Abstract
Studies with rat genetic models of hypertension pointed to roles for the CYP2C and CYP4A arachidonic acid epoxygenases and ω-hydroxylases in tubular transport, hemodynamics, and blood pressure control. Further progress in defining their physiological functions and significance to human hypertension requires conclusive identifications of the relevant genes and proteins. Here we discuss unequivocal evidence of roles for the murine Cyp4a14, Cyp4a10, and Cyp2c44 genes in the pathophysiology of hypertension by showing that: a) Cyp4a14(-/-) mice develop sexually dimorphic hypertension associated with renal vasoconstriction, and up-regulated expression of Cyp4a12a and pro-hypertensive 20-hydroxyeicosatetraenoic acid (20-HETE) levels, and b) Cyp4a10(-/-) and Cyp2c44(-/-) mice develop salt sensitive hypertension linked to downregulation or lack of the Cyp2c44 epoxygenase, reductions in anti-hypertensive epoxyeicosatrienoic acids (EETs), and increases in distal sodium reabsorption. Based on these studies, the human CYP4A11 and CYPs 2C8 and 2C9 genes and their products are identified as potential candidates for studies of the molecular basis of human hypertension.
Keywords: P450, arachidonic acid, epoxygenase, 20-HETE, EET, hypertension
Cytochrome P450 (P450) hemeproteins belong to a complex gene superfamily expressed from bacteria to man, with approximately 57 genes identified in the human genome. The pharmacological and toxicological importance of mammalian P450s is well established, however, less is known regarding their physiological and/or pathophysiological relevance. The demonstration of roles for microsomal P450s in the metabolism of arachidonic acid (AA) and other polyunsaturated fatty acids suggested functional roles for the enzymes involved in these reactions. The subsequent characterization and chemical synthesis of most of the products generated from AA metabolism by the P450 enzymes led to the identification of the important biological activities associated with several of them (1-4). These discoveries opened new avenues for studies of the physiological/pathophysiological relevance of P450 and roles in diseases such as hypertension, cancer, and inflammation (5-7).
The P450 Monooxygenase metabolizes AA primarily to: a) four regioisomeric epoxyeicosatrienoic acids (EETs)(Epoxygenase), and/or b) 19- and 20-hydroxyeicosatetraenoic acids (19- and 20-HETE)(ω/ω-1 hydroxylase), with members of the CYP2 and CYP4 gene subfamilies identified as the predominant epoxygenases and ω-hydroxylases, respectively, in most rodent and human tissues (1) (Figure 1).
Figure 1. The Arachidonic Acid Monooxygenase and its Epoxygenase and ω-Hydroxylase Branches.
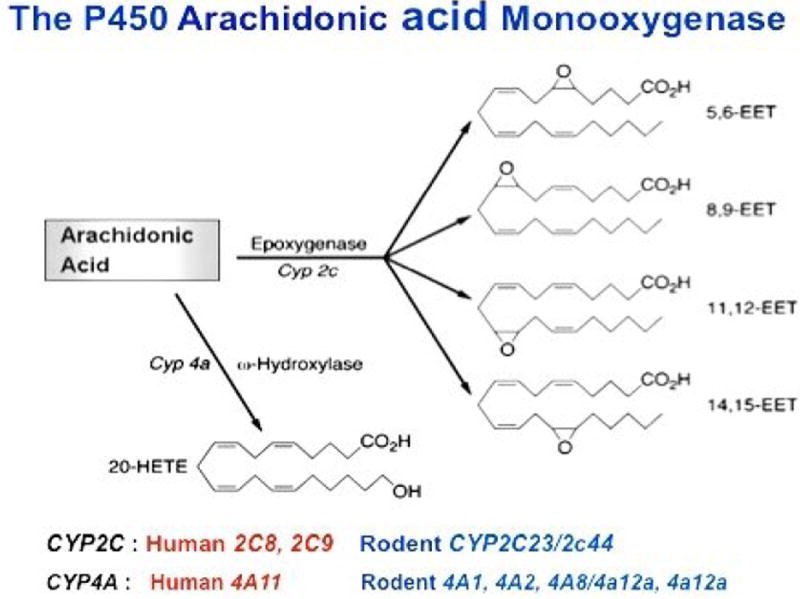
The scheme emphasizes members of the murine Cyp2c and Cyp4a gene subfamilies as functionally relevant kidney epoxygenases and ω-Hydroxylases. Human and rodent CYP2C and CYP4A isoforms known to catalyze renal AA metabolism are indicated at the bottom of Figure 1.
The enzymatic hydration of 8,9-, 11,12-, and 14,15-EET to dihydroxyeicosatrienoic acids (8,9-, 11,12-, and 8,9-DHET) was shown to be predominantly catalyzed by soluble (cytosolic) epoxide hydrolase (sEH) in 1983 (8). Subsequently, roles for sHE in the in vivo hydration of EET were proposed based on its steroselectivity for the EET enantiomers found endogenously in organ tissues (9). Since then, extensive inhibitor studies characterized sHE as a key regulator of EET organ levels and functional responses, as well as target for drug development (reviewed in references 10 and 11).
The identification of EETs and 20-HETE as components of human and rodent organs, urine, and plasma established the epoxygenase and ω-hydroxylase branches of the AA Monooxygenase as formal metabolic pathways (Figure 1), and suggested that their metabolites were functionally relevant (1-7). While the EETs have been characterized as vasodilator and pro-angiogenic lipids and as mediators of peptide hormonal release and signaling, nociception, and distal sodium excretion (3-6); 20-HETE has been identified as inhibitor of Na/K-ATPase and proximal tubule transport, and as a potent vasoconstrictor (2,5,7). Nonetheless, the identification of the epoxygenase and ω-hydroxylase P450 isoforms responsible for the in vivo biosynthesis of bioactive metabolites has been complicated by a multiplicity of P450 isoforms that share extensive amino acid sequence homology, metabolize AA to similar products, and often show common immunological determinants. The identification of the functionally significant enzymes is urgently needed to define their physiological contributions, mechanism(s) of action, regulatory control, and genetic properties. Several lines of evidence indicated that members of the CYP2C gene subfamily could be responsible for the biosynthesis of functionally important EETs in renal and vascular tissues, including: a) the characterizations of rat CYP2C23 and its murine homologue, Cyp2c44, as stereo selective epoxygenases and as the predominant epoxygenases in rat and mouse kidney, b) the identification of renal CYP2C23 and Cyp2c44 as dietary salt regulated epoxygenases, and c) the demonstration of reduced CYP2C23 expression and EET biosynthesis in the kidneys of hypertensive Dahl salt sensitive rats (2-6,12-15). Similarly, roles for rat CYP4A and mouse Cyp4a isoforms in the biosynthesis of functionally relevant 20-HETE were indicated by: a) the documentation of up-regulated renal CYP4A expression and 20-HETE biosynthesis during the onset of hypertension in the SHR/WKY rat model of spontaneous hypertension, b) differences in CYP4A2 expression and 20-HETE biosynthesis between salt resistant and sensitive Dahl rats (DR and DS genotypes, respectively), and c) antisense nucleotide inhibition of renal CYP4A1/CYP4A2 expression and normalization of the blood pressures of hypertensive SHR rats (2,5-7,16,17). Based on the above, as well as their tubular and vascular effects, anti- or pro-hypertensive properties were proposed for EETs and 20-HETE, and their corresponding CYP2C and CYP4A isoforms (16).
The availability of rat models of genetically determined hypertension opened the door to studies of gene-phenotype associations between products of the CYP2C and CYP4A genes and blood pressure control (2,5-7,16,17). However, the multi-genic and complex nature of the SHR/WKY and Dahl genetic models of hypertension precluded an unequivocal identification of roles for distinct P450s genes in blood pressure control. The advent of gene targeting techniques and the development of mouse models of monogenic dysfunction allows now studies of the physiological and pathophysiological significance of specific P450 isoforms. To date, mouse lines carrying disrupted copies of the genes coding for Cyp2c44, Cyp2j5, Cyp4a10, Cyp4a14, and Cyp4f4 have been generated and characterized to variable extents (18-22). There is published evidence for the involvement of Cyp2j5 in estrogen, but not AA metabolism (19), and for Cyp4f4 in vitamin E catabolism (21). We summarize herein studies performed in wild type (WT) and in mice carrying globally disrupted copies of the Cyp4a10 (4a10 KO), Cyp4a14 (4a14 KO), or Cyp2c44 (2c44 KO) genes (in isogenic 129SeV backgrounds) that identify roles for these P450 genes on the control of blood pressures. Although not discussed, recent studies with 2c44 KO mice also revealed that lack of the Cyp2c44 epoxygenase blunts tumor angiogenesis and growth (23).
A) Roles of the murine Cyp4a genes in blood pressure regulation
The mouse genome contains four Cyp4a genes: Cyp4a10, Cyp4a12a, Cyp4a12b, and Cyp4a14 localized in chromosome 4. The Cyp4a12a and Cyp4a12b genes share extensive intron/exon sequence identity (24) and code for the only mouse Cyp4a proteins with significant 19-and 20-HETE synthase activity (Figure 1)(18,24). Cyp4a12a is expressed in male kidneys in an androgen sensitive manner (18,24). Two highly homologous CYP4A genes, CYP4A11 and CYP4A22, are present in chromosome 1 of the human genome (Figure 1)(25). While CYP4A11 metabolizes AA to mostly 20-HETE, an abnormal heme prosthetic group in CYP4A22 renders it catalytically inactive (26).
a) Cyp4a14(-/-) mice, a model of androgen sensitive hypertension
A link between the Cyp4a14 gene and blood pressure regulation was established by the demonstration that its disruption causes a type of hypertension that is sexually dimorphic, male specific, and associated with: a) increases in plasma androgens, b) up-regulated kidney expression of Cyp4a12a, and c) increased urinary excretion of 20-HETE (18). Castration lowers renal Cyp4a12a expression and normalizes the pressures of hypertensive 4a14 KO mice. Testosterone or dehydrotestosterone administration restores renal Cyp4a12a expression and the hypertensive phenotype of castrated 4a14 KO mice (18). The pro-hypertensive role of Cyp4a12a and 20-HETE was confirmed when it was shown that overexpression of Cyp4a12a in transgenic mice raises renal 20-HETE levels and causes androgen-independent hypertension (27). The demonstration that hypertensive 4a14 KO mice display increased renal vascular resistance and impaired microvascular auto-regulatory capacity, suggested that increased afferent arteriole resistance compromises the animal’s tubular excretory capacity (18). Augmented proximal tubule fluid reabsorption was also implicated in the hypertensive phenotype of 4a14 KO mice (28).
b) Cyp4a10(-/-) mice show salt sensitive hypertension
Male and female 4a10 KO mice on low salt diets (0.05% NaCl) are normotensive but become hypertensive when fed diets containing normal (0.3%)(NS) or high salt (8% NaCl)(HS)(20). Compared to WT, 4a10 KO mice show similar kidney Cyp4a12a expression and urinary 20-HETE levels but decreased renal Cyp2c44 epoxygenase expression and EET biosynthetic capacity (20). Hypertensive 4a10 KO mice have a hyperactive collecting duct (CD) epithelial sodium channel (ENaC) and become normotensive when administered amiloride, an inhibitor of ENaC gating activity (20). Inasmuch as EETs, but not 20-HETE, inhibit ENaC gating (29), these results: a) pointed to roles for the Cyp4a10 gene in the regulation of kidney Cyp2c44 expression and EET biosynthesis by dietary salt, and b) suggested that deficiencies in EET-mediated regulation of ENaC activity and distal Na reabsorption were responsible for the salt sensitive phenotype of 4a10 KO animals (20). The transcriptional regulation of CYP4A and CYP2C gene expression by peroxisomal proliferator activated nuclear receptor alpha (PPARα) ligands is established (23,30,31). Wyeth 14,643, a selective PPARα ligand, increases renal Cyp2c44 expression and EET biosynthesis and normalizes the pressures of hypertensive 4a10 KO mice (20).
Summarizing, the hypertensive phenotypes resulting from disruption of murine Cyp4a14 and Cyp4a10 pointed to pro-hypertensive and anti-hypertensive roles for products of these genes, respectively, and provided unequivocal evidence for their roles in the control of systemic blood pressures (18,20). Importantly, associations between hypertension and a variant of the human CYP4A11 gene coding for a reduced 20-HETE synthase activity have been identified in different population studies (26,32-34) and linked to salt sensitive hypertension (35).
B) Roles of the murine Cyp2c44 gene in blood pressure regulation
The demonstration that EETs inhibited sodium reabsorption in dissected rabbit cortical CDs (5,6), that 11,12-EET is a powerful inhibitor of ENaC gating (29), and that the hypertensive phenotype of 4a10 KO mice was associated with down-regulated expression of renal Cyp2c44 (20) implicated the Cyp2c44 epoxygenase in the biosynthesis of antihypertensive EETs. The murine Cyp2c gene subfamily is composed of a complex group of genes and pseudo-genes (non-coding sequences) localized in chromosome 19. Approximately five Cyp2c genes code for epoxygenases with variable degrees of catalytic turnover and/or regio- and stereoselectivities (36). Among these, Cyp2c44 is unique in that: a) it catalyzes the stereo-selective epoxidation of AA to 14,15-, 11,12-EET and minor amounts of 8,9-EET (13), b) its amino acid sequence shows borderline homology to other Cyp2c proteins, and c) the gene is separated from the Cyp2c gene cluster by a 4.1 Mb interval, suggesting an structural basis for its unique responses to dietary salt and PPARα ligands. Four CYP2C genes are present in chromosome 10 of the human genome: CYP2C8, CYP2C9, CYP2C18 and CYP2C19, with only CYP2C8 and CYP2C9 coding for proteins with significant AA epoxygenase activity (Figure 1)(37). Rodent Cyp2c44 and 2C23 are catalytic homologues of the human CYP2C8 and 2C9 epoxygenases (12,13,37).
Cyp2c44(-/-) mice. A model of monogenic salt sensitive hypertension. EETs as natriuretic lipids
Key roles for the Cyp2c44 epoxygenase in the control of systemic blood pressure were unequivocally identified by the demonstration that lack of a functional Cyp2c44 gene causes a type of hypertension that is, like most human hypertension, sensitive to dietary salt intake (22). As shown in Figure 2A, male WT and 2c44 KO mice fed NS salt diets are normotensive (systolic pressures of 120 ±3 and 124 ±3 mm Hg, respectively)(22). In contrast, while WT mice on HS diets remain essentially normotensive, under similar conditions, 2c44 KO animals become severely hypertensive (systolic pressures of 134 ±2 and 168 ±4 mm of Hg for WT and 2c44 KO mice, respectively)(Figure 2A)(22).
Figure 2. Global disruption of the Cyp2c44 gene or inhibition of EGF-receptor signaling causes salt sensitive hypertension.
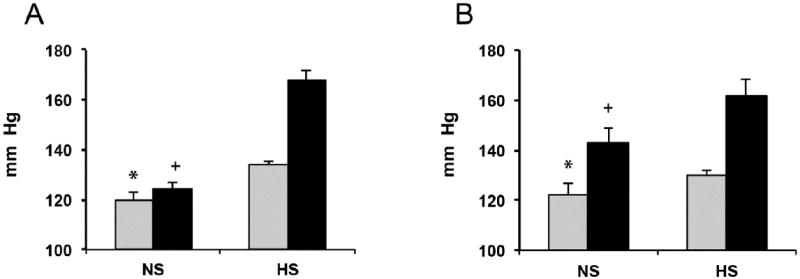
The systolic pressures of adult male mice fed normal (NS) or high salt (HS) diets for 4 weeks were measured as described in references 20,22. Shown are averages ± SE changes in blood pressure (in mm of Hg) for: A) WT and 2c44 KO mice (grey and black bars, respectively)(groups of 15 mice for WT in NS and HS and 2c44 KO on NS; and of 25 mice for 2c44 KO on HS)(25 measurements/animal), and B) WT mice on NS or HS were left untreated (5 and 6 mice respectively) (grey bars) or given injections of Cetuximab 8-10 days before blood pressure analyses (6 and 9 mice, respectively; every 48 hours, 12 mg/kg body weight)(black bars) as reported (55). A) Different from: * WT HS p<10-80, KO NS p<10-8; + KO HS p<10-270. The differences were between WT on NS and KO HS were non-significant (p>0.05). B) Different from: * untreated on HS p<10-3, Cetuximab treated on NS or HS p<10-5 or p<10-6, respectively; + Cetuximab treated on HS p<10-4, untreated NS p<10-5.
Measurements of total urinary epoxygenase metabolites (EETs + DHETs), as estimates of in vivo kidney epoxygenase activity, showed that while WT mice on a HS diet increased the excretion of epoxygenase products by approximately 3 fold (from 0.8 ±0.2 to 2.4 ±0.3 ng/mg of creatinine) and remained normotensive, 2c44 KO mice lacking a salt inducible Cyp2c44 epoxygenase were unable to do so and became hypertensive (2c44 KO: 0.9 ±0.3 ng/mg of creatinine)(22). The dietary salt regulated nature of the Cyp2c44 epoxygenase is indicated by the fact that WT and 2c44 KO mice on NS show similar levels of urinary epoxygenase metabolites (0.8 ±0.2 and 0.7 ±0.1 ng/mg of creatinine, respectively) suggesting that they are the product of alternate, non-salt sensitive, epoxygenases such as Cyp2c29 and/or Cyp2c38 (36). These studies revealed that the Cyp2c44 genotype-dependent effects of HS on blood pressure were paralleled by changes in urine excretion of epoxygenases metabolites (22). The determining role played by EETs in the hypertensive phenotype of 2c44 KO mice was confirmed when administration of a synthetic EET analog reversed the 2c44 KO phenotype by increasing sodium excretion and normalizing the pressures of salt loaded 2c44 KO mice (38).
As with 4a10 KO mice (20), amiloride lowered the blood pressures of salt loaded 2c44 KO mice to levels comparable to WT mice thus, uncovering the decisive impact of ENaC dysfunction upon the hypertensive phenotype of these animals (22). Electrophysiology studies in dissected CDs showed that disruption of the Cyp2c44 gene caused a constitutive activation of ENaC and increased inward sodium currents (22); a phenotype similar to what it is seen in Liddle’s Syndrome, a Mendelian form of human hypertension in which a hyperactive ENaC increases distal sodium reabsorption and blood pressure (39). As seen in Figure 3, AA inhibits ENaC activity in the CD of WT mice but it has no effects on 2c44 KO mice channels. In contrast, 11,12-EET inhibits the 2c44 KO channel, and reverses its constitutive activation (22). These results show that the effects of AA on ENaC activity require its Cyp2c44 epoxygenase mediated conversion to EETs. Of note, 11,12-EET inhibits ENaC activity regardless of mouse Cyp4a10 or Cyp2c44 genotype confirming that disruption of these genes does not affect ENaC’s intrinsic properties, but rather its EET-mediated regulation (20,22). An analysis of the regioisomeric composition of epoxygenase metabolites in the urine of WT and 2c44 KO mice on HS diets showed that 11,12-epoxygenase derived metabolites were 5.6- fold lower in 2c44 KO animals (22). In summary, these and published studies (5,6,20,22,29) identify: a) ENaC as a molecular target of the hypertensive phenotype resulting from lack of a functional, dietary salt regulated Cyp2c44 epoxygenase, and b) altered distal sodium transport as a causative agent of the 2c44 KO hypertensive phenotype.
Figure 3. ENaC is hyperactive in the collecting ducts of global Cyp2c44 KO mice.
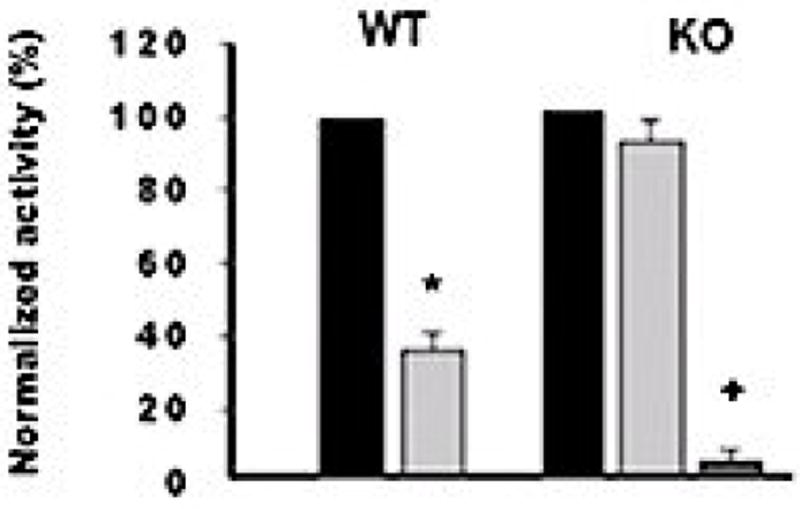
ENaC gating activity was determined in patches of CDs dissected from WT and Cyp2c44 KO mice (20,22). Normalized ENaC activity (NPo) under control conditions (black bars), in the presence of 10 μM AA (light gray bars) or 0.2 μM 11,12-EET (dark gray bar). NPo, calculated from 60 s data samples during steady state according to: Npo = Σ (t1 + 2t2 + … iti) (ti = fractional open time at a given current level). Conductance was calculated from currents recorded at ≥3 holding potentials. Values (as a percentage of control activity) are averages ±S.E. from ≥5 experiments. Different from controls: *p <0.01; … p <0.001.
A recent characterization of mice with a CD specific deletion of the Cyp2c44 gene (CD-2c44KO) showed that, at difference with global 2c44 KOs, these animals become hypertensive when fed high potassium (HK) (2.5-5% KCl) but not HS diets (40). Real time quantitative PCR analysis of Cyp2c44 expression in nephron segments dissected from WT mice indicated that: a) HS diets up-regulated Cyp2c44 expression in the thick ascending limb and the distal convoluted and connecting tubule segments of the aldosterone sensitive nephron and had little or no effects on CDs, and b) the effects of HK diets were limited to proximal connecting tubules and CD segments (40). The fact that amiloride also normalizes the blood pressures of hypertensive CD-2c44 KO mice on HK suggests that lack of a HK sensitive Cyp2c44 epoxygenase in the CD enhances ENaC activity, increases sodium absorption, and raises blood pressure (40). Therefore, it appears that: a) up-regulated expression of Cyp2c44 in the distal convoluted and connecting tubules is sufficient to prevent excessive sodium absorption during a high sodium intake, b) conversely, when mice are fed a high potassium diet, Cyp2c44 is up-regulated in the CD and ENaC in that segment becomes a major player in maintaining sodium homeostasis (40,41), and c) the HS sensitive phenotype of mice carrying a globally disrupted Cyp2c44 gene is associated with a hyperactive ENaC in the distal convoluted and/or connecting tubule portions of the aldosterone sensitive nephron (22,40,41). As expected from the discussed roles of HK diets on the CD expression of the Cyp2c44 epoxygenase and the inhibition of ENaC by its EET metabolites (22,29), 2c44 KO mice lacking a Cyp2c44 epoxygenase along the extent of the aldosterone sensitive nephron, become hypertensive when fed HK diets but, under similar conditions, WT animals remain normotensive (40,41)(Figure 4)
Figure 4. Global Cyp2c44 KO mice are hypertensive when fed high potassium diets.
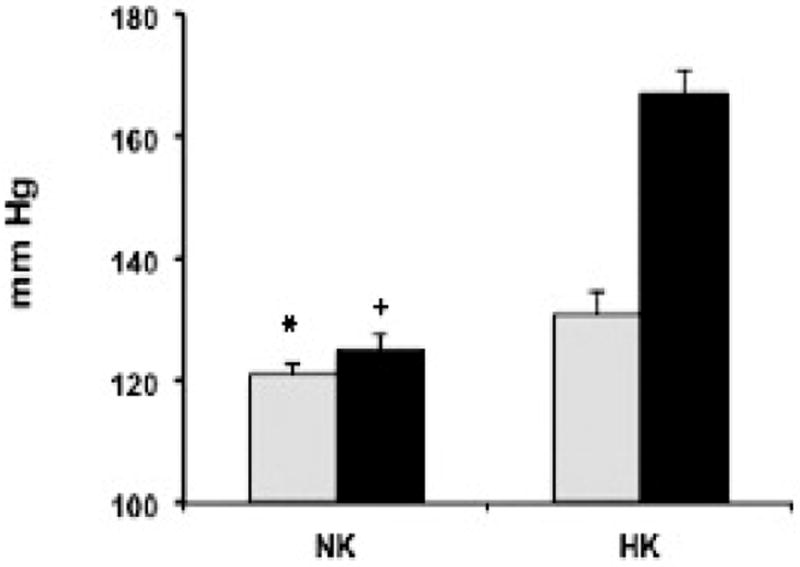
The systolic pressures of WT and Cyp2c44 KO male mice (grey and black bars, respectively)(8 mice each group) fed diets containing 0.3% NaCl and normal (NK) or high potassium (HK) (1.0 and 2.5% KCL, respectively)(40) for 2 weeks were measured as described (20,22). Shown are average blood pressures ± SE (in mm of Hg) for WT and 2c44 KO mice (grey and black bars, respectively). Calculated from ≥ 30 measurements/animal. Different from: * KO on NK p<10-4, WT on HK p<10-20, KO on HK p<100-100; + WT or KO on HK p<10-22 or HK p<10-100.
Differential roles in sodium transport for ENaC along the aldosterone sensitive distal nephron have been reported (42), as well as the predominant roles played by this channel in the distal convoluted and connecting tubules during high sodium and low potassium intake, and for ENaC in the CD during a high potassium low salt intake (42). Studies with mice carrying a CD-selective disruption of the gene coding for the alpha subunit of ENaC showed that low sodium diets increased distal sodium reabsorption even in the absence of a functional ENaC in the animal’s CDs (43). Of special relevance to this duality of functions in the aldosterone sensitive nephron is the suggestion that the amount of salt or potassium intake determines a segment selective up-regulation of Cyp2c44 and thus, its role in sodium transport and blood pressure regulation.
Several mechanisms have been identified as regulators of ENaC activity, including changes in channel proteolysis, translocation, membrane assembly, Nedd4-2 phosphorylation, ubiquitination and retrieval and degradation, as well as negative and positive effects by protein kinases including extracellular regulated kinase 1 and 2 (ERK1/2) among others (44-51). Added to that list is the identification of EETs, and 11,12-EET in particular, as a new class of in vivo regulators of ENaC activity (20,22,29). Furthermore, the demonstration that genetically determined or experimentally induced changes in the expression and/or activity of the Cyp2c44 epoxygenase causes salt sensitive hypertension identifies the EETs as a new class of endogenous natriuretic agents and as a compelling target for the development of new strategies for blood pressure management.
C) Mechanism(s) of EET-mediated regulation of ENaC activity and sodium excretion
Roles for epidermal growth factor (EGF) and mitogenic kinases in the regulation of ENaC activity by EETs were suggested by the conjunction of two apparently unrelated lines of studies. On the one hand, the EETs were shown to participate in EGF-mitogenic signaling and ERK1/2 activation (52,53) and, on the other hand, the demonstration that EGF inhibits ENaC activity by an ERK1/2 catalyzed threonine phosphorylation of the ENaC beta and gamma subunits (ENaCβ and ENaCγ)(45,46,54,55). The in vivo relevance of these data was indicated by Western blot analyses showing that: a) salt loaded hypertensive 2c44 KO mice show reduced kidney levels of anti-phospho-ERK1/2 and anti-threonine immunoreactive protein(s) with the electrophoretic mobility of ENaCγ (22), and b) compared to untreated controls, mice on HS and administered Cetuximab, an inhibitor of EGF receptor binding, show reductions in ENaCγ threonine phosphorylation (55). Importantly, a mechanistic commonality between the inhibitory effects of EGF and EETs on ENaC was further supported by the demonstration that inhibition of EGF receptor signaling (55) or disruption of the Cyp2c44 gene and lack of the kidney Cyp2c44 epoxygenase (22) led to comparable HS mediated effects on ENaC activity, systemic BP (Figure 2A and 2B) and kidney levels of anti-phosphothreonine and -ENaCγ immunoreactive proteins (22,55). Based on these studies and similarities between EETs and EGF as inhibitors of ENaC activity and activators of mitogenic kinase pathways (22,29,45,46,52,54,55), it was proposed that: a) EGF and Cyp2c44 epoxygenase-derived EETs regulate ENaC activity by common ERK1/2-mediated mechanisms, b) the EETs mediate the effects of EGF on ENaC activity, and c) impairments in Cyp2c44 mediated ERK1/2 activation and reduced ENaC phosphorylation contribute to the hypertensive phenotype of 2c44 KO mice. In summary, these results characterized functional roles for mitogen kinases and ERK members of mitogen activated kinase signaling cascades in renal sodium transport that appear distinct and independent of their more traditional roles on cell proliferation. The proposed participation of the Cyp2c44 epoxygenase in the in vivo regulation of mitogenic growth factor-ERK1/2-mediated signaling provides a common experimental platform that could explain the seemingly unrelated roles reported for the enzyme and its EET metabolites in channel activity, cell proliferation, and vasodilation (1-7,22,29,52,53). In this regard, in a xenograph model of tumorigenesis, 2c44 KO mice show reductions in endothelial cell proliferation, tumor vascularization, and growth (23). Furthermore, in a mouse orthotopic model of human lung cancer, peroxisomal proliferator activated receptor alpha (PPARα) ligands down-regulate endothelial Cyp2c44 expression and reduce tumor vascularization, growth, and metastatic potential (56).
Summarizing the preceding studies, the blood pressure changes resulting from disruption of the Cyp4a10, Cyp4a14 and Cyp2c44 genes are mechanistically different and mediated by either indirect or direct gene-dependent effects:
Indirect: these are associated with effects of the targeted gene on the expression of alternate genes. Examples of this are: 1) Cyp4a10(-/-) mice in which salt sensitive hypertensive is accompanied by down-regulated kidney expression of the anti-hypertensive Cyp2c44 epoxygenase, reduced urinary EET levels, and a hyperactive ENaC, and 2) Cyp4a14(-/-) mice in which sexually dimorphic hypertension is linked to androgen-mediated increases in the kidney expression of pro-hypertensive Cyp4a12a, increased 20-HETE excretion, and changes in renal hemodynamics and tubular excretion.
Direct: resulting from a lack of the protein(s) encoded by the targeted gene. This is seen with salt sensitive hypertensive Cyp2c44(-/-) mice where lack of a functional kidney Cyp2c44 epoxygenase causes reductions in EET biosynthesis resulting in increases in ENaC activity and sodium reabsorption.
The mechanism(s) by which the Cyp4a14 gene regulates plasma androgens and the expression of the Cyp4a12a 20-HETE synthase remains undetermined. However, sexual dimorphisms are seen in the SHR/WKY rat model of genetically determined hypertension (57), as well as in humans where hypertension is usually more prevalent and severe in males (58). It remains to be determined how the Cyp4a10 or Cyp4a14 genes, or for that matter HS, controls renal expression of the Cyp2c44 epoxygenase or Cyp4a12a 20-HETE synthase. In all these examples, changes in Cyp4a12a or Cyp2c44 expression are accompanied by changes in the corresponding mRNA levels (7,18,20,22,27,40) suggesting transcriptional mechanisms, however, changes in mRNA stability can be not be discarded as potential contributors. The transcriptional regulation of P450 genes, including the CYP2C and CYP4A subfamilies, by specific promoters has been reported (30,59). In this regard, rat CYP4A isoforms actively metabolize the 8,9-, 11,12-, and 14,15-EET to their corresponding 20-hydroxy-epoxides (Table 1), all of which bind and activate PPARα with high affinity and efficiency (60). As mentioned, PPARα ligands such as Wyeth14,643 induce the kidney expression of Cyp2c44 and normalize the pressures of hypertensive 4a10 KO mice (20). An interesting possibly is that these epoxyalcohols could be involved in a PPARα-mediated up-regulation of kidney Cyp2c44 by HS diets, and that the hypertensive phenotype of 4a10 KO is due to deficits in Cyp4a10 catalyzed EET ω–hydroxylation.
Table 1. Rates of EET ω-hydroxylation by purified recombinant rat CYP4A proteins.
Enzymatic activities were reconstituted in the presence of dilauroylphosphatidylcholine (50 mg/ml), by mixing each P450 enzyme with cytochrome b5 and cytochrome P450 oxido-reductase in 1:1:10 molar ratio. Reactions were carried out at 35° in Tris-Cl buffer pH 7.4 containing 10 mM MgCl2, 0.1 M KCl, and 1 mM NADPH. Organic soluble products were resolved by reversed phase HPLC and quantified by on-line β detection (60). Shown are average Kcat values ±SE (in min-1) calculated from time courses of product formation vs incubation time.
| Protein | 8,9-EET | 11, 12-EET | 14, 15-EET |
|---|---|---|---|
| CYP4A1 | 15.0 ±0.9 | 10.0 ±0.5 | 4.0 ±0.1 |
| CYP4A2 | 4.0 ±0.2 | 7.0 ±0.4 | 3.0 ±0.3 |
| CYP4A8 | 0.8 ±0.2 | 1.3 ±0.1 | 0.8 ±0.1 |
Figure 5 summarizes potential interrelationships between the regulatory effects of the Cyp4a14 and Cyp4a10 genes on the kidney expression of pro-hypertensive Cyp4a12a and anti-hypertensive Cyp2c44 enzymes, the know effects of 20-HETE and 11,12-EET metabolites on renal vasoconstriction and sodium transport and, ultimately, on the control of kidney hemodynamics and/or tubular transport (2-7,18,20). The dashed arrow indicates the close interrelationship that exists between these endpoints in the regulation of systemic blood pressures. The scheme is intended to suggest an explanation for the duality of functions attributed to the CYP4A enzymes as pro- and anti-hypertensive proteins (2,7). Thus, as suggested in Figure 5, their pro-hypertensive effects will be associated with up-regulated expression of rat CYP4A1/4A2 or mouse Cyp4a12a and increased biosynthesis of vasoconstrictor 20-HETE. Their anti-hypertensive responses will be associated with a CYP4A/Cyp4a10 gene mediated upregulation of CYP2C23 or Cyp2c44 expression and EET biosynthesis in the thick ascending limb and aldosterone sensitive nephron segments. Rat CYP4A1, 4A2, 4A3, and 4A8 isoforms (Table 1)(60) as well as Cyp4a10 and Cyp4a14 proteins (unpublished data) are capable of generating putative PPARα ligands by EET ω-hydroxylation. Of interest, the salt sensitive hypertensive phenotype of DS rats has been attributed to reductions in kidney CYP4A expression and 20-HETE formation (2), or to reduced CYP2C23 expression and EET biosynthesis (5,6,14). In both scenarios, PPARα ligands ameliorate or reverse the hypertensive phenotypes of salt loaded DS rats and 4a10 KO mice (2,20).
Figure 5. Schematic illustration of proposed mechanisms of blood pressure control by murine Cyp4a10 and Cyp4a14 genes.
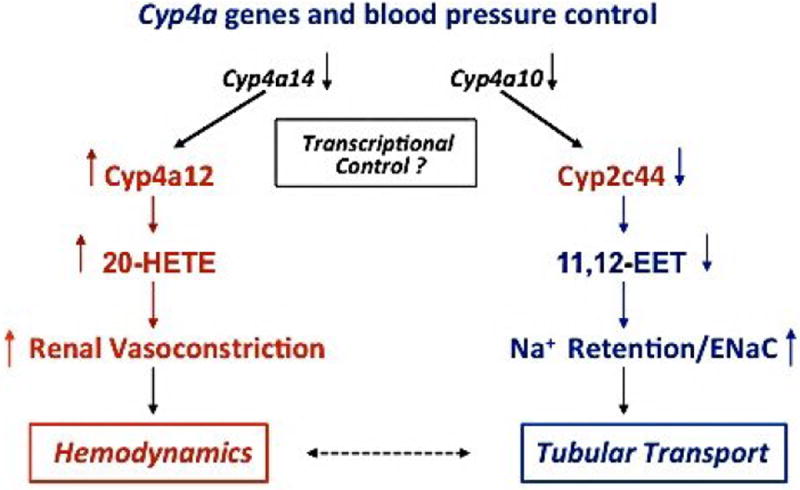
Shown are proposed hemodynamic and tubular transport responses resulting from disruption of the Cyp4a10 and Cyp4a14 genes and their effects on Cyp2c44 and Cyp4a12a expression, the biosynthesis of natriuretic 11,12-EET, vasoconstrictor 20-HETE, and blood pressure. Downward black arrows denote reductions or lack of gene product and/or function. Downward blue arrows indicate reductions in protein expression or product biosynthesis. The upward blue arrow signifies increases in ENaC activity and sodium retention. Upward red arrows indicate increases in protein and product formation, and renal vasoconstriction. A bidirectional dashed arrow is intended to illustrate the potential for pro- and anti-hypertensive effects of the Cyp4a isoforms as direct and/or indirect regulators renal hemodynamics and tubular transport.
With few exceptions, our understanding of the molecular basis of prevalent forms of human hypertension remains limited. Consequently, its treatment and diagnosis continues to be challenging, particularly since its multifaceted etiology is the likely result of a multiplicity of environmental and genetic factors and coexisting conditions. A better understanding of the pathophysiological mechanisms and genetic basis of the human disease should contribute to improved clinical management and early diagnosis, and to ameliorate the devastating consequences of untreated chronic hypertension. The identification of roles for the Cyp2c44 epoxygenase in the regulation of ENaC activity, as well as the rapid nature of the inhibitory response elicited by its EET metabolites (22,29,40,41), introduces a new mechanistic paradigm to account for the regulation of this channel and distal sodium transport. Recognition that: a) the Cyp4a14 gene and Cyp4a12a participate in the regulation of renal hemodynamics, b) the Cyp4a10 and Cyp2c44 genes influence tubular transport, and c) genetic or experimentally induced alterations in their function causes hypertension, offers fresh approaches for the understanding of mechanism(s) by which the kidney regulates renovascular tone, sodium excretion and, ultimately, blood pressure. Furthermore, the data discussed: a) provides an experimental foundation for future studies of the functional roles of the human CYP4A11 20-HETE synthase and CYP2C8/CYP2C9 epoxygenases in human hypertension, and b) suggest that maneuvers designed to target the expression of these genes, the biosynthesis of their metabolites, or to antagonize or mimic the functional properties of 20-HETE or 11,12-EET, respectively, could serve as a basis for the development of novel anti-hypertensive therapies. In support of these concepts, functional 20-HETE antagonists and EET analogs have been developed and their anti-hypertensive potential documented (7,38). The administration of a 20-HETE antagonist to hypertensive 4a14 KO and androgen treated WT mice lowers their blood pressures, and prevents the onset of hypertension in doxycycline–treated Cyp4a12a transgenic mice (7,27). Similarly, orally available EET analogs lower blood pressures and increase natriuresis in salt loaded 2c44 KO mice and ameliorate the onset of hypertension in SHR rats (38). In summary, it is expected that these studies will stimulate efforts to develop novel: a) CYP2C/EET and CYP4A/20-HETE based anti-hypertensive drugs, and b) strategies for the early detection and diagnosis of hypertension.
The studies summarized bring into consideration two important issues: Firstly, the implication of a role for EETs in tumor angiogenesis and growth raises pertinent questions regarding long term risks that might be associated with efforts to use inhibitors of EET metabolism as anti-hypertensive, anti- inflammatory drugs (61,62). Inhibition of enzymatic EET hydration could potentiate their mitogenic and tumorigenic activities; a possibility supported by tumorigenic studies with 2c44 KO mice (23,56). Secondly, several drugs in current clinical use are metabolized by human CYP2C8 and/or CYP2C9 (63) with yet undetermined effects on the regulation and/or epoxygenase activity of these enzymes. It is therefore, advisable that current methods for drug evaluation take into consideration the potential physiological and/or pathophysiological consequences of interfering with the activity and/or expression of P450s involved in endogenous metabolic pathways.
D) Unresolved issues, perspective for the future
The current state of knowledge regarding this pathway of AA metabolism and bioactivation, and the increasing interest in its biomedical potential and/or clinical relevance, positions this area of research at the threshold of a new chapter in the studies of P450-eicosanoids and their relationship to human disease. This new chapter should include renewed efforts to identify physiologically relevant human CYP2 and CYP4 genes and enzymes, their tissue specific expression, regulatory control, signaling mechanism, and pathophysiological roles. Ultimately these efforts will serve as prelude to medicinal chemistry efforts to target human epoxygenases and ω-hydroxylases for the development of novel therapies for the treatment of cardiovascular disease, hypertension, renal injury, CNS disorders, and inflammation. Among unresolved issues we include:
A) A need for unequivocal identifications of the P450 genes and proteins responsible for the endogenous synthesis of bioactive EETs and 20-HETE
Abundant studies identified members of the CYP2C and CYP2J, and CYP4A and CYP4F gene subfamilies as functionally significant AA epoxygenase or ω-hydroxylases, respectively. However, most of these assignments are based on in vitro enzymatic activities, immunological and/or RNA based studies of expression levels, genetic associations, inhibitor studies, or functional responses to transgene induced changes in expression (2-7,41). It is expected that improvements in gene targeting and editing methods will hasten future developments of models of monogenic P450 dysfunction and lead to further progress in this important area. The identity of the human homologues of functionally relevant EET/20-HETE synthases is needed to define meaningful correlations between pathophysiological conditions and alterations in the genes coding for these enzymes and/or involved in their expression, and for the development of novel strategies for disease diagnosis and treatment.
B) The definition of EET signaling mechanism
After attempts by different groups (3,4), selective binding and trans-membrane signaling has been reported only for 14(R),15(S)-EET in guinea pig mononuclear or human U937 cells (64,65). While G-proteins, mitogenic kinases/phosphatases, cAMP-kinase A, prostanoids, PPAR nuclear receptors; as well as effects on ion channel activity have been identified as potential mediators of EET responses (2-6), a membrane bound receptor, capable of selective EET binding and trans-membrane signaling, has yet to be identified. Exogenously added EETs readily cross cell membranes as evidenced by their rapid esterification into cellular glycerolipid pools (66); therefore, the possibility that their functional effects are mediated by extracellular or intracellular events needs to be considered.
As proposed in Figure 6: a) extracellular signaling would involve EET interactions with cognate receptors capable of trans-membrane signaling and amplification or, alternatively, by direct EET effects on the signaling properties of hormone receptors such the EGF-receptor (53); b) Intracellular signaling will require either EET crossing the cell membrane barrier by simple diffusion, carrier plasma lipoprotein uptake, or yet to be determined mechanisms. Otherwise, they could originate from phospholipase A2 catalyzed AA release followed by P450-mediated epoxidation. Intracellular EETs could signal by direct: a) interaction with intracellular receptors such as mitogen activated protein kinases, protein phosphatases, adenylyl- and/or guanylylcyclase, G proteins, etc., b) effects on the activities of enzymatic pathways such as those catalyzed by mitogenic kinases, or c) effects on ion channel/transporter phosphorylation/de-phosphorylation. If documented, these type of intracellular actions will add EET to the list of known intracellular signal effector molecules such as cAMP and cGMP nucleotides, inositol-phosphates, NO and, in addition to a conceptual breakthrough, help rationalize many of their multiple and pleotropic functional effects.
Figure 6. Potential mechanisms of EET signaling/action.
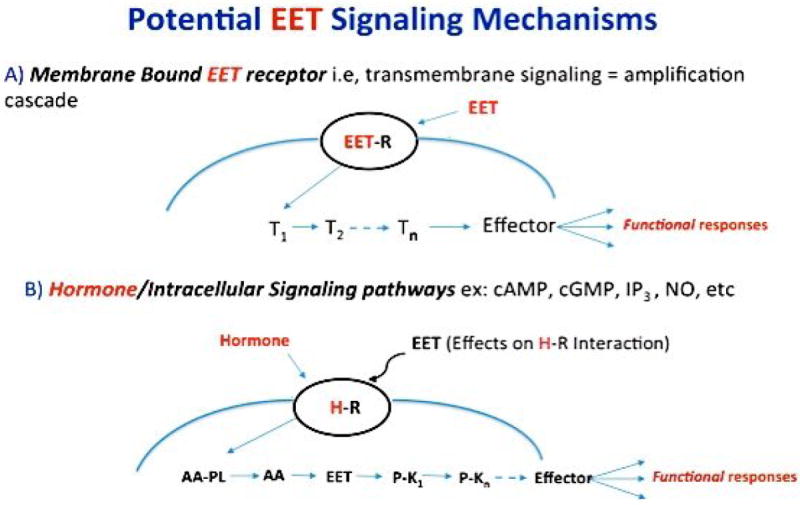
Shown are: A) involvement of a membrane bound EET receptor capable of trans-membrane signaling/amplification, and B) roles for the EETs as intracellular signaling molecules capable of, for example, directly regulate protein kinase activity. Curved blue lines housing an EET (A) or hormone receptor (B) (black ovals) represent a plasma membrane barrier. A) T1 to Tn symbolizes potential transducers leading to a final effector(s) and functional response. B) Hormone mediated intracellular release of AA and conversion to EETs capable of activating a cascade of protein kinases resulting in a functional response(s). Not shown is the possibility that EETs are released from endogenous EET containing phospholipids thus obviating a need for AA epoxidation.
Less is known regarding mechanisms of 20-HETE signaling involving plasma membrane bound selective 20-HETE receptors and/or an intracellular signaling mechanism(s). As with EETs, 20-HETE shows pro-mitogenic and pro-angiogenic properties (2,7), however, more often these eicosanoids elicit opposing biological responses. For example, while EETs activate vascular smooth muscle calcium sensitive 256 pS maxi potassium channels or BKCa causing cell hyperpolarization and vasodilation, 20-HETE inhibits the BKCa channel causing cell depolarization and vasoconstriction (2,3,7). It is not understood how these two lipids regulate BKCa in opposing ways but, electrophysiology studies suggested G-protein mediation of EET responses (3). Whether unique intracellular mechanisms, G-proteins, or membrane bound receptors are involved in the channel responses to 20-HETE remains unknown.
C) The nature and source of the active EET-mediators
Although rarely recognized, between 90 to 95% of the EETs present in rodent and human tissues and plasma are found as esters of glycerophospholipids (1,4,66). These EET containing phospholipids, generated by stereoselective acylation of EET-CoA derivatives into lysolipids (66), raise important issues such as: a) their functional roles as a source of bioactive EETs made available independently of P450 catalyzed AA epoxidation, b) the existence of selective epoxy-phospholipid phospholipases A2 responsible for EET release from distinct hormonally sensitive glycerophospholipid stores, c) whether the EET moiety confers unique functional properties to these phospholipids, d) whether epoxidation serves as a cellular mechanism to select functionally important phospholipids from merely bulk or structural pools, and e) are the EET-containing phospholipids or their di- or monoglyceride metabolic products signaling molecules or simple reservoirs of bioactive EETs? In this regard, the 2-(11,12- and/or 14,15-epoxyeicosatrienoyl)-glycerols present in rat brain, kidney, and spleen bind and activate the CB1 and CB2 subtypes of the cannabinoid receptor (67).
D) The characterization of mechanisms by which sHE controls physiological responses
As a participant in cellular EET metabolism (1,4), roles for sHE in the pathophysiology of hypertension, renal injury, inflammation, and diabetes have been identified based mostly on inhibitor studies (10,11). However, studies with an sEH(-/-) mouse lacking the enzyme hydrolase and phosphatase activities yielded variable results with regards to the effects of gene disruption on EET levels, vasoactivity and blood pressures (68). Among issues in need of further study we emphasize: a) the accepted concept that EET hydration leads to functionally inactive DHETs (10,11), b) the bi-functional nature of sEH, and the coexistence of phosphatase and epoxide hydrolase domains at the protein N- and C-terminals, respectively (69), and c) the effects of sEH inhibitors on the phosphatase domain of the enzyme (68,69). Inasmuch as phosphatases play recognized physiological roles as modifiers of the functional properties of products of protein kinases, and/or phosphorylated lipid mediators this raises relevant questions regarding the contribution of the phosphatase domain to some of the functional roles described for sEH (70-72).
E) The characterization of mechanisms controlling the expression of selected AA epoxygenases and ω-hydroxylases in an organ/tissue/cell specific manner
The direct and indirect mechanisms by which P450 genes control functional phenotypes (18, 20) illustrates the importance of a gene-dependent regulation of different (and at times unrelated) genes in the development of functional phenotype(s). This added level of complexity could be of importance for the interpretation of associations between pathophysiological conditions and P450 gene variants. Furthermore, gene-mediated dysfunction can be masked or enhanced by co-existent morbidities (26,73) or be detectable under stress or non-stress conditions. Under a normal dietary salt intake Cyp2c44(-/-) mice show no overt blood pressure phenotypes; however, roles for this gene in blood pressure control or tumor angiogenesis are evidenced after the stress imposed by a high salt or potassium intake (22,40) or processes associated with tumorigenesis (23). At difference with Cyp2c44(-/-) mice, the hypertensive phenotypes of Cyp4a10(-/-) and Cyp4a14(-/-) mice are evident in the absence of dietary or other experimental manipulations (18,20).
It is of interest that the blood pressures of carriers of the CYP4A11 8590C allele are dietary salt sensitive (35) as is with Cyp4a10(-/-) mice (20), however, a relationship between this genotype, blood pressure and urinary 20-HETE excretion is yet to be established (73). Also, it remains to be determined: a) whether CYP2C8 and/or CYP2C9 derived EETs play a role in the hypertensive phenotype identified for carriers of the CYP4A11 8590C allele, and b) the prevalence and severity of hypertension in double carriers of the CYP4A11 8590C allele and CYP2C8*3/*3 or CYP2C9*2/*2 reduced EET synthase activity variants (74). A guanine to adenine (G/A) polymorphism in the human CYP4F2 gene has been associated with increases in blood pressure and urinary 20-HETE excretion (7,75). However, the variant gene codes for a protein with reduced 20-HETE synthase activities due to a valine to methionine substitution at residue 433 (75). This discrepancy between a reduced 20-HETE synthase activity and increased 20-HETE urinary excretion in carriers of the CYP4F2 G/A variant is reminiscent of what has been reported for hypertensive Cyp4a14(-/-) mice (18), and suggests roles for the CYP4F2 gene in the regulation of alternate pro- or antihypertensive gene(s).
Finally, there is a paucity of information regarding regulatory mechanisms that determines organ, tissue, and cell type selective expression of functionally significant P450 isoforms. This is relevant to their roles in kidney physiology/pathophysiology since the functional and biochemical segmentation along the length of the nephron is well known. Most of the data regarding zonal expression of CYP2C, CYP2J, CYP4A and CYP4F isoforms in rodent kidneys is based on either immunological, mRNA in-situ hybridization, or PCR amplification data (1-7). Although useful in distinguishing P450s from different gene subfamilies, these methods are limited when applied to P450s of the same gene subfamily since, in most cases, they share extensive open reading frame and amino acid sequence homology. Nevertheless, these limitations can be addressed by, for example, targeting mRNA untranslated segments, developing peptide antibodies against unique protein segments, performing differential analyses before and after gene deletion or antisense nucleotide inhibition or, when the methodology becomes more widely available, diagnostic peptide imaging of tissue sections by mass spectroscopy (23).
Highlights.
A dysfunctional murine Cyp4a14 gene causes androgen sensitive hypertension associated with increases in 20-HETE biosynthesis and renal vasoconstriction.
A dysfunctional murine Cyp4a10 or Cyp2c44 gene causes salt sensitive hypertension associated with decreases in urinary EETs and a hyperactive epithelial sodium channel (ENaC).
Roles for the Cyp2c44 arachidonic acid epoxygenase and mitogen activated kinases in the EET-mediated regulation of ENaC activity are proposed.
Acknowledgments
Work conducted in the authors’ laboratories was supported by NIDDKP01-038226 (to JHC and JRF), HLP01-34300 (to WHW) and a Robert A. Welch Foundation grant (I-0011 to JRF).
Dedicated to Professor John C. McGiff, M.D. (1927-2013) as testimony of his fundamental contributions to the studies of the Arachidonic Acid Monooxygenase. It was thanks to his efforts, foresight, and courage that this field of research gained the recognition that enjoys nowadays. He was an excellent scientist, a superb teacher and, most of all a wonderful colleague and friend. His contributions and his vision continue to inspire the research efforts of younger generations, and have served as an example to many, including the authors.
Footnotes
Authors Contribution: In addition to their key intellectual input in all the studies carried out by the authors and summarized in the manuscript:
Jorge H. Capdevila: developed and characterized Cyp4a and Cyp2c44 knockout mice lines, and wrote substantial portions of the manuscript.
Wen-Hui Wang: Identified and characterized roles of Cyp4a and Cyp2c and EETs in ENaC gating, and of high salt or high potassium diets in sodium excretion. Review the manuscript.
John R. Falck: Synthesized all P450 eicosanoids, EET analogs, and 20-HETE antagonist described in this work and/or published studies by the authors and collaborators. Review the manuscript.
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. and cited references. [PubMed] [Google Scholar]
- 2.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. and cited references. [DOI] [PubMed] [Google Scholar]
- 3.Pfister SL, Gauthier KM, Campbell WB. Vascular Pharmacology of epoxyeicosatrienoic acids. Advances Pharmacol. 2010;60:1–33. doi: 10.1016/B978-0-12-385061-4.00002-7. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. and cited references. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Falck JR, Imig JD. Roles of cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney International. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. and cited references. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila JH. Regulation of ion transport and blood pressure by cytochrome P450 moooxygenases. Curr Opin Nephrol Hypertens. 2007;16:465–470. doi: 10.1097/MNH.0b013e32827ab48c. and cited references. [DOI] [PubMed] [Google Scholar]
- 7.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and Blood Pressure Regulation. Clinical Implications. Cardiology in Review. 2014;22:1–12. doi: 10.1097/CRD.0b013e3182961659. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacos N, Capdevila J, Falck JR, Manna S, Wixtrom C, Gill SS, et al. The reaction of arachidonic acid epoxides with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 9.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, et al. Regio and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993;268:6402–6407. [PubMed] [Google Scholar]
- 10.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Ann Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karara A, Makita K, Jacobson HR, Falck JR, Guengerich FP, DuBois RN, et al. Molecular cloning, expression and enzymatic characterization of the rat kidney cytochrome P450 arachidonic acid epoxygenase. J Biol Chem. 1993;268:13565–13570. [PubMed] [Google Scholar]
- 13.DeLozier TC, Tsao CC, Coulter SJ, Foley J, Bradbury JA, Zeldin DC, et al. CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products. J Pharmacol Exp Ther. 2004;310:845–854. doi: 10.1124/jpet.104.067819. [DOI] [PubMed] [Google Scholar]
- 14.Holla VR, Makita K, Zaphiropoulus PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest. 1999;104:751–760. doi: 10.1172/JCI7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94:2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGiff JC. Cytochrome P-450 metabolism of AA. Annu Rev Pharmacol Toxicol. 1991;31:339–369. doi: 10.1146/annurev.pa.31.040191.002011. and references therein. [DOI] [PubMed] [Google Scholar]
- 17.Wang MH, Guan H, Nguyen X, Zand BA, Nasjletti A, Schwartzman ML. Contribution of Cytochrome P450 4A1 and 4A2 to vascular 20-hydroxyeicosatetraenoic acid synthesis in rat kidneys. Am J Physiol Renal Physiol. 1999;276:F247–F253. doi: 10.1152/ajprenal.1999.276.2.F246. [DOI] [PubMed] [Google Scholar]
- 18.Holla VR, Adas F, Imig JD, Zhao Y, Price E, Olsen N, et al. Alterations in the regulation of androgen-sensitive Cyp4a monooxygenases cause hypertension. Proc Natl Acad Sci USA. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, et al. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB. 2008;22:4096–4108. doi: 10.1096/fj.08-114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, et al. Salt sensitive hypertension is associated with a dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS. Disruption of mouse cytochrome P450 4f4 (Cyp4f4 gene) causes severe perturbations in vitamin E metabolism. J Biol Chem. 2012;287:26077–2608. doi: 10.1074/jbc.M112.373597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capdevila JH, Pidkovka N, Mei S, Gong Y, Falck JF, Imig JD, et al. The Cyp2c44 epoxygenase regulates epithelial sodium channel activity and the blood pressure responses to increase dietary salt. J Biol Chem. 2014;289:4377–4386. doi: 10.1074/jbc.M113.508416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozzi A, Popescu V, Yang S, Mei S, Shi M, Puolitaival SM, et al. The anti-tumorigenic properties of the peroxisomal proliferator-activated receptor α are arachidonic acid epoxygenase mediated. J Biol Chem. 2010;285:12840–12850. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, et al. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellamine A, Wang Y, Waterman M, Gainer JV, Dawson E, Brown N, et al. Characterization of the CYP4A11 gene, a second CYP4A gene in humans. Arch Biochem Biophys. 2003;409:221–227. doi: 10.1016/s0003-9861(02)00545-3. [DOI] [PubMed] [Google Scholar]
- 26.Gainer JV, Bellamine A, Dawson E, Womble KE, Grant SW, Wang Y, et al. A functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 27.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, et al. Androgen sensitive hypertension associated with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quigley R, Chakravarty S, Zhao X, Imig JD, Capdevila JH. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol. 2009;113:23–28. doi: 10.1159/000235774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, et al. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase dependent pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson EF, Hsu MH, Savas U, Griffin KJ. Regulation of P450 4A expression by peroxisomal proliferator activates receptors. Toxicology. 2002;181-182:203–206. doi: 10.1016/s0300-483x(02)00282-2. and cited references. [DOI] [PubMed] [Google Scholar]
- 31.Corton JC, Fan L-Q, Brown S, Anderson SP, Bocos C, Cattley RC, et al. Down-Regulation of Cytochrome P450 2C Family Members and Positive Acute-Phase Response Gene Expression by Peroxisome Proliferator Chemicals. Mol Pharm. 1998;54:463–473. doi: 10.1124/mol.54.3.463. [DOI] [PubMed] [Google Scholar]
- 32.Mayer B, Lieb WQ, Gotz A, Koning A, Aherrahrou Z, Thiemig A, et al. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg Echocardiographic Substudy. Hypertension. 2005;46:766–771. doi: 10.1161/01.HYP.0000182658.04299.15. [DOI] [PubMed] [Google Scholar]
- 33.Lacks C, Cardenas L, Devos A, Toure A, Cardenas-Garcia J, Kenani A. Arachidonic acid ω-hydroxylase CYP4A11: inter-ethnic variations in the 8590T>C loss-of-function variant. Mol Biol Rep. 2012;39:1503–1508. doi: 10.1007/s11033-011-0888-x. [DOI] [PubMed] [Google Scholar]
- 34.Liang JQ, Yan MR, Yang L, Suyila Q, Cui HW, Su XL. Association of a CYP4A11 polymorphism and hypertension in the Mongolian and Ham populations of China. Genet Mol Res. 2014;13:508–517. doi: 10.4238/2014.January.21.20. [DOI] [PubMed] [Google Scholar]
- 35.Williams JS, Hopkins PN, Jeunemaitre X, Brown NJ. CYP4A11 T8590C polymorphism, salt-sensitive hypertension, and renal blood flow. J Hypertens. 2011;29:1913–1918. doi: 10.1097/HJH.0b013e32834aa786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsao CC, Coulter SJ, Chien A, Luo G, Clayton NP, Maronpot R, et al. Identification and localization of five CYP2Cs in murine extrahepatic tissues and their metabolism of arachidonic acid to region- and stereoselective products. J Pharmacol Exp Ther. 2001;310:845–854. [PubMed] [Google Scholar]
- 37.Zeldin DC, DuBois RN, Falck JR, Capdevila JH. Molecular cloning and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys. 1995;322:76–86. doi: 10.1006/abbi.1995.1438. [DOI] [PubMed] [Google Scholar]
- 38.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive Actions. Front Physiol. 2010;1:1–8. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 40.Wang WH, Zhang C, Lin DH, Wang L, Yue P, Capdevila JH, et al. Cyp-epoxygenase in the connecting tubule(CNT)/cortical collecting duct (CCD) is essential for high potassium (K) intake-induced antihypertensive effect. Am J Physiol Renal Physiol. 2014;307:F453–F460. doi: 10.1152/ajprenal.00123.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capdevila JH, Wang WH. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens. 2013;22:163–169. doi: 10.1097/MNH.0b013e32835d911e. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol. 2004;287:F593–F601. doi: 10.1152/ajprenal.00454.2003. and cited references. [DOI] [PubMed] [Google Scholar]
- 43.Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D. Collecting duct-specific gene inactivation of αENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest. 2003;112:554–565. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder PM. The epithelial sodium channel, Cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocrine Reviews. 2002;23:258–275. doi: 10.1210/edrv.23.2.0458. [DOI] [PubMed] [Google Scholar]
- 45.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, et al. Interactions of β and ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 46.Shen JP, Cotton CU. Epidermal growth factor inhibits amiloride-sensitive sodium absorption in renal collecting duct cells. Am J Physiol Renal Physiol. 2003;284:F57–F64. doi: 10.1152/ajprenal.00028.2002. [DOI] [PubMed] [Google Scholar]
- 47.Zhang YH, Alvarez de la Rosa D, Canessa CM, Hayslett JP. Insulin-induced phosphorylation of ENaC correlates with increased sodium channel function in A6 cells. Am J Physiol Cell Physiol. 2005;288:C141–C147. doi: 10.1152/ajpcell.00343.2004. [DOI] [PubMed] [Google Scholar]
- 48.Hu JC, Bengrine A, Lis A, Awayda MS. Alternative mechanisms of activation of the epithelial Na+ channel by cleavage. J Biol Chem. 2009;284:36334–36345. doi: 10.1074/jbc.M109.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butterworth MB, Weisz OA, Johnson JP. Some assembly required. Putting the epithelial sodium channel together. J Biol Chem. 2008;283:35305–35309. doi: 10.1074/jbc.R800044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butterwork MB, Edinger RS, Fizzel RA, Johnson JP. Regulation of epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296:F10–F24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamm LL, Feng Z, Hering-Smith KS. Regulation of sodium transport by ENaC in the kidney. Curr Opin Nephrol Hypertens. 2010;19:98–105. doi: 10.1097/MNH.0b013e328332bda4. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen JK, Wang D-W, Falck JR, Capdevila JH, Harris RC. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicated that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J Biol Chem. 1999;274:4764–4769. doi: 10.1074/jbc.274.8.4764. [DOI] [PubMed] [Google Scholar]
- 53.Chen JK, Capdevila JH, Harris RC. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc Natl Acad Sci USA. 2002;99:6029–6034. doi: 10.1073/pnas.092671899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falin RA, Cotton CU. Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site. J Gen Physiol. 2007;130:313–328. doi: 10.1085/jgp.200709775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pidkovka N, Rao R, Mei S, Gong Y, Harris RC, Wang WH, et al. Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal- regulated kinase 1 2 (ERK1/2)-mediated phosphorylation. J Biol Chem. 2013;288:5223–5231. doi: 10.1074/jbc.M112.407981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skrypnyk N, Chen X, Hu W, Su Y, Mont S, Yang S, et al. Activation of peroxisomal proliferator activated receptor (PPAR)α is beneficial for the prevention and treatment of non-small cell lung cancer. Cancer Res. 74:621–631. doi: 10.1158/0008-5472.CAN-13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci. 1991;48:85–96. doi: 10.1016/0024-3205(91)90428-e. and cited references. [DOI] [PubMed] [Google Scholar]
- 58.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. and cited references. [DOI] [PubMed] [Google Scholar]
- 59.Riddick DS, Lee C, Bathena A, Timsit YE, Cheng PY, Morgan ET, et al. Transcriptional regulation of cytochrome P45 genes by endogenous and exogenous chemicals. Drug Metabolism Disposition. 2004;32:367–375. doi: 10.1124/dmd.32.4.367. and cited references. [DOI] [PubMed] [Google Scholar]
- 60.Cowart LA, Wei S, Hsu MH, Johnson EF, Krishna MV, Falck JR, et al. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisomal proliferator-activated receptor ligands. J Biol Chem. 2002;277:35105–35112. doi: 10.1074/jbc.M201575200. [DOI] [PubMed] [Google Scholar]
- 61.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutical target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 63.Guengerich FP. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006;8:101–111. doi: 10.1208/aapsj080112. and cited references. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong PY-K, Lai P-S, Shen S-Y, Belosludtsev YY, Falck JR. Post-receptor signal transduction and regulation of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. Journal of Lipid Mediators and Cell Signaling. 1997;16:155–169. doi: 10.1016/s0929-7855(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 65.Wong PY-K, Lai P-S, Falck JR. Mechanism and signal transduction of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostag Other Lipid Med. 2000;62:321–333. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 66.Karara A, Dishman E, Falck JR, Capdevila JH. Endogenous epoxyeicosatrienoyl-phospholipids: A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J Biol Chem. 1991;266:7561–7569. [PubMed] [Google Scholar]
- 67.Chen JK, Chen J, Imig JD, Wei S, Hachey DL, Guthi JS, et al. Identification of novel endogenous cytochrome P450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283:24514–24524. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris TR, Hammock BD. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene. 2013;526:61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cronin A, Mowbray S, Durk H, Homburg S, Fleming I, Fisslthaler B. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA. 2003;100:1552–1557. doi: 10.1073/pnas.0437829100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.EnayetAllah AE, Luria A, Luo B, Tsai H-J, Sura P, Hammock BD, et al. Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J Biol Chem. 2008;283:36592–36598. doi: 10.1074/jbc.M806315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oguro A, Imaoka S. Lysophosphatidic acids are new substrates for the phosphatase domain of soluble epoxide hydrolase. J Lipid Res. 2012;53:505–512. doi: 10.1194/jlr.M022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou H-H, Hammock BD, Su K-H, Morisseau C, Kou YR, Imaoka S, et al. N-terminal domain of soluble epoxide hydrolase negatively regulates the VEGF-mediated activation of endothelial nitric oxide synthase. Cardiovasc Res. 2012;93:120–129. doi: 10.1093/cvr/cvr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laffer CL, Gainer JV, Waterman MR, Capdevila JH, Laniado-Schwartzman M, Nasjletti A, et al. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicoatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lundblad MS, Stark K, Eliasson E, Oliw E, Rane A. Biosynthesis of epoxyeicosatrienoic acids varies between polymorphic CYP2C enzymes. Biochem Biophys Res Commun. 2005;327:1052–1057. doi: 10.1016/j.bbrc.2004.12.116. [DOI] [PubMed] [Google Scholar]
- 75.Ward NC, Tsai I-J, Barden A, van Bockxmeer FM, Puddey IB, Hogson JM, Croft KD. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51:1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]


