Abstract
The innate immune system provides an immediate and relatively non-specific response to infection with the aim of eliminating the pathogen before an infection can be fully established. Activation of innate immune response is achieved by production of pro-inflammatory cytokines and type I interferon (IFN). The IFN response in particular is one of the primary defenses utilized by the host innate immune system to control pathogen infection, like virus infection. Hence, viruses have learned to manipulate host immune control mechanisms to facilitate their propagation. Due to this, much work has been dedicated to the elucidation of the Kaposi’s sarcoma-associated herpesvirus (KSHV)-mediated immune evasion tactics that antagonize a host’s immune system. This review presents our current knowledge of the immune evasion strategies employed by KSHV at distinct stages of its life cycle to control a host’s immune system with a focus on interferon signaling.
INTRODUCTION
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is a DNA tumor virus that has been identified as the etiological agent of Kaposi’s sarcoma (KS) (1) as well as B-cell associated lymphoproliferative disorders, namely, primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD) (2, 3). In order to efficiently establish lifelong persistency as well as their life cycle, KSHV display latent and lytic cycles. Once KSHV infects the host, it maintains its genome as a multicopy circular episomal DNA and only a minimal number of viral genes are expressed (4). Upon certain circumstances, the virus switch into lytic replication, leading to a temporally-regulated cascade of viral gene expression accompanied by replication of the viral genomic DNA (5). Importantly, mounting data indicates that modulation of host immune response is critical for these life cycles of KSHV. Thereby, KSHV encodes numerous genes for immunomodulatory proteins that subvert the host immune system (6).
Viral infection of host cells gives rise to type I interferon (IFN) and pro-inflammatory cytokines, which are essential for host immunity to viruses. Thus, innate immune signaling plays a key role in immune surveillance by sensing pathogens and initiating protective immune responses. Notably, the responsible receptors/sensors belong to one of five types of pattern-recognition receptors (PRRs): Toll-like receptors (TLRs), C-type lectin receptors (CLRs), Retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and the AIM2-like receptors (ALRs) (7–9). These PRRs recognize conserved molecular structures of pathogens called pathogen-associated molecular patterns (PAMPs) and trigger production of proinflammatory cytokines and IFNs for host defense (7–9). Such molecules are involved in direct inhibition of viral replication, elimination of viral components from infected cells, or induction of apoptosis in infected cells. Additionally, these innate immune signals can activate host adaptive immunity, therefore, are fundamental for clearance of pathogens (10).
To evade elimination via host immune response, KSHV thus targets key regulatory steps of the host innate immune responses, including IFN-mediated anti-viral immunity. Here, we present our field’s current knowledge of the immune evasion strategies employed by KSHV to control the type I IFN signaling cascade, with a specific focus on how KSHV modulates IFNs production.
1. IFN pathway
As we described above, one of the primary cellular responses to viral infection is expression of the type I IFNs (IFN-α and IFN-β) that result in the expression of genes that suppress cell growth, promote apoptosis, enhance antigen presentation, and modulate several signal transduction pathways. These genes are upregulated by interferon regulatory factors (IRFs), a family of transcription factors that are activated by IFN signaling through their cognate type I receptor (IFNAR). All IRFs share homology in the C-terminal region, which contains the IRF-association domain (IAD), and the N-terminal region has the DNA-binding domain (DBD), which is characterized by the presence of five tryptophan repeats. In brief, activation of IFN by viral infection leads to phosphorylation, dimerization, and translocation of IRFs, thereby it produces type I IFNs (IFN-α and IFN-β). Hence, it is not surprising that several KSHV proteins, in the form of viral IRFs (vIRFs), have evolutionarily developed various tactics to subvert these pathways to the advantage of their life cycles.
vIRF1 (K9)
vIRF1 was the first vIRF found to effectively repress cellular type I and type II IFN responses (11, 12). One of its known mechanisms is by inhibiting IRF1 transactivation independently of competition with IRF1 for DNA binding (12). Alternatively, vIRF1 binds to transcriptional cofactor p300 and interferes with CBP/p300-IRF3 complex formation, along with decreasing p300 histone acetyltransferase (HAT) activity, thus preventing IRF3-mediated transcriptional activation (13, 14). Recently, it was shown that vIRF1, vIRF2, vIRF3 have different capabilities to block Toll-like receptor 3 (TLR3)-mediated IFN induction (15). First, vIRF1 and vIRF2 inhibit transcription and translation of IFN-β upon TLR3 activation (15). Second, only vIRF1 but not vIRF2 nor vIRF3 reduce phosphorylation and nuclear translocation of IRF3 upon TLR3 activation (15). Overall, it implies that vIRFs might selectively inhibit TLRs in order to inhibit TLR-mediated IFN production.
vIRF2 (K11/K11.1)
Full-length vIRF2, which is translated from exons K11 and K11.1, represses IRF3 mediated IFN-β transcriptional activity via stimulation of IRF3 degradation and inhibition of IRF3 transactivation (16). vIRF2 inhibits IFN-α/β driven signaling as well as signaling induced by IFN-λ (17). The underlying mechanism, however, has yet to be defined. In addition, vIRF2 reduces the activation of the IFN-induced interferon-response element (ISRE) promoter through the deregulation of IFN-stimulated gene factor-3 (ISGF-3) (18). It is suggested that vIRF2 possesses pleiotropic activity to inhibit early type I IFN (IFN enhanceosome-dependent) and delayed type I IFN (ISGF-dependent) responses. Furthermore, previous studies have shown that the first exon of vIRF2 (K11.1) prevents PKR kinase activity (19), reducing protein synthesis and blocking IFN-α/β signaling to decrease the ability of the cells to respond to viral infections (20, 21). Based on a binding assay, this short form of K11.1 interacts with cellular IRF1, IRF2, IRF8, RelA, and p300, but not IRF3.
vIRF3 (K10.5)
vIRF3 interaction with cellular IRF7, suppresses IRF7 DNA binding, therefore, inhibits IFN-mediated immunity through the inhibition of IFN-α production (22). Remarkably, a putative double α-helix motif of vIRF3 (residues 240–280) that has been shown to be responsible for the interaction of vIRF3 with IRF7 is also sufficient to bind to IRF5 (23). As a result of this interaction, vIRF3 inhibits IRF5-mediated ISRE and IFN-β promoter activity (23). It was recently shown that vIRF3 is required for the survival of PEL cells. RNA interference (RNAi) knockdown of vIRF3 in PEL cells reduced cell proliferation by releasing IRF5 from p21 promoter transcription complexes.
In summary, the downregulation of the IFN regulatory pathway is a common characteristic of the three vIRFs whose functions have been well studied. While it remains to be discovered whether vIRF4, the most recently identified member of the vIRF family, affects IFN-mediated innate immunity as well, we expect that it would as IRF3 and IRF7 are key initiation factors of the host immune surveillance program against viral infection.
ORF45
ORF45, like the vIRFs, blocks phosphorylation and nuclear translocation of IRF7, resulting in suppression of IRF7 (24, 25). Ultimately, it decreases type I IFN mRNA levels. More interestingly, ORF45 interacts with the inhibitory domain of IRF7, thus maintaining the IRF7 in a closed form and hindering it from being activated by KSHV infection (26). Indeed, ORF45 deletion recombinant KSHV has an enhanced immune response, associated with an increase in transcription of type I IFNs (27). Collectively, it is indicated that ORF45 plays a crucial role in the abrogation of type I IFN response, which results in either promoting efficient viral replication or facilitating viral persistence.
RTA (ORF50)
The replication and transcription activator (RTA) is a master regulator for a switch from latency to lytic replication (28). An early study shows that RTA leads to proteasome-dependent degradation of IRF7, subsequently blocking IFN-α/β production (29). Addition of RTA also promotes polyubiquitination of IRF7 in an in vitro assay, suggesting that RTA acts as an ubiquitin E3 ligase (29). More interestingly, further study provides detailed evidence that RTA, a viral ubiquitin E3 ligase, cooperates with cellular E3 RTA-associated ubiquitin ligase (RAUL), also known as UBE3C, to enhance the proteasomal degradation of both IRF3 and IRF7 (30). Given its crucial role in type I IFN regulation, control of RAUL function by RTA allows IFN restraint.
K-bZIP (K8)
K-bZIP, a leucine zipper-containing transcription factor, is an immediate-early gene that is turned on by RTA. Interestingly, it represses RTA-mediated transactivation of it own promoter, thereby functioning as a transcriptional repressor (31). Therefore, K-bZIP negatively regulates IFN-β production by blocking IRF3 occupancy on the IFN-β promoter region, impairing formation of the enhanceosome IRF3-CBP/p300 (31, 32).
RIF (ORF10)
RIF, KSHV ORF10, is another regulator of IFN function that is classified as a delayed-early gene (33). So far, we have summarized evidence that several KSHV viral genes impair an amplification loop in type I IFN production in order to describe how KSHV subverts host IFN production. However, RIF possesses the unique capability to affect the state of type I IFN signaling. RIF blocks IFN signaling by forming inhibitory complexes that contain IFNAR subunits, the Janus kinases Jak1 and Tyk2, and the STAT2 transcription factor (33). Additionally, RIF mediates the aberrant recruitment of STAT2 to IFNAR1 despite inhibition of Tyk2 activity, which impairs the phosphorylation of STAT2, which subsequently prevents ISGF3 accumulation in the nucleus (33). Hence, RIF seems to be a pleiotropic regulator of IFN signaling pathway, exerting its effects within the signaling cascade.
2. TLR-mediated pathway
TLRs are transmembrane proteins that contain luminal leucine-rich repeats (LRRs), which contribute to ligand recognition and cytoplasmic Toll/interleukin-1 (IL-1) receptor homology (TIR) domains that signal through down-stream adaptors (8). To date, 11 human TLRs and 13 murine TLRs have been identified. TLRs involved in the detection of viral nucleic acids are located on the cell surface (TLR3) or in endosomal compartments (TLR3, TLR7, TLR8, and TLR9) (34). Accumulating evidence in relation to TLR signal transduction has demonstrated that TLR3 recognizes double-stranded RNA (dsRNA), which constitutes the genome of dsRNA viruses and is also an intermediate during replication of single-strand RNA (ssRNA) viruses (35). TLR7 and TLR8 recognize ssRNA (35) and TLR9 recognizes unmethylated CpG-containing DNA, which is commonly found in the genomes of DNA viruses like KSHV (8, 36).
Ligand recognition by TLRs leads to the recruitment of various TIR domain-containing adaptors, such as MyD88, TRIF, TRAM, and more (35). This recruitment of adaptors triggers the signaling pathway cascade, leading to activation of factors such as NF-κB and IRFs. Ultimately, it induces inflammatory cytokines and type I IFN signaling. Thus, the TLR signaling pathway is categorized into MyD88-dependent and TRIF-dependent pathways (8, 37, 38). The MyD88-dependent pathway is utilized by all TLRs except TLR3. Stimulation with the TLR ligand recruits MyD88 and IRAK, which leads to the activation of TRAF6. TRAF6 causes activation of TAK1, which results in activation of NF-κB and AP-1 through the IKK complex and MAP kinases, respectively. On the other hand, TLR3 and TLR4 initiate a TRIF-dependent pathway to induce inflammatory cytokines and type I IFN signaling. The TRIF-dependent pathway activates NF-κB via two independent pathways: the N-terminal domain of TRIF interacts with TRAF3 and activates NF-κB, while the C-terminal domain of TRIF interacts with RIP1 and activates TAK1 (8, 37–39). Given the role of TLRs in the activation of IRFs in response to viral infection, a recent growing body of information is beginning to shed light on TLR recognition of KSHV.
RTA (ORF50)
RTA targets several key components (IRF7, TRIF, TLR2, and TLR4) to effectively regulate IFN production as part of the host defense mechanism. RTA, viral E3 ubiquitin ligase, effectively inhibits TLR2 and TLR4 signaling in THP-1 monocytes by causing their degradation (40). In addition, RTA indirectly degrades TRIF (41). Given TRIF is absolutely required for TLR-3 signaling, RTA-mediated reduction of TRIF downregulates TLR-3 signaling (41). Consequently, RTA blocks IFN production by targeting cellular TRIF. More interestingly, TRIF enhances not only TLR3 activation but also KSHV RTA protein expression (42). Collectively, there is a potential regulatory loop: the activation of TRIF leads to enhanced KSHV RTA protein expression and RTA degrades TRIF to block innate immunity induced by TLR activation in order to either evade or utilize the host innate immune system for its own benefits.
As mentioned earlier, MyD88 is another adaptor that initiates signaling transduction pathway through all human TLRs except TLR3 (8, 37, 38). Very recently, Zhao et al. found that RTA also induces the proteasomal degradation of MyD88 through its direct interaction with MyD88, thereby repressing TLR4 signaling mediated IFN production and NF-κB activity (43). Furthermore, MyD88 was downregulated during the early stages of de novo infection and lytic reactivation. Overall, multiple strategies are adopted by RTA to avoid TLR-mediated signaling effectively. This indicates both that RTA is a crucial virulent factor and that antagonizing the TLR-mediated type I IFN signaling is important for immune evasion.
vIRFs
At first, KSHV infection increases TLR3, CXC chemokine ligand 10 (CXCL10), and IFN-β transcripts (44). In contrast, at later time points postinfection, TLR3 and CXCL10 transcripts are decreased (15). Notably, expression of KSHV vIRF1, -2, or -3 blocks TLR3-mediated activation of IFN-responsive promoter activity (15). Remarkably, both vIRF1 and vIRF2 inhibit IFN--β production upon TLR3 activation. However, it seems that vIRF1 and vIRF2 block TLR3-mediating signaling via different mechanisms: expressing vIRF1 but not vIRF2 led to decreased phosphorylation and nuclear translocation of IRF3 in response to TLR3 activation (15). It is indicated that each vIRF might have a unique capability to block TLR3-mediated antiviral immune response.
KSHV miRNAs (microRNAs)
Thus far, 12 KSHV pre-miRNAs, encoding 18 mature miRNAs, have been identified and these miRNAs are located in the KSHV latency locus (45, 46). Abend et al. demonstrated that the KSHV-encoded miRNAs miR-K9 and miR-K5 regulate the TLR/IL-1R signaling cascade by targeting two important components, IRAK1 and MyD88 (47). Especially, miR-K9-mediated downregulation of IRAK1 blocks IL-1α and TLR7/8 agonist-induced NF-κB activation (47).
Cell Type Dependency
Infection of endothelial cells with KSHV causes suppression of TLR4 through the activation of the extracellular signal-regulated kinase (ERK) MAPK pathway via viral gene expression-independent mechanisms and viral gene expression-dependent mechanisms by way of vIRF1 and vGPCR (48). In addition, stimulation of TLR7 and TLR8 reactivates KSHV in latently infected B lymphocytes (49). A later study showed that KSHV infection of plasmacytoid dendritic cells (pDCs) activates the TLR9 signaling pathway, leading to the upregulation of CD83 and CD86, and secretion of IFN-α (50). Overall, there is evidence that KSHV has developed different ways to escape TLR-mediated detection throughout their lifecycle to limit KSHV lytic replication and facilitate the establishment of latency.
3. NLR-mediated pathway
NLRs comprise a family of more than 22 members of cytoplasmic receptor proteins that are characterized by a conserved NOD motif (51, 52). Another feature of the NLR family is the presence of a LRR domain with the proposed function of detecting PAMPs, thus leading to NLR activation (53). NLRs can be divided into 4 subfamilies, based on different N-terminal effector domains: caspase recruitment domain (CARD), pyrin domain (PYD), baculoviral inhibitor of apoptosis repeat (BIR) domain, and the transactivation domain. Once activated, NLRs expose the N-terminal effector domains. This effector domain allows for homotypic protein-protein interactions with adaptor proteins and also interacts directly with enzymes such as procaspase- 1. Oligomerization of NLRs is energy-dependent and requires ATP. NLR activation consequently turns on multiple signaling pathways, including inflammasomes. Furthermore, the NLR proteins NOD1 and NOD2 interact with RIP2 and induce the activation of IRF3, IRF7, and the NF-κB and MAPK signaling pathways (52). Thus, NLR proteins can regulate both inflammatory and IFN pathways. A growing body of evidence shows some crosstalk between these two arms of the innate immune response.
In an effort to suppress the NLR-mediated host defense mechanism, remarkably, KSHV encodes for a protein named ORF36, a viral homolog of human NLRP1 without the CARD and PYD effector domains of its cellular counterpart, which blocks NLRP1-dependent caspase-1 activation and processing of IL-1β and IL-18 (49). More interestingly, ORF36 also inhibits NLRP3 activity even though it does not have significant similarity to NLRP3 (49), which suggests that ORF36 may broadly inhibit NLR-mediated signaling during the KSHV life cycle.
4. RLR-mediated pathway
RLRs are cytoplasmic proteins that detect the presence of foreign RNA, such as viral products within the cytosol. There are currently three RLR family members: retinoic acid-inducible gene-I (RIG-I), melanoma differentiation gene 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2) (54, 55). Both RIG-I and MDA5 contain a DExH/D box helicase domain that binds dsRNA and two N-terminal CARDs involved in signaling (8, 55). Specifically, RIG-I contains a C-terminal repressor domain that blocks signaling in the absence of ligand binding (34). RIG-I binds preferentially to ssRNA bearing 5’-triphosphate and short dsRNA (56, 57). MDA5 recognizes long dsRNA and does not require 5’-phosphorylation (57, 58). Once RIG-I recognizes viral RNA, it initiates a signaling cascade that results in production of type I IFN and proinflammatory cytokines (54–56, 59). One of the key components of this signaling cascade is mitochondrial antiviral signaling protein (MAVS). MAVS is a membrane-bound adaptor protein that transmits signals from RIG-I to downstream signaling molecules, most notably TBK-1 and the IκB kinase (IKK) family proteins (60).
Initially, KSHV ORF64, a tegument protein with deubiquitinase (DUB) activity, was shown to downregulate RIG-I-mediated IFN signaling by reducing the ubiquitination of RIG-I, ultimately decreasing RIG-I activation (61). Furthermore, West et al. recently showed that in cells depleted of either MAVS or RIG-I, KSHV infection increases global transcription of the viral genome during de novo infection (62). This indicates that both MAVS and RIG-I inhibit KSHV transcription after primary infection. Indeed, whole-genome transcriptome analysis discovered that many additional long and short noncoding RNAs are transcribed from the KSHV genome together with viral miRNAs (63, 64). Thus, given the importance of RIG-I in the activation of IRFs and type I IFNs, an efficient and immediate downregulation of its signaling pathway following both KSHV infection and reactivation helps the virus to effectively evade host antiviral immune responses.
5. ALR-mediated pathway
The ALRs participate in the detection of intracellular DNA. These receptors have a PYRIN domain allowing for protein-protein interactions and a DNA-binding HIN-200 domain. The first member of this family discovered, AIM2, interacts with the adaptor ASC and promotes inflammasome formation following the detection of intracellular DNA (8, 65–67). A more diverse set of functions has been attributed to a second member, gamma interferon-inducible protein 16 (IFI16), including the activation of STING-dependent IFN production and inflammasome formation (8, 68, 69). For the first time, Kerur et al. described that during de novo KSHV infection, KSHV induces caspase-1 activation by targeting IFI16 and ASC (68). Interestingly, IFI16-mediated inflammasome induction by KSHV was associated with sub-cellular redistribution of ASC, caspase-1, and IFI16 from the nucleus to the cytoplasm (68). Later, they showed that KSHV latency-associated viral FLIP (vFLIP) gene induced the expression of IL-1β, IL-18, and caspase-1 transcripts in a NF-κB dependent manner. Furthermore, IFI16 colocalizes with multiple copies of the latent KSHV genome (70). Overall, this indicates that the constant activation of inflammasomes against KSHV during latency could be one of the driving forces for the inflammatory and angiogenic responses detected in KSHV-associated diseases such as KS and PEL.
CONCLUDING REMARKS
As we have seen, viruses are masters of trickery. They mimic, manipulate, and usurp cellular functions, but always with an attractive twist to the multifaceted roles of virus-host interactions. The research discussed herein focuses primarily on how KSHV efficiently manipulates production of type I IFN. Hence, studies that add to our growing knowledge of viral immunomodulatory proteins might help us uncover new human genes that control immunity. Their characterization will increase our understanding of not only viral pathogenesis, but also normal immune mechanisms. Furthermore, mechanisms used by viral proteins suggest strategies of immune modulation that might have therapeutic potential.
KSHV, a sub-family of gamma-herpesvirus, contains a large double stranded DNA genome that encodes for over eighty open reading frames. Thus, as we described in the main text, different PRRs, such as TLRs, NRLs, RLRs, and ALRs are activated upon KSHV infection in different cell types. DNA was known to be a potent immune stimulant, thereby, the presence of cytosolic DNA through viral infection triggers a robust immune response including inflammasome activation and type I IFNs induction. Even though host immunity is activated in this way upon KSHV infection, the virus is still capable of establishing latency in infected cells, indicating that KSHV encodes for proteins that dysregulate this pathway. Current several important lines of evidence support cyclin-GMP-AMP (cGAMP) synthase (cGAS) as the long-sought-after STING-dependent cytosolic DNA sensor, which activates STING through the synthesis of the second message 2’-3’-cGAMP (71). Given STING’s critical role in host protection and disease, there is currently a strong interest in understanding the role of KSHV in cGAS-mediated signaling.
Although there are proven mechanisms for numerous immune evasion strategies employed by KSHV, they might only be the tip of the iceberg. Therefore, an understanding of the complexity and specificity of KSHV’s strategies to subvert host immune mechanisms and how they are integrated with KSHV-associated pathogenesis has the potential to inform appropriate therapeutic strategies and effective clinical management.
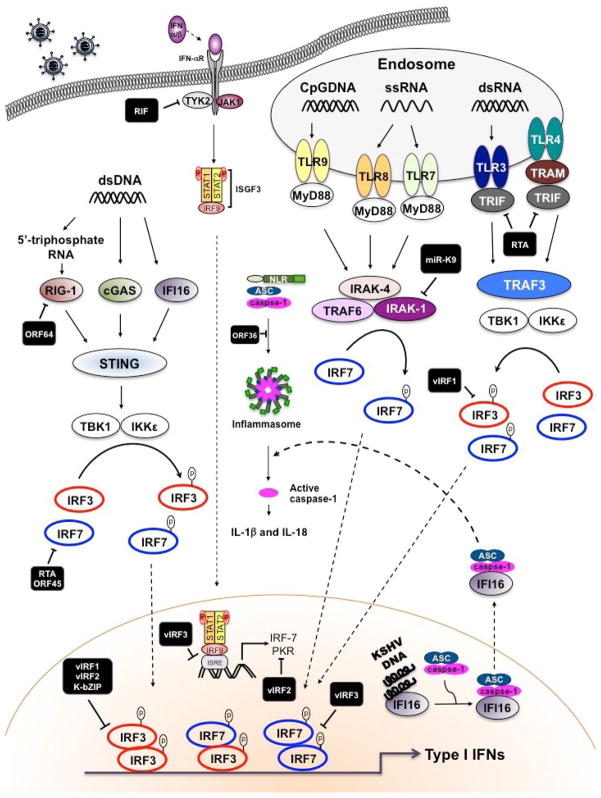
Figure 1.
An outline of the inhibition of type I IFN signaling pathways by viral proteins of KSHV. Following KSHV infection of cells, specific TLRs are activated, initiating the recruitment of adaptor proteins, MyD88 and TRIF. Subsequently, it leads to the activation of TBK1 and IKKε, which phosphorylate IRF3. IRF3 then dimerizes and translocates to the nucleus and participates in the transcriptional activation of the IFN-β promoter. Binding of newly secreted IFN-β to the type I IFN receptor (IFNAR1) leads to the activation of the JAK-STAT, resulting in forming thee ISGF3 complex. ISGF3 binds to ISRE found in numerous IFN-induced gene promoters, like IRF7. Newly synthesized IRF7 is phosphorylated by TBK1 and IKKε, which can then homodimerize or heterodimerize with IRF3 before binding to the promoters of the genes that encode IFN-α/β. Moreover, dsDNA accumulates in the cytoplasm after infection by KSHV. The intracellular DNA recognized by cytoplasmic receptor, IFI16 and cGAS. Alternatively, dsDNA is transcribed into dsRNA by polymerase III in cell-type specific manner. Generated dsRNA is recognized by RIG-1 and production of type I IFNs are induced. Following DNA stimulation, an ER protein STING translocates from the ER to the cytoplasmic punctate structure and subsequently it recruits TBK1 and IKKε. Among the KSHV-encoded proteins, numerous proteins target this pathway. Black squares indicate KSHV proteins.
Highlights.
Activation of innate immune response.
Type I interferon response to control pathogen infection.
Kaposi’s sarcoma-associated herpesvirus (KSHV)-mediated immune evasion tactics
Acknowledgments
This work was partly supported by NIH CA82057, CA31363, CA115284, CA180779, HL110609, DE023926, AI105909, AI073099, AI116585, Hastings Foundation, Fletcher Jones Foundation, GRL Program (K20815000001) and KRIBB (JUJ). Finally, we thank all of JJ’s lab members for their discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang Y, et al. Science. 1994 Dec 16;266:1865. [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. N Engl J Med. 1995 May 4;332:1186. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, et al. Blood. 1995 Aug 15;86:1276. [PubMed] [Google Scholar]
- 4.Zhong W, Wang H, Herndier B, Ganem D. Proc Natl Acad Sci U S A. 1996 Jun 25;93:6641. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renne R, et al. Nat Med. 1996 Mar;2:342. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 6.Lee HR, Brulois K, Wong L, Jung JU. Front Microbiol. 2012;3:44. doi: 10.3389/fmicb.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann J, Akira S. Curr Opin Immunol. 2013 Feb;25:1. doi: 10.1016/j.coi.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Annu Rev Immunol. 2015 Jan 2; doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan K, Bowie AG. Curr Opin Microbiol. 2010 Aug;13:503. doi: 10.1016/j.mib.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Nie Y, Wang YY. Protein Cell. 2013 Jan;4:1. doi: 10.1007/s13238-012-2122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao SJ, et al. Oncogene. 1997 Oct 16;15:1979. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- 12.Zimring JC, Goodbourn S, Offermann MK. J Virol. 1998 Jan;72:701. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, et al. Mol Cell Biol. 2000 Nov;20:8254. doi: 10.1128/mcb.20.21.8254-8263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin R, et al. Oncogene. 2001 Feb 15;20:800. doi: 10.1038/sj.onc.1204163. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs SR, et al. J Virol. 2013 Jan;87:798. doi: 10.1128/JVI.01851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Areste C, Mutocheluh M, Blackbourn DJ. J Biol Chem. 2009 Aug 28;284:23272. doi: 10.1074/jbc.M109.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuld S, Cunningham C, Klucher K, Davison AJ, Blackbourn DJ. J Virol. 2006 Mar;80:3092. doi: 10.1128/JVI.80.6.3092-3097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutocheluh M, et al. J Gen Virol. Oct;92:2394. doi: 10.1099/vir.0.034322-0. [DOI] [PubMed] [Google Scholar]
- 19.Burysek L, Pitha PM. J Virol. 2001 Mar;75:2345. doi: 10.1128/JVI.75.5.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burysek L, et al. J Virol. 1999 Sep;73:7334. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burysek L, Yeow WS, Pitha PM. J Hum Virol. 1999 Jan-Feb;2:19. [PubMed] [Google Scholar]
- 22.Joo CH, et al. J Virol. 2007 Aug;81:8282. doi: 10.1128/JVI.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wies E, et al. J Biol Chem. 2009 Mar 27;284:8525. doi: 10.1074/jbc.M809252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Q, et al. J Virol. 2012 Sep;86:10162. doi: 10.1128/JVI.05224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. Proc Natl Acad Sci U S A. 2002 Apr 16;99:5573. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sathish N, Zhu FX, Golub EE, Liang Q, Yuan Y. J Biol Chem. 2011 Jan 7;286:746. doi: 10.1074/jbc.M110.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu FX, Sathish N, Yuan Y. PLoS One. 2010;5:e10573. doi: 10.1371/journal.pone.0010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun R, et al. Proc Natl Acad Sci U S A. 1998 Sep 1;95:10866. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Wang SE, Hayward GS. Immunity. 2005 Jan;22:59. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Hayward GS. Immunity. 2010 Dec 14;33:863. doi: 10.1016/j.immuni.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao W, Tang Y, Lin SF, Kung HJ, Giam CZ. J Virol. 2003 Mar;77:3809. doi: 10.1128/JVI.77.6.3809-3815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefort S, Soucy-Faulkner A, Grandvaux N, Flamand L. J Virol. 2007 Oct;81:10950. doi: 10.1128/JVI.00183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisson SA, Page AL, Ganem D. J Virol. 2009 May;83:5056. doi: 10.1128/JVI.02516-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCartney SA, Colonna M. Immunol Rev. 2009 Jan;227:87. doi: 10.1111/j.1600-065X.2008.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasaki A, Medzhitov R. Nat Immunol. 2004 Oct;5:987. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 36.Bauer S, et al. Proc Natl Acad Sci U S A. 2001 Jul 31;98:9237. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akira S, Uematsu S, Takeuchi O. Cell. 2006 Feb 24;124:783. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Beutler BA. Blood. 2009 Feb 12;113:1399. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar H, Kawai T, Akira S. Biochem Biophys Res Commun. 2009 Oct 30;388:621. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 40.Bussey KA, et al. J Virol. 2014 Aug;88:9245. doi: 10.1128/JVI.00841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad H, et al. J Biol Chem. 2011 Mar 11;286:7865. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer F, et al. J Biol Chem. 2013 Jul 12;288:20435. doi: 10.1074/jbc.M113.487421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Q, et al. J Virol. 2015 Jan 1;89:415. doi: 10.1128/JVI.02591-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West J, Damania B. J Virol. 2008 Jun;82:5440. doi: 10.1128/JVI.02590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellare P, Ganem D. Cell Host Microbe. 2009 Dec 17;6:570. doi: 10.1016/j.chom.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walz N, Christalla T, Tessmer U, Grundhoff A. J Virol. 2010 Jan;84:716. doi: 10.1128/JVI.01302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abend JR, et al. J Virol. 2012 Nov;86:11663. doi: 10.1128/JVI.01147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagos D, et al. Cell Host Microbe. 2008 Nov 13;4:470. doi: 10.1016/j.chom.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregory SM, et al. Science. 2011 Jan 21;331:330. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West JA, Gregory SM, Sivaraman V, Su L, Damania B. J Virol. 2011 Jan;85:895. doi: 10.1128/JVI.01007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harton JA, Linhoff MW, Zhang J, Ting JP. J Immunol. 2002 Oct 15;169:4088. doi: 10.4049/jimmunol.169.8.4088. [DOI] [PubMed] [Google Scholar]
- 52.Lupfer C, Kanneganti TD. Immunol Rev. 2013 Sep;255:13. doi: 10.1111/imr.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monie TP. Trends Biochem Sci. 2013 Mar;38:131. doi: 10.1016/j.tibs.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Yoneyama M, et al. Nat Immunol. 2004 Jul;5:730. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 55.Yoneyama M, et al. J Immunol. 2005 Sep 1;175:2851. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 56.Pichlmair A, et al. Science. 2006 Nov 10;314:997. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 57.Saito T, Gale M., Jr J Exp Med. 2008 Jul 7;205:1523. doi: 10.1084/jem.20081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato H, et al. Nature. 2006 May 4;441:101. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 59.Gack MU, et al. Nature. 2007 Apr 19;446:916. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald KA, et al. Nat Immunol. 2003 May;4:491. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 61.Inn KS, et al. J Virol. 2011 Oct;85:10899. doi: 10.1128/JVI.00690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.West JA, et al. J Virol. 2014 May;88:5778. doi: 10.1128/JVI.03226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Nat Rev Immunol. 2011 Feb;11:143. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chandriani S, Xu Y, Ganem D. J Virol. 2010 Aug;84:7934. doi: 10.1128/JVI.00645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. Nature. 2009 Mar 26;458:509. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hornung V, et al. Nature. 2009 Mar 26;458:514. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burckstummer T, et al. Nat Immunol. 2009 Mar;10:266. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 68.Kerur N, et al. Cell Host Microbe. 2011 May 19;9:363. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unterholzner L, et al. Nat Immunol. 2010 Nov;11:997. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh VV, et al. J Virol. 2013 Apr;87:4417. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai X, Chiu YH, Chen ZJ. Mol Cell. 2014 Apr 24;54:289. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]