Abstract
Type 2 helper T (TH) cells produce interleukin 13 (IL-13) when stimulated by papain or house dust mites (HDM) and induce eosinophilic inflammation. This innate response is dependent on IL-33 but not T cell antigen receptors (TCRs). While type 2 innate lymphoid cells (ILC2s) are the dominant innate producers of IL-13 in naïve animals, we show here that in helminth-infected mice, TH2 cell numbers increased and became major mediators of innate type II responses. TH2 cells made important contributions to HDM-induced antigen–non-specific eosinophilic inflammation and protected mice recovering from Ascaris suum infection against subsequent infection with the phylogenetically distant nematode Nippostrongylus brasiliensis. Our findings reveal a previously unappreciated role of effector TH2 cells during TCR-independent innate-like immune responses.
Introduction
Host immunity is comprised of the non-specific innate immune system and the specific adaptive immune system1, 2. Innate lymphoid cells (ILCs) are lymphocyte-like innate immune cells, lacking T or B cell receptors, that make robust effector cytokine responses early in infection and often contribute to the resolution of such infections3. CD4+ T lymphocytes respond in an antigen-specific manner to infectious agents and can release the same set of cytokines that are produced by ILCs4. Previously, we have shown that in vitro primed helper TH1, TH2 and TH17 CD4+ T cells produce many of their signature cytokines not only in response to their cognate antigen but also to certain inducing cytokines5, much in the manner that ILCs respond, thus constituting “innate” immune responses by cells of the adaptive immune system.
The inducing cytokine requirements for CD4+ TH cells to produce their signature cytokines are stimulation with an interleukin 1 (IL-1) family member and a STAT-activator5. For TH1 cells, the IL-1 family member is IL-18 and the STAT-activating cytokine is IL-12, an activator of STAT4; for TH2 cells, the pair is IL-33 and IL-2, IL-7 or TSLP, all STAT5 activators; and for TH17 cells, IL-1β and IL-23, a STAT3 activator. ILCs use similar stimuli to produce their effector cytokines. For ILC2 cells, ILCs that express GATA-3 and produce the type 2 cytokines IL-13 and IL-5, IL-33 is a principal stimulant; TSLP can enhance that response.
The competence of memory phenotype CD4+ T cells to mount innate-like cytokine production in response to cytokine stimulation raises the question of the relative contribution of ILCs and CD4+ TH cells to innate-like cytokine responses. We sought to test this in models of ILC2 and TH2 responses. TH2 cells are quite rare in naïve mice so that it would be anticipated that ILC2 cells would dominate innate cytokine responses in such animals. The relative importance of the two cell types could be quite different in mice that have mounted vigorous type 2 immune responses and that have relatively large numbers of memory phenotype TH2 cells.
To test the relative importance of expanded ILC2 and TH2 cells in early innate cytokine responses, we made use of the 4C13R reporter mice previously reported6. These mice report IL-4 production by expression of AmCyan and IL-13 production by expression of DsRed and thus allow the determination of in situ production of IL-4 and IL-13 without ex vivo stimulation. We demonstrated that TH2 cells could produce IL-13 in vivo in response to the combination of IL-33 and a STAT5 activator and that ovalbumin (OVA)-specific (OT-II) TH2 cells produced IL-33-dependent IL-13 when challenged intratracheally with papain.
In mice recovering from Nippostrongylus brasiliensis (N. brasiliensis) infection, TH2 cells outnumbered ILC2 cells. These N. brasiliensis-induced TH2 cells responded to challenge with papain or house dust mites (HDM) with the production of IL-13 and mediation of type 2 inflammatory responses, independent of TCR stimulation. TH2 cells induced in response to infection with the Clade III parasitic nematode Ascaris suum (A. suum) contributed to host protection against a phylogenetically distant Clade V nematode N. brasiliensis7. Taken together, these results indicate that TH2 cells function actively in innate immune defense, promoting type II inflammation and protecting the mucosal barrier. The relative importance of TH2 and ILC2 cells in innate immunity depends largely on the relative abundance of the two cell types and therefore varies in naïve and antigen-experienced animals.
Results
Transferred TH2 cells respond to cytokine administration
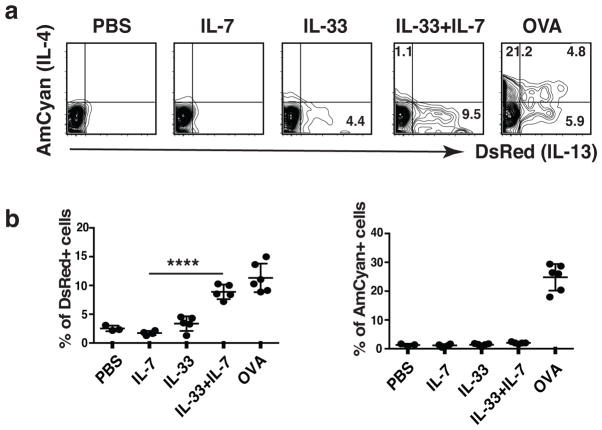
Expression of fluorescent proteins DsRed and AmCyan in 4C13R dual reporter mice were faithful indicators for the production of cytokine IL-13 and IL-4, respectively (Supplementary Fig. 1a, b, c). OVA-specific CD4+ TH2 cells were prepared from naïve T cells of OT-II–4C13R reporter mice and injected intravenously (i.v.) into C57BL/6 (B6) mice that were then challenged intratracheally with either cytokines or OVA. 24 h after the last challenge with cytokines, 26% of the OT-II cells harvested from the lung expressed AmCyan/IL-4 and 12% expressed DsRed/IL-13 (Fig. 1a, b). Administration of IL-33 and IL-7 led 1% of the cells to express AmCyan and 9% to express DsRed; the mean fluorescence intensity (MFI) of DsRed-expression induced by the cytokines was comparable to that induced by OVA. Administration of IL-33 or IL-7 alone caused only a modest (IL-33) or no (IL-7) increase in DsRed positive cells over that observed with PBS challenge. Administration of IL-33 and TSLP similarly induced significant DsRed but no AmCyan expression (Supplementary Fig. 2a, b). Thus, adoptively transferred TH2 cells can respond to administration of IL-33 plus the STAT5 activators IL-7 or TSLP to produce IL-13.
Figure 1. Administration of IL-33 and IL-7 induces TH2 cells to produce IL-13.
(a) Naïve CD4 T cells were sorted from OT-II–4C13R reporter mice and cultured under TH2 conditions for 3 rounds. Cells were cultured in IL-2-containing medium for 10 days and then injected i.v. into C57BL/6 recipient mice. 24h later, mice were intratracheally challenged with PBS, or indicated cytokines (150 ng IL-33 and/or 100 ng IL-7 each mouse) for 3 consecutive days, or OVA (100 μg endotoxin-free OVA in PBS) once. Lungs were collected and cytokine production was examined 24h after last cytokine administration (72h after OVA challenge). Cells shown were gated on transferred OT-II TH2 cells. (b) Statistical analysis of the cytokine production. Error bars represent standard deviation from the mean. ****, P <0.0001 by two tailed student’s t-test. Data are representative of three independent experiments with 3–6 mice in each group (a, b).
OT-II TH2 cells respond to papain to produce IL-13
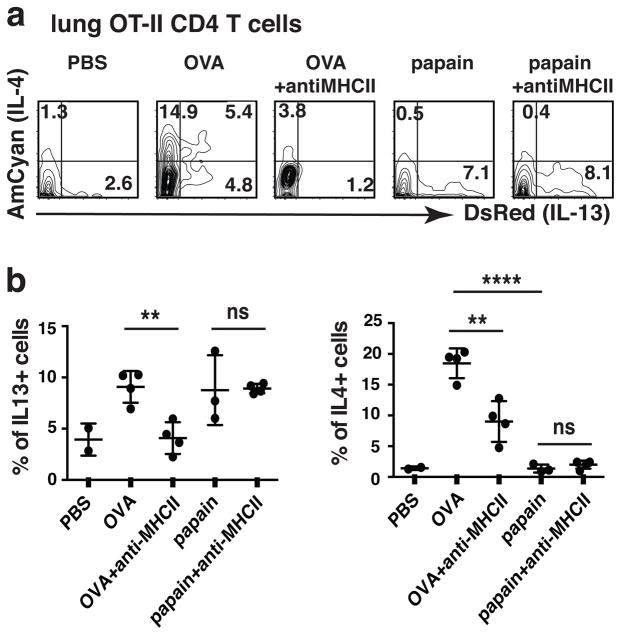
Papain has been reported to induce both IL-33 and TSLP production by epithelial cells8. We asked whether in vivo-generated TH2 cells would respond to papain challenge with the production of IL-13 and, if responding, whether the response would be MHC class II-independent. Naïve OVA-specific CD4+ T cells from OT-II–4C13R reporter mice were injected i.v. into B6 recipients that were then infected with N. brasiliensis third-stage larvae (L3) and, at the same time, immunized with endotoxin-free OVA (Fig. 2a, b). They received an intratracheal OVA “boost” five days later. Twenty five days after the N. brasiliensis infection and OVA priming, the mice were challenged intratracheally with endotoxin-free OVA once, PBS, or papain for three consecutive days in the presence or absence of an anti-MHC II antibody; lung cells were analyzed 24 h later. In response to OVA challenge, ~19% of the OT-II cells expressed AmCyan and ~9% expressed DsRed (Fig. 2a, b). Treatment with antibodies against major histocompatibility complex class II molecules (MHCII) diminished DsRed expression to basal amounts and substantially inhibited AmCyan expression in OVA-challenged mice. In response to challenge with papain, 8% of OT-II cells expressed DsRed; this frequency was not affected by anti-MHCII treatment. Papain did not induce AmCyan expression.
Figure 2. In vivo generated OVA-specific TH2 cells respond to papain to produce IL-13 in an MHC-independent manner.
(a, b) 0.5×106 sorted naïve CD4 T cells from OT-II–4C13R reporter mice were injected i.v. into C57BL/6 mice. One day after cell transfer, mice were immunized subcutaneously with a mixture of 500 N. brasiliensis (N.b.) infective larvae L3 and 100 μg endotoxin-free OVA and then boosted intratracheally 5 days later with OVA (100 μg) in PBS. Twenty-five days after N. brasiliensis infection, mice were challenged intratracheally with PBS, OVA (100 μg, endotoxin-free) once, or papain (25 μg) for 3 consecutive days with or without an anti-MHCII antibody. 500 μg anti-MHCII antibody was administered i.v. on day 1 and day 3 of papain challenge. Lungs were collected 24h after last papain challenge; DsRed and AmCyan expression by transferred OT-II TH2 cells were analyzed. Statistical analysis of the cytokine production by OT-II TH2 cells. **, P<0.01; ns, not significant, P>0.05; ****, P<0.0001. Data are representative of three independent experiments with 2–4 mice in each group.
Since the diminution of DsRed and AmCyan expression in the MHCII antibody-treated, OVA-challenged group might conceivably have been caused by depletion of MHCII-expressing cells rather than by blocking of TCR stimulation, a similar experiment was done by using FcRγ-deficient recipients, where the MHCII antibody is less likely to interfere with other cell types. Treatment with anti-MHCII inhibited responses to OVA, indicating that the MHCII antibody-mediated inhibition of antigen-driven responses was unlikely to have been caused by depletion of antigen-presenting cells (Supplementary Fig. 3). Thus, in vivo generated OVA-specific OT-II Th2 cells respond to papain with production of IL-13 but not IL-4 and such cytokine production is independent of MHCII.
Papain-induced IL-13 production requires IL-33
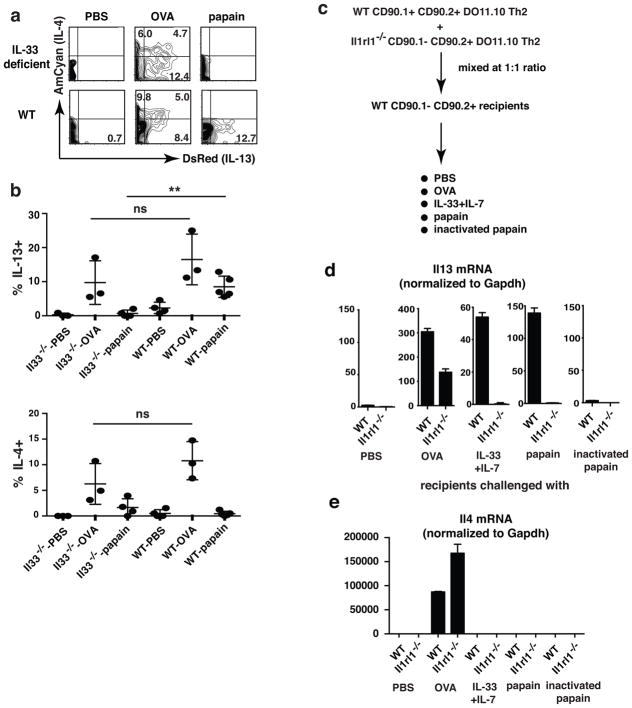
Naïve OVA-specific CD4+ T cells from OT-II–4C13R reporter mice were injected i.v. into IL-33-deficient or wild-type recipients. Mice were infected with N. brasiliensis, immunized with OVA and boosted as in the experiment described above. To exclude the possible impairment of TH2 priming in IL-33-deficient recipients, exogenous IL-33 (150 ng) was given i.v. on day 1 and day 5. Intratracheal challenge with papain caused ~9% of OT-II cells in wild-type recipients to express DsRed, but fewer than 2% were DsRed positive in IL-33-deficient mice (Fig. 3a, b). OVA challenge-induced AmCyan and DsRed expression in wild-type and IL-33-deficient recipients were not statistically different, indicating no intrinsic defect in priming of OT-II cells to TH2 cells in IL-33-deficient mice. We conclude that it was the absence of IL-33 at the time of challenge that was responsible for the lack of response of TH2 cells to papain in the IL-33-deficient recipients.
Figure 3. IL-33 acts directly on in vivo generated TH2 cells causing them to produce IL-13 in response to papain.
(a, b) 0.5×106 naïve CD4 cells from OT-II–4C13R reporter mice were transferred i.v. into wild-type (WT) recipient mice or IL-33-deficient (Il33−/−) recipient mice. Mice were immunized and challenged as described in 2a. 150 ng IL-33 was administered twice i.v. on Day 1 and Day 5 of infection. Lungs were collected 24h after last papain challenge; DsRed and AmCyan expression by transferred OT-II TH2 cells were analyzed. Statistical analysis of the cytokine production by the transferred OT-II TH2 cells. ns, not significant, P>0.05; **, P<0.01. (c–e) Naïve CD4 T cells were sorted from wild-type (WT) CD90.1+CD90.2+ DO11.10 mice or Il1rl1−/−CD90.1−CD90.2+ DO11.10 mice. Cells were cultured under TH2 conditions with OVA peptide and APC for three rounds. After being cultured in IL-7-containing medium for 7 days, wild-type TH2 cells and Il1rl1−/− TH2 cells were mixed at 1:1 ratio and i.v. injected into wild-type CD90.1−CD90.2+ BALB/c recipients. 24h later, mice were intratracheally challenged with IL-33 (150 ng), IL-7 (100 ng), papain (25 μg), heat-inactivated papain (25 μg) or PBS for 3 consecutive days, or with endotoxin-free OVA (100 μg) once. 24h after IL-33 plus IL-7, papain or PBS challenge or 4h after OVA challenge, lungs were isolated and single cell suspensions were prepared. Transferred WT and Il1rl1−/−DO11.10 TH2 cells were sorted from lung cell suspensions. The amounts of Il13 mRNA and Il4 mRNA were measured by real time PCR. Il13 (d) and Il4 (e) mRNA amount in wild-type cells in PBS-treated recipients were set as 1. Data are representative of two (a, b) or one (c–e) independent experiments with 2–5 mice in each group.
TH2 cells are the direct targets of IL-33
Naïve CD4+ cells were sorted from CD90.1−CD90.2+IL-33 receptor-deficient (Il1rl1−/−) DO11.10 mice and CD90.1+CD90.2+ wild-type DO11.10 mice and primed under TH2 conditions in vitro (Supplementary Fig. 4a, b). Such TH2 cells were mixed at a ratio of 1:1 and transferred i.v. into BALB/c recipients (Fig. 3c–e). In recipients that had been intratracheally challenged with OVA, sorted Il1rl1−/− DO11.10 TH2 cells and wild-type DO11.10 TH2 cells showed similar antigen-induced Il4 and Il13 mRNA upregulation, indicating no intrinsic defect in TH2 differentiation of Il1rl1−/−DO11.10 cells (Fig. 3d, e). In recipients that had been challenged with IL-33 plus IL-7, wild-type TH2 cells expressed ~50-fold more Il13 mRNA than did Il1rl1−/− TH2 cells. Indeed, Il1rl1−/− TH2 cells challenged with IL-33 plus IL-7 showed no more Il13 mRNA than did such cells challenged with PBS. Similarly, papain induced 140-fold Il13 mRNA induction in wild-type TH2 cells but not in Il1rl1−/− TH2 cells. Inactivated papain did not induce Il13 mRNA upregulation. Il4 mRNA upregulation in response to IL-33 plus IL-7 or papain did not occur in TH2 cells from either donor. Thus, IL-33 and papain, through IL-33, act directly on TH2 cells.
Analysis of endogenous polyclonal TH2 cells in vivo
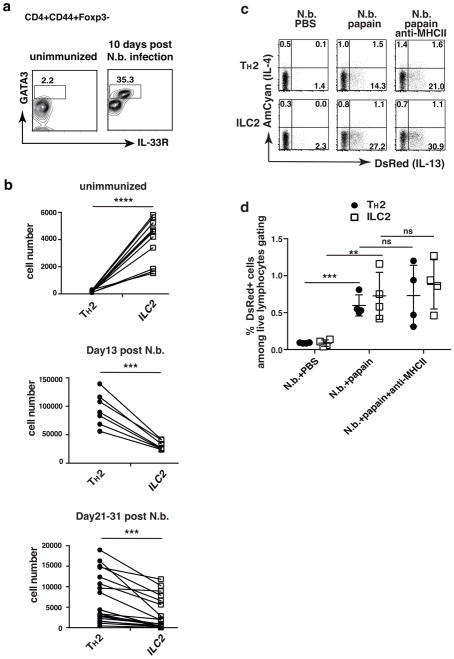
Endogenous TH2 cells were identified as CD4+CD44+Foxp3−GATA-3+IL-33R+ cells while Lin−CD44+GATA-3+IL-33R+CD127+Thy1+ marked ILC2 cells. Only a few hundred TH2 cells were detectable in the lungs of naïve wild-type mice while several thousand ILC2 cells were present (Fig. 4a, b). TH2 cell numbers increased during N. brasiliensis infection resulting in ~100,000 lung TH2 cells 13 days post inoculation with parasitic larvae. ILC2 cells increased to a more modest degree so that there were 2–4 fold more lung TH2 cells than ILC2 cells on day 13 post-inoculation. Numbers of TH2 and ILC2 cells declined proportionally thereafter but there were still significantly more TH2 than ILC2 cells 3 to 4 weeks post inoculation with N. brasiliensis.
Figure 4. TH2 cells generated by infection with N. brasiliensis respond to papain to produce TCR-independent IL-13.
(a) Lung TH2 cells expanded dramatically upon N. brasiliensis infection. Lungs were harvested from mice either unimmunized or 10 days after N. brasiliensis infection. TH2 cells were identified as CD4+CD44+Foxp3−GATA3+IL-33R+. Markers for ILC2 cells were Lin−CD44+GATA3+IL-33R+CD127+Thy1+. Data are representative of at least three independent experiments with 3 mice in each group. (b) Number of lung-resident TH2 and ILC2 cells in individual mice that were unimmunized or 13 days or 21–31 days post-N. brasiliensis infection. ****, P<0.0001; ***, P<0.001 by paired t-test. Data are compiled from multiple independent experiments. (c, d) Twenty-five days after N. brasiliensis infection, 4C13R reporter mice were challenged intratracheally with PBS, or papain (25 μg in PBS) for 3 consecutive days with or without anti-MHCII antibody. 500 μg anti-MHCII antibody was administered i.v. on day 1 and day 3 of papain challenge. DsRed and AmCyan expression by lung-resident TH2 and ILC2 cells were analyzed 24h after last papain challenge. (d) Comparison of percent of DsRed+ TH2 and ILC2 cells among total live lymphocytes. ns, not significant, P>0.05; **, P<0.01; ***, P<0.001. Data are representative of three independent experiments with 4 mice in each group (c–d).
4C13R reporter mice were inoculated with N. brasiliensis. Twenty-five days later, they were challenged with papain intratracheally for three consecutive days in the presence or absence of anti-MHCII. In response to papain, 16% of TH2 cells and 28% of ILC2s expressed DsRed; anti-MHCII administration did not diminish the frequency of DsRed-positive cells among either cell population (Fig. 4c). Although the proportion of DsRed-expressing TH2 cells among total TH2 cells was lower than those among ILC2s, the total number of TH2 cells and of ILC2 cells in the lung that produced IL-13 in response to papain were comparable (Fig. 4d). Anti-MHCII treatment did not diminish the frequency of DsRed expressing TH2 or ILC2 cells. Thus, both endogenous lung-resident Th2 cells and ILC2s respond to papain to produce IL-13.
HDM induces eosinophilic inflammation in previously-infected mice
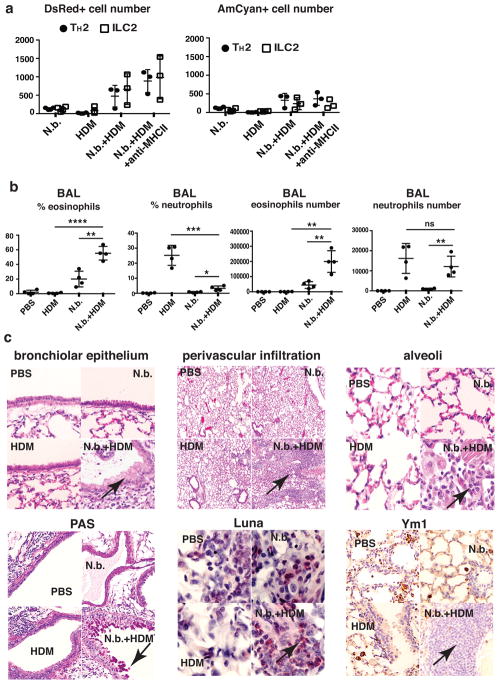
There were few DsRed-expressing cells in the lungs of mice 23 days after inoculation with N. brasiliensis (Fig. 5a). Three daily challenges of uninfected mice with HDM also led to very few DsRed-expressing cells. However, three challenges with HDM in mice recovering from N. brasiliensis infection resulted in substantial DsRed, but not AmCyan, expression by TH2 and ILC2 cells (Fig. 5a). DsRed-expressing TH2 cell number was comparable to DsRed-expressing ILC2 cells. Treatment with anti-MHCII antibody did not diminish DsRed expression by either cell population.
Figure 5. Short-term HDM challenge induces prompt eosinophilic airway inflammation in mice recovering from N. brasiliensis infection.
(a, b) C57BL/6 mice were uninfected or infected with N. brasiliensis. Twenty-three days later, mice were challenged intratracheally with PBS or HDM (25 μg in PBS) daily for 3 consecutive days. 24h after the last challenge, BAL fluids and lungs were collected and analyzed. N.b., N. brasiliensis only; HDM, HDM challenge in uninfected mice; N.b.+HDM, HDM challenge 23 days after N. brasiliensis infection; N.b.+HDM+anti-MHCII, 500 μg anti-MHCII antibody was injected i.v. on day 1 and day 3 of HDM challenge. Percentages shown were calculated as a percent of the live cells. ****P<0.0001; **, P<0.01; ***, P<0.001; *, P<0.05; ns, not significant, P>0.05. (c) Lung histology analysis. Top 3 panels, lung sections were stained with H&E; left panel arrow - mucus metaplasia; center panel arrow - perivascular infiltration; right panel arrow - alveolar infiltration. Lower left panel, PAS staining arrow - mucus metaplasia; lower middle, Luna staining arrow - eosinophils; and lower right, Ym1 staining arrow – Ym1+ macrophages. Data are representative of two (a, c) or three (b) independent experiments with 3–4 mice in each group.
Three daily challenges of previously uninfected mice with HDM caused an increase in neutrophils (Gr1+CD11b+ cells) but not eosinophils (SiglecF+CD11c− cells) in broncho-alveolar lavage (BAL) fluid (Fig. 5b). BAL fluid from mice recovering from N. brasiliensis infection contained ~3 × 104 eosinophils, comprising ~20% of BAL fluid cells, reflecting residual airway eosinophilia in mice recovering from a helminth infection. In such mice, HDM challenge induced a substantial cellular response dominated by eosinophils; the number of eosinophils in BAL fluid was ~2 × 105.
HDM challenge of mice recovering from N. brasiliensis infection caused massive lung eosinophilic and lymphocytic infiltration, bronchial and bronchiolar mucus metaplasia, and numerous large alveolar macrophages with Ym1-positive eosinophilic crystals (Fig. 5c). Such pathologic changes were not observed in the other groups. Thus, in contrast with neutrophils recruitment by HDM in unimmunized mice, three doses of HDM challenges induced massive eosinophil migration and recruitment into both the lung tissues and BAL fluid in mice previously exposed to parasites as well allergic inflammatory responses.
Mechanism of induced eosinophil airway inflammation
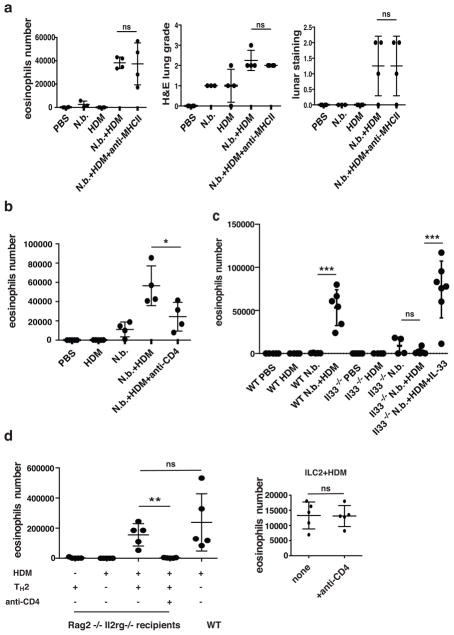
Treatment with anti-MHCII did not diminish the number of eosinophils in BAL fluid or the degree of lung inflammation in HDM-challenged mice recovering from N. brasiliensis infection (Fig. 6a). To determine whether CD4+ T cells played an important role in the development of HDM-induced eosinophilic airway inflammation in mice recovering from N. brasiliensis-infection, such mice were rendered CD4+ T cell-deficient by injecting a CD4-depleting antibody i.v. during the 3-day HDM challenge. Depletion of CD4+ T cell led to a statistically significant reduction of ~50% in the number of BAL eosinophils (Fig. 6b).
Figure 6. HDM-induced eosinophilic airway inflammation is independent of TCR, partially dependent on CD4 T cells, and mediated through IL-33.
(a) Mice were infected with N. brasiliensis and challenged with HDM as described in Fig. 4a. Anti-MHCII antibodies were injected i.v. at the time of 1st and 3rd HDM challenge. BAL fluid was collected for cellular constituents analysis. Lungs were harvested and lung sections were analyzed by H&E staining and lunar staining. ns, not significant, P>0.05. (b) Mice were infected and challenged as in Fig. 4a. 500 μg of anti-CD4 neutralizing antibody was injected i.v. on day 1 and day 3 of HDM challenge. *, P<0.05. (c) Wild-type mice or IL-33-deficient (Il33−/−) mice were infected with N. brasiliensis and challenged with HDM as described in Fig. 4a. 150 ng IL-33 was injected i.v. into IL-33-deficient mice on day 1 and day 5 of N. brasiliensis infection. One group of IL-33-deficient mice infected with N. brasiliensis was challenged with HDM without supplemental IL-33 while a second group received IL-33 supplementation (100 ng per mouse on day 1 of HDM challenge). BAL cellular constituents were analyzed. ***, P<0.001; ns, not significant, P>0.05. (d) Left, 5×106 in vitro cultured OT-II TH2 cells were injected i.v. into Rag2−/−Il2rg−/− recipient mice. Some groups of mice were challenged intratracheally with HDM (25 μg in PBS) daily for 4 weeks. 500 μg anti-CD4 antibody was injected into one group on days 2, 8 and 15. BAL cellular constituents were analyzed 24h after the last challenge. Right, 0.5×106 ILC2 derived from IL-25-injected mice and cultured for 3 days in IL-7 plus IL-33 were injected i.v. into Rag2−/−Il2rg−/− recipient mice. Mice were similarly treated and analyzed as those receiving primed OT-II TH2 cells. ns, P>0.05; ***, P<0.001. Data are representative of three (a–c) or two (d) independent experiments with 3–7 mice in each group.
To assess whether HDM-induced eosinophilia was mediated through IL-33, IL-33-deficient mice were inoculated with third-stage N. brasiliensis larvae and treated with IL-33 on days 1 and 5 of infection. Mice were challenged with HDM 25 days later (Fig. 6c). In contrast to marked eosinophilia in wild-type N. brasiliensis-infected mice receiving HDM challenge, eosinophil numbers in BAL fluid recovered from IL-33-deficient N. brasiliensis-infected mice remained low. Administration of IL-33 (100 ng) at the time of challenge with HDM restored eosinophil recruitment. Thus, HDM-induced eosinophilia was independent of MHC, partially dependent on CD4 T cells and mediated through IL-33.
CD4 T cells alone can induce eosinophilic inflammation
In vitro cultured OT-II TH2 cells were injected i.v. into mice deficient for Rag2 and the common cytokine receptor gamma chain (γc) (Rag2−/−Il2rg−/−) mice, lacking endogenous T cells, B cells and ILCs. Daily HDM administration for one month caused BAL eosinophilia comparable to that in similarly HDM-challenged wild-type mice (Fig. 6d). Treatment with anti-CD4 completely abolished BAL eosinophilia, whereas similar treatment showed no impact on BAL eosinophils recruitment in recipients that had received ILC2 cells rather than TH2 cells, and were challenged with HDM (Fig. 6d), indicating anti-CD4 treatment did not deplete eosinophils or other cells that may be involved in eosinophil recruitment. Thus, OVA-specific TH2 cells are sufficient to induce eosinophilia in mice challenged with HDM.
A. suum protects against N. brasiliensis
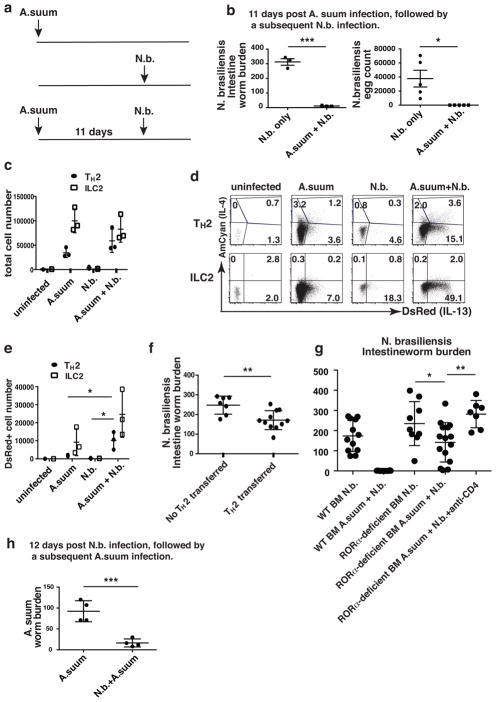
Next we asked whether TH2 cells generated in response to infection with one helminth will produce innate IL-13 and offer protection during infection with a different helminth. Two evolutionarily distant nematodes, A. suum and N. brasiliensis, were chosen to minimize antigenic cross reactivity. A. suum larvae derived from oral inoculation of infective eggs optimally migrate to the lungs by day 7 and are immediately cleared from the intestine following further migration. Mice that had been inoculated orally with A. suum eggs were inoculated subcutaneously with N. brasiliensis 11 days later (Fig. 7a). No adult N. brasiliensis worms and no egg production were detected in the intestine of such mice eight days after inoculation with N. brasiliensis, which differed from the response seen in N. brasiliensis-infected naïve mice (Fig. 7b). Full protection was also observed when N. brasiliensis was inoculated 25 days after A. suum infection (Supplementary Fig. 5). Thus, a prior A. suum infection caused prompt clearance of N. brasiliensis.
Figure 7. Tissue-resident TH2 cells contribute to innate host defense against helminths.
(a) Mice were inoculated with A. summ eggs orally and 11 days later received a subsequent subcutaneous inoculation with 500 N. brasiliensis L3. The mice in control groups were only inoculated subcutaneously with 500 N. brasiliensis L3 or only A. suum eggs. (b) N. brasiliensis adult worms in the small intestine and eggs in the fecal pellets were determined on day 8 post N. brasiliensis inoculation. ***, P<0.001; *, P<0.05. (c) Total number of lung-resident TH2 and ILC2 cells. (d) Lungs were harvested on day 6 post N. brasiliensis inoculation. DsRed and AmCyan expression by lung-resident TH2 and ILC2 cells were analyzed by flow cytometry. (e) Statistics of cell number of DsRed-expressing TH2 and ILC2 cells. *, P<0.05. (f) 5×106 in vitro cultured OT-II TH2 cells were injected i.v. into Rag2−/−Il2rg−/− recipient mice. 24h later, mice were inoculated with N. brasiliensis and adult worms in the small intestine were determined on day 8 after N. brasiliensis inoculation. Data were compiled from two independent experiments. **, P<0.01. (g) BM chimeras were generated by injecting wild-type or RORα-deficient BM cells into Rag2−/−Il2rg−/− recipient mice. 8 weeks after reconstitution, chimeras were inoculated with N. brasiliensis L3 alone or with A. summ eggs followed by N. brasiliensis as described in Fig. 7a. Adult worms in small intestine were determined on day 8 after N. brasiliensis inoculation. Some mice received 500 μg anti-CD4 antibodies i.v. on day 0 and day 4 after inoculation with N. brasiliensis. RORα-deficient BM chimeras that have less than 3% ILC2 cells in lung with and without injection of anti-CD4 antibody are shown. Data were compiled from three independent experiments with 4–5 mice in each group. *, P<0.05; **, P<0.01. (h) Mice were inoculated with N. brasiliensis L3 and 12 days later with A. summ eggs. The mice in the control group were only inoculated with A. suum eggs. The parasitic third-stage larvae in lung were determined 8 days after A. summ inoculation. ***, P<0.001. Data are representative of five (b) or two (c–e, h) independent experiments with 3–5 mice in each group.
Seventeen days after A. suum infection, the numbers of both TH2 cells and ILC2s had greatly increased, indicating a robust A. suum-induced type 2 response (Fig. 7c). Approximately 5% of the TH2 cells in these mice expressed DsRed and ~ 4% expressed AmCyan, among which some expressed both (Fig. 7d). ~7% of the ILC2s expressed DsRed and almost none AmCyan. Upon subsequent N. brasiliensis infection, the total TH2 cell number on day 6 of infection (day 17 after inoculation with A. suum) had increased two-fold (Fig. 7c); ~19% of these TH2 cells were DsRed positive (Fig. 7d). The increased percent of DsRed positive TH2 cells was matched by an increase in the number of DsRed-expressing TH2 cells, from ~103 in mice that had recovered from A. suum infection to ~104 in such mice subsequently inoculated with N. brasiliensis L3 (Fig. 7e). Subsequent N. brasiliensis infection did not increase ILC2 cell numbers but the frequency of DsRed-positive ILC2s increased to 50% and the number to ~25,000 (Fig. 7c–e). Thus, TH2 cells made up ~30% of the total number of IL-13-producing cells in N. brasiliensis-challenged mice that had recovered from A. suum infection (Fig. 7e).
In contrast to DsRed, AmCyan expression by TH2 cells from A. suum recovering mice remained unchanged or only modestly increased upon N. brasiliensis-challenge (Fig. 7d), suggesting that the observed TH2 cytokine production is not driven by TCR engagement but rather, mediated through IL-33 or IL-25. Six days after N. brasiliensis-inoculation of previously uninfected mice, numbers of TH2 cells and ILC2 were still low, indicating that the increased numbers of cells and their cytokine production was dependent on the prior infection with A. suum (Fig. 7c, d).
TH2 cells partially protect against N. brasiliensis infection
In vitro cultured OT-II TH2 cells were injected i.v. into Rag2−/−Il2rg−/− mice that were then inoculated with N. brasiliensis (Fig. 7f). At 8 days post inoculation, intestinal adult worm burden was significantly reduced in mice that had received OT-II TH2 cells compared to control mice. Thus, OVA-specific TH2 cells provided partial protection against N. brasilienis infection.
In wild-type mice infected with N. brasiliensis after recovery from A. suum, endogenous IL-13-producing ILC2s outnumbered IL-13–producing TH2 cells (Fig. 7e). We wished to determine whether the TH2 cells made a significant contribution to the innate protection against N. brasiliensis infection in mice previously infected with A. suum. Bone marrow (BM) chimeras were generated by injecting wild-type or RORα-deficient BM cells into Rag2−/−Il2rg−/− recipient mice (Fig. 7g) as RORα has been reported to be critical for ILC2 development9. Both uninfected RORα-deficient BM chimeras and those infected with N. brasiliensis without prior A. suum infection had very few ILC2 cells (Supplementary Fig. 6a, b and Supplementary Fig. 7a), consistent with the previous report9. In vitro primed TH2 cells from both wild-type and RORα-deficient BM chimeras produced comparable amounts of cytokines in response to PMA plus ionomycin or IL-33 and IL-7 (Supplementary Fig. 8), indicating that in vitro TH2 differentiation is unimpaired in the absence of RORα.
Wild-type and RORα-deficient BM chimeras were inoculated with N. brasiliensis L3 only or with A. suum eggs followed by N. brasiliensis. In wild-type BM chimeras, previous A. suum infection resulted in expulsion of N. brasiliensis by day 8 (Fig. 7g), consistent with the full protection observed in wild-type mice (Fig. 7b). Interestingly, in the RORα-deficient BM chimeras, prior A. suum infection led to induction of as many as 7% ILC2 cells in some animals (Supplementary Fig. 7b), while similarly infected wild-type BM chimeras had ILC2 cells ranging from 2–20% (Supplementary Fig. 7d). We examined worm burden in RORα-deficient BM chimeras that had fewer than 3% ILC2 cells. In those animals, a statistically significant, ~50% diminution of the intestinal N. brasiliensis worm burden was observed and some completely eliminated the N. brasiliensis (Fig. 7g). The protection of RORα-deficient BM chimeras with fewer than 3% ILC2 cells was eliminated by treatment with anti-CD4 antibody during N. brasiliensis inoculation (Fig. 7g and Supplementary Fig. 7c). Thus, A. suum-generated TH2 cells were sufficient to partially protect against a N. brasiliensis infection.
We also observed that infection with N. brasiliensis protected against a subsequent A. suum infection by substanially reducing the number of migrating A. suum larvae detected in the lungs (Fig. 7h). Because of the dominance of TH2 cells in N. brasiliensis-infected mice, this result strengthens the contention that innate action of TH2 cells can play an important role in protection against infection with a phyogenetically distant helminth.
Discussion
IL-33 is found in the nucleus of epithelial barrier tissues and lymphoid organs and is released into the extracellular space as an alarmin after cell damage or cellular stress10, 11, 12, 13, 14. TSLP is also an epithelial derived cytokine11, 15. Previously, we have shown the combination of these two alarmins induces TH2 cells to produce IL-13 but not its closely related TH2 cytokine IL-4 in vitro5. This suggests that in response to helminth invasion or allergen exposure, innate IL-13 production is mounted by tissue resident TH2 cells in response to IL-33 produced by epithelial cells and likely TSLP produced by similar cells.
Although the closely linked TH2 cytokines IL-4 and IL-13 have many similarities, they show distinct physiologic functions. IL-13, but not IL-4, is essential for expulsion of N. brasiliensis helminths16, 17. Anti-IL-13 but anti-IL-4 inhibits elicitation of airway hypersensitivity responses18, 19. Thus, IL-33 plus TSLP-induced selective production of IL-13 from TH2 cells is consistent with the role of IL-13 as an effector molecule and suggests that T helper effector cells could, like ILCs, exert innate immune functions especially at mucosal sites20. To test this hypothesis, we utilized IL-4 and IL-13 dual reporter mice, which allow us to determine the in situ production of IL-4 and IL-13 without ex vivo stimulation, and focused on lung TH2 cells during airway inflammation and during defense against helminth infection.
Adoptively transferred in vitro-primed OVA-specific TH2 cells and in vivo generated TH2 cells produced IL-13 in response to administration of IL-33 and IL-7 or of papain, respectively. In the latter case, IL-13 production was not inhibited by anti-MHCII antibody treatment but was IL-33-dependent, implying it was TCR-independent, cytokine-dependent. In unimmunized mice, there are very few TH2 cells but thousands of ILC2 cells, consistent with the dominant role of ILC2 cells during early type 2 responses in naïve mice. After infection by N. brasiliensis, TH2 cells preferentially differentiated and expanded with frequencies reaching 105 by day 13 post inoculation, now outnumbering ILC2s. After expulsion of parasites, TH2 and ILC2 cell number declined proportionally so that TH2 cell frequency remained greater than that of ILC2 cells at 4 weeks after infection. Thus, it might be anticipated that TH2 cells would play a more important role in cytokine-dependent cytokine production in mice recovering from responses that induce strong TH2 immunity than they would in naïve mice.
Three daily exposures of HDM in mice recovering from N. brasiliensis infection induced a striking TCR-independent eosinophilia in BAL fluid and lung tissues, whereas in unimmunized mice such challenge caused accumulation of neutrophils in BAL fluid but no lung eosinophilic inflammation. Depletion of TH2 cells by treatment with anti-CD4 antibody led to a marked reduction in BAL eosinophilia in mice recovering from N. brasiliensis infection. BAL eosinophilia was induced by HDM in mice lacking endogenous T cells and ILCs that had received in vitro cultured OT-II TH2 cells. Thus, innately activated TH2 cells play an important role in the induction of eosinophilic airway inflammation in response to HDM exposure and TH2 cells alone are able to mediate such antigen non-specific airway inflammation.
The interplay of helminth infection and allergic disorders is complex21, 22. While there are reports that prior helminth infection of humans can protect against allergic responses, there are many reports that helminth infection can potentiate such inflammation23, 24, 25, 26. Infection intensity and induced immunomodulation are thought to be the most important factors accounting for such differential effects22, 27, 28, 29. Intense and chronic helminth infections are associated with protection against allergic disease whereas mild and transient infections have been linked to potentiation of allergic responses.
While intense helminth infection causes strong Th2 responses, it also results in induction of regulatory T cells and M2 macrophages, and production of the anti-inflammatory cytokine IL-1021, 30, 31, 32, 33. Such immunomodulation does not occur during mild helminth infection22, 27. Under these circumstances, the large number of resultant tissue resident Th2 cells and ILC2 could trigger more frequent and more severe allergic diseases, representing innate immunologic memory. This could explain our observation that HDM-induced robust eosinophilic airway inflammation in mice 3–4 weeks after inoculation with N. brasiliensis, but not in uninfected mice. N. brasiliensis infection is self-curing and parasites are expelled 10–12 days post inoculation with infective L3 larvae.
We also examined the importance of effector TH2 cells in the innate defense against helminth infection. A previous infection with A. suum led to full protection against a subsequent infection with the evolutionarily distant helminth N. brasiliensis; complete clearance of N. brasiliensis from the intestine was observed as early as six days after exposure, far earlier than in non-A. suum-exposed mice. Earlier studies have suggested that eosinophils34, 35, basophils36, and macrophages37 play roles in protection during secondary N. brasiliensis infection. IL-13 is crucial for effective expulsion of N. brasiliensis as it coordinates various effector systems16. Here we examined the cellular sources of IL-13 production in mice 6 days after inoculation with N. brasiliensis L3 larvae. Mice previously infected with A. suum demonstrated a marked increase in IL-13 production by both TH2 and ILC2 cells. IL-13-producing lung resident TH2 cells made up to 30% of the total number of IL-13-producing cells. Adoptive transfer of OVA-specific TH2 cells into mice lacking endogenous T cells, B cells and ILCs conferred partial protection against N. brasiliensis infection, implying that lung resident TH2 cells contribute to protection against helminth infection.
Collectively our results indicate that both TH2 and ILC2 cells express IL-33R and respond to epithelial-derived IL-33 by producing the effector cytokine IL-13. Under physiological or pathological circumstances where both TH2 and ILC2 cells are present, their relative contribution to cytokine production, allergic inflammation and protection against subsequent helminth infection appears to be proportional to their cell numbers, the amount of GATA-3 they express and their IL-33R level. Thus, tissue-resident TH2 cells can exert innate functions upon encounter with appropriate stimuli and their innate responses can contribute to both airway inflammation and protection against helminth infection. Our results suggest that humans in areas where poly-parasitism from phylogenetically diverse helminths is endemic may display a substantial degree of protective Th2-based innate immunity.
Methods
Mice
Generation of the 4C13R mice on C57BL/6 background was described previously6. OT-II–4C13R mice were generated by breeding OT-II C57BL/6 mice with the 4C13R mice. WT CD90.1+ CD90.2+ DO11.10 mice were generated by crossing CD90.2+ Rag2−/− DO11.10 mice to CD90.1+ WT BALB/c mice. IL-33R-deficient (Il1rl1−/−) CD90.1−CD90.2+ DO11.10 mice were generated by crossing CD90.2+ Rag2−/− DO11.10 mice to CD90.2+ IL-33R-deficient mice. FcRγ−/− mice, Rag2−/−Il2rg−/− recipient mice, CD90.2+ Rag2−/− DO11.10 mice, BALB/c and OT-II TCR transgenic mice were purchased from Taconic. BALB/c CD90.1+ mice were purchased from Jackson Laboratory. C57BL/6 mice were purchased from Jackson Laboratory, Taconic Biosciences or NCI Mouse Repository. Il1rl1−/− mice38 were kindly provided by A. N. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK) and have been backcrossed to BALB/c to 10 generations. IL-33-deficient mice10 on C57BL/6 background were kindly provided by J.-P. Girard (Institute de Pharmacology et de Biologies Structurale-Centre National de la Recherche Scientifique-Universite de Toulouse, France). RORα–deficient mice on C57BL/6 background were purchased from Jackson Laboratory. Heterozygous RORα-deficient mice were used for breeding; pups were identified by tattooing the plantar surface of the paws of 6-day-old neonates. Tail clipping and PCR were performed for genotyping. The primers used for genotyping mice were: WT-F: 5′-TCTCCCTTCTCAGTCCTGACA-3′; WT-R: 5′-TATATTCCACCACACGGCAA-3′; RORα–deficient-F: 5′-GATTGAAAGCTGACTCGTTCC-3′; and RORα–deficient-R: 5′-CGTTTGGCAAACTCCACC3′. All the mice were bred and/or maintained in the NIAID specific pathogen-free animal facility and all animal experiments were performed under the approval of NIAID Animal Care and Use Committee, National Institutes of Health.
Bone Marrow Chimeras
RORα-deficient mice and WT littermate within 10 days old were used as donor mice. Rag2−/−Il2rg−/− mice of 6–10 weeks old were used as recipients. Recipient mice received sublethal irradiation (single dose of 450 Rads) prior to transplantation of 1 × 107 whole bone marrow cells from donor mice by retro-orbital injection on the same day. Mice were treated with trimethoprim (0.67 mg/ml) and sulfamethoxazole (0.13 mg/ml) via their drinking water for 5 weeks. Reconstitution was examined 8 weeks later.
Helminth infection
Mice of at least 8 weeks of age were used for infection with N. brasiliensis and A. suum. 500 infective N. brasiliensis L3 were inoculated subcutaneously into the mice. Worm expulsion was determined by counting adult worms in the small intestine on day 8 after inoculation. Eggs in fecal pellets were counted 8–10 days post inoculation using McMaster counting chambers.
A suspension of embryonated A. suum eggs containing 1,500 infective larvae29 were inoculated orally into mice and parasitic L3 were detected 8 days later in lungs after processing. Briefly, explanted lungs were finely minced with scissors and suspended in tubes containing 8 ml of RPMI-1640 medium for 3h in a 37°C water bath. The suspension was transferred to a 6-well culture plate and parasitic L3 were counted using an inverted microscope with 200× lens magnification.
Adoptive cell transfer
0.5 × 106 sorted Lin−CD25−CD45RBhi cells from the OT-II–4C13R reporter mice, 0.5 × 106 ILC2 cells, or 5 × 106 in vitro cultured TH2 cells that have been maintained in IL-7-containing medium for 10 d were used for transfer. After extensive washes in PBS, cells were suspended in 200 μl of PBS and injected i.v. into retro-orbital sinuses of recipient mice.
Administration of cytokines, papain, house dust mites and antibodies
Mice were anesthetized with volatile isoflurane and held on an intubation stand by hooking its upper incisors on it. Materials were dissolved in 40 μl of sterile PBS and administrated intratracheally into mice through a catheter inserted into the trachea. Reagents used were: IL-33 (100 or 150 ng) (PeproTech), IL-7 (100 ng) (PeproTech), TSLP (150 ng) (R&D Systems), EndoFit Ovalbumin (100 μg) (InvivoGen), papain (25 μg) (Merck Millipore), and house dust mite extracts (Dermatophagoides farina) (25 μg) (Greer Laboratories). To inactivated papain, papain was heated at 100 °C for 10 min. In some experiments, 500 μg of anti-CD4 (GK1.5, BioXCell) or 500 μg of anti-MHCII (Y-3P, BioXCell) antibody were injected into mice through retro-orbital injection.
BAL fluid cytology
BAL was performed by instilling 1 ml PBS into lung through a tracheal cannula and then gently aspirating the fluid. The lavage was repeated four times; BAL fluids from 4 washes were pooled, centrifuged (337 × g, 4 °C, 5min) to collect the cells. Cells were stained and BAL cellular composition was analyzed by flow cytometry. Neutrophils were identified as live SSChiCD3−B220−Gr-1+CD11b+SiglecF−CD11cloMHCII−. Eosinophils were identified as live SSChiCD3−B220−SiglecF+CD11cloGr-1−CD11b+MHCII−.
In vitro TH2 and TH17 cell culture
CD62LhiCD44lo naïve CD4+ T cells were sorted from OT-II, DO11.10, or C57BL/6 mice. Antigen-presenting cells (APCs) were purified from splenocytes by treatment with anti-Thy1.2 and low-Tox M rabbit complement at 37 °C for 45 min and irradiated at 30 Gray. For Th2 culture, CD4 T cells were primed with OVA peptide (0.2 μM) or anti-CD3 (3 μg/ml) CD28 (3 μg/ml) and APC in the presence of IL-2 (100 units/ml), IL-4 (1000 units/ml), anti-IL-12 (10 μg/ml) (Harlan Bioproducts for Science) and anti-IFN-γ (10 μg/ml) (Harlan Bioproducts for Science). After a 4 day-priming period, TH2 cells were washed and cultured in IL-7 (10 ng/ml)-containing medium. Cells were cultured under TH2 conditions for 3 rounds of four days each. For TH17 culture, CD4+ T cells were primed with anti-CD3 (3 μg/ml), anti-CD28 (3 μg/ml) and APC in the presence of IL-6 (10 ng/ml) (PeproTech), TGF-β (10 ng/ml) (PeproTech), IL-1β (10 ng/ml) (PeproTech), anti-IL-4 (10 μg/ml) (Harlan Bioproducts for Science), anti-IL-12 (10 μg/ml) (Harlan Bioproducts for Science) and anti-IFN-γ (10 μg/ml) (Harlan Bioproducts for Science).
In vitro ILC2 cells
IL-25 (200 ng) (R&D systems) was administrated intraperitoneally to mice for three consecutive days. Mesenteric lymph nodes were collected and Lin−KLRG+ cells were sorted and cultured in medium containing IL-33 (5 ng/mL) and IL-7 (5 ng/mL).
Isolation of lymphocytes from lung tissues
Mice were euthanized and lungs were perfused with cold PBS. Afterwards, lung tissue was dissected, digested in RPMI medium containing 4 μg/mL of Liberase TL (Roche) and 4 U/mL of DNase I (Roche) at 37 °C for 20min, and dissociated into single cell suspensions by gentle MACS Dissociator (Miltenyi Biotec). Cell suspensions were incubated with ACK lysis buffer (Life Technology) for 15 s at 20°C and then washed extensively with cold PBS. Lung single cell suspension was ready for staining and flow cytometry analysis.
Flow cytometry
Cell surface staining were performed in PBS containing 0.1% BSA. For intracellular staining, cells were stained with cell surface markers, extensively washed, fixed in 4% paraformaldehyde, permeabilized in PBS supplemented with 0.1% BSA, 0.1% Triton X-100, and then stained with specific antibodies in intracellular staining buffer (0.1% BSA, 0.1% Triton X-100 in PBS). For all the staining, cells were incubated with anti-Fc receptor blocking antibody (anti-CD16) before staining with a cocktail of fixable viability dye (eBioscience) and various antibodies. Antibodies are shown below. Cells were acquired on a BD™ LSRII flow cytometer and analyzed by FlowJo software (Tree Star). FACS Aria cell sorter (BD Biosciences) was used for cell sorting.
| Experiment | Ab | Clone ID | Vendor |
|---|---|---|---|
| Fig. 2,3a,4,5a,7c,7d,7e,s3,s6,s7 | anti-CD19 | 1D3 | eBiosciences |
| Fig. 2,3a,4,5a,7c,7d,7e,s3,s6,s7 | anti-CD8 | 53-6.7 | eBiosciences |
| Fig. 2,3a,4,5a,5b,6,7c,7d,7e,s3,s6,s7 | anti-CD11b | M1/70 | eBiosciences |
| Fig. 2,3a,4,5a,5b,6,7c,7d,7e,s3,s6,s7 | anti-CD11c | N418 | eBiosciences |
| Fig. 2,3a,4,5a,7c, 7d,7e,s3,s6,s7 | anti-NK1.1 | PK136 | eBiosciences |
| Fig. 2,3a,4,5a,5b,6,7c,7d,7e,s3,s6,s7 | anti-Gr-1 | RB6-8c5 | eBiosciences |
| Fig. 2,3a,4,5a,7c,7d,7e,s3,s6,s7 | anti-CD49b | DX5 | eBiosciences |
| Fig. 2,3a,4,5a,7c,7d,7e,s3,s6,s7 | anti-TER119 | Ter-119 | eBiosciences |
| Fig. 2,3a,4,5a,7c,7d,7e,s3,s6,s7 | anti-FcεR1 | MAR-1 | eBiosciences |
| Fig. 2,3a,4,5a,7c,7d,7e,s3,s6,s7 | anti-TCRγδ | eBioGL3 | eBiosciences |
| Fig. 4,s6 | anti-IL-7Rα | A7R34 | eBiosciences |
| Fig. 4 | anti-Thy-1.2 | 53-2.1 | eBiosciences |
| Fig. 5b,6 | anti-B220 | RA3-6B2 | eBiosciences |
| Fig. 5b,6 | anti-CD3ε | 45-2C11 | eBiosciences |
| Fig. 1,2,3a,3c,4,5a,7c,7d,7e,s2,s3 | anti-CD4 | RM4-5 | eBiosciences |
| Fig. 5b,6,7g,s4,s7 | anti-CD4 | RM4-4 | eBiosciences |
| Fig. 1,2,3a,3c,4,5a,7c,7d,7e,s2,s3,s4,s6,s7 | anti-CD44 | IM7 | eBiosciences |
| Fig. 3c,s4,s8 | anti-CD62L | MEL-14 | eBiosciences |
| Fig. 5b,6 | anti-MHCII | M5/114.15.2 | eBiosciences |
| Fig. 5b,6 | anti-siglecF | E50-2440 | BD Biosciences |
| Fig. 2,3a,s3 | anti-CD45RB | C363.16A | eBiosciences |
| Fig. 2,3a,s3 | anti-CD25 | PC61.5 | eBiosciences |
| Fig. 3c,s4 | anti-CD90.1 | HIs51 | eBiosciences |
| Fig. 3c,s4 | anti-CD90.2 | 30-H12 | eBiosciences |
| Fig. 3c,s4 | anti-DO11.10 TCR | KJ126 | eBiosciences |
| Fig. 1,2,3a,s2,s3 | anti-TCR Vα2 | B20.1 | eBiosciences |
| Fig. 1,2,3a,s2,s3 | anti-TCR Vβ5 | MR9-4 | eBiosciences |
| Fig. 4,5a,7c,7d,7e,s4 | streptavidin | Biolegend | |
| Fig. 4,5a,7c,7d,7e,s4 | Biotin-anti-IL-33R | DJ8 | MD Bioproducts |
| Fig. 4,5a,7c,7d,7e,s7 | PE-anti-IL-33R | DJ8 | MD Bioproducts |
| Fig. 4,5a,7c,7d,7e,s6,s7 | anti-GATA3 | L50-823 | BD Biosciences |
| Fig. 4,5a,7c,7d,7e | anti-Foxp3 | MF23 | BD Biosciences |
| Fig. s6 | anti-RORγt | AFKJs-9 | eBiosciences |
| Fig. s1a,s4,s8 | anti-IL-13 | eBio13A | eBiosciences |
| Fig. s1a,s4,s8 | anti-IL-4 | 11B11 | Biolegend |
| Fig. s1c | anti-IL17A | eBio17B7 | eBiosciences |
Lung histopathology
For fixation, the trachea was exposed and ligature was attached loosely around the trachea. The trachea was severed above the ligature and cannulated with a 21 gauge blunt needle. The lungs were infused with 1 ml of 10% neutral buffered formalin (NBF) (Sigma) and ligature was tightened to hold formalin within the lungs. The lungs were dissected and stored in NBF for 24h and then transferred into 70% ethanol until histologic processing. Embedding, sectioning, and staining (H&E staining, lunar staining, PAS staining, Ym1 or CHI3L3 staining) were performed by Histoserv Inc. (Germantown, MD). Mouse anti-Chitinase 3-like 3 or ECF-L (R&D, 1:400, without antigen retrieval) was used for Ym1 staining.
Quantitative PCR (qPCR)
Total RNA was isolated using RNeasy mini kit (Qiagen); first strand cDNA was prepared using Superscript III (Invitrogen). All PCR was performed on 7900HT sequence detection systems (Applied Biosystems). The TaqMan® universal PCR SuperMix and the primer and probe sets for murine Il4, Il13, and Gapdh were purchased from Applied Biosystems.
Statistical analysis
Statistical significance was determined by application of two-tailed Student’s t test.
Supplementary Material
Acknowledgments
We thank A. N. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom) for kindly providing us Il1rl1−/− mice; J.-P. Girard (Centre National de la Recherche Scientifique, Institute de Pharmacology et de Biologie Structurale) for kindly providing us Il33-LacZ Gene Trap Reporter mice; J. Ward at Global Vet Pathology for excellent help in pathology analysis; and J. Edwards and K. Weng (Lab of Immunology, National Institute of Allergy and Infectious Diseases (NIAID)) for their help in cell sorting. This work is supported by the Division of Intramural Research of the NIAID, National Institute of Health NIH (NIH).
Footnotes
Contributions
L.G. designed, performed and interpreted experiments, and wrote the manuscript; Y.H. assisted with some experiments; X.C. assisted with some experiments; J.H.-L. assisted with some experiments; J.F.U. helped to design, perform and interpret experiments with A. suum and N. brasiliensis infection, provided N. brasiliensis, and read the manuscript; W.E.P. designed and interpreted the experiments, wrote the manuscript, and supervised the whole study.
Completing financial interests
The authors declare no competing financial interests.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YT, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA., Jr Medzhitov R Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, et al. IL-25-responsive, lineage-negative KLRG1 cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Martin J, Abubucker S, Yin Y, Gasser RB, Mitreva M. Systematic analysis of insertions and deletions specific to nematode proteins and their proposed functional and evolutionary relevance. BMC Evol Biol. 2009;9:23–36. doi: 10.1186/1471-2148-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 11.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Junttila IS, Paul WE. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erb KJ. Can helminths or helminth-derived products be used in humans to prevent or treat allergic diseases? Trends Immunol. 2009;30:75–82. doi: 10.1016/j.it.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Palmer LJ, Celedon JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Resp Crit Care. 2002;165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 24.Buijs J, Borsboom G, Renting M, Hilgersom WJ, van Wieringen JC, Jansen G, et al. Relationship between allergic manifestations and Toxocara seropositivity: a cross-sectional study among elementary school children. Eur Respir J. 1997;10:1467–1475. doi: 10.1183/09031936.97.10071467. [DOI] [PubMed] [Google Scholar]
- 25.Pinelli E, Brandes S, Dormans J, Gremmer E, van Loveren H. Infection with the roundworm Toxocara canis leads to exacerbation of experimental allergic airway inflammation. Clin Exp Allergy. 2008;38:649–658. doi: 10.1111/j.1365-2222.2007.02908.x. [DOI] [PubMed] [Google Scholar]
- 26.Lynch NR, Palenque M, Hagel I, DiPrisco MC. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am J Resp Crit Care. 1997;156:50–54. doi: 10.1164/ajrccm.156.1.9606081. [DOI] [PubMed] [Google Scholar]
- 27.Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22:372–377. doi: 10.1016/s1471-4906(01)01958-5. [DOI] [PubMed] [Google Scholar]
- 28.Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, et al. Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastr. 2006;3:275–284. doi: 10.1038/ncpgasthep0471. [DOI] [PubMed] [Google Scholar]
- 29.Schopf L, Luccioli S, Bundoc V, Justice P, Chan CC, Wetzel BJ, et al. Differential modulation of allergic eye disease by chronic and acute ascaris infection. Invest Ophth Vis Sci. 2005;46:2772–2780. doi: 10.1167/iovs.04-0899. [DOI] [PubMed] [Google Scholar]
- 30.Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol. 2010;10:278–284. doi: 10.1038/nri2730. [DOI] [PubMed] [Google Scholar]
- 31.Anthony RM, Rutitzky LI, Urban JF., Jr Stadecker MJ, Gause WC Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 33.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 34.Shin EH, Osada Y, Chai JY, Matsumoto N, Takatsu K, Kojima S. Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int Arch Allergy Imm. 1997;114:45–50. doi: 10.1159/000237717. [DOI] [PubMed] [Google Scholar]
- 35.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz C, Turqueti-Neves A, Hartmann S, Yu P, Nimmerjahn F, Voehringer D. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci USA. 2014;111:E5169–5177. doi: 10.1073/pnas.1412663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014;15:938–946. doi: 10.1038/ni.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.