Abstract
Post-injury multiple organ failure results from an inappropriate, overwhelming immune response to injury. During trauma and hemorrhagic shock (T/HS), mesenteric ischemia causes gut mucosal breakdown with disruption of the intestinal barrier. It has been proposed that this releases the gut microbiota systemically via post-shock mesenteric lymph, engendering infectious complications. Despite extensive investigation, no clear evidence has been presented for gut bacterial translocation after resuscitation from T/HS. However, such previous studies were limited by available technologies. More sensitive methods, such as quantitative polymerase chain reaction (PCR), have since emerged for detection of bacterial presence and danger-associated molecular patterns (DAMPs). Quantitative PCR was applied to post-shock mesenteric lymph (PSML) derived from a rat model of T/HS. No bacterial presence was detected in a series of 12 samples, whereas multiple lymph samples showed presence of DAMPs after T/HS. Thus, we confirmed that bacterial translocation does not exist in PSML following resuscitation from T/HS-associated mesenteric ischemia. However, T/HS does increase the presence of mitochondrial DAMPs in PSML. These results support our current position that PSML elaborates remote organ injury by multiple inflammatory mechanisms, including lipid-mediated pro-inflammatory stimuli, and by contribution from gut-derived DAMPS.
Keywords: trauma, hemorrhagic shock, bacterial translocation, systemic inflammatory immune response, danger-associated molecular patterns, mesenteric lymph
INTRODUCTION
Advances in pre-hospital care, resuscitation strategies, operative techniques and surgical critical care have improved the survival of critically injured patients. However, patients surviving the initial injury and resuscitation (>24 hours after injury) remain at significant risk for subsequent development of systemic inflammatory response syndrome (SIRS) and multi-organ failure (1). While the overall incidence of multi-organ failure (MOF) is decreasing, the rates of MOF-related resource utilization, morbidity and death remain unchanged (2). Furthermore, despite decades of investigation, the mechanism driving these late complications continues to be the subject of debate.
Post-traumatic SIRS and elaboration to MOF often occur in absence of infectious etiology. It is proposed that trauma patients experience a “first hit” from interrupted flow-dependent oxygen consumption resulting in ischemia/reperfusion injury. This insult primes the immune system during the early phase following injury, potentiating a pro-inflammatory environment that increases susceptibility to subsequent “second hit” inflammatory stimuli (3). While a number of early mechanisms for this “first hit” immune priming event have been proposed, the precise pathophysiology remains elusive.
Many have suggested the gastrointestinal tract as the major source of bioactive components with the potential to enact the immune response leading to post-injury MOF. One of the earliest and still touted theories proposes that mesenteric ischemia causes gut mucosal breakdown and resulting failure of the intestinal barrier. This potentiates translocation of gut microbiota systemically and leads to infectious complications (4). As post-injury patients with MOF often succumb to infections and secondary sepsis from microorganisms consistent with a gastrointestinal tract origin, bacterial translocation is a plausible mechanistic etiology. Animal models initially focused on portal venous flow as the conduit by which these bacteria were conveyed systemically. However, early clinical investigations that evaluated bacterial cultures of both portal venous and systemic blood samples from critically injured human patients demonstrated no significant bacterial presence correlating with clinical septic sequelae (5).
A sensitive method has emerged for detection and enumeration of bacteria through broad-range quantitative PCR assay of 16S ribosomal RNA (rRNA) genes (6, 7). PCR using 16S rRNA has demonstrated presence of bacteria in systemic blood from patients with acute pancreatitis and concurrent negative blood cultures (8). Similarly, PCR has established early presence of mitochondrial danger-associated molecular patterns (DAMPs) in trauma patients’ plasma (9). Combined, these techniques have been applied to successfully differentiate sterile systemic inflammatory response syndrome from sepsis in a baboon model (10). We have applied this technology to further investigate the hypothesis that mesenteric ischemia-reperfusion from T/HS promotes gut bacterial translocation to PSML.
MATERIALS AND METHODS
Trauma/Hemorrhagic Shock/Resuscitation (T/HS/R) Model
All animal care and procedures were conducted under protocol as approved by the University of Colorado Denver Institutional Animal Care and Use Committee. T/HS was simulated per our established model (11). Briefly, Sprague-Dawley rats weighing 350g to 400g (Harlan Labs, Indianapolis, IN) were housed in a climate controlled barrier facility with 12-hour light/dark cycles with free access to food and water for a period of at least 1 week prior to experimental procedures. Anesthesia was performed with intraperitoneal injection of 50 mg/kg sodium pentobarbital. Local anesthesia was performed with subcutaneous injection of 1% lidocaine. The femoral artery and vein were then cannulated with PE 50 tubing and blood pressure was monitored using a ProPaq invasive monitoring device. A separate skin puncture was created to tunnel the catheters prior to closure of the groin incision. A 3 cm midline laparotomy was performed to mimic tissue injury with trauma. The bowel was eviscerated and rotated to the left, and the mesenteric duct and accessory duct (located adjacent to the superior mesenteric artery) were isolated by blunt dissection. The main lymphatic duct was cannulated with PE 100 tubing, secured with 7-0 prolene suture and the catheter was tunneled posteriorly through the skin. The accessory duct was then ligated with suture. The laparotomy incision was closed in a two layer fashion and lymph collection took place in half-hour intervals. After a 1 hour period of pre-shock lymph collection, shock was induced by controlled hemorrhage to a MAP of 30mmHg which was sustained for 40 min by withdrawing or returning shed blood (SB). Body temperature was monitored rectally and euthermia was maintained with a heat lamp. Resuscitation was performed by infusing 2x Shed Blood volume in NS over 30 min, followed by ½ Shed Blood volume over 30 min, then completed with 2x Shed Blood volume in NS over 60min via the femoral vein. Lymph collection continued for one hour following the completion of resuscitation (3rd hour lymph). The animals were then euthanized via pentobarbital overdose. Lymph collected during the 1 hour of pre-shock, and pooled samples of lymph collected for 3 consecutive hours following shock were centrifuged at 5000g for 7 min. The supernatants were collected and frozen in liquid nitrogen prior to storage at -80°C.
Quantitative PCR
Microbial and eukaryotic DNA was extracted from 300 μL of lymph using an UltraClean Microbial DNA extraction kit according to the manufacturer’s protocol (MoBio Laboratories, Carlsbad, CA). Total bacteria were assayed by detection of the microbial 16S rRNA gene using the TaqMan primer/probe set (Life Technologies, Carlsbad, CA). Each PCR reaction included 10 μL DyNAmo Colorflash Probes mastermix (Thermo Fisher, Pittsburg, PA), 1 μL of combined primer/probe at 20x (Integrated DNA Technologies, Inc., Coralville, IA), 5 μL of DNA template, and 4 μL of PCR water. Negative PCR control and positive fecal control were assayed to verify accuracy of the probe (see Figure, Supplemental Digital Content 1). The mitochondrial DNA was quantified using the genes for rat cytochrome C oxidase subunit III (cox3) and rat cytochrome B (cytB). The mitochondrial genes were normalized to the total DNA input, by measuring the eukaryotic 18S rRNA gene. Mitochondrial and 18S PCR reactions contained 10 μL DyNAmo Colorflash SYBR mastermix (Thermo Fisher, Pittsburg, PA), 1 μL of each forward and reverse primers at 4 μM (Integrated DNA Technologies, Inc., Coralville, IA), 5 μL of DNA template, and 3 μL of PCR water. For all qPCR assays, thermocycling was performed on the Bio-Rad CFX96 C1000 using the following conditions: 7 minutes at 95°C, then 40 cycles of 15 seconds at 95°C and 1 minute at 60°C with a final 5 minute extension at 60°C.
RESULTS
Detection of bacterial presence
A total of 12 rats underwent T/HS with successful collection of pre- and post-shock mesenteric lymph. All pre-shock mesenteric lymph samples demonstrated no 16S rRNA gene amplification to support bacterial presence. Additionally, there was no detected amplification of the 16s rRNA gene in all post-shock mesenteric lymph samples as detected by PCR.
Detection of mitochondrial DAMPs
Mitochondrial DAMPs via cytB amplification were detected in 11/12 pre-shock samples with significant amplification, whereas 9/12 PSML samples demonstrated amplification. Expression of cytB in pre-shock samples was 326.9 ± 284.6 fold-change higher than the comparative housekeeping gene, and cytB expression in post-shock samples was 973.4 ± 863.9-fold higher. While the post-shock samples had on average higher fold-change in expression, this was not statistically significant (p=0.4889). (Figure 1)
Figure 1.
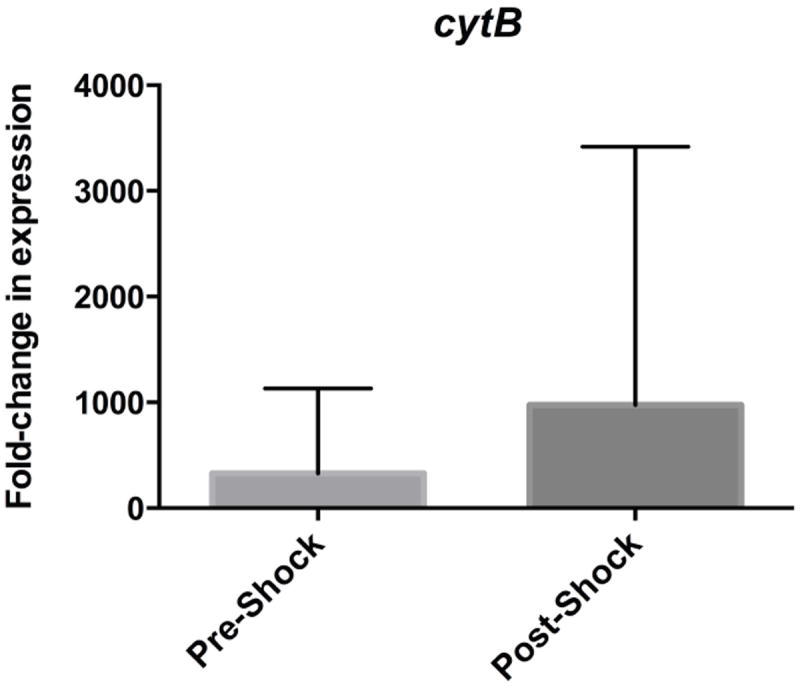
Relative fold-change in expression of mitochondrial DAMPs cytB in pre-shock and post-shock mesenteric lymph as compared to the housekeeping gene 18s.
Similarly, comparison of relative expression of cox3 did not demonstrate a significant difference between pre- and post-shock lymph samples (p=0.8007). The relative fold-change in expression of cox3 in pre-shock samples was 46.24 ± 45.51 greater than housekeeping gene expression. In post-shock samples, the relative fold-change in expression of cox3 was 65.29 ± 58.40 greater than housekeeping gene. (Figure 2)
Figure 2.
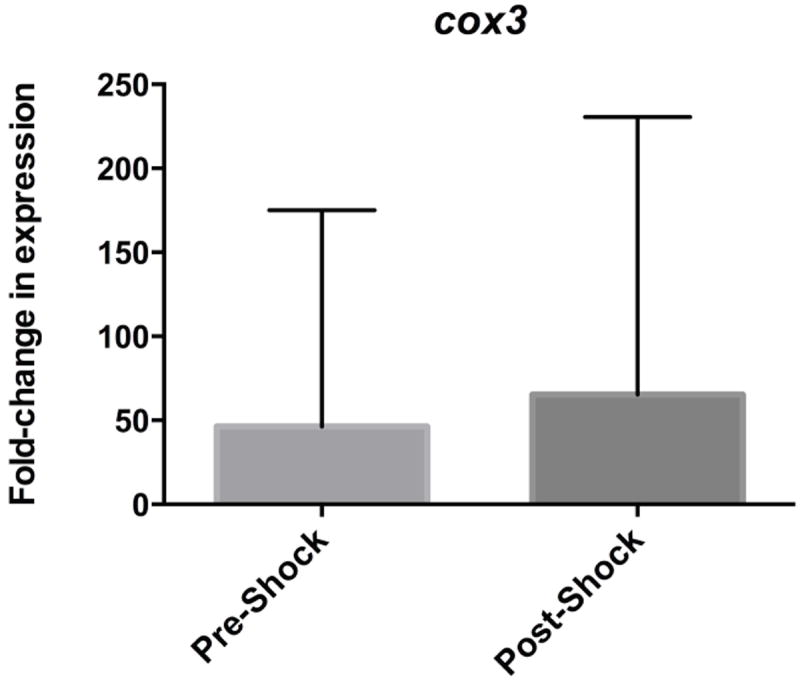
Relative fold-change in expression of mitochondrial DAMPs cox3 in pre-shock and post-shock mesenteric lymph as compared to the housekeeping gene 18s.
Lastly, paired analysis of each animal’s pre- and post-shock lymph samples were compared to determine if within experimental individuals there was a consistent trend of increased mitochondrial DAMPs following shock. However, this statistical analysis did not reach significance. Of note, some paired samples showed a trend towards decreasing mitochondrial DAMPs following shock and resuscitation.
DISCUSSION
Reperfusion of ischemic mesenteric tissue beds leads to systemic elaboration of immunologically active substances that can evoke MOF, systemic infection and sepsis. Abundant evidence supports PSML as the conduit by which culprit mediators are conveyed systemically. Because of this observation, it has been hypothesized that bacteria from the gut microbiome translocate into PSML during resuscitation following ischemic insult and ultimately mediate post-shock systemic sequelae. However, human studies have demonstrated conflicting evidence in support of this theory. To date, continued support for bacterial translocation in PSML is sustained by limited animal models with investigative technologic constraints.
With lack of evidence to implicate the portal vein, investigators considered mesenteric lymphatics draining the reperfused gut as a potential bacterial conduit. In 1998, Magnotti et al had confirmed that mesenteric lymph carried gut-derived mediators of endothelial permeability and lung injury following hemorrhagic shock (12). Post-shock mesenteric lymph (PSML) was subsequently shown to be a bioactive body fluid capable of inciting remote organ dysfunction in the lung (11), kidney (13), and heart (14, 15). Further, mesenteric lymph serves to prime quiescent neutrophils for enhanced respiratory burst (16) potentially via mechanisms related to the release of inflammatory lipid mediators such as phospholipase A2 and 5-lipoxygenase products (13, 17). PSML has also been demonstrated to enhance leukocyte sequestration as elaborated by up-regulation of both neutrophil and endothelial cell adhesion molecules (18-20). Moreover, cross-transfusion of PSML into naïve rats elicited acute lung injury without the presence of T/HS (11), while mesenteric duct ligation prior to T/HS mitigated this injury (14, 18, 21).
With experimental agreement that post-shock mesenteric lymph is central in the genesis of remote organ injury, investigations sought to demonstrate bacterial translocation from the reperfused gut into this transudate. Attempts to do so in either portal blood or lymph have been limited by technologies with poor sensitivity and specificity. Further complicating the debate, studies have suggested differing definitions of “bacterial translocation”. Detection of endotoxemia in portal and systemic blood via limulus assay has been used as a corollary of gram-negative bacteremia (22, 23). However, the limulus assay is flawed as its response varies quantitatively to different bacterial species. Substances other than bacterial endotoxin can trigger positive reactions, and no clinical correlation has been consistently shown in post-injury MOF patients (22, 24, 25). Microbial culture of tissue specimens including portal blood, systemic blood, and mesenteric lymph nodes have also been performed to establish migration of gut-origin bacteria beyond the intestines (5, 26, 27). Moore et al performed blood and lymph node cultures prospectively on trauma patients undergoing laparotomy, and found no parallels between positive cultures and clinical outcomes such as sepsis, organ failure, or survival (5). Doty et al utilized a swine model of abdominal compartment syndrome and found equivocal results, wherein both control and treatment animals had positive culture results with no significant difference between the two groups (27). As such, culture-based definitions of bacterial translocation are similarly flawed in their accuracy and may not translate to clinical outcomes. Ultimately, the bacterial translocation hypothesis persists due to conflicting evidence and technically limited methodologies throughout this investigative history.
This study provides evidence that bacterial translocation as measured by presence of bacterial 16S rRNA genes does not exist in PSML following resuscitation from T/HS-associated mesenteric ischemia. We have demonstrated previously that within three hours of hemorrhagic shock, a significant inflammatory response with systemic effects occurs (11, 13, 28). This study confirms that both trauma and hemorrhagic shock can increase the presence of mitochondrial DAMPs as demonstrated by increased mitochondrial DNA in mesenteric lymph. Mitochondrial DAMPs serve as both markers of injury as well as bioactive components that are conveyed to the systemic circulation, supporting our current position that PSML elaborates remote organ injury by multiple inflammatory stimuli. The bioactivity of mitochondrial DAMPs has been shown in vitro via activation of human neutrophils through p38 mitogen-activated protein kinase phosphorylation (29). Previously described non-infectious mechanisms of mesenteric ischemia-induced remote injury further include lipid mediators such as phospholipase A2 (17), leukotriene pathway modulation and arachidonate derivatives (28), potentiation of endothelial cell adhesion molecule expression (18, 19), neutrophil priming (16, 19, 30), endothelial cell toxicity mediated by modified albumin (31) and nuclear factor-κB (32), and gut-derived DAMPs (9).
As mitochondrial DAMPs were found to have generally increasing amplification in post-shock samples, pairwise analysis was attempted within experimental individuals. Interestingly, this demonstrated decreased measured amplification of mitochondrial DAMPs in post-shock samples as compared to pre-shock lymph in some animals. This is a hypothesis-generating observation. DAMPs may be released during laparotomy (trauma) and diluted in increased volume of lymph produced during resuscitation. We have previously demonstrated significant increases in lymph production during resuscitation from hemorrhagic shock (33). This increase in effluent could then result in an observed dilution of substrates elaborated within lymph as a response to trauma. Pooled post-shock lymph represents a technical limitation of the current study. Future investigation of DAMPs in PSML would benefit from sequential collection of lymph specimens (hourly) through resuscitation which would allow for analysis of the time course of DAMP’s elaboration and potential hourly dilution as lymph volume increases. Fold-change in DAMPs could then be correlated with concurrent volume of lymph production to better delineate a potential dilutional effect seen in the current investigation.
Investigations of mesenteric lymph inherently requires a traumatic insult (i.e. laparotomy) to provide access for sampling of the biological specimen. Thus, our model has no true “sham” as even pre-shock specimens will reflect some level of insult as evidenced by the presence of mitochondrial DAMPs in pre-shock mesenteric lymph. Our PSML specimens were also pooled over 3 hours. This time point was based on our previous research demonstrating a systemic inflammatory response within this time period. While bacterial translocation may occur at a later time point and contribute to delayed systemic inflammatory sequelae, it is evident that bacterial translocation is not the inciting event during acute ischemia/reperfusion based on these data. Post-shock lymph was pooled for this analysis with the intent of optimizing opportunity to identify or exclude bacterial 16s subunits in PSML. Analysis based on sequentially collected specimens, at hourly intervals, may have better demonstrated an increase of mitochondrial DAMPs in PSML, representing a technical limitation of analyzing PSML in this investigation.
Lastly, the use of PCR has been demonstrated to be highly sensitive for the detection of bacterial DNA and mitochondrial DAMPs in prior studies. PCR amplifies a genetic message to confirm its presence within biological samples. However, a genetic message that is not being actively transcribed does not translate into a biologically active process. Thus, PCR data can be further enhanced when utilized in concert with a secondary method that can confirm biological end-products. Unfortunately, many of these research methods have been previously discussed as flawed techniques. This study examined lymph, an ultrafiltrate, for products of cell lysis; such samples are prone to biological variability and RNA degradation, which can decrease the sensitivity and accuracy of PCR. In this regard, the detection of positive fecal controls for bacterial DNA and eukaryotic 18s rRNA in our lymph samples add validity to the methods and confirms the presence of detectable biological products in our lymph specimens.
Further investigation of PSML continues to reveal its complex nature, implicating increasingly more bioactive compounds capable of inducing tissue injury. Proteomic analyses in animal models and human subjects have shown the complex nature of PSML as compared to plasma and its dynamic changes throughout the resuscitative period (34, 35). These studies revealed depletion of coagulation cascade factors, increased lipid mediators, altered protease/anti-protease balance, and increased extracellular matrix-related proteins. Mechanistically, the processes by which PSML elicit systemic inflammation and remote injury re complex and no longer include bacterial translocation as a principal etiology. Future directions of research should therefore focus on defining the bioactive components of PSML and methods of intervention to improve patient outcomes.
Supplementary Material
Figure, Supplementary Digital Content 1. Relative fold-change in expression of 16s rRNA gene to represent bacterial presence, with no measured amplification of pre- or post-shock samples. Positive and negative fecal controls were also performed for test validity assurance.
Acknowledgments
Statement of support: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers P50GM049222 and T32GM008315. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40(9):912–8. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, Maier RV, Burlew CC. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76(3):582–92. doi: 10.1097/TA.0000000000000147. discussion 592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotstein OD. Modeling the two-hit hypothesis for evaluating strategies to prevent organ injury after shock/resuscitation. J Trauma. 2003;54(5 Suppl):S203–6. doi: 10.1097/01.TA.0000064512.62949.92. [DOI] [PubMed] [Google Scholar]
- 4.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1(2):141–5. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM, Abernathy CM, Parsons PE. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. The Journal of trauma. 1991;31(5):629–36. doi: 10.1097/00005373-199105000-00006. discussion 636-8. [DOI] [PubMed] [Google Scholar]
- 6.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148(Pt 1):257–66. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 8.Pearce CB, Zinkevich V, Beech I, Funjika V, Ruiz AG, Aladawi A, Duncan HD. Using the polymerase chain reaction coupled with denaturing gradient gel electrophoresis to investigate the association between bacterial translocation and systemic inflammatory response syndrome in predicted acute severe pancreatitis. World J Gastroenterol. 2005;11(45):7142–7. doi: 10.3748/wjg.v11.i45.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39(1):55–62. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wohlauer MV, Moore EE, Harr J, Eun J, Fragoso M, Banerjee A, Silliman CC. Cross-transfusion of postshock mesenteric lymph provokes acute lung injury. J Surg Res. 2011;170(2):314–8. doi: 10.1016/j.jss.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228(4):518–27. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stringham JR, Moore EE, Gamboni F, Harr JN, Fragoso M, Chin TL, Carr CE, Silliman CC, Banerjee A. Mesenteric lymph diversion abrogates 5-lipoxygenase activation in the kidney following trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2014;76(5):1214–21. doi: 10.1097/TA.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambol JT, Lee MA, Caputo FJ, Kawai K, Badami C, Kawai T, Deitch EA, Yatani A. Mesenteric lymph duct ligation prevents trauma/hemorrhage shock-induced cardiac contractile dysfunction. J Appl Physiol (1985) 2009;106(1):57–65. doi: 10.1152/japplphysiol.90937.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambol JT, Lee MA, Jiang M, Dosi G, Dong W, Deitch EA, Yatani A. Mesenteric lymph from rats with trauma-hemorrhagic shock causes abnormal cardiac myocyte function and induces myocardial contractile dysfunction. J Appl Physiol (1985) 2011;111(3):799–807. doi: 10.1152/japplphysiol.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Johnson JL, Silliman CC. Mesenteric lymph is responsible for post-hemorrhagic shock systemic neutrophil priming. J Trauma. 2001;51(6):1069–72. doi: 10.1097/00005373-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez RJ, Moore EE, Ciesla DJ, Meng X, Biffl WL, Silliman CC. Post-hemorrhagic shock mesenteric lymph lipids prime neutrophils for enhanced cytotoxicity via phospholipase A2. Shock. 2001;16(3):218–22. doi: 10.1097/00024382-200116030-00008. [DOI] [PubMed] [Google Scholar]
- 18.Xu DZ, Lu Q, Adams CA, Issekutz AC, Deitch EA. Trauma-hemorrhagic shock-induced up-regulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32(3):760–5. doi: 10.1097/01.ccm.0000114815.88622.9d. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez RJ, Moore EE, Ciesla DJ, Nieto JR, Johnson JL, Silliman CC. Post-hemorrhagic shock mesenteric lymph activates human pulmonary microvascular endothelium for in vitro neutrophil-mediated injury: the role of intercellular adhesion molecule-1. J Trauma. 2003;54(2):219–23. doi: 10.1097/01.TA.0000047807.12644.95. [DOI] [PubMed] [Google Scholar]
- 20.Partrick DA, Moore FA, Moore EE, Barnett CC, Jr, Silliman CC. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New horizons. 1996;4(2):194–210. [PubMed] [Google Scholar]
- 21.Badami CD, Senthil M, Caputo FJ, Rupani BJ, Doucet D, Pisarenko V, Xu DZ, Lu Q, Feinman R, Deitch EA. Mesenteric lymph duct ligation improves survival in a lethal shock model. Shock. 2008;30(6):680–5. doi: 10.1097/SHK.0b013e318173edd1. [DOI] [PubMed] [Google Scholar]
- 22.Koike K, Moore EE, Moore FA, Read RA, Carl VS, Banerjee A. Gut ischemia/reperfusion produces lung injury independent of endotoxin. Crit Care Med. 1994;22(9):1438–44. doi: 10.1097/00003246-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Plachouras D, Stamatakos M, Baziaka F, Giamarellos-Bourboulis E, Tsaganos T, Giamarellou H, Safioleas M. Portal and systemic endotoxemia in abdominal operations: the significance of acute abdomen. J Surg Res. 2006;134(1):133–7. doi: 10.1016/j.jss.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Brandenburg K, Howe J, Gutsman T, Garidel P. The expression of endotoxic activity in the Limulus test as compared to cytokine production in immune cells. Curr Med Chem. 2009;16(21):2653–60. doi: 10.2174/092986709788682001. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J, McConnell JS. Limulus assay in prediction of septic shock. Lancet. 1988;1(8595):1165. doi: 10.1016/s0140-6736(88)91977-0. [DOI] [PubMed] [Google Scholar]
- 26.Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989;124(6):699–701. doi: 10.1001/archsurg.1989.01410060065013. [DOI] [PubMed] [Google Scholar]
- 27.Doty JM, Oda J, Ivatury RR, Blocher CR, Christie GE, Yelon JA, Sugerman HJ. The effects of hemodynamic shock and increased intra-abdominal pressure on bacterial translocation. J Trauma. 2002;52(1):13–7. doi: 10.1097/00005373-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silliman CC, Banerjee A. Arachidonic acid in postshock mesenteric lymph induces pulmonary synthesis of leukotriene B4. J Appl Physiol (1985) 2008;104(4):1161–6. doi: 10.1152/japplphysiol.00022.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34(1):55–9. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 30.Caruso JM, Feketeova E, Dayal SD, Hauser CJ, Deitch EA. Factors in intestinal lymph after shock increase neutrophil adhesion molecule expression and pulmonary leukosequestration. J Trauma. 2003;55(4):727–33. doi: 10.1097/01.TA.0000037410.85492.77. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, Xu DZ, Deitch EA. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock. 2005;23(5):417–25. doi: 10.1097/01.shk.0000160524.14235.6c. [DOI] [PubMed] [Google Scholar]
- 32.Damle SS, Moore EE, Nydam TL, Banerjee M, Gamboni-Robertson F, Su X, Banerjee A. Postshock mesenteric lymph induces endothelial NF-kappaB activation. J Surg Res. 2007;143(1):136–40. doi: 10.1016/j.jss.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83(2):83–8. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 34.D’Alessandro A, Dzieciatkowska M, Peltz ED, Moore EE, Jordan JR, Silliman CC, Banerjee A, Hansen KC. Dynamic changes in rat mesenteric lymph proteins following trauma using label-free mass spectrometry. Shock. 2014;42(6):509–17. doi: 10.1097/SHK.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peltz ED, Moore EE, Zurawel AA, Jordan JR, Damle SS, Redzic JS, Masuno T, Eun J, Hansen KC, Banerjee A. Proteome and system ontology of hemorrhagic shock: exploring early constitutive changes in postshock mesenteric lymph. Surgery. 2009;146(2):347–57. doi: 10.1016/j.surg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplementary Digital Content 1. Relative fold-change in expression of 16s rRNA gene to represent bacterial presence, with no measured amplification of pre- or post-shock samples. Positive and negative fecal controls were also performed for test validity assurance.


