscle microvascular perfusion was determined using CEU, a significant distribution of small vessels arises from the saphenous artery (Kochi et al. 2013). As shown in Fig.1, gAd dose-dependently relaxed the arterial ring isolated from LFD rats but this effect was significantly blunted (by ∼50%, P < 0.05) in arteries from HFD rats, confirming that HFD indeed induces resistance to gAd’s direct vasodilatory action. Pretreatment of the arterial rings with l-NAME abolished gAd’s vasodilatory action in both LFD and HFD animals. Insulin induced a dose-dependent vasodilatation in LFD rat arterial rings but similarly this effect was impaired by HFD (∼50% less, P < 0.05). Pretreatment of the HFD rat arterial rings with gAd fully restored the vascular response to insulin and this action was totally blocked by AMPK inhibition with Compound C. Similarly gAd pretreatment improved the arterial vasodilatory response to ACh but not in the presence of Compound C (Fig.2A). Endothelium independent vasodilatation was not affected by HFD feeding (Fig.2B). Neither l-NAME nor Compound C altered arterial resting tension (Fig.1).
Figure 1.
gAd improves vascular response to insulin in HFD rats via AMPK-mediated pathway
Each rat was fed with either a HFD or LFD for 4 weeks. The distal saphenous artery was isolated, pre-constricted with phenylephrine (PE) and the vasodilatory responses to gAd (A and B) or insulin (C and D) with or without the pretreatment with gAd (1 μg ml−1) and/or Compound C (5 μm) were determined. *P < 0.05 compared with HFD control (ANOVA). #P < 0.05 compared with gAd + HFD. n = 6 each.
Figure 2.
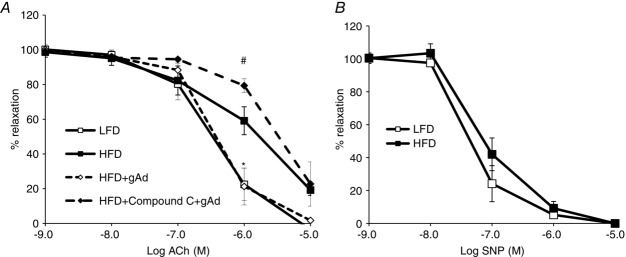
gAd restores endothelial responses to ACh in HFD rats
Each rat was fed either a HFD or LFD for 4 weeks. The distal saphenous artery was isolated, pre-constricted with phenylephrine, and the vasodilatory responses to ACh (A) in the presence or absence of gAd and/or Compound C, or sodium nitroprusside (SNP) (B) were determined. *P < 0.05 compared with LFD control (ANOVA). #P < 0.05 compared with HFD + gAd. n = 4–6 each.
HFD feeding abolishes gAd-induced microvascular recruitment
We have recently reported that gAd potently recruits muscle microvasculature in healthy insulin sensitive rats, which leads to increased insulin delivery to, and action in, muscle (Zhao et al. 2013). The above ex vivo findings led us to examine whether gAd’s microvascular actions were also blunted by HFD feeding. After 4 weeks of HFD feeding, rats received gAd intraperitoneally at a dose that potently recruits muscle microvasculature in chow-fed rats (Zhao et al. 2013). HFD feeding completely abolished gAd’s microvascular actions as evidenced by a lack of changes in muscle MBV (0.91 ± 0.15, 0.98 ± 0.07 and 0.98 ± 0.15 times baseline at 30, 60 and 120 min, respectively, P = 0.944), MFV (0.33 ± 0.03, 0.33 ± 0.02, 0.35 ± 0.03 and 0.31 ± 0.02 S−1 for 0, 30, 60 and 120 min, respectively, P = 0.764) and MBF (0.97 ± 0.23, 1.06 ± 0.16 and 0.97 ± 0.13 times baseline at 30, 60 and 120 min, respectively, P = 0.704), indicating microvascular resistance to gAd in these animals.
Adiponectin restores microvascular and improves metabolic responses of insulin in HFD fed rats via an AMPK-dependent pathway
As our ex vivo myograph study showed that the vasodilatory action of gAd was reduced but gAd remained effective in improving the vasodilatory effect of insulin in small resistance arteries isolated from rats fed a HFD, we next examined whether administration of gAd to HFD fed rats could restore microvascular insulin responses. Compared to LFD, 4 weeks of HFD feeding did not alter body weight, blood pressure, plasma cholesterol, triglycerides or glucose levels, but did significantly increase plasma insulin concentrations and decrease plasma adiponectin concentrations (Table1). Injection of gAd raised plasma adiponectin concentrations from 4.0 ± 0.3 μg ml−1 (0 min) to 6.7 ± 0.4 μg ml−1 (120 min) in HFD rats. Insulin infusion raised plasma insulin concentrations to 670.1 ± 26.8, 718.6 ± 27.4 and 692.9 ± 25.1 pm, respectively, in LFD, HFD and gAd + HFD groups (n = 5 each, P = 0.455).
Table 1.
Animal characteristics
| Body weight (g) | MAP (mmHg) | Glucose (mm) | Cholesterol (mm) | Triglyceride (mm) | Insulin (pm) | Adiponectin (μg ml−1) | |
|---|---|---|---|---|---|---|---|
| LFD | 434.33 ± 10.53 | 111.86 ± 1.99 | 4.92 ± 0.10 | 1.38 ± 0.11 | 0.46 ± 0.09 | 102.0 ± 10.92 | 5.74 ± 0.52 |
| HFD | 459.50 ± 15.39 | 112.43 ± 1.78 | 4.91 ± 0.10 | 1.59 ± 0.11 | 0.55 ± 0.08 | 139.8 ± 7.52* | 4.00 ± 0.31* |
Values are means ± SEM. LFD: low-fat diet; HFD: high-fat diet; MAP: mean arterial blood pressure. n = 5–6.
P < 0.05 compared with LFD group.
Similar to previous reports (Vincent et al. 2004; Zhao et al. 2013), insulin increased muscle MBV by ∼60% without altering MFV in LFD rats, which led to a marked increase in muscle MBF (P < 0.05). However, this insulin-mediated microvascular recruitment was totally abolished by HFD feeding (Fig.3), confirming microvascular insulin resistance in these animals. Administration of a single dose of gAd intraperitoneally before the insulin clamp completely restored insulin-mediated microvascular recruitment in HFD rats. Pretreatment with Compound C or l-NAME did not alter the microvascular responses to insulin, but totally abolished gAd-induced restoration of insulin responses in muscle microvasculature in HFD rats (Fig.3).
Figure 3.
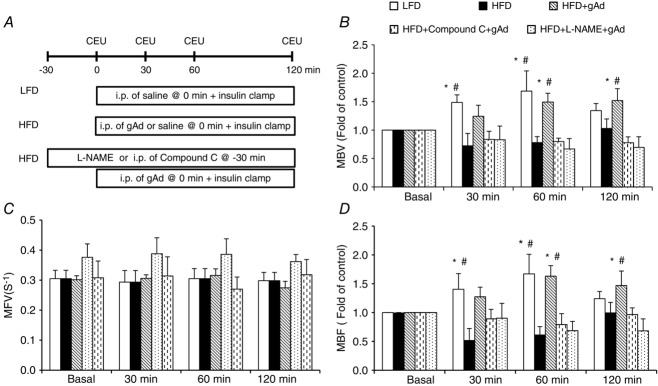
gAd restores microvascular response to insulin in HFD rats via AMPK-mediated pathway
Each rat was fed a HFD for 4 weeks and then received an i.p. injection of either gAd (0.4 μg (g body wt)−1) or saline followed by a 2 h euglycaemic hyperinsulinaemic clamp (3 mU kg−1 min−1) in the absence or presence of Compound C pretreatment (i.p. 10 μg (g body wt)−1) or l-NAME infusion. A, Study protocol. B, MBV. C, MFV. D, MBF. *P < 0.05 compared with LFD baseline (0 min); #P < 0.05 compared with HFD baseline (0 min). n = 4–6 each.
As shown in Fig.4, HFD feeding for 4 weeks decreased insulin-mediated whole body glucose disposal by ∼60% (P < 0.001). Giving gAd before the insulin infusion increased insulin-mediated whole-body glucose disposal by ∼30% over the entire clamp period (P < 0.001, ANOVA) and at the steady state (90–120 min; P < 0.05). This effect was again inhibited by Compound C pretreatment or simultaneous infusion of l-NAME.
Figure 4.
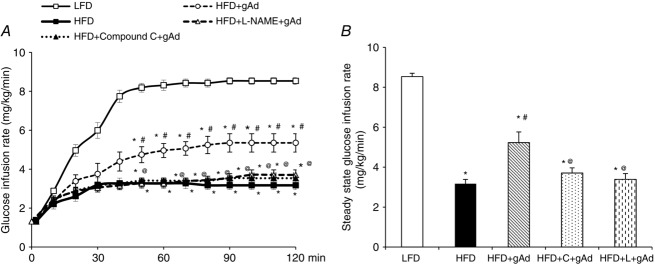
Administration of gAd ameliorates HFD-induced metabolic insulin resistance
Each rat was fed a HFD for 4 weeks and then received an i.p. injection of either gAd (0.4 μg (g body wt)−1) or saline followed by a 2 h insulin clamp (3 mU kg−1 min−1) in the absence or presence of compound C or l-NAME. LFD fed rats were used as control. A, time course of glucose infusion rate. B, steady state glucose infusion rate (C: Compound C; L: l-NAME). *P < 0.05 compared with control; #P < 0.05 compared with HFD; @P < 0.05 compared with gAd + HFD. n = 5–6.
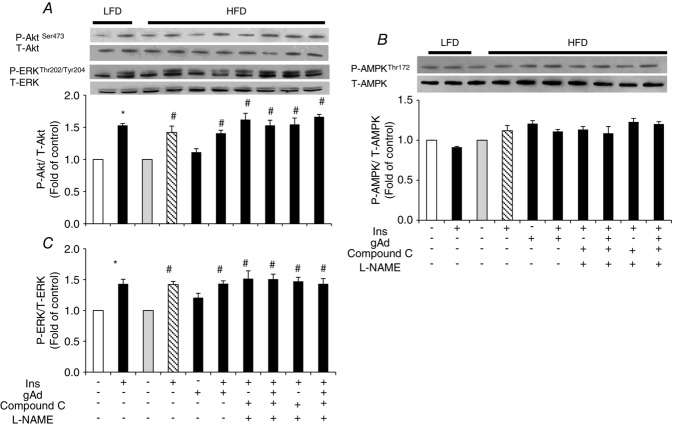
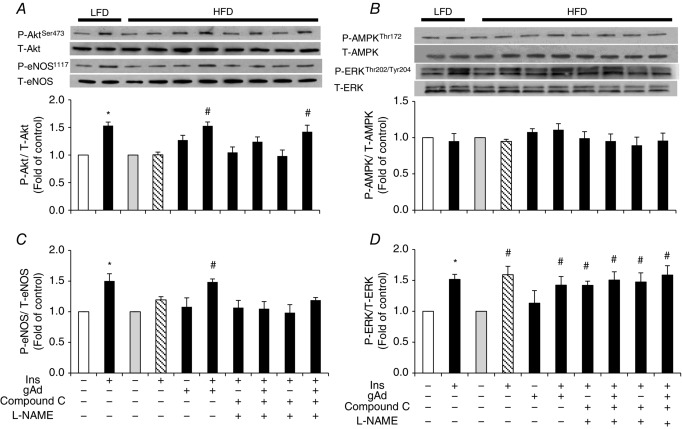
Adiponectin restores insulin-stimulated Akt and eNOS phosphorylation in aorta but not in muscle in HFD fed rats
The above results confirm the involvement of AMPK and NO in adiponectin restoration of insulin’s vascular responses in both the ex vivo small resistance artery and the in vivo microvascular studies. To further examine the underlying molecular mechanism, we compared the adiponectin and insulin signalling pathways in both metabolic tissue (muscle) and vasculature (aorta) between animals fed either a HFD or LFD. The protein expressions of AdipoR1, AdipoR2 and APPL1 were not altered by HFD feeding in either skeletal muscle or aorta (Fig.5). The insulin-stimulated Akt phosphorylation was not affected by HFD in muscle (Fig.6), but the insulin-stimulated Akt and eNOS phosphorylation was blunted in aorta (Fig.7) in HFD animals. gAd alone did not alter the phosphorylation of Akt, ERK1/2 or AMPK in either muscle or aorta from HFD rats. However, administration of a single dose of gAd before the insulin clamp markedly enhanced Akt and eNOS phosphorylation in aorta which is totally blocked by Compound C pretreatment. The insulin-stimulated ERK pathway was intact in both muscle and aorta tissue in HFD fed rats. Realizing that NO signalling may differ in small vs. large arteries, we also measured plasma NO concentrations in our in vivo studies. Insulin increased plasma NO concentrations in LFD rats by ∼33% (30.9 ± 2.4 μm vs.40.4 ± 2.0 μm, baseline vs. 120 min, P < 0.05). While insulin failed to increase plasma NO concentrations in HFD rats (28.6 ± 2.5 μm vs. 29.9 ± 3.2 μm, baseline vs. 120 min, P = 0.72), administration of gAd restored insulin-stimulated NO production to the levels seen in LFD rats (27.5 ± 1.1 μm vs. 43.9 ± 5.1 μm, baseline vs. 120 min, P < 0.05).
Figure 5.
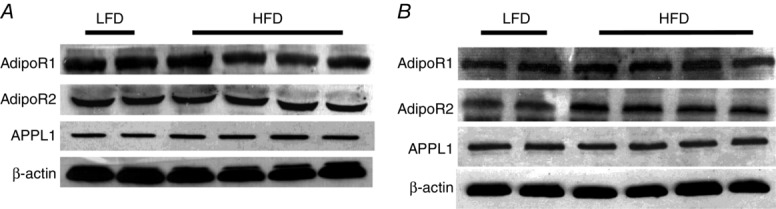
HFD has no effect on Adipo R1/2 and APPL1 protein expression in either muscle (A) or aorta (B)
Figure 6.
Protein phosphorylation in muscle of high-fat fed rats
A, Akt. B, AMPK. C, ERK1/2. *P < 0.05 compared with control; #P < 0.05 compared with HFD. n = 3–4. (ANOVA.) P = Phosphorylated; T = Total.
Figure 7.
Protein phosphorylation in aorta of HFD fed rats
A, Akt. B, AMPK. C, eNOS. D, ERK1/2. *P < 0.05 compared with control; #P < 0.05, compared with HFD. n = 3–4. (ANOVA.)
Discussion
In the current study, we aimed to examine the relationship between vascular function and metabolic insulin responses and how adiponectin’s vascular actions would affect insulin’s metabolic actions in muscle in the insulin resistant state. We used muscle to assess insulin’s metabolic action, muscle microvasculature to examine insulin’s microvascular action which actively regulates insulin’s metabolic action in muscle, and the distal saphenous artery, which feeds to the microvasculature, to examine vascular reactivity. We demonstrated that HFD feeding engenders both adiponectin and insulin resistance in the resistance arterioles and microvasculature while acute administration of gAd improves metabolic insulin sensitivity by improving AMPK-mediated restoration of microvascular insulin responses in HFD rats. These findings extend our previous report that gAd enhances insulin’s metabolic action in the insulin sensitive state via muscle microvascular recruitment and increased muscle delivery and action of insulin (Zhao et al. 2013), and provide a logical explanation to adiponectin’s insulin sensitization action in the insulin resistant state. It is important to note that variations according to sex exist in vascular function (Sader & Celermajer, 2002) and whether our findings obtained in male rats can be extrapolated to females warrants further investigation.
Adiponectin resistance is well recognized in liver, adipose tissue and muscle, and is closely associated with insulin resistance. Indeed, gAd responsiveness is significantly reduced in skeletal muscle from obese/diabetic patients (Bruce et al. 2005; Chen et al. 2005) and animals fed a HFD (Mullen et al. 2007, 2009), as well as in liver tissue from insulin receptor transgenic/knockout mice (Lin et al. 2007) or heart tissue from HFD-induced diabetic mice (Yi et al. 2011). Reduced vascular responsiveness to adiponectin has also been reported in the aorta from hyperlipidaemic rats (Li et al. 2007) and resistance arteries of Zucker diabetic fatty rats (Schmid et al. 2011). The significantly reduced vasodilatation in isolated saphenous artery and the lack of microvascular recruitment in vivo in HFD fed rats in response to gAd administration in the current study confirm the presence of adiponectin resistance in small resistance artery as well as the microvascular bed. The lack of gAd-induced AMPK phosphorylation in HFD animals in our study lends further evidence of adiponectin resistance in the insulin resistant state. Indeed, Mullen et al. found that HFD feeding could totally abolish the acute effect of gAd on AMPK phosphorylation in rat skeletal muscle in as early as 3 days (Mullen et al. 2009). It is very likely that in addition to hypoadiponectinaemia this adiponectin resistance in the microvasculature contributes to endothelial dysfunction and insulin resistance in diabetic humans and animals. Inasmuch as adiponectin activates AMPK, and we have in the current study shown that Compound C totally blocked adiponectin’s effects both ex vivo and in vivo (Figs1 and 3) without affecting microvascular parameters or blood pressure, we cannot entirely rule out the involvement of other kinase pathways in the gAd and HFD interaction due to the non-specific effect of Compound C on other kinases.
The mechanisms underlying vascular adiponectin resistance remain unclear. Adiponectin exerts its vasodilatory action via AdipoR1 and AdipoR2-mediated NO production through the AMPK–eNOS pathway (Kadowaki & Yamauchi, 2005; Nedvídková et al. 2005). It does not appear that HFD-induced vascular adiponectin resistance is due to altered AdipoR1 or AdipoR2 expression as these were unchanged in either muscle or aorta in our study. Others have also noted that muscle AdipoR1 expression did not change after 4 weeks of a HFD (Mullen et al. 2009) and that aortic AdipoR1/R2 expression did not change until 16 weeks of HFD feeding (Li et al. 2010). A previous study showed that HFD-treated mice had similar plasma adiponectin levels to those of control mice and AdipoR2 expression was lower in aorta of db/db mice compared with db/m+ mice (Wong et al. 2011). This discrepancy probably resulted from differences in species (rats vs. mouse) and disease models (diet model vs. genetic diabetic model). In addition, the expression of APPL1, an adaptor protein that is critical in the crosstalk between the adiponectin and insulin signalling pathways (Mao et al. 2006), was also not affected. This, coupled with the failure to increase AMPK and eNOS phosphorylation in aorta after gAd administration, strongly suggests that vascular adiponectin resistance occurs downstream of APPL1 and warrants further investigation. Caution should be exercised when extrapolating aortic protein expression data to small resistance arteries due to vascular heterogeneity in protein expression.
Despite the fact that the HFD induced resistance to the direct vasodilatory action of gAd in isolated distal saphenous artery, administration of gAd was able to restore the vasodilatory response to insulin. This is consistent with many previous reports demonstrating that hypoadiponectinaemia is closely associated with endothelial dysfunction, and adiponectin supplementation improves endothelial function in the insulin resistant states (Ohashi et al. 2006; Li et al. 2007; Lee et al. 2012). This ex vivo study also helps explain our in vivo findings that acute administration of gAd in HFD rats did not recruit muscle microvasculature but completely restored muscle microvascular insulin sensitivity, as insulin exerts its microvascular actions via an endothelium dependent pathway (Vincent et al. 2003). A recent study has also shown that addition of gAd significantly attenuates impaired perivascular adipose tissue control of insulin-induced vasoreactivity in muscle in db/db mice (Meijer et al. 2013).
Our findings that 4 weeks of HFD feeding significantly raised plasma insulin concentrations and decreased plasma adiponectin concentrations again confirm a reverse association of insulin sensitivity and hypoadiponectinaemia. It has also been reported by Mullen et al. that plasma adiponectin concentration is decreased after 3 days of HFD feeding in rats (Mullen et al. 2009). The finding that giving gAd prior to insulin infusion significantly increased insulin-mediated glucose disposal is certainly consistent with the literature suggesting that adiponectin is an insulin sensitizer. Indeed, replenishment of adiponectin via either injection or adenovirus-mediated overexpression improves insulin sensitivity in rats (Fruebis et al. 2001; Yamauchi et al. 2001, 2002, 2003; Satoh et al. 2005; Palanivel et al. 2007). Together with the observations that gAd administration restores insulin’s muscle microvascular responses and that inhibition of NO production abolishes insulin-mediated increase in whole body glucose disposal in HFD animals, our data strongly support our previous conclusion that gAd enhances insulin action in muscle via enhanced microvascular recruitment.
Though gAd administration completely restored insulin-mediated microvascular recruitment, insulin-stimulated whole body glucose disposal increased by only ∼30% and remained significantly lower than that in the LFD fed rats. This is certainly consistent with a previous report demonstrating that insulin’s microvascular action could contribute up to 40% of insulin-mediated glucose disposal during insulin clamp (Vincent et al. 2003). Indeed, insulin-mediated Akt phosphorylation did not change in muscle but significantly increased in aorta in HFD fed rats received gAd. This may reflect muscle adiponectin resistance as ample data have confirmed a direct action of gAd on muscle cells to increase GLUT4 translocation and glucose uptake and improve insulin actions in cultured muscle cells (Ceddia et al. 2005; Wang et al. 2007). Whether chronic administration of gAd directly improves the metabolic insulin sensitivity of muscle remains to be clarified.
Our observations that 4 weeks of HFD abolished insulin-mediated microvascular recruitment and decreased insulin-stimulated glucose disposal by ∼60% confirm the presence of both microvascular and metabolic insulin resistance in those animals. The close association of the microvascular and metabolic insulin resistance during HFD feeding once again demonstrates an important role of muscle microvasculature in mediating insulin’s metabolic action. We and others have confirmed that muscle microvasculature actively regulates insulin delivery and action by providing an endothelial exchange surface area (Vincent et al. 2003, 2004; Chai et al. 2011, 2012; Fu et al. 2013; Zhao et al. 2013). In addition, insulin stimulates its own trans-endothelial transport via its own signalling pathways in the endothelial cells (Wang et al. 2008). In the presence of insulin resistance, as seen in obesity or during systemic lipid or tumour necrosis factor α infusion, microvascular insulin responses are blunted (Clerk et al. 2006; Wang et al. 2011, 2013). This could significantly decrease insulin delivery to, and thus action in, muscle. Our findings that gAd improves endothelial function and restores insulin’s microvascular action are potentially clinically significant and provide a logical explanation to the insulin-sensitizing action of gAd in the insulin-resistant states.
In conclusion, HFD induces resistance to the vasodilatory actions of both gAd and insulin but administration of gAd restores endothelial function and improves metabolic insulin sensitivity in HFD animals via an AMPK-mediated restoration of microvascular insulin responses (Fig.8). Though acute adiponectin effects may bear no relation to chronic outcomes, and it is impractical to use adiponectin administration as a therapeutic modality, our results suggest that increasing endogenous adiponectin levels and/or enhancing the crosstalk between the adiponectin and insulin signalling pathways might have a therapeutic potential for improving metabolic insulin resistance and preventing cardiovascular complications associated with diabetes via modulating endothelial function and microvascular insulin sensitivity.
Figure 8.
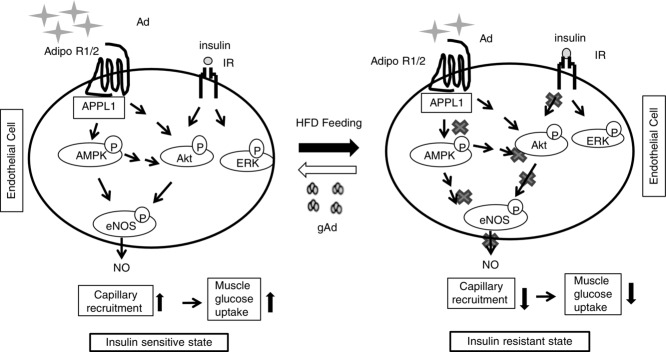
Schematic diagram showing the improvement of insulin sensitivity by gAd replenishment via
AMPK-mediated restoration of microvascular insulin response
Ad, adiponectin; AdipoR1/R2, adiponectin receptor 1/2; IR, insulin receptor. In the insulin sensitive state, adiponectin and insulin are able to stimulate endothelial NO production through the AdipoR1/2–APPL1–AMPK–eNOS and IR–Akt–eNOS pathways, respectively, leading to capillary recruitment and increased insulin delivery to muscle and thus increased muscle glucose uptake. In the insulin-resistant state, both adiponectin and insulin actions on endothelial cells are impaired, which results in reduced capillary response and decreased muscle insulin delivery and action (muscle glucose uptake). Replenishment of adiponectin could restore endothelial response to insulin.
Glossary
- AdipoR1
adiponectin receptor 1
- AdipoR2
adiponectin receptor 2
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- APPL1
adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1
- CEU
contrast-enhanced ultrasound
- eNOS
endothelial nitric oxide synthase
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- gAd
globular adiponectin
- HFD
high-fat diet
- LFD
low-fat diet
- l-NAME
l-NG-nitroarginine methyl ester
- MAP
mean arterial blood pressure
- MBF
microvascular blood flow
- MBV
microvascular blood volume
- MFV
microvascular blood flow velocity
- NO
nitric oxide
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
L.Z.: conception and design, collection and assembly of data, data analysis and interpretation and manuscript writing; Z.L.: conception and design, financial support, administrative support, collection and assembly of data, data analysis and interpretation, and manuscript writing; Z.F., J.W. and K.W.A.: collection and assembly of data, data analysis and interpretation. E.J.B. and W.C.: conception and design, and data analysis and interpretation. All authors provided final approval of the manuscript. All experiments were carried out at the University of Virginia.
Funding
This work was supported by American Diabetes Association grants 1-11-CR-30 and 1-15-CE-32 (to Z.L.), and National Institutes of Health Grants R01HL094722 and R01DK102359 (to Z.L.).
Translational perspective.
Adiponectin is a well-known insulin sensitizer. Hypoadiponectinaemia is closely associated with endothelial dysfunction and insulin resistance in patients with, or animal models of, obesity and diabetes. Recent evidence suggests that microvascular dysfunction contributes significantly to the development of metabolic insulin resistance. Whether adiponectin ameliorates metabolic insulin resistance by affecting muscle microvascular recruitment remains unknown. In the present study, we found that high-fat diet feeding causes microvascular resistance to both globular adiponectin and insulin but replenishment of adiponectin improves endothelial function and ameliorates metabolic insulin resistance by restoring AMPK-mediated microvascular insulin responses. This suggests that globular adiponectin might have a therapeutic potential for improving insulin resistance and preventing cardiovascular complications in patients with diabetes via modulation of microvascular endothelial function and insulin sensitivity.
References
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T. Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W. Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M. Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Mertz VA, Heigenhauser GJ. Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G. Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48:132–139. doi: 10.1007/s00125-004-1609-y. [DOI] [PubMed] [Google Scholar]
- Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W. Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wang W, Dong Z, Cao W. Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes. 2011;60:2939–2946. doi: 10.2337/db10-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wu Y, Li G, Cao W, Yang Z. Liu Z. Activation of p38 mitogen-activated protein kinase abolishes insulin-mediated myocardial protection against ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2008;294:E183–E189. doi: 10.1152/ajpendo.00571.2007. [DOI] [PubMed] [Google Scholar]
- Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O’Brien PE, Dixon JB, Cameron-Smith D, Kemp BE. Steinberg GR. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–3672. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR. Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S. Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Obici S, Scherer PE. Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh RT, Serné EH, IJzerman RG, de Vries G. Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W. Liu Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E222–E228. doi: 10.1152/ajpendo.00473.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE. Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Zhao L, Chai W, Dong Z, Cao W. Liu Z. Ranolazine recruits muscle microvasculature and enhances insulin action in rats. J Physiol. 2013;591:5235–5249. doi: 10.1113/jphysiol.2013.257246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K. Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004;89:1391–1396. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T. Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Inyard AC, Clerk LH, Vincent MA. Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes. 2007;56:2194–2200. doi: 10.2337/db07-0020. [DOI] [PubMed] [Google Scholar]
- Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC. Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- Kadowaki T. Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Kochi T, Imai Y, Takeda A, Watanabe Y, Mori S, Tachi M. Kodama T. Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PLoS One. 2013;8:e84047. doi: 10.1371/journal.pone.0084047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang H, Chen J, Dellsperger KC, Hill MA. Zhang C. Adiponectin abates diabetes-induced endothelial dysfunction by suppressing oxidative stress, adhesion molecules, and inflammation in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2012;303:H106–H115. doi: 10.1152/ajpheart.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wang WQ, Zhang H, Yang X, Fan Q, Christopher TA, Lopez BL, Tao L, Goldstein BJ, Gao F. Ma XL. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- Li R, Xu M, Wang X, Wang Y, Lau WB, Yuan Y, Yi W, Wei X, Lopez BL, Christopher TA, Wang XM. Ma XL. Reduced vascular responsiveness to adiponectin in hyperlipidemic rats–mechanisms and significance. J Mol Cell Cardiol. 2010;49:508–515. doi: 10.1016/j.yjmcc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE. Accili D. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 2007;56:1969–1976. doi: 10.2337/db07-0127. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu J, Jahn LA, Fowler DE. Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J. Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F. Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B, Richter EA, Smulders YM, van Hinsbergh VW, Serné EH. Eringa EC. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes. 2013;62:590–598. doi: 10.2337/db11-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, Wright D. Dyck DJ. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–R251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- Mullen KL, Smith AC, Junkin KA. Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–E90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- Nedvídková J, Smitka K, Kopský V. Hainer V. Adiponectin, an adipocyte-derived protein. Physiol Res. 2005;54:133–140. [PubMed] [Google Scholar]
- Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T. Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M. Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- Palanivel R, Fang X, Park M, Eguchi M, Pallan S, De Girolamo S, Liu Y, Wang Y, Xu A. Sweeney G. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75:148–157. doi: 10.1016/j.cardiores.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Sader MA. Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:597–604. doi: 10.1016/s0008-6363(01)00473-4. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE. Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- Schmid PM, Resch M, Steege A, Fredersdorf-Hahn S, Stoelcker B, Birner C, Schach C, Buechler C, Riegger GA, Luchner A. Endemann DH. Globular and full-length adiponectin induce NO-dependent vasodilation in resistance arteries of Zucker lean but not Zucker diabetic fatty rats. Am J Hypertens. 2011;24:270–277. doi: 10.1038/ajh.2010.239. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Barrett EJ, Lindner JR, Clark MG. Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S. Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark AD. Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes. 2002;51:3492–3498. doi: 10.2337/diabetes.51.12.3492. [DOI] [PubMed] [Google Scholar]
- Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ. Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282:7991–7996. doi: 10.1074/jbc.M700098200. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang AX, Liu Z. Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 2008;57:540–547. doi: 10.2337/db07-0967. [DOI] [PubMed] [Google Scholar]
- Wang N, Chai W, Zhao L, Tao L, Cao W. Liu Z. Losartan increases muscle insulin delivery and rescues insulin’s metabolic action during lipid infusion via microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E538–E545. doi: 10.1152/ajpendo.00537.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ko SH, Chai W, Li G, Barrett EJ, Tao L, Cao W. Liu Z. Resveratrol recruits rat muscle microvasculature via a nitric oxide-dependent mechanism that is blocked by TNF. Am J Physiol Endocrinol Metab. 2011;300:E195–E201. doi: 10.1152/ajpendo.00414.2010. α. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE. Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Wong WT, Tian XY, Xu A, Yu J, Lau CW, Hoo RL, Wang Y, Lee VW, Lam KS, Vanhoutte PM. Huang Y. Adiponectin is required for PPARγ-mediated improvement of endothelial function in diabetic mice. Cell Metab. 2011;14:104–115. doi: 10.1016/j.cmet.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Xing W, Yan W, Liu P, Ji L, Li Y, Sun L, Tao L, Zhang H. Gao F. A novel mechanism for vascular insulin resistance in normotensive young SHRs: hypoadiponectinemia and resultant APPL1 downregulation. Hypertension. 2013;61:1028–1035. doi: 10.1161/HYPERTENSIONAHA.111.00728. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB. Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R. Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P. Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, Wang Y, Yuan Y, Wang X, Tao L, Li R, Koch W. Ma XL. Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antioxid Redox Signal. 2011;15:1779–1788. doi: 10.1089/ars.2010.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chai W, Fu Z, Dong Z, Aylor KW, Barrett EJ, Cao W. Liu Z. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circ Res. 2013;112:1263–1271. doi: 10.1161/CIRCRESAHA.111.300388. [DOI] [PMC free article] [PubMed] [Google Scholar]