Abstract
Sepsis is an enormous public health issue and the leading cause of death in critically ill patients in intensive care units (ICU). Overwhelming inflammation, characterized by cytokine storm, oxidative threats, and neutrophil sequestration is an underlying component of sepsis-associated organ failure. Despite recent advances in sepsis research, there is still no effective treatment available beyond the standard of care and supportive therapy. To reduce sepsis-related mortality, a better understanding of the biological mechanism associated with the sepsis is essential. Endoplasmic reticulum (ER), a subcellular organelle is responsible for the facilitation of protein folding and assembly and involved in several other physiological activities. Under the stress and inflammation condition, ER loses the homeostasis in its function, which is termed as ER stress. During ER stress, unfolded protein response (UPR) is activated to restore ER function to its normal balance. However, once the stress is beyond the compensatory capacity of UPR or protracted, the apoptosis would be initiated by triggering cell injuries, even to cell death. As such, ER stress and UPR are reported to be implicated in several pathological and inflammatory conditions. Although the detrimental role of ER stress during infections has been demonstrated, there is growing evidences that ER stress participate in the pathogenesis of sepsis. In this review, we summarize the current research in the context of ER stress and UPR signaling associated with sepsis and its related clinical conditions, such as trauma- hemorrhage, and ischemia/reperfusion (I/R) injury. We also discuss the potential implication of ER stress as a novel therapeutic target and prognostic marker in patients with sepsis.
Keywords: ER stress, UPR, sepsis, inflammation, apoptosis
Introduction
Sepsis and septic shock are serious healthcare issue, resulting from severe inflammatory response to infection and injury that lead to multiple organ failure [1-3]. Sepsis progresses quickly and general public is ‘little’ aware of its threat. Sepsis referred as ‘equal-opportunity’ threat since it does not respect the age, sex, race or economic status of its victims and has been a scourge of the human race for thousands of years. It is estimated that 18 million cases of sepsis occur each year in the world, with mortality rates ranging as high as 30% to 50% [4]. Those septic patients who initially survive, experience functional deficits and impaired quality of life, in addition to being at risk for higher mortality. In the United States, the annual cost of providing care to septic patients is approximately $24 billion [5, 6]. Currently, there is no effectively therapeutic intervention against sepsis approved by the Food and Drug Administration (FDA). In the past 30 years, there has only been one FDA approved medicine (i.e., activated protein C or Xigris), but it was voluntarily withdrawn in 2011 by the manufacturer since follow-up studies did not show substantial improvement in the survival of septic patients [7]. Understanding the biological process involved in the pathogenesis of sepsis is an important first step in improving outcomes and treating the patients.
The endoplasmic reticulum (ER) is a vital intracellular organelle in the secretory pathways, commonly known as protein folding factory [8-10]. It is responsible for protein translocation, protein folding, and protein post-translational modifications that allow further transport of proteins to the Golgi body. Moreover, ER provides the place for calcium storage, lipid synthesis and carbohydrate metabolism. Certain pathological conditions, such as sepsis, trauma, ischemia and viral infection as well as few pharmacological agents including tunicamycin, thapsigargin, brefeldin A, lead to the accumulation of unfolded or misfolded proteins that alter the homeostasis of the ER and cause ER stress [11-14]. ER is equipped with three super specialized sentinel ER membrane-embedded proteins named inositol-requiring protein 1 (IRE1), double-stranded RNA-dependent protein kinase (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6) to sense the stress in ER [15-18]. When these sensors recognize the enhanced ER stress, the ER elicits a sophisticated and complex adaptive response, referred as unfolded protein response (UPR) to rebuilt cellular homeostasis [16, 19-21]. The aim of these signaling cascades is to reduce the accumulation of unfolded proteins in the ER; however, when these events are unresolved or protracted, it initiates apoptosis and other components of cell death [8, 10, 22, 23].
There is growing interest in investigating the regulatory mechanisms underlying ER stress and UPR signaling and the development of strategies to target this pathway, since there is substantial evidences for the participation of ER stress in many diseases, including inflammatory disorders, neurodegeneration, cancer, and diabetes [18, 24-26]. Interestingly, attenuation of ER stress with pharmacological or gene therapy strategies has been successful in reducing pathological features in various experimental models of inflammatory diseases [14, 24, 27, 28]. Thus, we demonstrate that investigation on ER stress will hold promise to reveal novel therapeutic targets for the inflammatory diseases including sepsis in human. This review highlights recent progress towards the understanding of the role of the ER stress in sepsis and its related clinical conditions. Specifically, we focus on the possible implication of ER stress and its constituents in sepsis, trauma-hemorrhage, and ischemia/reperfusion (I/R) injury and the potential use as prognostic markers and a novel therapeutic target under those clinical conditions.
ER Stress and UPR: an overview
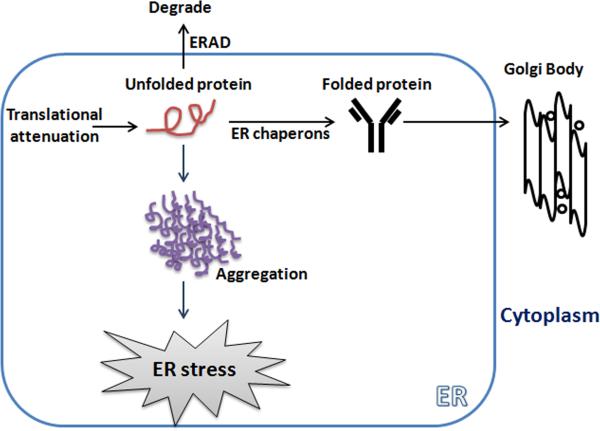
The ER is a complex network of tubules and flattened sacs that have a variety of physiological functions in the cell. The ER serves the role in the adequate folding of nascent protein and the transport of synthesized proteins to the Golgi body. To ensure proper protein folding, the ER maintains a unique environment by several ER related genes (Table) to establish a balance between the ER protein load and the ability to control this load. The proper folding of protein within the cell is mediated by several ER bounded chaperone proteins, including protein disulfide isomerase (PDI), the Hsp70 family member glucose related protein 78 (GRP78), also known as binding immunoglobulin protein (Bip), calnexin, and calreticulin [15, 16, 18]. Disturbances in redox regulation, inflammatory overloads, calcium homeostasis, or the over-expression of protein can lead to impairment of this protein folding machinery and this condition is referred as ER stress. To protect against such kind of stress, cells have an orchestrated and integrated UPR signaling to restore homeostasis and normal ER function. As a consequence, 1) ER sensors diminish the protein load by inhibiting protein translation and by limiting the pool of mRNAs ready to process in the ER, 2) induction of ER chaperones for proper folding of nascent proteins and allowing them to translocate into Golgi body 3) and activation of ER-associated degradation (ERAD) machine to trap the unfolded proteins (Fig. 1). However, if this signaling cascade is insufficient to restore the ER stress and cellular function is compromised, apoptosis is initiated [8, 18, 19].
Table.
ER Stress related genes implicated in sepsis
| ER Stress related genes | Function in | Possible role in Sepsis |
|---|---|---|
| Bip/GRP78 | Chaperon proteins, bind to the unfolded proteins for ERAD in order to reduce the loads of unfolded proteins | Up regulated in sepsis [14, 24, 81, 98] |
| IRE1 | XBP1 splicing, activation of stress kinase proteins leading to inflammation and apoptosis | Up regulated in sepsis [24, 83] |
| XBP1 | ERAD, inflammation and apoptosis | Up regulated in sepsis [14, 81, 98] |
| PERK | Inhibits general protein synthesis through eIF2α into the ER | Up regulated in sepsis [24, 83] |
| eIF2α | Inhibits general protein synthesis | Up regulated in sepsis [14, 83] |
| ATF6 | Transcription of ER chaperone genes | Up regulated in sepsis [14, 80] |
| ATF4 | Induces CHOP and proteins involved in amino acid metabolism | Up regulated in sepsis [14] |
| CHOP | Apoptosis and inflammation | Up regulated in sepsis [24,80, 81] |
| ERO1α | Promotes oxidative stress in ER | Up regulated in sepsis [8, 90] |
| Caspase-12 | Apoptosis | Up regulated in sepsis [24, 94] |
Figure 1.
An accumulation of unfolded proteins in the endoplasmic reticulum (ER) induces ER stress. ER stress responses start with: (1) translational attenuation to reduce the further influx of nascent protein; (2) expression of ER chaperones to translocation the folded protein to the Golgi complex; (3) enhanced ERAD for degradation of unfolded or misfolded protein; and if unresolved (4) apoptosis. These responses are regulated by the ER localized protein sensors.
The UPR is double edged sword, confers cytoprotection when activated to a moderate extent, but become a threat when it is protracted over and may lead to cellular dysfunction, death and disease. The UPR consists of two central components, a group of specialized stress sensors IRE1, PERK and ATF6 located in the ER membrane and downstream transcription factors eIF2- α (for PERK), fragmented ATF6 (for ATF6), and spliced XBP1 (for IRE1) that reprogram gene expression to enable adaptation to stress or the induction of apoptosis depend on the severity and extent of the damage. These factors directly activate the transcription of chaperones or proteins functioning in redox homeostasis, protein secretion, lipid biosynthesis, autophagy, inflammation or cell death programs (Fig. 2).
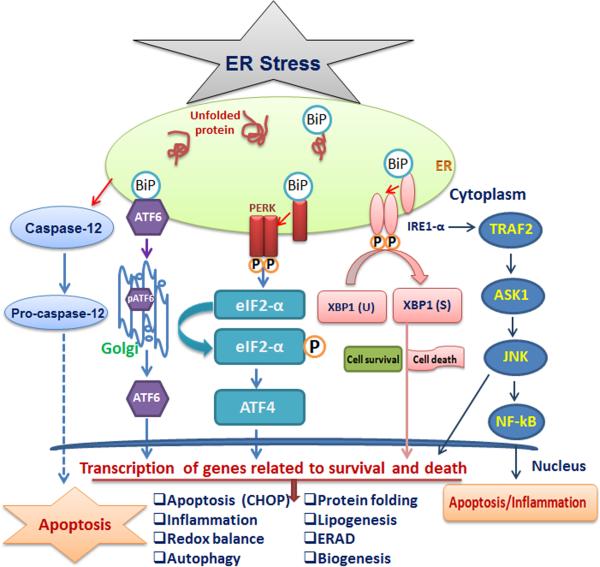
Figure 2.
ER stress and unfolded protein response (UPR) signaling. The ER stress response is mediated by three ER localized protein sensors: Inositol-requiring enzyme1 (IRE1) double-stranded RNA activated protein kinase (PKR)-like ER kinase (PERK) and activating transcription factor (ATF-6). During stress condition, these sensors dissociate from protein chaperones Bip and initiate a complex transcriptional cascade with different cytosolic functions. Upon activation, IRE1 splices the mRNA encoding XBP1 to generate spliced XBP1 (XBP1s). IRE1 also recruits TRAF2 and ASK1, leading to activation of JNK and NF-kB; this leads to induction of inflammation and apoptosis. PERK activation phosphorylates the initiation factor eIF2α to decrease the overall protein synthesis. Enhanced eIF2α phosphorylation increases translation of the ATF4 mRNA, which encodes a transcription factor that induces the expression of genes involved in autophagy, oxidative stress and apoptosis. Upon ER stress, ATF6 is translocated to the Golgi body and processed by proteases. Cleaved ATF6 fragment creates an active transcription factor that induces the expression of several components important for protein folding, degradation, and ER expansion. All three sensors try to restore the ER homeostasis or proceed to induce cell death, depending on the extent of damage and duration of the stress.
Under conditions of prolonged ER stress, UPR sensors shift their signaling towards cell death possibly through distinct overlapping signaling mechanisms. The first is transcriptional activation of the gene for C/EBP homologous protein (CHOP) mediated by PERK, IRE1 and ATF6 [29, 30]. The second is activation of the c-Jun N-terminal protein kinases (JNK) pathway, which is mediated by IRE1 and subsequently activation of TNF receptor-associated factor 2 (TRAF2) and apoptosis signal-regulating kinase1 (ASK1) [31]. The third is activation of ER-associated caspase-12 activation [32, 33]. Activated capase-12 is a representative caspase implicated in cell death-executing mechanisms correspond to ER stress [34]. During prolong ER stress, caspase-12 migrates from ER to cytosol and cleaves the caspase-9 and further activate caspase-3. A study by Nagakawa et al. reported that caspase-12 deficient mice were resistant to amyloid-beta induced apoptosis in experimental model of Alzheimer's disease and proposed that murine caspase-12 serves as a key mediator of ER stress and suggested human orthologue of caspase-12 as a potential therapeutic target [34].
Thus, ER is an organelle of great importance. The UPR pathway is valuable for cellular homeostasis and other important physiological activities. Therefore, investigating the UPR pathway and monitoring ER stress in the experimental models system is important to study the pathogenic mechanisms associated with sepsis and other related clinical condition in human. Nevertheless, it is also worth investigating to measure the stress in other organelles as well during pathological condition.
ER Stress and UPR Signaling
There are three types of UPR signaling associated with ER stress, which control the expression of specific transcription factors, and UPR downstream pathways. Here, we explain the ER stress mediated UPR signaling and discuss the possible mechanism involved in the transition of the UPR from a protective to a pro-apoptotic phase during prolonged ER stress in disease condition.
IRE-1 and XBP1 Axis
The IRE1/XBP1 axis has been documented as the conserved core of the UPR, which largely exists from yeast to human and is essential for mammalian developmental processes [13]. IRE1 is an ER membrane protein whose ER domain is involved in the sensing of unfolded or misfolded proteins, whereas the cytoplasmic domain serves as a protein kinase and endoribonuclease activities. Two different isoforms of IRE1 have been presented in mammalian cells: IRE1α and IRE1β. IRE1α is ubiquitously expressed, while IRE1β is tissue-specific, mostly localized in the gut [35]. Accumulation of unfolded proteins in the ER results in the release of bounded GRP78 from IRE1, stimulates IRE1α oligomerization in the ER membranes and autophosphorylation of IRE1α's cytosolic domain [15, 36]. Activated IRE1 splices a 26-nucleotide intron from the mRNA encoding XBP1, which generates a more stable and potent transcriptional factor of UPR genes, known as spliced XBP1 [37]. The XBP1s target genes and downstream effects are cosmopolitan, depending on the cell type and the nature of the stress stimuli.
The XBP-1 protein binds to promoters of several genes involved in UPR and ERAD to restore protein homeostasis and provide cytoprotection. Deficiency of XBP1 has been shown to result in the impairment of pancreatic cells, hepatocytes, and plasma B-cells, all of which produce large amounts of secretory proteins, suggesting an important role for XBP1 in preserving the protein secretory machinery. In addition, XBP1s indirectly regulates the biogenesis of the ER and Golgi by enhancing the activity of enzymes related to phospholipid biosynthesis [38]. XBP1s also binds estrogen receptor-α in a ligand-independent manner [39]. However, the relevance of these interactions remains unclear. Recent studies identified an interaction between the p85α regulatory subunit of phosphatidylinositol 3-kinase (PI3K) with XBP1s in an ER stress-dependent manner [40, 41]. This association is linked with the regulation of metabolic control in diabetes [42]. In addition, XBP1s was documented to negatively regulate the expression levels of the transcription factor Forkhead box O1 (FOXO1), which also modulated glucose metabolism [43]. Moreover, a study by Ye and his coworker demonstrated that TLR4 promotes liver disease by inducing ROS dependent XBP1 activation in Kupffer cells, suggesting the role of XBP1 in the immune response [44]. Discrepancies in the role of XBP1 in immune response reflects the multifaceted function and in regulation of its target gene. This is an active area of research, and recent works have begun to uncover the precise mechanisms by which IRE1α governs these pathways.
IRE1α controls the initiation of several downstream signaling pathways in addition to the processing of XBP1 mRNA. In addition to endoribonuclease, activated IRE1 also serves as a kinase and binds to TRAF2, which recruits ASK1 and activates the phosphorylation of JNK and p38 MAPK [31]. IRE1-dependent JNK activation is an important signaling mechanism that activates the transcription factor CHOP and NF-kB which causes changes in gene expression that favor apoptosis and inflammatory response [45]. In addition, IRE1 is also implicated in the activation of autophagy [10]. TRAF2-dependent activation of IRE1 and c-Jun JNK reportedly result in the phosphorylation of Bcl-2, allowing the dissociation of Beclin-1, activation of the PI3K complex and autophagy [46, 47]. A study by Hetz and his coworkers reported that proapoptotic proteins Bax and Bak activate the IRE1α signaling in the ER and established the association between apoptosis and UPR [48]. Similarly, another study by Klee and his colleagues suggested that the enforced expression of the pro-apoptotic BH3-only proteins in the ER initiate the activation of the JNK pathway in IRE1α and Bak-dependent manner [49]. In contrast, the cytosolic chaperone heat shock protein 72 (Hsp72) was recently shown to reduce the cell damage under ER stress conditions [50]. These results provide for the first time an interconnection between cytosolic chaperones and the UPR. Beyond its role in the maintenance of secretory cells and lipid metabolism, IRE1/XBP1 axis was shown to regulate immune responses. XBP1 is important for the development and survival of dendritic cells and in the immune response to challenge by pathogens that activate the Toll-like receptors (TLRs) [51, 52]. Qiu et al demonstrated that IRE1α is required for production of pro-inflammatory cytokines as evidenced by impaired TLR-induced cytokine production in IRE1α-null macrophages and neutrophils, suggesting IRE1α is a potential therapeutic target for autoimmune disease [53]. Recently, Lerner and his coworker demonstrated that IRE1 induces thioredoxin-interacting protein (TXNIP) which subsequently activates the inflammasome complex mediated cell death, suggesting ER stress and TXNIP axis could be a target for effective treatment [54].Taken together, the IRE1/XBP1 axis is the key in maintaining the various physiological processes and plays a bifunctional role in cell death and adaptation to stress, depending on cell types and extent of the stress stimuli.
PERK Pathway
PERK is a type I transmembrane protein located in the ER that senses the accumulation of misfolded or unfolded proteins in the ER [36]. Like IRE1, the ER domain of PERK senses unfolded proteins, whereas the cytoplasmic domain possesses kinase activity. In the absence of ER stress, GRP78 binds to the ER domain of PERK and inhibits its activation. Under ER stress condition, PERK separates from GRP78 and activated through oligomerization and transphosphorylation. Activation of PERK inhibits general protein translation into the ER through the inactivation of the initiation factor eIF2α by serine 51 phosphorylation. This phosphorylation suppresses the guanine nucleotide exchange factor eIF2β, a complex that recycles eIF2α to its active GTP-bound form. This inhibitory effect of translation helps to reduce the ER stress by decreasing the further influx of misfolded or unfolded proteins into ER.
Furthermore, the phosphorylation of PERK is regulated through the feedback mechanism by specific phosphatases, such as a constitutive repressor of eIF2a phosphorylation (CReP) and its regulatory subunit GADD34 (growth arrest and DNA damage-inducible protein-34) [55]. In contrast to global translational attenuation, phosphorylation of eIF2α enhances the translation of activating transcription factor 4 (ATF4). ATF4 is a transcription factor, induces another transcription factor CHOP, which involved in the induction of apoptosis that upregulates a pool of UPR genes that function preferentially in amino acid import, glutathione biosynthesis, and combating oxidative stress [29, 56]. Interestingly, a study by Cullinan et al identified the nuclear factor (erythroid-derived 2)-like 2 (NRf2), a transcription factor that controls the regulation of oxidative stress as a novel PERK substrates [57]. It has also been demonstrated that PERK may also control the expression of NF-κB in an ATF4-independent manner [58].
ATF-6 Pathway
ATF6 is a type II ER transmembrane protein located in the ER. There are two genes for ATF6, called ATF6α and ATF6β, which have a similar function and are ubiquitously expressed [59]. Similar to IRE1 and PERK, the ER domain of ATF6 is responsible for the sensing the unfolded or misfolded proteins, however, cytoplasmic portion has a DNA-binding domain containing the basic-leucine zipper motif (bZIP) and a transcriptional activation domain [60]. In normal condition, ATF6 is synthesized as an inactive precursor and binds to the GRP78. As such, the binding association with GRP78 impedes the Golgi localization signal and inhibits the translocation to the Golgi apparatus [15].
Under ER stress condition, ATF6 is separated from GRP78 and translocates to the Golgi apparatus by vesicular transport. In the Golgi apparatus, it is cleaved by a pair of processing proteases, called site 1 protease (S1P) and site 2 protease (S2P). This proteolysis results the release of its cytoplasmic domain, ATF6f (a fragment of ATF6), an active form of ATF6. The active fragment of ATF6 translocates into the nucleus, and enhance molecular chaperones and several genes associated with ERAD and protein folding, such as GRP78, GRP94, and calreticulin as well as the ER stress response element (ERSE) [61-63]. Interestingly, recently several other putative ATF6 homologs have been identified, which are modulated by ER stress in specific tissues, including CREBH, OASIS, CREB4 , LUMAN/ CREB3, and BBF2H7 [64-66]. The cleavage of ATF6 is conserved and different, especially as the second cleavage by S2P occurs in the ER transmembrane. This process is called regulated intramembrane proteolysis (RIP), which is well conserved from bacteria to mammals. The known substrate of RIP is sterol response element-binding protein (SREBP), a transcription factor [67]. Similar to ATF6, SREBP is also located in the ER membrane. In the condition of sterol deficiency, SREBP is transported to the Golgi apparatus, cleaved by S1P and S2P, and activates the transcription of genes associated with biosynthesis of sterol [18].
UPR Independent ER Stress
A key signal transduction pathway connecting apoptosis to ER–mitochondrial interactions is an alteration in intracellular calcium (Ca2+) homeostatic mechanisms. The ER is the major site for Ca2+ storage. In the ER, Ca2+-binding chaperones mediate the proper folding of proteins and regulates a diversity of cellular responses and signaling transduction pathways. Ca2+ transfer between the ER and mitochondria represents a critical signal in the induction of apoptosis. Several studies demonstrated that acute release of Ca2+ from the ER can trigger a variety of signaling mechanisms that promote cell death mainly by Ca2+-mediated mitochondrial cell death [68, 69]. There are increasing reports that Bax and Bak are involved in Ca2+-mediated apoptosis in the ER [70, 71]. Over-expression of Bax results in release of ER Ca2+, with a subsequent increase in mitochondrial Ca2+ that leads to release of cytochrome c from mitochondria to the cytoplasm. A family of Bcl-2 proteins such as Bcl-2 and Bcl-xL also located in the ER membrane and are reported to protective against ER stress [8]. It is believed that this cytoprotective function is mainly due to the ability of Bcl-2 to lower steady-state levels of ER Ca2+. The protective effect of Bcl-2 in regulation of ER Ca2+ is inhibited by phophorylation of JNK pathway [72]. Phosphorylated Bcl-2 loses its anti-apoptotic function and increase the Ca2+ release from the ER, which is associated with mitochondria Ca2+ uptake and apoptosis [68].
Calreticulin, a major Ca2+ binding ER chaperone plays an important role in the adequate folding of newly synthesized proteins [73]. Therefore, fluctuations or alterations in Ca2+ level in the ER might impact folding capacity and initiate the components of cell death. A study by Lim et al reported that enhanced calreticulin expression in mature cardiomyocytes disrupts intracellular calcium regulation, leading to calcium-dependent apoptosis [74]. Thus, alterations in Ca2+ dynamics seem to play a key role in the ER stress-associated mechanisms of cell death.
Recently, a new signaling pathway associated with ER stress has been identified where Cdk5 and MEKK1 induce apoptosis in a Drosophila model of retinitis pigmentosa, independently of the three traditional UPR branches, IRE1α, PERK and ATF6 [75]. In this study, Kang and his coworkers have shown that Cdk5 phosphorylates MEKK1 (human ortholog; MAP3K4) and activates the JNK signaling pathway for apoptosis followed by ER stress. Ablation of this pathway delayed age-associated retinal degeneration in the Drosophila model of retinitis pigmentosa. Recently, Giorgi and his colleagues revealed that p53 activation increases the mitochondrial Ca2+ loads and subsequently enhanced the apoptosis in a Ca2+-dependent manner. Interestingly, they found thatpharmacological inhibition of p53 or naturally occurring p53 missense mutants inhibits Ca2+-signaling from ER to the mitochondria [76]. These findings define a novel function of p53 in regulating Ca2+-dependent apoptosis. Murine caspase-12 is a member of the caspase family and has been reported to play an important role in ER stress mediated cell death [32, 77]. Murine caspase-12 shows similar characteristics to the human caspase-4 which is located within caspase-1/ICE genes [77]. A study by Morishima and his coworkers have suggested that caspase-12 activation is not dependent on mitochondrial or any death receptor activation and its activation triggers the ER specific caspase cascade that leads to apoptotic death [78]. This theory was further supported by another finding by Rao and his colleagues where they investigated that inhibition of caspase-12 reduced the thapsigargin-induced cell death, suggesting an important role of caspase-12 in ER stress mediated cell death [79]
ER Stress in Sepsis
Improving the quality of life and reducing the mortality from sepsis remains one of the most significant unmet medical needs of the current era. New researches on ER stress signaling have recently revealed a new and fascinating interaction between ER stress and sepsis associated cell death [80-83]. Recently, a number of investigators have reported that the suppression of ER stress stabilizes protein conformation, facilitates the trafficking of mutant proteins, and improves ER folding capacity and suggested that ER stress is a potential therapeutic target for various diseases including diabetes, cystic fibrosis, sepsis, trauma and hemorrhage and ischemic injuries of brain, kidney spinal cords and liver [84-87]. This has been demonstrated in CLP model of sepsis, where ER stress contributes to abnormal lymphocyte apoptosis during sepsis in mice, suggesting ER stress-mediated apoptosis pathway may be a novel target in clinical prevention and therapy of sepsis-induced lymphocyte apoptosis [81, 88]. Myocardial depression is a well-recognized manifestation of organ dysfunction in sepsis, and myocardial apoptosis is a key step for this progression. A very recent study reported that components of ER stress (GRP94, CHOP, and caspase-12) were up regulated in the hearts of septic rats and inhibition of ER stress protected the myocardial from ER stress induced apoptosis in rats [24]. CHOP/GADD153 (growth arrest/DNA damage) plays a convergent role in the UPR and has been identified as one of the most important mediators of ER stress-induced apoptosis [29, 30, 89, 90]. There are several targets of the CHOP including GADD34; DR5 (TRAIL Receptor-2), a caspase-activating cell-surface death receptor of the TNF receptor family and Ero1α (endoplasmic reticulum oxidoreductase-1), which hyperoxidizes the ER and promotes apoptotic cell death [8]. A study by Li et al has reported that Ero1α activates the inositol triphosphate receptor (IP3R)-induced excessive Ca2+ transport from the ER to the mitochondria, and initiate the apoptosis in macrophages [91]. This finding sheds new light on how the CHOP pathway of apoptosis triggers calcium-dependent apoptosis through an ERO1-alpha-IP3R pathway and small interfering RNA of ERO1-α suppresses apoptosis [91]. The significance of ER stress in apoptosis was reported in CHOP-deficient mice where in the embryonic fibroblasts revealed that CHOP deficiency provides partial resistance to ER stress induced apoptosis [92]. A study by Gupta et al. have stated that Hsp72 protects rat pheochromocytoma PC12 cells from ER stress-induced apoptosis via enhancement of IRE1α-XBP1 signaling, suggesting an important and critical role of ER stress [50]. Discrepancies in the finding the role ER stress from prodeath to prosurvival warrant further deep understanding which could help to develop the therapeutic intervention.
During sepsis, uncontrolled inflammation and activation of the innate immune system may contribute to tissue damage and ultimately cell death. Transcription factor CHOP is a major inducer of apoptosis in response to ER stress, however, recent evidences suggested an inflammatory role of CHOP as a mediator of the inflammatory response in sepsis. Recently, Ferlito and his colleagues reported that septic mice exhibited increased expression of CHOP and treatment with H2S increased the survival rate in an experimental model of sepsis by inhibiting the CHOP expression, suggesting the participation of ER stress in sepsis [80]. They have highlighted a major role for CHOP, which act as an amplifier of the inflammatory response in the pathogenesis of sepsis, and the ability of H2S treatment to counter CHOP signaling via upregulation of Nrf2 [80]. Moreover, the ER stress pathway involving CHOP is activated in immunostimulated macrophages, suggesting an important role for this response in the pathogenesis of LPS-induced inflammation. This pathway is also activated in the lungs of LPS- treated mice, where damage could be inhibited in CHOP knockout mice [93]. These findings further demonstrate that the CHOP mediated ER stress plays an essential role in the pathogenesis of septic injury in mice. Moreover, Kim and his colleagues have reported that LPS injection in mice induces ER stress and up regulate the UPR-related markers including ATF-6, XBP-1, phospho-eIF2α, and ATF-4 along with Bip and CHOP and recognized the possibility that inhibition of ER stress using a specific ER stress inhibitor 4-phenylbutyrate (4-PBA) attenuates LPS-induced lung inflammation in mice [14]. This study has provided a new concept in the pathogenesis of LPS-induced lung inflammation, which is associated with ER stress that may be one of the promising therapeutic targets for LPS-related diseases.
Signaling pathways in response to ER stress that lead to inflammation and apoptosis are intertwined through a variety of mechanisms, including Ca2+ influx, ROS generation, caspase activation, and phophorylation of NF-κB. A study by Toltl et al has investigated that inhibition of ER stress by activated protein C suppressed the inflammation and apoptosis in human blood monocytes and this may partly explain the protective effects of APC treatment in severe sepsis [94]. Recently, caspase-12 was reported to negatively regulate the inflammasome activation by recruiting caspase-1 and impeding the complex formation [95]. Interestingly, they also reported that caspase-12 deficient mice were shown to resistant to septic shock in an experimental mouse model of sepsis, suggesting the critical role of caspase-12 in sepsis condition. Fernandez and Lamkanfi suggested that caspase-12 directly binds to the microbial components and hampers the replication of pathogens by inducing pyroptosis and confers the resistance against sepsis [96]. Moreover, the association of human caspase-12 with susceptibility to sepsis in individuals of African and Indian population has been documented by previously published studies [97, 98]. Saleh and his colleagues demonstrated that caspase-12 deficiency confers protection in septic mice and the presence of caspase-12 leads to enhanced vulnerability to bacterial infection and septic mortality, suggesting a detrimental role of caspase-12 in sepsis [95].
Inhibitions of ER stress by treatment with chemical chaperones, such as 4-PBA or tauroursodeoxycholic acid (TUDCA), suppresses LPS-induced inflammatory and apoptotic expression in mice and normal human bronchial epithelial cells, suggesting a role of ER stress in sepsis associated cell death [14]. A very recent study by Diao et al reported that burn plus LPS promotes the inflammasome and ER stress in rat liver as evidence with increased level of CHOP and sXBP1[99]. Although, these observations increase our knowledge of the biological mechanisms in the context of ER stress and sepsis and simultaneously shed light on new targets and suggest novel strategies for the treatment of this condition. Further research is warranted to elucidate the exact mechanism how ER stress contributes the sepsis associated cell death.
ER Stress after Trauma and Hemorrhage
Traumatic hemorrhagic is a pathological condition resulting from traumatic insult and characterized by rapid and significant blood loss. The trauma-hemorrhage-induced immunosuppression is associated with an increased susceptibility to sepsis, organ dysfunction and ultimately death [9, 100]. There is growing evidence that ER stress initiates the deleterious cascade and eventually contribute to organ dysfunction after trauma and hemorrhage [9, 86, 101, 102]. Increased oxidative stress, hypoxia, and proinflammatory cytokines, hallmarks of the posttrauma-hemorrhage condition, can initiate ER stress [17]. Moreover, altered ATP and glucose levels disturb the complex ER machinery as well as the posttranslational modification of newly synthesized proteins or lipids [103]. These deleterious events alter the redox environment of the cell and are supposed to interfere with the protein-folding machinery of the ER, leading to the accumulation of unfolded or misfolded proteins inside the ER.
A study by Duvigneau and his colleagues recognized the possibility of contribution of ER stress associated cell death in rats after trauma hemorrhage [102]. Jian et al have demonstrated the role of ER stress and subsequent activation of the UPR and the elevated apoptosis in a murine model of trauma and hemorrhage [9]. Sodhi et al investigated the involvement of ER stress in trauma/hemorrhage induced lung injury [104]. In this study they reported that activation of epithelial TLR4 and HMGB1 enhance the lung injury and inhibition of TLR4 signaling by TLR4 inhibitor reduced the ER stress, decreased the HMGB1 and protected lung injury. Similarly Kozlov and coworkers demonstrated that ER stress contributes the pathogenesis of trauma and hemorrhagic shock in rats, suggesting an important and deleterious role of ER in propagation of components of cell death [101]. Moreover, sustained ER stress and ROS expedite Ca2+ release of ER, which can activate both mitochondria dependent and mitochondria-independent caspase cascades, ultimately leading to apoptosis or necrosis [102, 105]. A recent study by Begum and colleagues investigated that sustained ER stress plays a key role in the progression of neuronal damage in rat model of traumatic brain injury and suggested that inhibition of ER stress reduces the abnormal protein accumulation, and neurological deficits [86]. Moreover, He et al suggested that inhibition of CHOP following hemorrhage in rats reduces the apoptosis and vascular injury, suggesting the role of ER stress component in the hemorrhagic induced cell death [106].
Blast-induced trauma causes the blood brain barrier damage and induces inflammatory cascades that promote intracellular Ca2+ accumulation [107, 108]. Ca2+ perturbations are known to cause ER stress and trigger the UPR. Blast-induced ER stress mediated CHOP elevation, along with increased caspase-12 and caspase-3 cleavage, suggests a neuronal shift from the repair response to the apoptosis in rats [108]. Moreover, modulation of the ER stress response with ER stress modulator Salubrinal has been shown to attenuate PERK dependent CHOP expression and limit apoptosis, suggesting suppression of ER stress could be an effective therapy related to trauma and hemorrhage. Recently, Yu et al demonstrated that prolonged stress induced ATF6-dependent ER stress and its components in the rat brain, indicating the participation of ER stress in post trauma induced apoptosis [109]. Further studies are needed to dissect the possible mechanism how ER stress contributes trauma-hemorrhage-induced cell death and to develop new therapeutic approaches for the prevention and treatment of trauma-hemorrhage.
ER Stress after Ischemia/Reperfusion (I/R) Injury
ER stress plays an important and essential step in the progression of ischemia-reperfusion (I/R) injury in rodents and human [110-115]. I/R injury affects the integrity of the ER, the site of synthesis and folding of numerous proteins. Following I/R injury, ER recognize any perturbation caused by increased oxidative stress, alteration in calcium homeostasis, accumulation of unfolded proteins and hypoxia and leads to the activation of the UPR signaling pathway (Fig. 3). A study by Miyazaki et al reported that the ER stress-induced CHOP-mediated pathway has a deleterious effect on the development of myocardial I/R injury and deficiency of CHOP attenuated the myocardial apoptosis in mouse myocardial I/R injury [111]. Terai et al presented the evidence that the UPR is activated in neonatal rat cardiomyocytes in response to hypoxia and may play a role in the pathogenesis of heart disease [116]. Tao and his colleagues demonstrated that apelin, an endogenous ligand for the G protein-coupled APJ receptor, protects the heart in rat model of I/R injury via modulation of ER stress-mediated apoptosis in time dependent fashion, suggesting deleterious role of ER stress in cardiac I/R injury [110]. Several studies have investigated and reported that inhibition of ER stress provides protection against organs I/R injury. A recent study by Mizukami and his coworkers suggested that inhibition of ER stress by chemical chaperone 4-PBA reduces the loads of unfolded proteins in the ER and protects the cell from spinal cord ischemia in rabbits [85]. Dong et al described the deleterious role of CHOP in apoptosis following mouse renal I/R injury, and suggested that targeting CHOP expression may be promising in the treatment of renal I/R [87]. A study by Sun et al. suggested that N-acetyl cysteine treatment provides protection against mouse liver I/R injury and reduces the ER stress in mouse hepatocytes cell [117]. Vilabota and his coworkers suggested that chemical chaperone 4-PBA reduced the loads of unfolded protein in the ER and ameliorated the apoptosis via inhibition of CHOP, caspase 12 and peIF2α in rodents model of liver I/R injury [118]. Recently, Rao and his coworkers reported that alleviation of ER stress by chemical chaperone 4-PBA or ATF6 small interfering RNA (siRNA) diminished the pro-inflammatory response in mouse Kupffer cells, leading to the inhibition of liver immune response and protection of livers from I/R injury [27]. In contrast, up-regulation of ER chaperones protected mouse cardiomyocytes from ER stress-induced apoptosis [119]. These findings suggest that the ER stress response is essential for the homeostasis of cardiomyocytes. All the above findings in the context of ER stress and I/R injury presented the evidences that ER stress participates and contributes in I/R injury associated cell death. Thus, targeting the ER-associated cell death pathway might offer a novel approach to reduce I/R injury. Further studies and better understanding of the reciprocal interaction of ER stress and I/R injury is essential to shed new insights into the possible mechanism and new therapeutic approaches for the prevention of I/R injury.
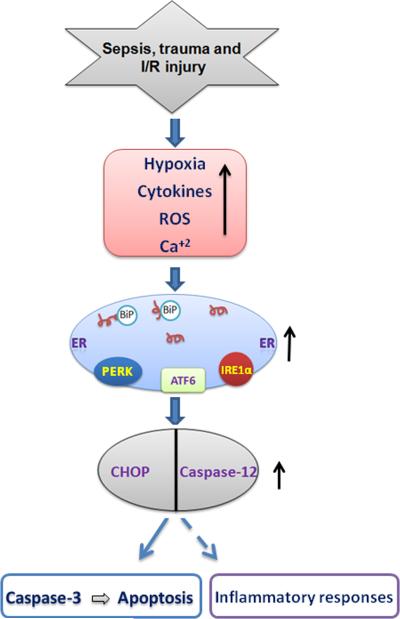
Figure 3.
Schematic depiction of ER stress signaling in sepsis, trauma and I/R injury. Abundant production of cytokines, hypoxia, ROS and calcium influx are the hallmark of these diseases. ER stress and UPR are initially an adoptive response and aim to protect the cells from these threats. If the stress is prolonged, or the adaptive response fails, sustained ER stress leads to activation of UPR and up regulation of inflammatory and proapoptotic signaling pathway via activation of CHOP and caspase-12. Upregualtion of CHOP and caspase-12 could lead to cell death and organ dysfunction.
Perspectives and Future Directions
The ER plays a critical and dynamic role in cellular physiology by ensuring the correct folding of secretory and transmembrane proteins. However, the precise molecular mechanisms by which ER stress leads to cell survival/death remains enigmatic. Over the past decades, new research has provided exciting evidences that activation of the UPR not only maintains a homeostatic environment in the ER, but also involved in the regulation of a wide variety of other cellular processes, including cellular proliferation and differentiation, inflammation, apoptosis, and angiogenesis. Moreover, as documented by several investigators, activation of the UPR appears to be a dynamic process closely correlated with the duration and severity of ER stress. Accordingly, increased ER stress and UPR activation have been reported in many human diseases, including cancer, ischemia, neurodegenerative diseases, diabetes and metabolic disorders. In this review, we highlighted that, during normal conditions, concerted action of ER stress components is required to maintain cellular homeostasis following sepsis, trauma and I/R injury. Imbalance in these regulatory components which could lead to apoptosis (Fig. 3), as often seen in several pathological conditions, presents a challenge to develop therapies to restore such homeostasis.
From a therapeutic perspective, it will be of great importance to understand how the UPR could be pharmacologically manipulated to switch from prodeath to prosurvival pathway. In recent years, pharmacological compounds targeting PERK, IRE1 and ATF6 have been used in disease models and are beginning to show promising properties. Understanding the crosstalk among the various components of the UPR as well as how these signaling pathways are intertwined with initiation of inflammatory and apoptotic cascade will provide new treatment options for various pathologies including sepsis, trauma and hypoxia. Moreover, recent findings on caspase-12 activation during sepsis, trauma and I/R injury open a new field for therapeutic strategies focused on modulation of caspase-12 and shed new light on its physiological role.
In summary, ER is an external stimuli sensing device which could sense any alterations in the microenvironment of the cells from toxins to pathogens. It could serve as a rich platform for understanding the interactions between environmental signals and the biological response. Hence, investigation of ER stress and its associated UPR signaling represents a new and exciting area to explore in the field of sepsis, trauma and I/R injury.
Acknowledgments
Source of Funding:
This study was supported by a grant from NIH R01GM053008 and R01GM057468 to PW.
Abbreviation
- ASK1
apoptosis signal regulating kinase 1
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- Bip
binding immunoglobulin protein
- CHOP
C/EBP homologous protein
- eIF2
eukaryotic initiation factor 2
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- Ero1α
endoplasmic reticulum oxidoreductase-1
- I/R
ischemia/reperfusion
- GRP78
glucose-regulated protein
- IRE1
inositol-requiring protein 1
- JNK
c-Jun N-terminal protein kinases
- NRf2
nuclear factor (erythroid-derived 2)-like 2
- PDI
Protein disulfide isomerase
- PERK
double-stranded RNA-dependent protein kinase (PKR)-like ER kinase
- 4-PBA
4-phenylbutyrate
- ROS
reactive oxygen species
- TRAF2
TNF receptor-associated factor 2
- UPR
unfolded protein response
- XBP1
X-box binding protein1
Footnotes
Conflicts of Interest
The authors have no conflict of interest.
References
- 1.Chong J, Dumont T, Francis-Frank L, Balaan M. Sepsis and septic shock: a review. Crit Care Nurs Q. 2015;38:111–120. doi: 10.1097/CNQ.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 2.Aziz M, Jacob A, Wang P. Revisiting caspases in sepsis. Cell Death Dis. 2014;5:e1526. doi: 10.1038/cddis.2014.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guirgis FW, Khadpe JD, Kuntz GM, Wears RL, Kalynych CJ, Jones AE. Persistent organ dysfunction after severe sepsis: a systematic review. J Crit Care. 2014;29:320–326. doi: 10.1016/j.jcrc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 5.Camicia G, Pozner R, de Larranaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 6.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247–1288. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Group P-SS: Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 8.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jian B, Hsieh CH, Chen J, Choudhry M, Bland K, Chaudry I, Raju R. Activation of endoplasmic reticulum stress response following trauma-hemorrhage. Biochim Biophys Acta. 2008;1782:621–626. doi: 10.1016/j.bbadis.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 12.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 13.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ, Lee YC. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-kappaB/HIF-1alpha signaling pathway. Sci Rep. 2013;3:1142. doi: 10.1038/srep01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu G, Lee AS. Role of the Unfolded Protein Response, GRP78 and GRP94 in Organ Homeostasis. J Cell Physiol. 2014 doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluquet O, Pourtier A, Abbadie C. The unfolded protein response and cellular senescence. A Review in the Theme: Cellular Mechanisms of Endoplasmic Reticulum Stress Signaling in Health and Disease. Am J Physiol Cell Physiol. 2015;308:C415–425. doi: 10.1152/ajpcell.00334.2014. [DOI] [PubMed] [Google Scholar]
- 21.Bettigole SE, Glimcher LH. Endoplasmic Reticulum Stress in Immunity. Annu Rev Immunol. 2014 doi: 10.1146/annurev-immunol-032414-112116. [DOI] [PubMed] [Google Scholar]
- 22.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 23.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Liu Y, Zhang JS, Zhang XH, Chen WJ, Yin XH, Qi YF. Cortistatin protects myocardium from endoplasmic reticulum stress induced apoptosis during sepsis. Mol Cell Endocrinol. 2015;406:40–48. doi: 10.1016/j.mce.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Adhikary A, Chakraborty S, Bhattacharjee P, Mazumder M, Putatunda S, Gorain M, Chakraborty A, Kundu GC, Das T, Sen PC. Cross-talk between endoplasmic reticulum (ER) stress and the MEK/ERK pathway potentiates apoptosis in human triple negative breast carcinoma cells: role of a dihydropyrimidone, nifetepimine. J Biol Chem. 2015;290:3936–3949. doi: 10.1074/jbc.M114.594028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Xie X, Pan H, Wu Z, Lu W, Yang G. Role of Endoplasmic Reticulum Stress in Brain Damage after Cardiopulmonary Resuscitation in Rats. Shock. 2015 doi: 10.1097/SHK.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 27.Rao J, Yue S, Fu Y, Zhu J, Wang X, Busuttil RW, Kupiec-Weglinski JW, Lu L, Zhai Y. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am J Transplant. 2014;14:1552–1561. doi: 10.1111/ajt.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiu F, Catapano M, Diao L, Stanojcic M, Jeschke MG. Prolonged ER Stressed-Hepatocytes Drives an Alternative Macrophage Polarization. Shock. 2015 doi: 10.1097/SHK.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46:629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 30.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 31.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 32.Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 33.Groenendyk J, Michalak M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim Pol. 2005;52:381–395. [PubMed] [Google Scholar]
- 34.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 35.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 38.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding LH, Ye QN, Yan JH, Zhu JH, Lu QJ, Wang ZH, Huang CF. [XBP-1 interacts with estrogen receptor alpha (ERalpha)]. Sheng Wu Gong Cheng Xue Bao. 2004;20:332–336. [PubMed] [Google Scholar]
- 40.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SW, Herrema H, Salazar M, Cakir I, Cabi S, Basibuyuk Sahin F, Chiu YH, Cantley LC, Ozcan U. BRD7 regulates XBP1s' activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 2014;20:73–84. doi: 10.1016/j.cmet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, Lee J, Fisher SJ, White MF, Biddinger SB, Ozcan U. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058–1067. doi: 10.1136/gutjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- 45.Szczesna-Skorupa E, Chen CD, Liu H, Kemper B. Gene expression changes associated with the endoplasmic reticulum stress response induced by microsomal cytochrome p450 overproduction. J Biol Chem. 2004;279:13953–13961. doi: 10.1074/jbc.M312170200. [DOI] [PubMed] [Google Scholar]
- 46.Cheng X, Liu H, Jiang CC, Fang L, Chen C, Zhang XD, Jiang ZW. Connecting endoplasmic reticulum stress to autophagy through IRE1/JNK/beclin-1 in breast cancer cells. Int J Mol Med. 2014;34:772–781. doi: 10.3892/ijmm.2014.1822. [DOI] [PubMed] [Google Scholar]
- 47.Criollo A, Vicencio JM, Tasdemir E, Maiuri MC, Lavandero S, Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy. 2007;3:350–353. doi: 10.4161/auto.4077. [DOI] [PubMed] [Google Scholar]
- 48.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 49.Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol. 2010;8:e1000410. doi: 10.1371/journal.pbio.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osorio F, Tavernier SJ, Hoffmann E, Saeys Y, Martens L, Vetters J, Delrue I, De Rycke R, Parthoens E, Pouliot P, Iwawaki T, Janssens S, Lambrecht BN. The unfolded-protein-response sensor IRE-1alpha regulates the function of CD8alpha+ dendritic cells. Nat Immunol. 2014;15:248–257. doi: 10.1038/ni.2808. [DOI] [PubMed] [Google Scholar]
- 52.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu Q, Zheng Z, Chang L, Zhao YS, Tan C, Dandekar A, Zhang Z, Lin Z, Gui M, Li X, Zhang T, Kong Q, Li H, Chen S, Chen A, Kaufman RJ, Yang WL, Lin HK, Zhang D, Perlman H, Thorp E, Zhang K, Fang D. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. EMBO J. 2013;32:2477–2490. doi: 10.1038/emboj.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiramatsu N, Messah C, Han J, LaVail MM, Kaufman RJ, Lin JH. Translational and posttranslational regulation of XIAP by eIF2alpha and ATF4 promotes ER stress-induced cell death during the unfolded protein response. Mol Biol Cell. 2014;25:1411–1420. doi: 10.1091/mbc.E13-11-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91:1219–1243. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 59.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen J, Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem. 2004;279:43046–43051. doi: 10.1074/jbc.M408466200. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Zhang SX, Ma JH, Bhatta M, Fliesler SJ, Wang JJ. The unfolded protein response in retinal vascular diseases: Implications and therapeutic potential beyond protein folding. Prog Retin Eye Res. 2015;45C:111–131. doi: 10.1016/j.preteyeres.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 65.Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, Iseki K, Wanaka A, Imaizumi K. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005;7:186–194. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- 66.Kondo S, Saito A, Hino S, Murakami T, Ogata M, Kanemoto S, Nara S, Yamashita A, Yoshinaga K, Hara H, Imaizumi K. BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol. 2007;27:1716–1729. doi: 10.1128/MCB.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 68.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 69.Shanmughapriya S, Rajan S, Hoffman NE, Zhang X, Guo S, Kolesar JE, Hines KJ, Ragheb J, Jog NR, Caricchio R, Baba Y, Zhou Y, Kaufman BA, Cheung JY, Kurosaki T, Gill DL, Madesh M. Ca2+ signals regulate mitochondrial metabolism by stimulating CREB-mediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci Signal. 2015;8:ra23. doi: 10.1126/scisignal.2005673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 71.Kaufman RJ, Malhotra JD. Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1843:2233–2239. doi: 10.1016/j.bbamcr.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 73.Li WH, Li YZ, Song DD, Wang XR, Liu M, Wu XD, Liu XH. Calreticulin protects rat microvascular endothelial cells against microwave radiation-induced injury by attenuating endoplasmic reticulum stress. Microcirculation. 2014;21:506–515. doi: 10.1111/micc.12126. [DOI] [PubMed] [Google Scholar]
- 74.Lim S, Chang W, Lee BK, Song H, Hong JH, Lee S, Song BW, Kim HJ, Cha MJ, Jang Y, Chung N, Choi SY, Hwang KC. Enhanced calreticulin expression promotes calcium-dependent apoptosis in postnatal cardiomyocytes. Mol Cells. 2008;25:390–396. [PubMed] [Google Scholar]
- 75.Kang MJ, Chung J, Ryoo HD. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nat Cell Biol. 2012;14:409–415. doi: 10.1038/ncb2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Galindo Ramirez F, Rizzuto R, Di Virgilio F, Zito E, Pandolfi PP, Wieckowski MR, Mammano F, Del Sal G, Pinton P. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 2015;112:1779–1784. doi: 10.1073/pnas.1410723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 79.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferlito M, Wang Q, Fulton WB, Colombani PM, Marchionni L, Fox-Talbot K, Paolocci N, Steenbergen C. Hydrogen sulfide [corrected] increases survival during sepsis: protective effect of CHOP inhibition. J Immunol. 2014;192:1806–1814. doi: 10.4049/jimmunol.1300835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma T, Han L, Gao Y, Li L, Shang X, Hu W, Xue C. The endoplasmic reticulum stress-mediated apoptosis signal pathway is involved in sepsis-induced abnormal lymphocyte apoptosis. Eur Surg Res. 2008;41:219–225. doi: 10.1159/000135631. [DOI] [PubMed] [Google Scholar]
- 82.Zhao P, Turdi S, Dong F, Xiao X, Su G, Zhu X, Scott GI, Ren J. Cardiac-specific overexpression of insulin-like growth factor I (IGF-1) rescues lipopolysaccharide-induced cardiac dysfunction and activation of stress signaling in murine cardiomyocytes. Shock. 2009;32:100–107. doi: 10.1097/SHK.0b013e31818ec609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calisto KL, Camacho AC, Mittestainer FC, Carvalho BM, Guadagnini D, Carvalheira JB, Saad MJ. Diacerhein attenuates the inflammatory response and improves survival in a model of severe sepsis. Crit Care. 2012;16:R158. doi: 10.1186/cc11478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Gorasia DG, Dudek NL, Veith PD, Shankar R, Safavi-Hemami H, Williamson NA, Reynolds EC, Hubbard MJ, Purcell AW. Pancreatic beta cells are highly susceptible to oxidative and ER stresses during the development of diabetes. J Proteome Res. 2015;14:688–699. doi: 10.1021/pr500643h. [DOI] [PubMed] [Google Scholar]
- 85.Mizukami T, Orihashi K, Herlambang B, Takahashi S, Hamaishi M, Okada K, Sueda T. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J Vasc Surg. 2010;52:1580–1586. doi: 10.1016/j.jvs.2010.06.172. [DOI] [PubMed] [Google Scholar]
- 86.Begum G, Yan HQ, Li L, Singh A, Dixon CE, Sun D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J Neurosci. 2014;34:3743–3755. doi: 10.1523/JNEUROSCI.2872-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong B, Zhou H, Han C, Yao J, Xu L, Zhang M, Fu Y, Xia Q. Ischemia/reperfusion-induced CHOP expression promotes apoptosis and impairs renal function recovery: the role of acidosis and GPR4. PLoS One. 2014;9:e110944. doi: 10.1371/journal.pone.0110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma T, Han L, Hu WQ. [Study of the role of endoplasmic reticulum stress mediated apoptosis signal pathway in sepsis-induced splenic lymphocyte apoptosis]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21:48–50. [PubMed] [Google Scholar]
- 89.Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151:217–219. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 91.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138:501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- 94.Toltl LJ, Austin RC, Liaw PC. Activated protein C modulates inflammation, apoptosis and tissue factor procoagulant activity by regulating endoplasmic reticulum calcium depletion in blood monocytes. J Thromb Haemost. 2011;9:582–592. doi: 10.1111/j.1538-7836.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 95.Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 96.Fernandez DJ, Lamkanfi M. Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem. 2015;396:193–203. doi: 10.1515/hsz-2014-0253. [DOI] [PubMed] [Google Scholar]
- 97.Chen J, Wilson ES, Dahmer MK, Quasney MW, Waterer GW, Feldman C, Wunderink RG. Lack of association of the caspase-12 long allele with community-acquired pneumonia in people of African descent. PLoS One. 2014;9:e89194. doi: 10.1371/journal.pone.0089194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yavari M, Brinkley G, Klapstein KD, Hartwig WC, Rao R, Hermel E. Presence of the functional CASPASE-12 allele in Indian subpopulations. Int J Immunogenet. 2012;39:389–393. doi: 10.1111/j.1744-313X.2012.01107.x. [DOI] [PubMed] [Google Scholar]
- 99.Diao L, Marshall AH, Dai X, Bogdanovic E, Abdullahi A, Amini-Nik S, Jeschke MG. Burn plus lipopolysaccharide augments endoplasmic reticulum stress and NLRP3 inflammasome activation and reduces PGC-1alpha in liver. Shock. 2014;41:138–144. doi: 10.1097/SHK.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, Koenderman L, Kubes P, Lilford RJ. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kozlov AV, Duvigneau JC, Hyatt TC, Raju R, Behling T, Hartl RT, Staniek K, Miller I, Gregor W, Redl H, Chaudry IH. Effect of estrogen on mitochondrial function and intracellular stress markers in rat liver and kidney following trauma-hemorrhagic shock and prolonged hypotension. Mol Med. 2010;16:254–261. doi: 10.2119/molmed.2009.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duvigneau JC, Kozlov AV, Zifko C, Postl A, Hartl RT, Miller I, Gille L, Staniek K, Moldzio R, Gregor W, Haindl S, Behling T, Redl H, Bahrami S. Reperfusion does not induce oxidative stress but sustained endoplasmic reticulum stress in livers of rats subjected to traumatic-hemorrhagic shock. Shock. 2010;33:289–298. doi: 10.1097/SHK.0b013e3181aef322. [DOI] [PubMed] [Google Scholar]
- 103.Vishnu N, Jadoon Khan M, Karsten F, Groschner LN, Waldeck-Weiermair M, Rost R, Hallstrom S, Imamura H, Graier WF, Malli R. ATP increases within the lumen of the endoplasmic reticulum upon intracellular Ca2+ release. Mol Biol Cell. 2014;25:368–379. doi: 10.1091/mbc.E13-07-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sodhi CP, Jia H, Yamaguchi Y, Lu P, Good M, Egan C, Ozolek J, Zhu X, Billiar TR, Hackam DJ. Intestinal Epithelial TLR-4 Activation Is Required for the Development of Acute Lung Injury after Trauma/Hemorrhagic Shock via the Release of HMGB1 from the Gut. J Immunol. 2015;194:4931–4939. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boussabbeh M, Ben Salem I, Prola A, Guilbert A, Bacha H, Abid-Essefi S, Lemaire C. Patulin Induces Apoptosis through ROS-Mediated Endoplasmic Reticulum Stress Pathway. Toxicol Sci. 2015 doi: 10.1093/toxsci/kfu319. [DOI] [PubMed] [Google Scholar]
- 106.He Z, Ostrowski RP, Sun X, Ma Q, Tang J, Zhang JH. Targeting C/EBP homologous protein with siRNA attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Exp Neurol. 2012;238:218–224. doi: 10.1016/j.expneurol.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arun P, Abu-Taleb R, Oguntayo S, Tanaka M, Wang Y, Valiyaveettil M, Long JB, Zhang Y, Nambiar MP. Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: role of compromised cell membrane integrity. Neurosci Lett. 2013;552:87–91. doi: 10.1016/j.neulet.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 108.Logsdon AF, Turner RC, Lucke-Wold BP, Robson MJ, Naser ZJ, Smith KE, Matsumoto RR, Huber JD, Rosen CL. Altering endoplasmic reticulum stress in a model of blast-induced traumatic brain injury controls cellular fate and ameliorates neuropsychiatric symptoms. Front Cell Neurosci. 2014;8:421. doi: 10.3389/fncel.2014.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu B, Wen L, Xiao B, Han F, Shi Y. Single Prolonged Stress induces ATF6 alpha-dependent Endoplasmic reticulum stress and the apoptotic process in medial Frontal Cortex neurons. BMC Neurosci. 2014;15:115. doi: 10.1186/s12868-014-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tao J, Zhu W, Li Y, Xin P, Li J, Liu M, Li J, Redington AN, Wei M. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol. 2011;301:H1471–1486. doi: 10.1152/ajpheart.00097.2011. [DOI] [PubMed] [Google Scholar]
- 111.Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 112.Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B. Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int. 2014;68:18–27. doi: 10.1016/j.neuint.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 113.Xia JG, Xu FF, Qu Y, Song DG, Shen H, Liu XH. Atorvastatin post-conditioning attenuates myocardial ischemia reperfusion injury via inhibiting endoplasmic reticulum stress-related apoptosis. Shock. 2014;42:365–371. doi: 10.1097/SHK.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 114.Grall S, Prunier-Mirebeau D, Tamareille S, Mateus V, Lamon D, Furber A, Prunier F. Endoplasmic reticulum stress pathway involvement in local and remote myocardial ischemic conditioning. Shock. 2013;39:433–439. doi: 10.1097/SHK.0b013e31828e4f80. [DOI] [PubMed] [Google Scholar]
- 115.Duan SR, Wang JX, Wang J, Xu R, Zhao JK, Wang DS. Ischemia induces endoplasmic reticulum stress and cell apoptosis in human brain. Neurosci Lett. 2010;475:132–135. doi: 10.1016/j.neulet.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 116.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun Y, Pu LY, Lu L, Wang XH, Zhang F, Rao JH. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J Gastroenterol. 2014;20:15289–15298. doi: 10.3748/wjg.v20.i41.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, Contreras JL. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138:342–351. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 119.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]