Abstract
20-hydroxyeicosatetraenoic acid (20-HETE) is a metabolite of arachidonic acid that exhibits a myriad of biological effects in the vascular system. This review discusses the current knowledge related to the effects of 20-HETE on vascular reactivity, activation, and remodeling, as well as its role in vascular inflammation and angiogenesis. The information explaining how 20-HETE and the renin-angiotensin system interact to promote hypertension, vasoconstriction, and vascular dysfunction is summarized in this article. 20-HETE enhances vascular inflammation and injury in models of diabetes, ischemia/reperfusion, and cerebrovascular oxidative stress. Recent studies also established a role for 20-HETE in normal and pathological angiogenesis conditions. This review will also discuss the molecular mechanisms through which 20-HETE induces these vascular actions. Potential additional studies are suggested to address shortcomings in the current knowledge of 20-HETE in the vascular system.
Keywords: 20-HETE, renin-angiotensin system, vascular remodeling, vascular dysfunction, vascular inflammation, angiogenesis
Introduction
20-hydroxyeicosatetraenoic acid (20-HETE) is an eicosanoid that regulates a myriad of actions in the vascular system. It is synthesized through metabolism of arachidonic acid (AA) by cytochrome P450 (CYP) ω-hydroxylases. Several isoforms of CYP ω-hydroxylases, which are the main producers of 20-HETE, are expressed in humans, mice, and rats. A number of research groups have established a role for 20-HETE in the vascular system through the use of cell, animal, and human models.
The main focus of this review is to discuss the effects of 20-HETE in the vascular system and the mechanisms involved in these processes. 20-HETE has an integral interaction with the renin-angiotensin system leading to a feed-forward mechanism that perpetuates vascular dysfunction and hypertension. The mechanisms involving 20-HETE in vascular reactivity, activation, and remodeling have been extensively studied. Changes in 20-HETE production also regulate vascular inflammation in diabetes, ischemia/reperfusion and cerebrovascular oxidative stress injury. Several studies established that 20-HETE enhances angiogenesis in normal and pathological conditions. Here we summarize the literature related to these vascular actions of 20-HETE and what is known regarding the mechanisms through which 20-HETE regulates these processes. We also address currently unanswered questions that are of interest to further advance the understanding of the vascular actions of 20-HETE.
20-HETE Biosynthesis
20-HETE is derived from metabolism of AA by CYP ω-hydroxylases of the CYP4A and CYP4F subfamilies. Arachidonic acid is a polyunsaturated fatty acid that is a major component of membrane phospholipids. AA is liberated from the plasma membrane by phospholipase A2. To produce 20-HETE, the CYP ω-hydroxylases insert a hydroxyl group at the terminal sp3 carbon group of AA [1]. There are several isoforms of CYP4A/CYP4F responsible for the production of 20-HETE, which are summarized in Table 1. In humans, these isoforms are CYP4A11, CYP4A22, CYP4F2, and CYP4F3 [2–4]. The predominant 20-HETE synthase in humans is CYP4F2 followed by CYP4A11. CYP4F2 exhibits high activity in leukocytes and kidneys [4, 5]. In mice, the 20-HETE producing enzymes include CYP4A10 and CYP4A12 [6]; CYP4A12 is the primary 20-HETE synthase [6, 7]. In rats the 20-HETE producing enzymes include CYP4A1, CYP4A2, CYP4A3, and CYP4A8 [8–10].
Table 1.
Summary of CYP450 ω-hydroxylases that produce 20-HETE in humans, rats, and mice.
| Species | Cytochrome P450 ω-hydroxylases responsible for 20-HETE production |
| Human | CYP4A11 (CYP4A11); CYP4A22 (CYP4A22); CYP4F2 (CYP4F2); CYP4F3 (CYP4F3) |
| Rat | Cyp4a1 (CYP4A1); Cyp4a2 (CYP4A2); Cyp4a3 (CYP4A3); Cyp4a8 (CYP4A8) |
| Mouse | Cyp4a10 (CYP4A10); Cyp4a12 (CYP4A12) |
gene; (protein)
Various studies have revealed functional variants in both human CYP4F2 and CYP4A11. Population differences have been observed in the CYP4A11 loss-of-function variant 8590T>C with higher frequency being observed in African-American and some Japanese populations [11] . In vitro experiments have demonstrated that several human CYP4F2 variants result in reduced production of 20-HETE [12]. In contrast to these in vitro results, an in vivo study revealed that the CYP4F2 V433M polymorphism was associated with increased urinary excretion of 20-HETE [13]. These discrepancies could be due to different factors regulating 20-HETE production in humans as compared to isolated in vitro systems. Caution should be taken when comparing in vitro results to human populations.
Vascular synthesis and release of 20-HETE occurs primarily in vascular smooth muscle cells [14–20]. These cells are not the sole source of 20-HETE; it can arise from myeloid cells in the peripheral blood and bone marrow [21–23]. 20-HETE is also produced in human neutrophils and platelets [24]. Neutrophil and platelet 20-HETE production is increased by Ang II and endothelin-1 treatment [24]. Androgen is also a potent inducer of 20-HETE synthesis [25]. Interestingly, endothelial progenitor cells (EPC), which are involved in postnatal neovascularization, produce 20-HETE [26]. In contrast, vascular endothelial cells in most circulatory beds are devoid of 20-HETE synthase activity [27].
20-HETE and the Renin-Angiotensin System (RAS)
The renin-angiotensin system (RAS) serves a critical role in the regulation of blood pressure. The RAS is comprised of several components including renin, angiotensin-converting enzyme (ACE), and angiotensin II type 1 receptor (AT1R). Formation of the vasoactive octapeptide angiotensin II (Ang II) occurs through stepwise degradation of angiotensinogen. Angiotensinogen, which is primarily produced by the liver, is first converted to the decapeptide angiotensin I (Ang I) via the enzyme renin. Ang I is further cleaved by ACE to its vasoactive Ang II form. The vasomotor actions of Ang II are primarily via activation of the AT1R within the vasculature resulting in vasoconstriction and a variety of other vascular, renal, and fluid balance effects [28, 29].
Several studies document the complex interactions between the RAS and 20-HETE in hypertension. The release and synthesis of 20-HETE is induced by several autacoids including endothelin-1 [30–32] and Ang II [33, 34]. Ang II stimulates the synthesis and release of 20-HETE from isolated rat preglomerular microvessels to enhance the pressor effects of Ang II [35–37]. 20-HETE mediates the mitogenic [15, 33, 38–40] and vasoconstrictor effects of Ang II by mediating hypertrophy and hypertension through activation of the Ras/MAP kinase pathway [41]. Thus, inhibition of 20-HETE synthesis attenuates the renal pressure response to Ang II as well as inhibits the development of Ang II-dependent hypertension [42, 43]. Interestingly, Ang II’s actions on vascular cells parallel the biological actions of 20-HETE: stimulation of superoxide/ROS, NF-kB activation, and induction of inflammatory adhesion molecules (ICAM/VCAM) [44–52]. Conversely, recent studies identified 20-HETE as a potent inducer and transcriptional activator of endothelial ACE expression in microvascular endothelial cells [53, 54].
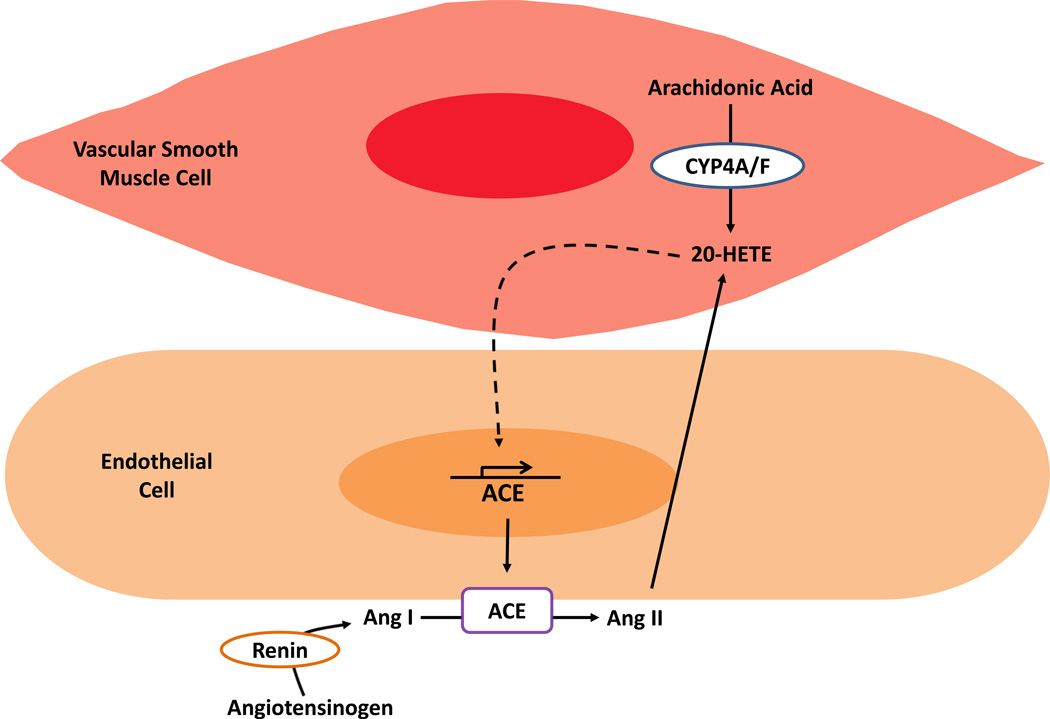
Animal models of hypertension that demonstrate increased vascular 20-HETE production are also RAS-mediated and –dependent. These models include the spontaneous hypertensive rat (SHR) [55, 56] and androgen-induced hypertension [17, 57, 58]. Androgen influences renal 20-HETE synthesis in spontaneously hypertensive rats [59]. Sprague-Dawley rats overexpressing CYP4A2 in the vascular endothelium exhibit increased 20-HETE production and hypertension [6, 17, 60]. The increase in blood pressure coincides with increased expression of vascular ACE and is normalized by ACE inhibition or AT1R blockade [53, 60]. These observations suggest a feed forward mechanism by which the 20-HETE axis and the RAS work in concert to promote vascular dysfunction and hypertension [61]. The interaction between the RAS and 20-HETE is depicted in Figure 1.
Figure 1. Diagram of the interaction between the Renin-Angiotensin System and 20-HETE.
Angiotensinogen is converted to Angiotensin I (Ang I) by the enzyme, renin. Ang I is cleaved by angiotensin-converting enzyme (ACE) to generate the vasoactive Angiotensin II (Ang II). 20-HETE is produced through the metabolism of arachidonic acid (AA) by enzymes of the CYP4A and CYP4F subfamilies. Ang II can also induce synthesis and release of 20-HETE. 20-HETE has also been shown to activate transcription of ACE in endothelial cells (dashed line). This 20-HETE/RAS interaction establishes a mechanism perpetuating Ang II and 20-HETE production.
Vascular Reactivity, Endothelial Dysfunction and Endothelial Activation in Response to 20-HETE
20-HETE sensitizes vascular smooth muscle cells to a variety of constrictor stimuli, including Ang II, phenylephrine and endothelin [18, 62, 63] through several mechanisms. 20-HETE sensitizes smooth muscle through inhibition of the large conductance Ca2+-activated K+ (BKCa) channels. Inhibition of BKCa channels depolarizes plasma membranes, increases Ca2+ entry through L-type Ca2+ channels, and elevates cytosolic [Ca2+] to potentiate vasoconstriction [15, 64, 65]. 20-HETE can also increase conductance of the L-type Ca2+ channels through PKC activation. In small coronary arteries, 20-HETE activates Rho kinase resulting in phosphorylation of myosin light chain (MLC20) to increase sensitivity of the vessel to Ca2+ [41]. 20-HETE not only induces vasoconstriction but also reduces vascular relaxation. For example, 20-HETE attenuates the relaxing response to acetylcholine in renal interlobar arteries pre-constricted with phenylephrine [66–68]. These vascular effects depend on the complex relationship between RAS and 20-HETE as concurrent pretreatment of vessels with 20-HETE and an ACE inhibitor (Lisinopril) or AT1R blocker (Losartan) attenuate 20-HETE’s effects [54].
Nitric oxide (NO) produced by the vascular endothelium is an important mediator in the defense against vascular injury and inflammation [69]. Nitric oxide, generated from the conversion of L-arginine by endothelial nitric oxide synthase (eNOS), is a potent endothelial-derived relaxing factor [70]. Diminished production or accelerated degradation of NO leads to endothelial dysfunction and directly increases vascular tone [71–73]. Several models of endothelial dysfunction linked to 20-HETE biosynthesis are known. Both androgen-induced, 20-HETE-dependent, hypertension and the endothelial overexpression of CYP4A2 in rats display endothelial dysfunction characterized by reduced vasodilation in response to acetylcholine, reduced NO levels, and increased superoxide anion levels [17, 58, 67]. These animal models are consistent with recent results from human studies. In patients with stable atherosclerotic cardiovascular disease, increased plasma 20-HETE levels were associated with reduced brachial artery flow mediated dilation [74] . These results are consistent with a previous study showing an inverse correlation between urinary excretion of 20-HETE and brachial artery flow-mediated dilation [75]. In hypertensive patients, a positive association was observed between urinary 20-HETE and oxidative stress [76]. The mechanisms behind these effects may be explained by in vitro experiments. In tissue culture, 20-HETE promotes endothelial dysfunction by uncoupling eNOS from its chaperone protein HSP90, decreasing NO production, and increasing superoxide anion [77]. The uncoupling of eNOS depends on the activation of an EGFR-, MAPK-, and IKK-dependent pathway. In contrast, 20-HETE increases NO production leading to vasodilation in the pulmonary vasculature, [78]. This difference may be attributed to intrinsic differences between the vascular beds. Further studies will be needed to elucidate the differences in these systems.
Gender differences in androgen levels have been linked to the development of hypertension and onset of cardiovascular disease in humans [79]. Data from mice suggests a complex relationship between androgens and ω -hydroxylases which result in androgen-induced hypertension. Male mice in which the androgen-sensitive Cyp4a14 gene has been disrupted have increased plasma androgen levels, Cyp4a12 expression, and urinary 20-HETE excretion. These mice exhibit androgen-sensitive hypertension due to increased CYP4A12-mediated 20-HETE production [80]. Androgens induce 20-HETE-dependent hypertension in Sprague-Dawley rats; however, concurrent treatment with 5a-dihydrotestosterone (DHT) and 20-HEDE, a 20-HETE antagonist, prevents the development of hypertension [81]. Further studies are needed to confirm a link between gender differences in androgens, 20-HETE, and cardiovascular disease in humans.
20-HETE promotes endothelial activation that involves the secretion of inflammatory cytokines (IL-8) [52, 82] and adhesion molecules (ICAM/VCAM) [44–52]. A recent study by Cheng et al. demonstrated that mice with endothelial specific overexpression of the human CYP4F2 have increased IL-6 levels, which is both NADPH oxidase- and 20-HETE- dependent [83]. Interestingly, aortas from Tie2-CYP4F2 transgenic mice displayed increased phenylephrine-induced vasoconstriction responses compared to WT mice, while the response to acetylcholine-induced relaxation remained unchanged [83].
20-HETE and Vascular Remodeling
Vascular remodeling is a structural reorganization of blood vessels that contributes to the development of hypertension. Remodeling is initiated by a variety of stimuli that induce collagen synthesis and deposition as well as reorganization of the vessel wall extracellular matrix (ECM). This process includes matrix metalloproteinases (MMPs) and inflammatory signals that render vessels stiffer and thicker to further exacerbate hypertension [84]. Increased media thickness and media-to-lumen ratio are two major hallmarks of vascular remodeling [85]. Arterial wall collagen synthesis increases as hypertension progresses in the spontaneous hypertensive rat (SHR) and DOCA-salt rat model. Ang II-induces an inflammatory program that includes the activation of transcription factors NF-kB and AP-1 to promote vascular remodeling [86–90]. In addition, Ang II induces expression of genes such as monocyte chemotactic protein 1 (MCP-1), ICAM-1 and VCAM-1 [91]. These proteins increase vascular remodeling by inducing recruitment and adhesion of monocytes/macrophages to the vascular wall.
Recent studies in rodents demonstrate that 20-HETE induces vascular remodeling in renal resistant arteries. The role of 20-HETE as a potent inducer of vascular remodeling is supported by results from a Cyp4a12 transgenic mouse. In this model, global expression of Cyp4a12, the dominant 20-HETE synthase in mice, is driven by a tetracycline-sensitive promoter. Treatment with doxycycline (DOX) drives CYP4A12 production and hypertension [92]. DOX-treated Cyp4a12 transgenic mice exhibit increased blood pressure and vascular remodeling, while mice treated with DOX+20-HEDGE (a 20-HETE antagonist) remain normotensive and lack any significant change in vascular remodeling [81]. A study by Ding et al. showed that 20-HETE-induced remodeling occurs independent of blood pressure elevation. DHT induces 20-HETE-dependent hypertension in Sprague-Dawley rats. However, the 20-HETE antagonist 20-HEDE prevents this hypertension and abrogates changes in media-to-lumen ratio, media thickness, and collagen IV deposition in renal interlobar arteries [81]. In this model, reserpine, an antihypertensive medicine, was able to reduce hypertension, but not reverse the vascular remodeling. Therefore, 20-HETE contributes to androgen-induced vascular remodeling independent of blood pressure elevation [81]. Further studies will be required to determine the role of the RAS in 20-HETE-dependent vascular remodeling and how induction of ACE serves to contribute to 20-HETE’s effects in the vasculature.
20-HETE and its Impact on Vascular Inflammation, Injury, and Disease
20-HETE is known to play a role in various aspects of vascular inflammation and injury. As previously mentioned, 20-HETE induces endothelial activation through upregulation of adhesion molecule and proinflammatory cytokine expression. Along with expression of proinflammatory cytokines, 20-HETE also impacts other aspects of vascular inflammation and injury in pathological/disease conditions.
In humans with diabetes associated with severe cardiac ischemia, a condition associated with endothelial damage and vascular inflammation, there is an increase in 20-HETE levels [93]. Diabetes is a disease in which blood glucose levels are high due to insufficient quantity of insulin or ineffective use of insulin to lower blood glucose. Interestingly, a recent article published by Li et al. showed that 20-HETE impairs endothelial insulin signaling by inducing phosphorylation of the insulin receptor substrate-1 (IRS-1) [94]. Aortas from mice treated with 20-HETE had increased ERK1/2 activation and impaired insulin-dependent activation of the IRS-1/PI3K/Akt/eNOS pathway that control the vasodilator effects of insulin [94]. Several studies have established a role of 20-HETE in diabetic nephropathy [95–97]; however, the specific mechanisms involving 20-HETE and its effect on the vascular system leading to progression of this disease are not completely understood. In a streptozotocin-induced diabetic rat model, renal hypertrophy was associated with increased CYP4A expression and 20-HETE production, increased fibronectin and TGF-β1 expression, as well as increased ROS and NADPH oxidase activity [96]. Gangadhariah et al. treated male mice with genetic disruption of Cyp4a14 with streptozotocin to induce diabetes [97]. This study determined that hypertension induced by 20-HETE is a key contributor to the progression of diabetes-induced kidney disease [97]. These studies enhance our understanding of how 20-HETE functions in insulin signaling and diabetes progression; however, further studies need to be performed to fully understand the role of 20-HETE in this disease.
The role of 20-HETE in vascular inflammation and/or injury has been studied with ischemia/reperfusion and balloon injury models. In a kidney ischemia/reperfusion injury model, treatment with the CYP4A/F inhibitor HET0016 or the 20-HETE antagonist 6,15,20-HEDE is protective; it reduces vascular inflammation, tubular injury, and loss of renal function [98]. The 20-HETE agonist 5,14-20-HEDE partially reversed these beneficial effects [98]. Het0016 also preserved organ function in a model of cerebral ischemia/reperfusion. Inhibition of 20-HETE biosynthesis increased blood-brain barrier function, reduced brain edema through decreased superoxide production and MMP-9/JNK pathway activation, and preserved tight junction integrity [99]. Another recent study used balloons to induce endothelial injury and neointimal growth in rat carotid arteries. It showed that the CYP4A enzyme and 20-HETE levels were increased in response to injury. Treatment of rats with HET0016 prior to balloon injury prevented the increase in 20-HETE levels, reduced vascular smooth muscle cell migration, and proliferation. These effects led to significant reduction of intimal hyperplasia and vascular remodeling [100]. These findings establish a role for 20-HETE in vascular injury; however, additional studies are needed to determine the mechanisms involved in 20-HETE mediated vascular inflammation and injury in these models.
20-HETE is also involved in cerebrovascular inflammation and injury. In a recent study, spontaneously hypertensive rats treated with HET0016 exhibited decreased oxidative stress in the middle cerebral arteries. Arteries showed reduced vascular NF-kB activation and reduced cerebrovascular inflammation as seen by reduced TNFα, IL-1β, and IL-6 mRNA expression [101]. These results suggest that inhibition of 20-HETE synthesis leads to a reduction in cerebrovascular inflammation. In vitro studies with cerebromicrovascular endothelial cells showed that 20-HETE treatment increased vascular ROS and NF-kB activation [101]. Together, these studies establish 20-HETE as a key regulator of vascular inflammation, injury, and disease.
20-HETE and its role in Angiogenesis
20-HETE is established as an initiator of angiogenesis in various vascular beds. Angiogenesis is the generation of blood vessels where endothelial cells sprout from an existing vessel and anastomose to form new complex vascular networks [102, 103]. This is an intricate process with interactions between tip cells, stalk cells, growth factors, and signaling pathways that coordinate and guide growing and migrating endothelial cells [104–106]. Coordination of blood vessel growth requires apical-basal polarization, cord hollowing, and lumen formation to generate an intricate vascular network [107–111]. The regulation of angiogenesis by CYP4A and 20-HETE was recognized several years ago [38, 39, 112]. More recent studies have determined the underlying mechanisms through which 20-HETE regulates angiogenesis that are summarized in Figure 2. The role of 20-HETE in both developmental and pathological neovascularization was recently described in an extensive review [113].
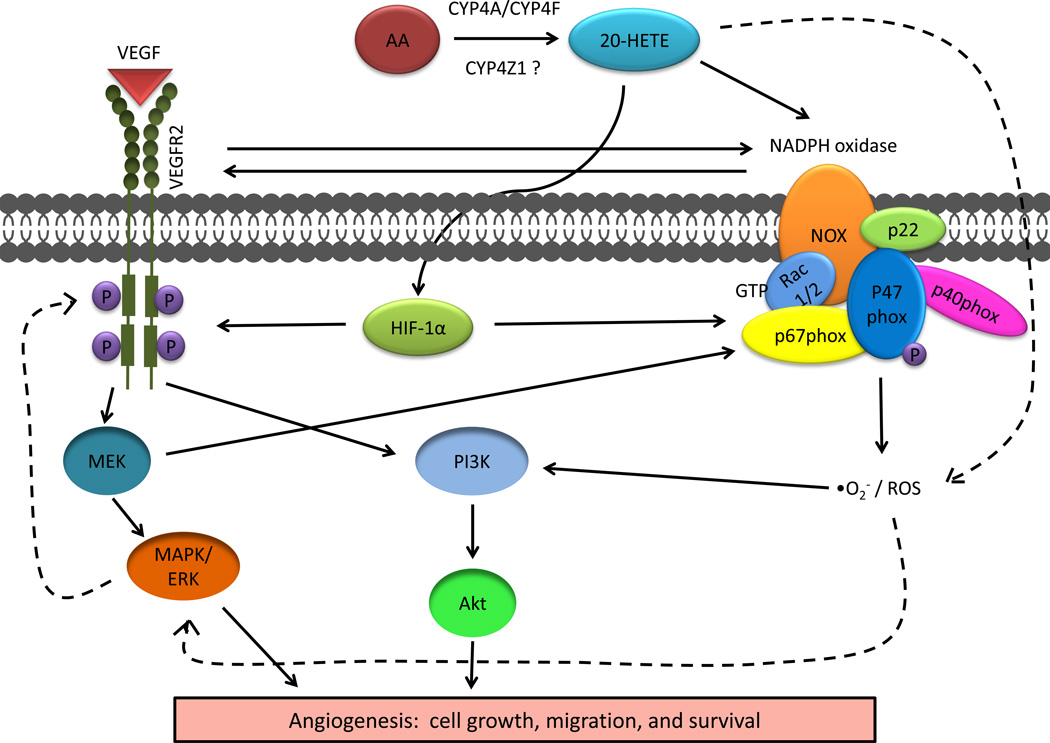
Figure 2. Schematic of signaling cascades involving 20-HETE in angiogenesis.
Arachidonic acid (AA) is metabolized to 20-HETE by cytochrome P450 (CYP) ω-hydroxylases of the CYP4A/CYP4F subfamilies. Recent evidence suggests that a novel family member, CYP4Z1, may also biosynthesize 20-HETE. 20-HETE acts through hypoxia-inducible factor (HIF)-1α and NADPH-oxidase via activation of Rac1/2 and phosphorylation of p47(phox) to increase vascular endothelial growth factor expression (VEGF) expression and phosphorylation of VEGF Receptor 2 (VEGFR2). VEGF signaling is activated leading to stimulated angiogenesis via MEK/MAPK/ERK and PI3K/Akt. In vitro evidence suggests that VEGF and MEK also partially regulate NADPH oxidase. Reactive oxygen species (ROS) are increased through a NADPH-dependent pathway resulting in activation of the PI3K signaling leading to angiogenesis. It is also thought that ROS are stimulated by a NADPH-independent mechanism resulting in activation of MAPK/ERK that leads to increased VEGF (dashed lines); however, this mechanism is not completely understood. The solid lines refer to well established pathways, whereas the dashed lines are indicative of pathways that are not completely characterized.
20-HETE induction of ROS is critical for initiating signaling cascades that regulate angiogenesis. In pulmonary arteries and pulmonary artery endothelial cells, 20-HETE enhances ROS via NADPH-dependent mechanisms to promote angiogenesis [114]. 20-HETE increases Rac1/2 activation and induces translocation and phosphorylation of the NADPH oxidase subunit p47(phox) to stimulate ROS formation [115].
Endothelial cell growth and survival are a critical components of angiogenesis that are regulated by 20-HETE. 20-HETE protects against apoptosis and increases cell survival in bovine pulmonary artery endothelial cells through activation of PI3-kinase and Akt pathways and ROS generation [114]. 20-HETE increases endothelial proliferation in both human macro- and microvascular endothelial cells through increased expression of key inducers of angiogenesis: vascular endothelial growth factor (VEGF) and phosphorylation of the VEGF Receptor 2 [44]. NADPH-independent activation of ROS by 20-HETE, in human macro- and microvascular endothelial cells, can also result in activation of MAPK to enhance VEGF expression [44]; however, the precise mechanism remains unclear.
The CYP4A/20-HETE system was recently identified as a novel regulator of endothelial progenitor cell (EPC) functions associated with angiogenesis [26]. In this study, VEGF and hypoxia induced CYP4A11 in endothelial progenitor cells leading to an increase in 20-HETE production in vitro. Inhibition of the 20-HETE system significantly reduced angiogenesis induced by endothelial progenitor cells in an in vivo matrigel plug assay [26]. Additional studies on the role of CYP4F-derived 20-HETE in this model could be performed to understand the contribution of these CYP ω-hydroxylase isoforms in angiogenesis.
Until recently, most studies have focused on understanding the angiogenic properties of CYP4A-derived 20-HETE, but ignored the role of CYP4F-derived 20-HETE. CYP4F2 is thought to be the enzyme responsible for the majority of 20-HETE production in humans [4]; therefore, it is important to study the impacts of this enzyme on 20-HETE induced angiogenesis. Cheng et al. showed that CYP4F2-derived 20-HETE also mediates angiogenic responses because mouse endothelial cells isolated from Tie2-CYP4F2 transgenic mice overexpressing human CYP4F2 exhibited increased growth and tube formation dependent on VEGF and NADPH oxidase [83]. Also, these endothelial cells exhibited a 20-HETE-dependent increase in HIF-1α and MAPK [83]. A VEGF neutralizing antibody partially decreased the level of NADPH oxidase in these cells suggesting that VEGF also regulates NADPH oxidase [83]. A MEK inhibitor decreased NADPH oxidase in the Tie2-CYP4F2 transgenic endothelial cells indicating that induction of NADPH is partially controlled by MEK signaling [83]. Additional studies are necessary to explore these angiogenic responses in vivo in mice with endothelial specific expression of human CYP4F2.
20-HETE increases angiogenesis to promote tumor growth and metastasis. CYP ω-hydroxylases facilitate growth and metastasis of human non-small cell lung cancer through PI3K signaling and angiogenic responses [116]. In human non-small cell lung cancer cells, a stable 20-HETE analog (WIT003) or overexpression of CYP4A11 induced invasion of the cells in a modified Boyden chamber assay associated with expression of VEGF and MMP-9 [116]. The induction of VEGF and MMP-9 were blocked by inhibitors of PI3K or ERK signaling. In an in vivo, tumor xenograft model, human non-small cell lung cancer cells expressing CYP4A11 showed an increase in tumor volume, microvessel density, and lung metastasis [116]. Another recent study indicated that breast cancer cells with overexpression of the novel CYP4 family member CYP4Z1 generated increased 20-HETE levels [117]. Media from CYP4Z1-expressing T47D and BT-474 breast cancer cells contained angiogenic factors: this media promoted proliferation, migration, and tube formation of human umbilical vein endothelial cells and promoted angiogenesis in zebrafish and chick embryos [116]. In human tumor xenografts, CYP4Z1 overexpression increased tumor weight and microvessel density [116]. Additional studies will need to be performed to definitively establish the connection between CYP4Z1 and 20-HETE in angiogenesis. While this review is more broadly associated with vascular components and 20-HETE, an extensive review summarizing the role of 20-HETE producing enzymes in cancer was published in 2013 [118].
Summary
Overall, 20-HETE, the product of ω-hydroxylation of AA, exhibits multiple effects on the vascular system through various signaling pathways. 20-HETE works in conjunction with the RAS to promote hypertension and vascular dysfunction. Vascular reactivity is enhanced by 20-HETE through sensitization to constrictor stimuli. 20-HETE promotes vascular remodeling through mechanisms independent of changes in blood pressure. The multiple signaling mechanisms affected by 20-HETE and the ability of 20-HETE agonists or antagonists to alter these pathways provide evidence that a 20-HETE receptor may exist; however, the receptor has yet to be identified. Studies have established a role for 20-HETE in vascular inflammation and injury in response to diabetes, ischemia/reperfusion, or cerebrovascular oxidative stress. Angiogenesis, including tumor angiogenesis, is promoted by 20-HETE. These studies have improved our understanding of 20-HETE and its effects on the vascular system; however, future studies will allow for refinement of our knowledge and enhanced understanding of the mechanisms involved in the vascular actions of 20-HETE. Additional studies are necessary to determine the role of RAS in 20-HETE-mediated vascular remodeling. Research related to the different CYP4A/F isoforms will give insight into the contributions of 20-HETE derived from the various isoforms on its actions in various vascular beds. Studies with human disease and animal models involving the vasculature will allow for further understanding of the mechanisms involved in 20-HETE signaling in the vascular system.
Highlights.
20-HETE acts in a feed forward mechanism with the renin-angiotensin system to perpetuate hypertension and vascular dysfunction.
20-HETE sensitizes smooth muscle to constrictor stimuli by inhibiting large conductance Ca2+-activated K+ channels and increasing Ca2+ entry through L-type Ca2+ channels.
20-HETE induces vascular remodeling through mechanisms independent of changes in blood pressure.
20-HETE enhances vascular inflammation and injury in diabetes and in models of ischemia/reperfusion and cerebrovascular oxidative stress.
20-HETE induces angiogenesis via increased production of reactive oxygen species (ROS). 20-HETE increases ROS though NADPH-oxidase and Rac1/2-dependent mechanisms and increased expression of VEGF and VEGFR2.
Acknowledgments
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES025034 to D.C.Z.), NIH HL034300 (to M.L.S.) and a Diversity Supplement Award HL34300-26A1S1 (to V.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts to declare.
References
- 1.O'Donnell VB, Maskrey B, Taylor GW. Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol. 2009;462:5–23. [PubMed] [Google Scholar]
- 2.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 3.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 4.Powell PK, et al. Metabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther. 1998;285(3):1327–1336. [PubMed] [Google Scholar]
- 5.Lasker JM, et al. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem. 2000;275(6):4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 6.Muller DN, et al. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403(1):109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa K, et al. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116(6):1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura S, et al. The rat clofibrate-inducible CYP4A gene subfamily. I. Complete intron and exon sequence of the CYP4A1 and CYP4A2 genes, unique exon organization, and identification of a conserved 19-bp upstream element. DNA. 1989;8(7):503–516. doi: 10.1089/dna.1.1989.8.503. [DOI] [PubMed] [Google Scholar]
- 9.Kimura S, et al. The rat clofibrate-inducible CYP4A subfamily. II. cDNA sequence of IVA3, mapping of the Cyp4a locus to mouse chromosome 4, and coordinate and tissue-specific regulation of the CYP4A genes. DNA. 1989;8(7):517–525. doi: 10.1089/dna.1.1989.8.517. [DOI] [PubMed] [Google Scholar]
- 10.Stromstedt M, et al. Cloning and characterization of a novel member of the cytochrome P450 subfamily IVA in rat prostate. DNA Cell Biol. 1990;9(8):569–577. doi: 10.1089/dna.1990.9.569. [DOI] [PubMed] [Google Scholar]
- 11.Lino Cardenas CL, et al. Arachidonic acid omega-hydroxylase CYP4A11: inter-ethnic variations in the 8590T>C loss-of-function variant. Mol Biol Rep. 2012;39(2):1503–1508. doi: 10.1007/s11033-011-0888-x. [DOI] [PubMed] [Google Scholar]
- 12.Stec DE, et al. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics. 2007;30(1):74–81. doi: 10.1152/physiolgenomics.00003.2007. [DOI] [PubMed] [Google Scholar]
- 13.Ward NC, et al. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51(5):1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 14.Gebremedhin D, et al. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507:771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JM, et al. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56(4):336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu F, et al. Catalytic activity and isoform-specific inhibition of rat cytochrome p450 4F enzymes. J Pharmacol Exp Ther. 2004;308(3):887–895. doi: 10.1124/jpet.103.059626. [DOI] [PubMed] [Google Scholar]
- 17.Singh H, et al. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50(1):123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 18.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41(4):175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 19.Harder DR, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266(5 Pt 2):H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 20.Parmentier JH, et al. Evaluation of cytochrome P450 4 family as mediator of phospholipase D activation in aortic vascular smooth muscle cells. Life Sci. 2005;77(9):1015–1029. doi: 10.1016/j.lfs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Christmas P, et al. Alternative splicing determines the function of CYP4F3 by switching substrate specificity. J Biol Chem. 2001;276(41):38166–38172. doi: 10.1074/jbc.M104818200. [DOI] [PubMed] [Google Scholar]
- 22.Hill E, Murphy RC. Quantitation of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) produced by human polymorphonuclear leukocytes using electron capture ionization gas chromatography/mass spectrometry. Biol Mass Spectrom. 1992;21:249–253. doi: 10.1002/bms.1200210505. [DOI] [PubMed] [Google Scholar]
- 23.Rosolowsky M, Falck JR, Campbell WB. Metabolism of arachidonic acid by canine polymorphonuclear leukocytes synthesis of lipoxygenase and omega-oxidized metabolites. Biochim Biophys Acta. 1996;1300(2):143–150. doi: 10.1016/0005-2760(95)00238-3. [DOI] [PubMed] [Google Scholar]
- 24.Tsai IJ, et al. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol. 2011;300(4):H1194–H1200. doi: 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96(1–4):45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, et al. 20-HETE regulates the angiogenic functions of human endothelial progenitor cells and contributes to angiogenesis in vivo. J Pharmacol Exp Ther. 2014;348(3):442–451. doi: 10.1124/jpet.113.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng J, et al. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J Pharmacol Exp Ther. 2010;332(1):57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le TH, Coffman TM. Targeting genes in the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2008;17(1):57–63. doi: 10.1097/MNH.0b013e3282f2fd39. [DOI] [PubMed] [Google Scholar]
- 29.Cvetkovic B, Sigmund CD. Understanding hypertension through genetic manipulation in mice. Kidney Int. 2000;57(3):863–874. doi: 10.1046/j.1523-1755.2000.057003863.x. [DOI] [PubMed] [Google Scholar]
- 30.Hercule HC, Oyekan AO. Cytochrome P450 omega/omega-1 hydroxylase-derived eicosanoids contribute to endothelin(A) and endothelin(B) receptor-mediated vasoconstriction to endothelin-1 in the rat preglomerular arteriole. J Pharmacol Exp Ther. 2000;292:1153–1160. [PubMed] [Google Scholar]
- 31.Oyekan A, Balazy M, McGiff JC. Renal oxygenases: differential contribution to vasoconstriction induced by ET-1 and ANG II. Am J Physiol. 1997;273(1 Pt 2):R293–R300. doi: 10.1152/ajpregu.1997.273.1.R293. [DOI] [PubMed] [Google Scholar]
- 32.Oyekan AO, McGiff JC. Cytochrome P-450-derived eicosanoids participate in the renal functional effects of ET-1 in the anesthetized rat. Am J Physiol. 1998;274(1 Pt 2):R52–R61. doi: 10.1152/ajpregu.1998.274.1.R52. [DOI] [PubMed] [Google Scholar]
- 33.Imig JD, et al. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270(1 Pt 2):R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 34.Carroll MA, et al. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol. 1996;271(4 Pt 2):R863–R869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- 35.Carroll MA, et al. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51:1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- 36.Croft KD, et al. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 37.Alonso-Galicia M, et al. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 38.Amaral SL, et al. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2003;284(5):H1528–H1535. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- 39.Jiang M, et al. Smooth muscle--specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circ Res. 2004;94(2):167–174. doi: 10.1161/01.RES.0000111523.12842.FC. [DOI] [PubMed] [Google Scholar]
- 40.Muthalif MM, et al. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1998;95(21):12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 42.Alonso-Galicia M, et al. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 43.Chabova VC, et al. Effects of chronic cytochrome P-450 inhibition on the course of hypertension and end-organ damage in Ren-2 transgenic rats. Vascul Pharmacol. 2007;47(2–3):145–159. doi: 10.1016/j.vph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Guo AM, et al. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321(1):18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- 45.Guo AM, et al. 20-HETE can act as a nonhypoxic regulator of HIF-1alpha in human microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(2):H602–H613. doi: 10.1152/ajpheart.00874.2008. [DOI] [PubMed] [Google Scholar]
- 46.Huang XC, Richards EM, Sumners C. Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J Biol Chem. 1996;271(26):15635–15641. doi: 10.1074/jbc.271.26.15635. [DOI] [PubMed] [Google Scholar]
- 47.Ishizaka N, et al. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation. 1994;96:1923–1929. doi: 10.1161/01.cir.96.6.1923. [DOI] [PubMed] [Google Scholar]
- 48.Landmesser U, Drexler H. Effect of angiotensin II type 1 receptor antagonism on endothelial function: role of bradykinin and nitric oxide. J Hypertens Suppl. 2006;24(1):S39–S43. doi: 10.1097/01.hjh.0000220405.38622.23. [DOI] [PubMed] [Google Scholar]
- 49.Mollnau H, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90(4):E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 50.Morgan-Boyd R, et al. Effects of bradykinin and angiotensin II on intracellular Ca2+ dynamics in endothelial cells. Am. J. Physiol. 1987;253:C588–C598. doi: 10.1152/ajpcell.1987.253.4.C588. [DOI] [PubMed] [Google Scholar]
- 51.Wolf G, Neilson EG. Angiotensin II as a hypertrophogenic cytokine for proximal tubular cells. Kidney Int Suppl. 1993;39:S100–S107. [PubMed] [Google Scholar]
- 52.Ishizuka T, et al. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324(1):103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 53.Sodhi K, et al. CYP4A2-Induced Hypertension Is 20-Hydroxyeicosatetraenoic Acid- and Angiotensin II-Dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng J, et al. Induction of Angiotensin-Converting Enzyme and Activation of the Renin-Angiotensin System Contribute to 20-Hydroxyeicosatetraenoic Acid-Mediated Endothelial Dysfunction. Arterioscler Thromb Vasc Biol. 2012;32(8):1917–1924. doi: 10.1161/ATVBAHA.112.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sacerdoti D, et al. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243(4889):388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 56.Dunn KM, et al. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295(6):H2455–H2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holla VR, et al. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98(9):5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu CC, et al. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension. 2011;57(4):788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishizuka T, et al. [Role of androgens in the renal production of 20-hydroxyeicosatetraenoic acid in spontaneously hypertensive rats] Nihon Jinzo Gakkai Shi. 2004;46(7):685–692. [PubMed] [Google Scholar]
- 60.Inoue K, et al. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol. 2009;297(4):F875–F884. doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imig JD. 20-hydroxyeicosatetraenoic acid and angiotensin: a positive feedback system to cause hypertension. Hypertension. 2010;56(5):822–823. doi: 10.1161/HYPERTENSIONAHA.110.156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, et al. Transfection of CYP4A1 cDNA decreases diameter and increases responsiveness of gracilis muscle arterioles to constrictor stimuli. Am J Physiol Heart Circ Physiol. 2004;287(3):H1089–H1095. doi: 10.1152/ajpheart.00627.2003. [DOI] [PubMed] [Google Scholar]
- 63.Zhang F, et al. Modulation by 20-HETE of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;38(6):1311–1315. doi: 10.1161/hy1201.096116. [DOI] [PubMed] [Google Scholar]
- 64.Zou AP, et al. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am.J.Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 65.Gebremedhin D, et al. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507(Pt 3):771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frisbee JC, Falck JR, Lombard JH. Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H1517–H1526. doi: 10.1152/ajpheart.2000.278.5.H1517. [DOI] [PubMed] [Google Scholar]
- 67.Wang JS, et al. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98(7):962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 68.Joly E, et al. Increased renal vascular reactivity to ANG II after unilateral nephrectomy in the rat involves 20-HETE. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R977–R986. doi: 10.1152/ajpregu.00401.2005. [DOI] [PubMed] [Google Scholar]
- 69.Schulz E, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10(6):1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 70.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 71.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 72.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100(9):2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frisbee JC, Falck JR, Lombard JH. Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol. 2000;278(5):H1517–H1526. doi: 10.1152/ajpheart.2000.278.5.H1517. [DOI] [PubMed] [Google Scholar]
- 74.Schuck RN, et al. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis. 2013;227(2):442–448. doi: 10.1016/j.atherosclerosis.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward NC, et al. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation. 2004;110(4):438–443. doi: 10.1161/01.CIR.0000136808.72912.D9. [DOI] [PubMed] [Google Scholar]
- 76.Ward NC, et al. Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med. 2005;38(8):1032–1036. doi: 10.1016/j.freeradbiomed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 77.Cheng J, et al. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294(2):H1018–H1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 78.Jacobs ER, et al. VEGF-induced relaxation of pulmonary arteries is mediated by endothelial cytochrome P-450 hydroxylase. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L369–L377. doi: 10.1152/ajplung.00265.2004. [DOI] [PubMed] [Google Scholar]
- 79.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013;125(7):311–318. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holla VR, et al. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding Y, et al. 20-HETE induces remodeling of renal resistance arteries independent of blood pressure elevation in hypertension. Am J Physiol Renal Physiol. 2013;305(5):F753–F763. doi: 10.1152/ajprenal.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103(5):398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng J, et al. Vascular characterization of mice with endothelial expression of cytochrome P450 4F2. FASEB J. 2014;28(7):2915–2931. doi: 10.1096/fj.13-241927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemarie CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010;48(3):433–439. doi: 10.1016/j.yjmcc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 85.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 86.Ooshima A, et al. Increased collagen synthesis in blood vessels of hypertensive rats and its reversal by antihypertensive agents. Proc Natl Acad Sci U S A. 1974;71(8):3019–3023. doi: 10.1073/pnas.71.8.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Udenfriend S, et al. Increased formation of collagen in the blood vessels of hypertensive rats. Ann N Y Acad Sci. 1976;275:101–103. doi: 10.1111/j.1749-6632.1976.tb43343.x. [DOI] [PubMed] [Google Scholar]
- 88.Spector S, et al. Increased vascular collagen biosynthesis by hypertension and reversal by antihypertensive drugs. Blood Vessels. 1978;15(1–3):176–182. doi: 10.1159/000158163. [DOI] [PubMed] [Google Scholar]
- 89.Ooshima A. Collagen metabolism in blood vessels of hypertensive rats. Jpn Circ J. 1977;41(8):912–914. doi: 10.1253/jcj.41.912. [DOI] [PubMed] [Google Scholar]
- 90.Ooshima A, et al. Collagen biosynthesis in blood vessels of brain and other tissues of the hypertensive rat. Science. 1975;190(4217):898–900. doi: 10.1126/science.171771. [DOI] [PubMed] [Google Scholar]
- 91.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29(7):367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Wu CC, et al. Androgen-Sensitive Hypertension Associates with Upregulated Vascular CYP4A12-20-HETE Synthase. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Issan Y, et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013;100–101:15–21. doi: 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, et al. 20-Hydroxyeicosatetraenoic acid impairs endothelial insulin signaling by inducing phosphorylation of the insulin receptor substrate-1 at Ser616. PLoS One. 2014;9(4):e95841. doi: 10.1371/journal.pone.0095841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eid S, et al. 20-HETE and EETs in diabetic nephropathy: a novel mechanistic pathway. PLoS One. 2013;8(8):e70029. doi: 10.1371/journal.pone.0070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eid S, et al. Involvement of renal cytochromes P450 and arachidonic acid metabolites in diabetic nephropathy. J Biol Regul Homeost Agents. 2013;27(3):693–703. [PubMed] [Google Scholar]
- 97.Gangadhariah MH, et al. Hypertension Is a Major Contributor to 20-Hydroxyeicosatetraenoic Acid-Mediated Kidney Injury in Diabetic Nephropathy. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoff U, et al. Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 2011;79(1):57–65. doi: 10.1038/ki.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, et al. The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 2014;1544:45–53. doi: 10.1016/j.brainres.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 100.Orozco LD, et al. 20-Hydroxyeicosatetraenoic acid inhibition attenuates balloon injury-induced neointima formation and vascular remodeling in rat carotid arteries. J Pharmacol Exp Ther. 2013;346(1):67–74. doi: 10.1124/jpet.113.203844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toth P, et al. Treatment with the cytochrome P450 omega-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. Br J Pharmacol. 2013;168(8):1878–1888. doi: 10.1111/bph.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 103.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 104.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 106.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445(7129):781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 107.Lee CY, Bautch VL. Ups and downs of guided vessel sprouting: the role of polarity. Physiology (Bethesda) 2011;26(5):326–333. doi: 10.1152/physiol.00018.2011. [DOI] [PubMed] [Google Scholar]
- 108.Lizama CO, Zovein AC. Polarizing pathways: balancing endothelial polarity, permeability, and lumen formation. Exp Cell Res. 2013;319(9):1247–1254. doi: 10.1016/j.yexcr.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horvat R, et al. Endothelial cell membranes contain podocalyxin--the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986;102(2):484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16(2):222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strilic B, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17(4):505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 112.Chen P, et al. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol. 2005;166(2):615–624. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen L, Ackerman R, Guo AM. 20-HETE in neovascularization. Prostaglandins Other Lipid Mediat. 2012;98(3–4):63–68. doi: 10.1016/j.prostaglandins.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Dhanasekaran A, et al. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2009;296(3):H777–H786. doi: 10.1152/ajpheart.01087.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Medhora M, et al. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L902–L911. doi: 10.1152/ajplung.00278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu W, et al. Cytochrome P450 omega-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancer. Cancer Chemother Pharmacol. 2011;68(3):619–629. doi: 10.1007/s00280-010-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu W, et al. Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol Appl Pharmacol. 2012;264(1):73–83. doi: 10.1016/j.taap.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alexanian A, Sorokin A. Targeting 20-HETE producing enzymes in cancer -rationale, pharmacology, and clinical potential. Onco Targets Ther. 2013;6:243–255. doi: 10.2147/OTT.S31586. [DOI] [PMC free article] [PubMed] [Google Scholar]