Abstract
We are witnessing the emergence of a novel type of biological regulation, namely, the communication between cells via their secreted substances, the secretome. This brief overview is based on the available published data and our own experience. We discuss 3 vignettes illustrating the importance of communication via the secretome: 1) the secretome of stem cells and its effects in sepsis and systemic inflammatory response; 2) the profibrotic secretomes partially responsible for development of fibrotic complications; and 3) the contribution of senescence-associated secretory products to the propagation of the senescence phenotype. Considering the richness of secretomes of different cells under diverse conditions, it becomes imperative to gain insights into their individual components in an attempt to harness cell secretomes for therapeutic purposes.
Keywords: proteome, sepsis, senescence, fibrosis
On this 50th anniversary of Ettore Majorana Foundation and Centre for Scientific Culture (EMFCSC), that has started as a meeting place for theoretical physicists, but later opened its doors to other sciences, it would be appropriate to discuss a subject of significant interdisciplinary interest, namely, the communication. The relationship between the local and the global regulatory factors is steadily gaining additional layers of complexity. This subject percolates through diverse fields of knowledge, from sociology to theoretical physics, where it is described as Mach’s Principle. In the field of biology the ideas of plants communicating with each other or the microbiome of the soil have deep historic roots and gave rise to the concept of allelopathy, study of plant-released chemicals that influence growth and survival of neighboring organisms. Positive and negative allelopathy is distinguished on the basis of whether these chemicals are supportive or harmful to the neighbors. Quite similar principles are operant in the field of communication in the animal kingdom and between various cells. The century-old ideas of the hierarchical regulation by the central nervous system and endocrine hormones describe the control mechanisms governing local functions through the central regulators. In parallel with those, there is growing awareness that the local regulators per se determine or modulate local functions and that these regulatory mechanisms can spill over to the distant organs to achieve the global impact. This latter trend was initiated by the discoveries of eicosanoids with their local and systemic organismal functions. More recently, a fledgling realization of the fact that secretory products of individual cells within an organ affect their own behavior, as well as that of the neighboring cells and distant organs has gained experimental recognition.
General concept of the secretome
In parallel with the completion of genome sequencing, a host of further “-omics” questions has emerged: What are the transcriptome, translatome or proteome, and how those affect the secretome of a particular cell (1). The authors of this 14-year-old original paper illustrated their case with the comparative analysis of transcriptome and translatome (http://bioinfo.mbb.yale.edu/what-is-it). The consensus based on this “–omics” tree defines the cell secretome as a portion of proteins secreted to the extracellular space, which in humans constitutes 13–20% of the entire proteome (2). More recently, proteins present in microvesicles (100nm-1um in diameter) and exosomes (<100nm in diameter), both organelles secreted by cells and containing up to 42% of the secretome (3) (see ExoCarta database of exosomal proteins in http://exocarta.ludwig.edu.au), have been incorporated into the original term. Obviously, the secretome of individual cells and tissues is specific, and this secretome signature changes in response to fluctuations in physiologic states or pathologic conditions. Not surprisingly, therefore, studies of the secretome are considered of significant importance as a way to discovery of diagnostic tools for cancer, infectious diseases, senescence-messaging, to name a few, and could serve as a pathfinder of therapeutic strategies and of stem cell adaptive transfer (4). The field, however, remains immature. Here, we shall describe a few examples of communication via the secreted substances (with the full understanding that these are mere vignettes representative of more numerous studies and our own interest) to illustrate recent advances in this type of information exchange. It should be mentioned that lipid mediators, as important as they are, do not strictly fit the definition of the secretome (part of the proteome) and are not discussed below.
Sepsis and effects of bone marrow stromal cells on macrophages
The switch of the cell secretome from a modest baseline to the activated state occurs in response to diverse pathological stressors. For instance, hypoxia induces a sharp increase in the release of several pro-angiogenic cytokines (HGF, VEGF, bFGF, PlGF and TGF-β) (5). LC-MS/MS analysis of the secretome of mesenchymal stem cells stimulated by TNF-α revealed increased expression of IL-6, IL-8, MCP-1, MMPs, PTX3 and cathepsin L (6). Some of the components of the secretome have cardioprotective effect, as judged by the improved ischemic heart function upon infusion of MSC-conditioned medium, even without MSC themselves (7). Administration of single cytokines, like G-CSF, GM-CSF, erythropoietin or IGF-1, however, was ineffective (8), arguing in favor of combination therapy.
Analysis of mechanisms responsible for the improved survival of mice with experimental sepsis and receiving transplantation of bone marrow-derived stromal cells revealed that these adoptively transferred cells secrete prodigious amounts of PGE2 (9). In turn PGE2 produced by transplanted cells activates prostaglandin EP1-EP4 receptors to reprogram infiltrating macrophages to increase their IL-10 production. This mechanism, according to Nemeth et al (9), underlies the protective effect of stem cell transplantation in sepsis.
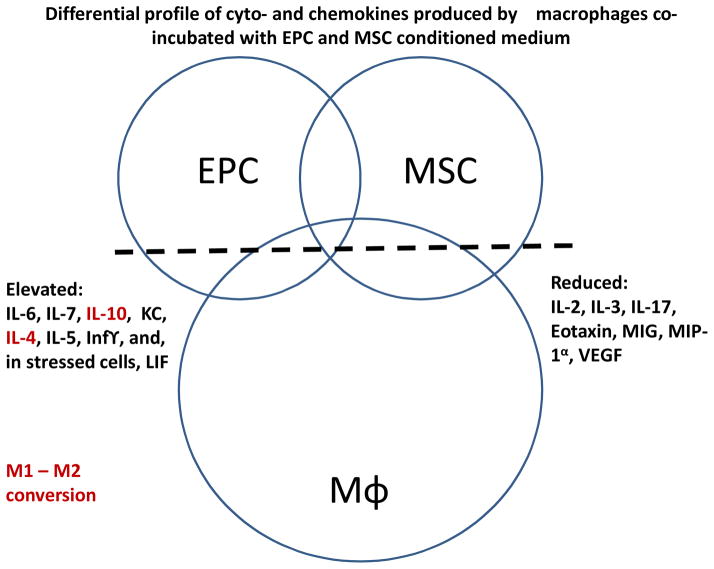
Our own observations (10) supplement this data with findings obtained in septic mice receiving a combination therapy with mesenchymal stem and endothelial progenitor cells, each acting via the secretome to elicit beneficial effect. The cyto-/chemokine release from embedded stem cells was examined including effects on modulating the polarization and release of proinflammatory molecules from macrophages (Fig. 1). EPC-MSC co-culturing improved stem cell viability during LPS exposure, an effect augmented by MSC hypoxic preconditioning. Delivery of co-embedded EPC with hypoxic preconditioned MSC to AKI mice demonstrated additive improvement (as compared to EPC delivery alone) in medullary RBF and proteinuria, with no differences observed for serum creatinine, MAP or angiogenesis. Exposure of proinflammatory M1 macrophages to EPC-MSC conditioned culture medium changed their polarization to anti-inflammatory M2 macrophages, while EPC-MSC delivery to endotoxemic mice elevated levels of circulating M2 macrophages. Incubation of co-embedded EPC-MSC with macrophages altered their release of cyto-/chemokines including enhanced release of anti-inflammatory IL-10 from macrophages (10).
Figure 1. Differential profile of the secreted cyto- and chemokines by macrophages alone or co-incubated with endothelial progenitor and mesenchymal stem cells.
Note that the secretion of interleukins -4 and -10 is elevated by co-incubation with stem cells, and this change in the secretome could be responsible for conversion of M1 to M2 macrophages.
Waves of secreted products in acute ischemic injury are responsible for the local and systemic inflammatory responses
Cell stress induces a rapid activation of xanthine oxidoreductase followed by increased generation of uric acid, one of the earliest “danger” signals (11, 12). Uric acid release after IRI unleashes a cascade of secondary events. The relatively fast damage response is accomplished via Toll-like receptors (TLR) -4 and -2-mediated exocytosis of Weibel-Palade bodies (12). Exocytosis of Weibel-Palade bodies represent the second wave of response to noxious stimuli. These endothelia-specific organelles contain von Willebrand factor, IL-8, angiopoietin-2, eotaxin, endothelin-1 and big endothelin-1 together with endothelin-converting enzyme, among other biologically active molecules. Exocytosis of Weibel-Palade bodies releases to the bloodstream these normally sequestered substances. Kuo et al documented release of angiopoietin-2, eotoxin and IL-8 to the systemic circulation. In general, exocytosis of Weibel-Palade bodies results in the release of components that possess either pro-inflammatory or pro-regenerative properties. The biological role of the pro-inflammatory components is dominant in the acute phase of an injury, whereas the role of the pro-regenerative components appears to play a role in the long-term outcome: inhibition of exocytosis of Weibel-Palade bodies, while ameliorating acute renal dysfunction after the insult, tends to exaggerate the chronic fibrotic consequences (13). Considering the plethora of biologically active constituents of Weibel-Palade bodies, it is possible that they can exert mutually opposing actions. It would be a future goal to analyze each of them separately and replenish those which are necessary for regeneration, while suppressing those which exacerbate inflammation.
Next, we reasoned that the surge in uric acid after acute renal injury may also lead to exocytosis of other internal storage pools and secretory lysosomes, the known vehicles for the release of high-mobility group box protein 1 (HMGB1). Previous findings (14–16) support the idea that HMGB1 is released from endothelial cells. HMGB1 is translocated from the nucleus to the cytoplasm and released from the cell upon stimulation by lipopolysaccharide (LPS) or TNF-α (14). We showed that in vivo intrarenal injection of uric acid, mimicking its post-ischemic surge, or renal ischemia-reperfusion insult lead to HMGB1 nucleo-cytoplasmic translocation and subsequent release from the cytosol into the circulation within one hour (17). Once released into the circulation, HMGB1 exerts potent cytokine-like pro-inflammatory effects (18). HMGB1 has been shown to interact with the receptor for advanced glycation end products, Toll-like receptors 4 and 2, TREM-1 and CD24 on target cells and lead to activation of NF-κB-mediated pathways (19.) HMGB1 is released passively from necrotic cells and actively by various stressed cells, such as monocytes, macrophages, and T-cells, all playing a role in the inflammatory response to injury (20). In studies of endotoxemia, neutralizing antibodies against HMGB1 prevented lethality (21). Release of HMGB1 has been reported after renal ischemia-reperfusion injury (IRI). Data from Lu’s laboratory (22) and neutralizing antibodies against HMGB1 offered significant protection against renal injury, as jusdged by the reduction in tubular cell apoptosis, blunted elevation of serum creatinine, BUN, TNFα expression, and pathologic manifestations of injury. We demonstrated (23) that the ischemic kidney is the primary source of a surge in HMGB1 in the circulation, that endothelial cells contribute to it, and that uric acid signaling can mimic this process both in vitro and in vivo. We showed that after ischemic insult, HMGB1 is released into the renal circulation within 1–3 hours (Fig 1b, c). Moreover, the surge in HMGB1 is capable of increasing several pro- and anti-inflammatory cytokines in the circulation and is in part responsible for aggravating acute kidney injury (AKI), as judged by the fact that prevention of nucleo-cytoplasmic translocation of HMGB1 ameliorates renal dysfunction. This is explained by the fact that once released into the circulation, HMGB1 acts as a DAMP. HMGB1 acts on macrophages to release TNF-α and IL-6 (21), stimulates the release of TNF-α, IL-1α, IL-1β, IL-6, IL-8, and MIP-1α (24). HMGB1 induces the release of TNFα, IL-8, G-CSF, and expression of adhesion molecules such as ICAM-1 and E-selectin in endothelial cells (25). One hour after infusion of exogenous HMGB1, levels of IL-6, IP-10 and MIP-1α surge in the plasma, followed, after 3 hours, by the increase in eotaxin and G-CSF plasma concentration; while IL-8 and IL-10 remain elevated during this time interval.
Recent reports have identified HMGB1 as a strong chemoattractant and a stimulator of proliferation for vessel-associated stem cells (i.e. EPC and mesoangioblasts) (26), as well as a mobilizing agonist for bone marrow stem cells, thus underscoring its potential pro-regenerative properties (27). Yet, the existing dichotomy of HMGB1 effects shows that its pro-inflammatory actions prevail over its pro-regenerative properties: inhibition of HMGB1 secretion by pretreatment with ethyl pyruvate ameliorates acute kidney injury (28).
Pro-fibrotic secretome
A decades-old observation that tissue fibrosis (i.e. the renal tubulointerstitial fibrosis) progresses hand-in-hand with the site-specific rarefaction of the microvasculature still has not found a molecular explanation. It is safe to assume that mutual communication, via secretory products, exists between fibroblasts and endothelial cells and this local mechanism maintains the viability and density of each compartment. Indeed, the most detailed so far proteomic studies by M. Mayr’s group (29) have revealed that up to 248 distinct proteins are secreted by cardiac fibroblasts and that activation by TGFβ affects secretion of 148 proteins. The follow-up studies from this group revealed that cardiac fibroblasts after myocardial infarction or activation by TGFβ show reduced expression of secreted microRNAs miR-29b leading to cardiac fibrosis. Normally, miR-29b is responsible for up-regulation of several proteins like multiple collagens, matrix metalloproteinses, leukemia-inhibitory factor, insulin-like growth factor-1, and pentraxin-3 and attenuates responses to TGFβ, all having a protective effect (29). In the case of liver sinusoidal endothelial cells, the balance between fibrotic and regenerative signaling is maintained by the expression of stromal-derived factor-1 receptors CXCR7 and CXCR4 (30). After an acute injury, upregulation of CXCR7 leads to Id-1-dependent induction of secretory angiocrine factors which stimulate regeneration, while chronic liver injury augments CXCR4 signaling and production of pro-fibrotic secretome. The role of microRNAs in promoting organ fibrosis (fibromiRs) is rapidly expanding (31). A list of fibromiRs includes miR-15 family, miR-21, miR-34a, miR-192, miR-199b, miR-208; whereas other miRs exhibit antifibrotic signaling, such as miR-1, miR-24, miR-29b, miR-101, miR-200b, among others.
In a series of recent studies our group has found that the extract of endothelial progenitor cells has the capacity to prevent TGFβ-induced activation of fibroblasts and their conversion to myofibroblasts, as well as prevent and reverse renal fibrosis in vivo (unpublished data). Multiplex analysis of the medium conditioned by endothelial progenitors revealed that it is highly enriched in the vascular endothelial growth factor and leukemia inhibitory factor. The contribution of each of these factors to halting transition to myofibroblasts is currently under the investigation.
Senescence-associated secretory phenotype
One of the sizable contributors to organismal aging is cell senescence. The direct confirmation of this thesis was obtained in progeroid mice subjected to elimination of p16Ink4a-positive senescent cells and exhibiting a delay in age-related dysfunction (32). One of the plausible explanations how a relatively few senescent cells can affect organ and organismal functions rests on the idea that these cells secrete substances which could be deleterious to neighboring cells by amplifying pro-senescence signaling – the concept of senescence-associated secretory phenotype (SASP) (33). The main components of SASP include IL-6, IL-8, TNF-α, TGF-β, matrix metalloproteinases, IGF-binding proteins, monocyte chemoattractant protein-1 (MCP-1), PAI-1, among many other components which vary depending on the cell type (34). SASP affords both the autocrine and the paracrine effects, which are important for diverse pathological processes from cancer progression to immunomodulation, changes in cell microenvironment, and propagation of cellular senescence (35). SASP is responsible at least in part for the sterile systemic inflammation, one of the cardinal features of aging. At the initial stages of this inflammatory response activation of inflammasomes leads to activation of caspase-1 (aka IL-1-converting enzyme) and amplification of IL-1 signaling (36). IL-1 in turn activates NF-kB and C/EBP-β transcription factors, which lead to the induction of IL-6 and IL-8. Importantly, Kuilman et al have shown that by depleting IL-6 it was possible to ameliorate inflammatory SASP network (37). Pharmacological design of future senolytic therapies should target elements of SASP (35.)
Interim conclusions
Earlier discoveries in the field of local and systemic signaling by secreted eicosanoids have recently been expanded to outline the broad spectrum of secreted cellular products capable of autocrine and paracrine regulation of diverse cellular functions. As with the plants, these secreted signals may affect neighboring cells in the positive and negative ways. Obviously, the functional usefulness of secretomes explains their evolutionary conservation. The authors clearly recognize that this review is by no means exhaustive. A few vignettes provided above (and selected based on their appeal to the authors and authors’ own contributions) illustrate the role of different secretomes in beneficial effects of stem/progenitor cells in sepsis, pro-inflammatory and pro-regenerative secretomes participating in “danger” signaling, senescence-associated secretory products participating in the propagation of cell senescence to neighboring cells, and secreted substances that can either facilitate or repress programs responsible for organ fibrosis. Considering the richness of secretomes of different cells under diverse conditions, it becomes imperative to gain insights into their individual components in an attempt to harness cell secretome for therapeutic purposes.
Highlights.
Studies of secreted products by individual cells under distinct conditions are in their infancy. Pioneered by the research in the field of prostaglandins, this field has moved toward studies of secreted protein components – the secretome. Here we illustrate some facets of the secretome of stem cells which insure their beneficial effect in endotoxemia, or anti-fibrotic effect of the secretome of endothelial progenitor cells, or influence of the secretome of senescent cells on their neighboring cells. There is increasing recognition that studies of the secretome hold a key to better diagnostic and therapeutic tools.
Acknowledgments
Studies in the authors’ laboratory were supported by NIH grants DK54602, DK052783 and DK45462 (MSG); AHA grant 12SDG9080006 and ASN grant 010973-101 (BR); and the Westchester Artificial Kidney Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LIST OF REFERENCES
- 1.Greenbaum D, Luscombe N, Jansen R, Quian J, Gerstein M. Genome Res. 2001;11:1463–1468. doi: 10.1101/gr.207401. [DOI] [PubMed] [Google Scholar]
- 2.Mathivanan S, Simpson R. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee P, Mani S. Methodologies to decipher the cell secretome. Biochem Biophys Acta. 2013;1834:2226–2232. doi: 10.1016/j.bbapap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranganath S, Levy O, Inamdar M, Karp J. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinnaird T, Stabile E, Burnett M, Lee C, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Kim J, Kim M, Bae Y, Ryu S, Lee T, Kim J. Proteomic analysis of TNF-α-induced secretome of human adipose tissue-derived mesenchymal stem cells. J Proteome Res. 2010;9:1754–1762. doi: 10.1021/pr900898n. [DOI] [PubMed] [Google Scholar]
- 7.Beohar N, Rapp J, Pandya S, Losordo D. Rebuilding the damaged heart: the potential of cytokines and growth factors in the treatment of ischemic heart disease. J Am Coll Cardiol. 2010;56:1287–1297. doi: 10.1016/j.jacc.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranganath S, Levy O, Inamdar M, Karp J. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth K, Leelahavanichkul A, Yuen P, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their IL-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zullo JA, Nadel EP, Rabadi MM, Baskind MJ, Rajdev MA, Demaree CM, Vasko R, Chugh SS, Lamba R, Goligorsky MS, Ratliff BB. The secretome of hydrogel co-embedded EPCs and MSCs instruct macrophage polarization. Stem Cells. doi: 10.5966/sctm.2014-0111. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol. 2007 May;18(5):1516–24. doi: 10.1681/ASN.2006070759. [DOI] [PubMed] [Google Scholar]
- 12.Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, Addabbo F, Goligorsky MS. Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. Journal of the American Society of Nephrology. 2008;19:2321–2330. doi: 10.1681/ASN.2007111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasuda K, Vasko R, Hayek P, Ratliff B, Bicer H, Mares J, Maruyama S, Bertuglia S, Mascagni P, Goligorsky MS. Functional consequences of inhibiting exocytosis of Weibel-Palade bodies in acute renal ischemia. American journal of physiology Renal physiology. 2012;302:F713–721. doi: 10.1152/ajprenal.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullins GE, Sunden-Cullberg J, Johansson AS, Rouhiainen A, Erlandsson-Harris H, Yang H, Tracey KJ, Rauvala H, Palmblad J, Andersson J, Treutiger CJ. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scandinavian journal of immunology. 2004;60:566–573. doi: 10.1111/j.0300-9475.2004.01518.x. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. European journal of biochemistry/FEBS. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi ME, Beltrame M. Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO reports. 2000;1:109–114. doi: 10.1093/embo-reports/kvd030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabadi MM, Kuo MC, Ghaly T, Rabadi SM, Weber M, Goligorsky MS, Ratliff BB. Interaction between uric acid and HMGB1 translocation and release from endothelial cells. American journal of physiology Renal physiology. 2012;302:F730–741. doi: 10.1152/ajprenal.00520.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. American journal of physiology Cell physiology. 2003;284:C870–879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of biological chemistry. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 20.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. The EMBO journal. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 22.Lu CY, Hartono J, Senitko M, Chen J. The inflammatory response to ischemic acute kidney injury: a result of the ‘right stuff’ in the ‘wrong place’? Current opinion in nephrology and hypertension. 2007;16:83–89. doi: 10.1097/MNH.0b013e3280403c4e. [DOI] [PubMed] [Google Scholar]
- 23.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol. 2013 Mar;24(4):529–36. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 24.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. The Journal of experimental medicine. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. The Journal of cell biology. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, Iinuma S, Saga K, Nimura K, Shimbo T, Umegaki N, Katayama I, Miyazaki J, Takeda J, McGrath JA, Uitto J, Kaneda Y. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6609–6614. doi: 10.1073/pnas.1016753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabadi MM, GT, Goligorsky MS, Ratliff BB. HMGB1 in Renal Ischemic Injury. American Journal of Physiology - Renal Physiology. 2012 Sep 15;303(6):F873–85. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abonnenc M, Nabeebaccus A, Mayr U, et al. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ Res. 2013;113:1138–1147. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- 30.Ding B-S, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pottier N, Cauffiez C, Perrais M, Barby P, Mari B. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol Sci. 2014;35:119–126. doi: 10.1016/j.tips.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays aging-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrinello S, Coppe J, Crtoloca A, Campisi J. Stromal=-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland J. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acosta J, Banito A, Wuestefeld T, Georgilis A, et al. A complex secretory program orchestrated by inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuilman T, Michaloglou C, Vredeleld L, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]