Abstract
Blood chimerism has been reported sporadically among visceral transplant recipients, mostly in association with graft-vs-host disease (GVHD). We hypothesized that a higher degree of mixed chimerism would be observed in multivisceral (MVTx) than in isolated intestinal (iITx) and isolated liver transplant (iLTx) recipients, regardless of GVHD. We performed a longitudinal prospective study investigating multilineage blood chimerism with flow cytometry in 5 iITx and 4 MVTx recipients up to one year post-transplant. Although only one iITx patient experienced GVHD, T-cell mixed chimerism was detected in 8 out of 9 iITx/MVTx recipients. Chimerism was significantly lower in the four subjects who displayed early moderate to severe rejection. Pre-formed high titer donor-specific antibodies, bound in vivo to the circulating donor cells, were associated with an accelerated decline in chimerism. Blood chimerism was also studied in 10 iLTx controls. Among non-sensitized patients, MVTx recipients exhibited greater T and B-cell chimerism than either iITx and iLTx recipients. Myeloid lineage chimerism was present exclusively among iLTx and MVTx (6/13) recipients, suggesting that its presence required the hepatic allograft. Our study demonstrates, for the first time, frequent T cell chimerism without GVHD following visceral transplantation and a possible relationship with reduced rejection rate in MVTx recipients.
Keywords: Intestinal, liver and multivisceral transplantation, mixed chimerism, rejection
Introduction
The concept of chimerism, the co-existence of donor and recipient hematopoietic elements, has captivated immunologists for over sixty years. Microchimerism (denoting <1% donor cells in this manuscript) is known to occur among solid organ transplant (SOT) recipients in the immediate post-operative period and has likewise been documented in some long-term transplant survivors, leading to speculation about its role in promoting tolerance (1). The mechanism behind the development of chimerism among SOT recipients is thought to be associated with the transfer of so-called “passenger lymphocytes” carried to the host within the transplanted donor tissue (1, 2). Several groups have investigated the role of microchimerism in facilitating allograft acceptance (3, 4), but failed to demonstrate that microchimerism was sufficient for tolerance induction in either animals or humans (5). However, in both animal studies and pilot clinical trials, we and others have demonstrated that durable or transient macrochimerism (≥1% donor cells) can be achieved without Graft-Versus-Host Disease (GVHD) and can lead to donor specific-tolerance (6–8). While these trials relied on myelosuppressive pre-treatment followed by bone marrow infusion, other studies of human SOT recipients have involved administration of donor bone marrow without myelosuppressive conditioning to promote engraftment. To date, none have been associated with successful immunosuppression withdrawal (9). The spontaneous development of macrochimerism, or mixed chimerism after organ transplantation has been less extensively studied. Individuals who receive densely lymphoid allografts, including liver, intestinal and multivisceral transplants, demonstrate a higher incidence of GVHD compared to recipients of other organs (10, 11). Studies of macrochimerism have largely focused on its potential role as a biomarker for the development of this life-threatening complication (12–15). Nonetheless, case reports have documented the rare capacity of liver transplant recipients in particular to spontaneously develop full peripheral blood chimerism without GVHD, facilitating withdrawal of immunosuppressive medications without rejection (16, 17). Large cohort studies have also shown that, for still poorly understood reasons, multivisceral transplant recipients whose allografts include hepatic tissue have improved outcomes compared to those who do not undergo concurrent liver transplantation (18–20). In the absence of donor bone marrow infusion, human studies of post-transplant macrochimerism in the absence of GVHD are minimal (21). We hypothesized that the large volume transfer of lymphocytes inherent to multivisceral transplantation (MVTx) would correlate with a higher degree of macrochimersim compared to intestinal transplant (iITx) or isolated liver (iLTx), and might account for the lower rate of severe intestinal rejection in this population.
We assessed lineage chimerism using a refined flow cytometric technique and examined the effect of increasing the donor antigenic and lymphoid load by comparing recipients of MVTx versus iITx and iLTx.
Materials and Methods
Recruitment of Subjects, Blood Collection and Processing
Approval was obtained from the Columbia University Institutional Review Board (IRB# AAAJ5056). Subjects were recruited by our center’s transplant biobank (Columbia Center for Translational Immunology, Columbia University Medical Center). All subjects or legal guardians provided their written, informed consent. The patients enrolled into this study received either a small intestine in isolation or as part of a multivisceral allograft. Multivisceral allografts included the liver, stomach, duodenum, pancreas and small intestine with or without colon and required recipient splenectomy at the time of the surgery. The nine intestinal transplant recipients received anti-thymocyte globulins (ATG) as induction followed by maintenance therapy that included tacrolimus and steroids. One of the MVTx recipients was highly sensitized before transplantation and additionally received prophylactic plasmapheresis, polyclonal immunoglobulin and rituximab. Blood was drawn and prospectively collected from iITx and MVTx, weekly for the first two months and then progressively less often, whenever possible, until 1 year post-transplant. All patients underwent pre- and post-transplant serum testing (on a weekly basis the first month and then progressively spaced out) for class I and II donor-specific antibodies (DSA) by Luminex ®SA (Luminex LABScreen® Single Antigen, One Lambda Inc., CA, USA). All beads showing a normalized Mean Fluorescence Intensity > 1000 were considered positive. Protocol biopsies were obtained in the initial post-transplant course in addition to biopsies for symptoms. Protocol biopsies were usually performed 2 times per week for the first month, 1 time per week for the following 2 months, and once a month until one year post-transplant. The pathologic scoring scheme used to grade acute cellular rejection at our institution relies mostly on the apoptotic body count, as previously reported (22)f. Antibody-mediated rejection (AMR) was characterized by the following features: presence of diffuse C4d capillary staining in the lamina propria, associated with vascular changes and circulating DSA. Mixed rejection referred to the co-existence of ACR and AMR.
Ten iLTx recipients were retrospectively enrolled in the study to act as a control group for comparing donor chimerism among recipients of highly lymphoid allografts. The immunosuppressive regimen in iLTx included no (n=8) or basiliximab (n=2) induction therapy followed by a combination of steroids, tacrolimus and mycophenolic acid. Blood samples were drawn from living liver donors and their respective liver recipients just prior to transplantation and from hepatic transplant recipients at roughly 7, 14, 30, 60, 180, 270 and 365 days post-transplant, whenever possible.
HLA Typing, Antibody Selection and Cellular Staining
We screened candidate monoclonal HLA class I allele-specific antibodies (mAb) for the ability to discriminate donor and pre-transplant recipient cells, based on molecular HLA typing information. mAb were purchased from OneLambda or BD Biosciences. Each mAb was quality control tested for specificity. Those that readily distinguished donor from the pretransplant recipient peripheral blood mononuclear cells (PBMCs) were used in the corresponding post-transplant chimerism assay(s) (Table S1). Just prior to staining, cryopreserved and thawed PBMCs were incubated with human AB serum to block nonspecific binding to Fc receptors. The following anti-human antibodies were used: CD3-Percp-Cy5 (SP34-2), CD8-APC-Cy7 (SK1), CD45-V500 (HI30), CD33-AF700 (WM53), CD11c-PE-Cy5 (B-ly6), CD14-Pacific Blue (M5E2), HLA-ABC-APC (G46-2.6), and HLA-A2-PE (BB7.2) from BD Pharmingen; CD4-AF700 (OKT4), and CD19-Pacific Blue or -PE (HIB19) from BioLegend; HLA A2, A28-FITC or-biotin (BIH0037), HLA A30, A31-biotin (BIH0087), HLA A9-FITC or –biotin (FH0964), HLA A11-biotin (BIH0084), HLA 25, 26-biotin (BIH0048), HLA A29-biotin (BIH0155), HLA B8-FITC or – biotin (FH0536A), HLA B7, B27-biotin (BIH1453), HLA B12-biotin (BIH0066), HLA B13-biotin (BIH0261), HLA Bw4-biotin (BIH007) from One Lambda.
Leukocytes were identified with anti-CD45. Lymphoid lineage panels included anti-CD3, CD4, CD8, and CD19. Myeloid lineage panels included anti-CD33, CD11c, and CD14. Both lineage panels included pan-HLA-ABC antibody in addition to anti-donor and/or anti-recipient HLA mAbs specifically selected to distinguish between a particular donor-recipient pair. Just prior to FCM analysis, DAPI stain was added to each FACS tube to allow for exclusion of dead cells.
Sensitivity Assays
Once an HLA allele group-specific antibody was selected to distinguish donor and recipient cells, cell dilution assays were carried out to test its sensitivity for detecting known percentages of donor PBMCs within a sample of liver transplant recipient PBMCs. Selected HLA class I antibodies were tested on artificial mixtures of known ratios of donor and recipient pre-transplant cells. Whenever sufficient cell numbers were available, known donor concentrations ranged from 0.1 to 50%; in other cases, serial dilutions ranged from 1.0 to 50%. The dilution assays were carried out with an anti-recipient or anti-donor antibody. DAPI was added prior to data collection to gate out dead cells.
Flow Cytometric Analysis and chimerism level comparison across different lineages and patients
Data was acquired using an LSR II flow cytometer (BD Biosciences) using DIVA software. Analysis was carried out using FlowJo software (TreeStar, Inc, Ashland, OR). CD3+/CD4+/CD8-, CD3+/CD4-/CD8+, CD3-/CD56-/CD19+, CD45+/CD33+, CD45+/CD11c+ and CD45+/CD14+ populations were individually assessed for staining with MHC pan-class I and specific anti-donor and/or anti-recipient antibodies. Cells that strongly stained with MHC pan-class I antibody and anti-donor antibody and/or not with anti-recipient antibody were determined to be of donor origin. For chimerism assessment in individual patients, Area Under the Curve (AUC) was analyzed and plotted using the software Mathematica® (Wolfram Research, Inc, Champaign, IL). The chimerism AUC was the area under the curve (mathematically known as integral) in a plot of percentage of blood chimerism (≥ 1%) against time. The trapezoidal rule was used to estimate AUC. As AUC resulted from the product of a unit of percentage by a unit of time, the results were given in % × days. Myeloid chimerism values were calculated from the sum of AUC for CD33+, CD14+ and CD11c+ lineages.
Statistical Methods
Comparisons were based on Fischer’s exact test for categorical data and the Mann Whitney U test for continuous data. Survival curves were analyzed using Kaplan-Meier analysis with log-rank testing. All statistical analyses were performed using GraphPad Prism Software (GraphPad Software Inc, La Jolla, CA). To estimate reliably the limit of quantification of donor macrochimerism, especially with low numbers of interrogated events, we used a one-sided Poisson test, which postulates that the number of donor cells, say X, given the total number of cells in a given lineage, say b, follows a Poisson distribution with mean bθ, where θ is the intensity parameter. We adopt the definition of θ ≤ 10 donor cells per 1000 base cells as “noise” or the “lower limit of detection” and have tested the null hypothesis H0: θ ≤ 10/1000 base cells against the alternative that H1: θ > 10/1000 base cells (the chimerism alternative). Note that the criterion for rejecting H0 depends upon the base b. Table S2 provides the minimum number of cells, say C=C(b), such that if X ≥ C, we will reject H0 with type I error rate no more than α=0.01 (probability of falsely declaring chimerism).
Results
Patient characteristics and clinical outcome
Four multivisceral (MVTx) and five isolated intestinal transplant recipients (iITx) were enrolled into the study (Table 1). In intestinal transplantation, more than 50% of acute cellular rejection episodes occur within 90 days after transplant and there is a known correlation between the grade of rejection and probability of graft survival (23). Of the nine intestinal allograft recipients in our study, four patients experienced moderate to severe rejection episodes (n=4) within 90 days post-transplant after a median interval of 20.5 [range: 13–39] days (Table 1). Among the other five patients, two experienced a mild rejection at postoperative day 14 and 58 and three remained rejection-free. Overall, ITx subjects who suffered moderate rejection received similar doses of ATG and had similar levels of tacrolimus exposure over the first three months, compared to those who experienced mild or no rejection (Table 2). Ultimately, those who experienced early moderate rejections were more likely to develop de novo DSA (Table 2).
Table 1.
Individual clinical characteristics and chimerism data in intestinal transplant recipients
| Pt# | Rec. Age (yrs) | Don. Age (yrs) | Indication for iITx/MVTx | Type of Tx | Post-Transplant Outcome and Anti-Rejection Therapies (within 90 days) | Peak of CD3+ T cell macrochim. (%) | Peak of myeloid macrochim. (%) |
|---|---|---|---|---|---|---|---|
| 1 | 53 | 6 | Gardner’s Syndrome | MVTx, including colon | No rejection, no DSA | 43.1 | CD33: 7.1 CD14: 0 CD11c: 0 |
| 2 | 47 | 48 | Short gut syndrome | iITx | Early moderate rejection Steroid IV pulses and oral taper | 0 | CD33: 0 CD14: 0 CD11c: 0 |
| 3 | 17 | 21 | Pseudo-obstruction | MVTx | No rejection, no DSA | 8.2 | CD33: 0 CD14: 1.4 CD11c: 0 |
| 4 | 6 | 2 | Dysmotility | iITx | Early moderate rejection Mixed rejection (de novo DSA) Steroid IV pulses and oral taper, ATG, rituximab, IVIG |
2.9 | CD33: 0 CD14: 0 CD11c: 0 |
| 5 | 27 | 13 | Short gut syndrome | iITx | No rejection, no DSA | 6.6 | CD33: 0 CD14: 0 CD11c: 0 |
| 6 | 3 | 3 | Short gut syndrome | iITx | Self-limited skin GVHD Mild rejection, no DSA Steroid IV pulses and oral taper | 23.9 | CD33: 0 CD14: 0 CD11c: 0 |
| 7 | 53 | 44 | Entire porto-mesenteric system thrombosis | MVTx | Mild rejection, No DSA Steroid IV pulses and oral taper | 16.3 | CD33: 0 CD14: 0 CD11c: 12.6 |
| 9 | 2 | 1 | Short gut syndrome | iITx | Early moderate rejection Mixed rejection (de novo DSA) Steroid IV pulses and oral taper, ATG, rituximab, IVIG, PE |
3.3 | CD33: 0 CD14: 0 CD11c: 0 |
| 10 | 32 | 2 | Extensive porto-mesenteric system thrombosis | MVTx | Early moderate rejection Mixed rejection (preformed DSA) Steroid IV pulses and oral taper, ATG, rituximab, IVIG, PE |
2.2 | CD33: 0 CD14: 0 CD11c: 0 |
Abbreviations: ATG, anti-thymoglobulin; Don, donor; DSA, donor-specific antibody; iITx, isolated intestinal transplantation; IVIG, intra-venous immunoglobulin; MVTx, multivisceral transplantation; PE, plasma exchanges; Rec, recipient; Tx, transplantation; yrs, years.
Table 2.
Clinical characteristics and chimerism data in intestinal transplant recipients with and without early (<3 months) moderate to severe rejection
| Moderate to severe rejection (n=4) | Mild or no rejection (n=5) | p value | |
|---|---|---|---|
| Recipient Age | 21.8 ± 21.5 | 30.6 ± 22.2 | ns |
| Donor Age | 13.3± 23.2 | 17.4 ± 16.4 | ns |
| Multivisceral Allograft | 1/4 | 3/5 | ns |
| Pre-Tx PRA>0 (CDC) | 1/4 | 0/5 | ns |
| Pre-Tx DSA (SPA) | 1/4 | 1/5 | ns |
| Cumulative ATG induction dose (mg/kg) | 8.0 ± 1.5 | 9.9 ± 1.4 | ns |
| Mean tacrolimus T0 levels [0–3 mo] (ng/mL) | 15.3 ± 2.1 | 16.8 ± 3.2 | ns |
| De novo DSA (SPA) | 3/4 | 0/5 | p<0.05 |
| GVHD | 0/4 | 1/5 | ns |
| T-cell macrochimerism | 3/4 | 5/5 | ns |
| B-cell macrochimerism | 1/4 | 3/5 | ns |
| Myeloid macrochimerism | 0/4 | 3/5 | ns |
| Median [range] duration of T-cell macrochimerism (days) | 28.5 [0–58] | 127 [21–378] | p=0.19 |
| Median [range] peak of CD3 chim. (days × %) | 2.6 [0–3.3] | 16.3 [6.6–43.1] | p<0.02 |
| Median [range] peak of CD4 chim. (days × %) | 4.8 [0–7.8] | 10.3 [8.3–29.4] | p<0.02 |
| Median [range] peak of CD8 chim. (days × %) | 1.8 [0–3.7] | 21.4 [6.8–54.8] | p<0.02 |
| Median [range] peak of CD19 chim. (days × %) | 0 [0–0] | 8.8 [0–61] | p=0.11 |
| Median [range] peak of CD33 chim. (days × %) | 0 [0–0] | 0 [0–7.1] | ns |
| Median [range] peak of CD11c chim. (days × %) | 0 [0–0] | 0 [0–12.6] | ns |
| Median [range] peak of CD14 chim. (days × %) | 0 [0–0] | 0 [0–1.4] | ns |
| Median [range] CD3 chim. AUC (days × %) | 34.5 [0–62] | 820 [101–9098] | p<0.02 |
| Median [range] CD4 chim. AUC (days × %) | 69.5 [0–225] | 750 [142–7653] | p<0.05 |
| Median [range] CD8 chim. AUC (days × %) | 24.7 [0–69] | 921 [105–8109] | p<0.02 |
| Median [range] CD19 chim. AUC (days × %) | 0 [0–0] | 165 [0–9157] | ns |
| Median [range] Myeloid chim. AUC (days × %) | 0 [0–0] | 202 [0–731] | ns |
| Median follow-up (days [range]) | 415 [132–941] | 524 [343–991] | ns |
| Death | 0/4 | 2/5 | ns |
Abbreviations: ATG, anti-thymoglobulin; AUC, Area Under the Curve; CDC, complement-dependent cytotoxicity; chim., chimerism; DSA, donor-specific antibody; GVHD, graft-vs-host disease; mo, months; PRA, panel reactive antibody; Pre-Tx, pre-transplantation; SPA, solid-phase assay; T0, trough levels
Over the course of follow-up (median 524 days, ranging from 132–991 days), only one iITx recipient had a self-limited rash from day 46 to day 54 consistent with biopsy-proven mild skin GVHD, which spontaneously resolved in association with a mild graft rejection episode. Two MVTx recipients died of post-transplant lymphoproliferative disorder (day 387) and fungal infection (day 343).
Assay Sensitivity and Accuracy
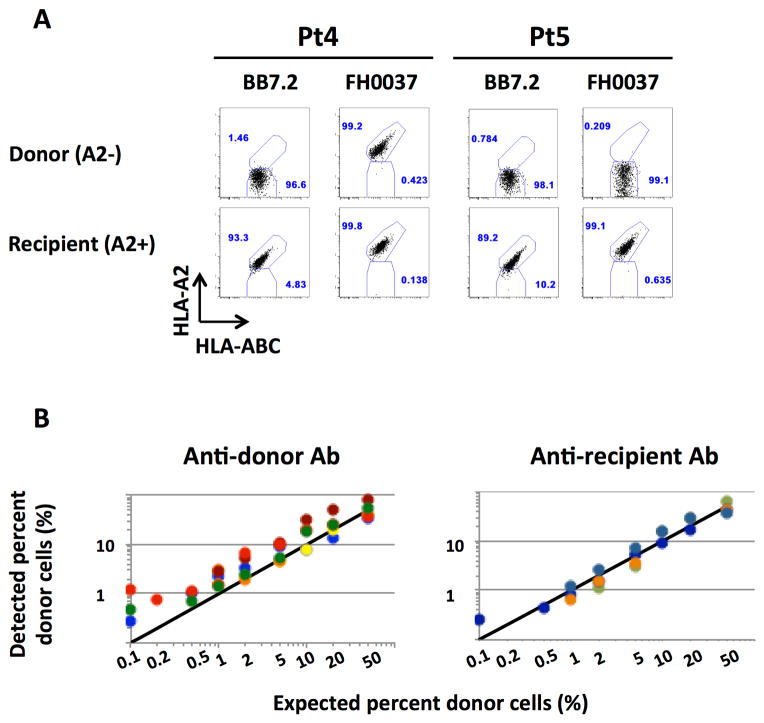
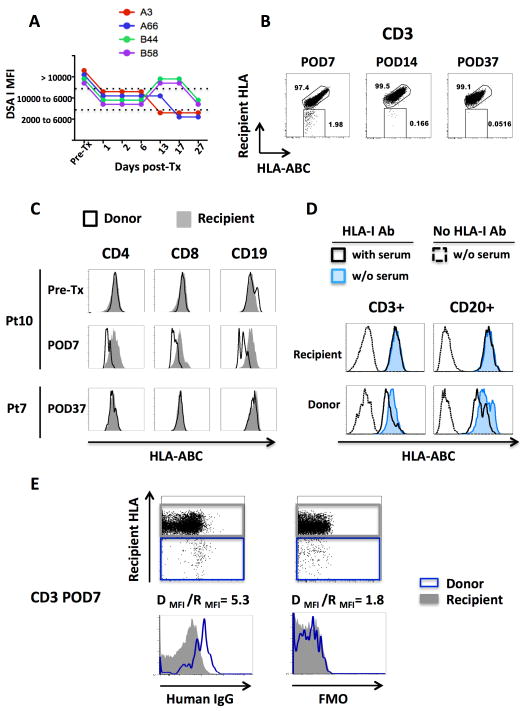
Identification of an HLA allele-specific mAb that distinguished donor and recipient cells in quality control assays was a prerequisite for inclusion in the study (Table S1). In the quality control studies, pan-HLA-ABC Ab enabled us to identify the class I MHC-expressing mononuclear cells that stained appropriately or inappropriately negative or positive for the mAb used to identify the recipient or donor population. Figure 1A depicts the reactivity of two different anti-HLA-A2 clones (BB7.2 and FH0037) with donor and recipient cells from two different donor-recipient pairs. In each case, the recipient and donor were HLA-A2 positive and HLA-A2 negative, respectively. Although each clone accurately differentiated recipient and donor cells in iITx #5, the clone FH0037 stained both donor and recipient cells in iITx #4. This example illustrates the cross-reactivity of the currently available monoclonal anti-HLA antibodies and emphasizes the importance of quality control assays prior to chimerism assessment with flow cytometry.
Figure 1. Assay Sensitivity and Accuracy.
(A): Dot plots demonstrating variable cross-reactivity of anti-HLA-A2 mAbs between two separate iITx donor / recipient pairs. (B): Summary of findings regarding the sensitivity of anti-donor and anti-recipient antibodies for detecting the chimeric cell population in the iLTx population. Each color represents a different subject. Each dot represents a different dilution.
Dilution assays employing known concentrations of donor and recipient cells demonstrated that the donor cell detection threshold varied, depending on the particular donor/recipient pair, yet could be as low as 0.2% in some cases. Importantly, this flow cytometry-based approach enabled us to accurately distinguish between donor and recipient cells in all patients at dilutions equal to or greater than 1% (Figure 1B). This finding confirmed the validity of this approach for studying macrochimerism.
Development of donor chimerism after intestinal and multivisceral transplantation in the absence of significant rejection
We used multicolor flow cytometry to prospectively monitor donor chimerism in lymphoid (CD3 and CD19 cells) and myeloid (CD33, CD14 and CD11c) lineages (e.g. Figure 2). Only the measurements of blood chimerism equal to or greater than 1%, assessed on an adequate number of interrogated cells for each given lineage, were included into the analysis (Table S2). T cell macrochimerism was detected in all but one (8/9) patient (Table 1). Furthermore, T cell chimerism peaked at a significantly higher level and was found, overall, to be more durable within the group that did not have episodes of moderate or severe rejection (Table 2, Figure 3). The peaks of both the percentage and absolute number of donor T-cells were significantly higher in the blood of patients who were free of rejection (Table 2, Figure 3A, B). Notably, the only patient (Pt6) who experienced a skin biopsy-proven GVHD had the highest absolute number of circulating donor T cells at 3 weeks post-transplant, 25 days before the occurrence of the GVHD-related skin rash (Figure 3B).
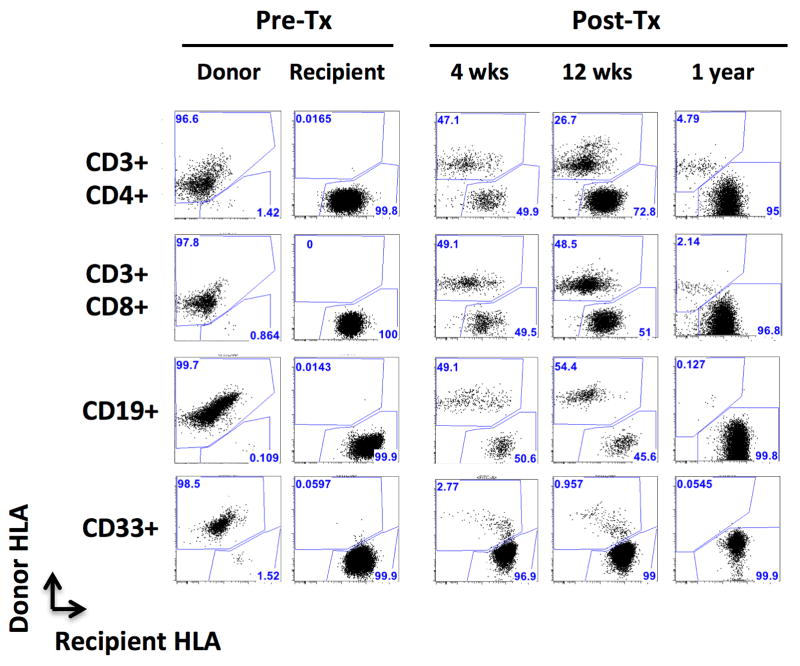
Figure 2. Sustained multilineage chimerism in a multivisceral transplant recipient.
Representative plots showing how donor and recipient cells were differentiated with a combination of donor- and recipient-specific antibodies (MVTx recipient #1). CD3+/CD4+ CD3+/CD8+, CD45+/CD19+, CD45+/CD33+, CD45+/CD11c+ and CD45+/CD14+ populations were individually assessed for staining with anti-donor (HLA-A2) and anti-recipient (HLA-A9) antibodies.
Abbreviations: Post-Tx, post-transplantation; Pre-Tx, pre-transplantation; wks, weeks
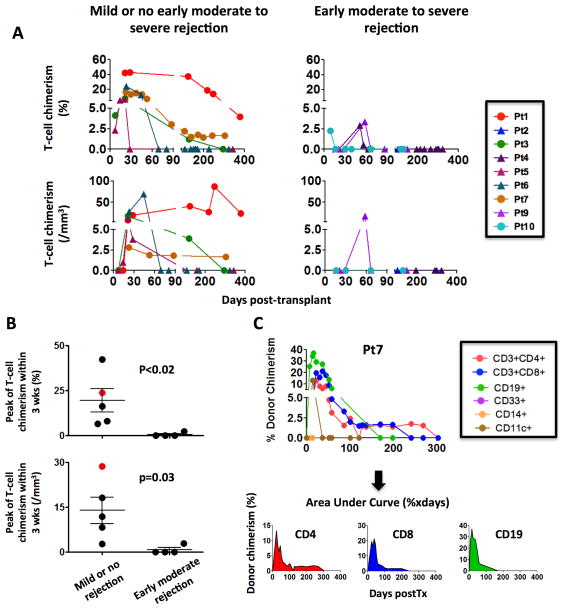
Figure 3. Analysis of T-cell (CD3+) chimerism over time in MVTx and iITx recipients according to the occurrence of an early moderate to severe rejection episode.
(A) Patients free of significant rejection had higher T cell chimerism both in frequency and absolute number. Circles and triangles indicate MVTx (Pts 1, 3, 7 and 10) and iITx (Pts 2, 4, 5, 6, 9) recipients, respectively (B) The early (within 3 weeks) peak of T-cell chimerism was significantly higher in the patients without moderate rejection. Pt6 (red dot), who experienced skin GVHD episode from day 46 to 54, displayed the highest absolute count of circulating donor T cells at 3 weeks. (C): Calculation of overall chimerism (Area Under Curve) by integrating the donor chimerism frequency over time.
Abbreviations: Pt, patient; wks, weeks
To better assess and compare chimerism levels among individual subjects who were not uniformly investigated on the same post-transplant days, we calculated the Area-Under-the-Curve (AUC) by integrating the percent donor chimerism over time for each lineage (Figure 3C). Overall the extent of CD3 T cell chimerism was significantly greater (Median [range]: 820 [101–9098] vs 34.5 [0–62], p<0.02) in the group with mild or no rejection than in the group that experienced moderate to severe rejection (Table 2). In addition, B cell and myeloid lineage chimerism greater than 1% was found to be present among 3/5 and 3/5 patients free of moderate rejection respectively, whereas no B-cell or myeloid macrochimerism was detected in patients with significant rejection (Tables 1 and 2). B cell chimerism AUC tended to be higher in the absence of moderate rejection (Median [range]: 165 [0–9157] vs 0 [0–0], p=0.11).
Lack of early T-cell chimerism following intestinal transplantation is associated with early moderate to severe rejection
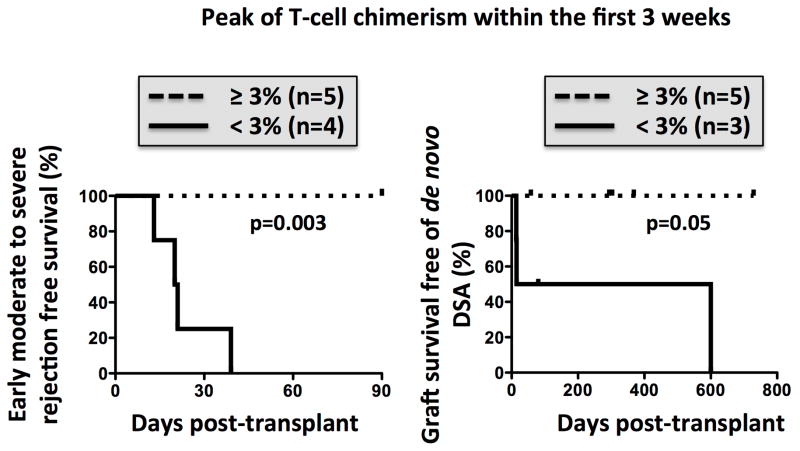
To investigate whether early assessment of chimerism might help to identify patients at greater risk of moderate rejection, we compared the clinical outcomes of subjects according to the peak of T cell chimerism during the first three weeks post-transplant. Remarkably, all subjects with peak T-cell chimerism less than 3% experienced an early moderate rejection, whereas those with early T cell-chimerism above 3% remained free of moderate rejection (p=0.003) (Figure 4). Of note, in two ITx recipients (Pt4 and Pt9), early, severe cellular rejection necessitated a second course of ATG. Once rejection had successfully resolved, T-cell chimerism transiently peaked at 2.9% (Pt4) and 3.3% (Pt9) on day 51 and 58, respectively (Figure 3A).
Figure 4. Low early chimerism associated with the development of significant rejection.
(A): Freedom from early (<90 days) moderate to severe rejection (left) and de novo DSA (right) after intestinal transplantation according to the peak of donor T-cell chimerism within 3 weeks post-transplant.
In addition to comparing chimerism and its association with clinical events, we investigated the development of de novo donor-specific antibodies (DSA) in relation to peak chimerism. A single pre-sensitized MVTx recipient was excluded from this portion of the study, since he already had preformed DSA against all 7 donor HLA-mismatches at HLA-A, B, DR and DQ loci, prior to the transplant. We found that a peak level of T-cell chimerism less than 3% differentiated the patients who developed de novo DSA from those who did not, as only the patients with less than 3% chimerism developed DSA (Figure 4).
Rapid clearance of circulating donor cells bound by preformed DSA
As noted above, one of the MVTx recipients was transplanted across seven high-titer DSA (MFI>10,000) and a positive cross-match. The transplanted liver was expected to adsorb part of the preformed DSA and reduce their titer synergistically with the desensitization regimen (24, 25). However, anti-class I DSA remained at a very high titer during the post-transplant course (Figure 5A), and an early mixed and moderate rejection occurred at day 21. Donor cells were readily detected in the blood at day 7 post-transplant (Figure 5B), but were barely detectable one week later and became undetectable shortly thereafter, in contrast to the other MVTx recipients who all had sustained T-cell chimerism (Figure 6). Donor chimerism was not only very short-lived but was also associated with an unusual flow cytometric pattern: post-transplant donor T and B cells displayed lower levels of HLA class I by flow cytometry than recipient cells (Figure 5B and C), whereas pre-transplant cells did not differ in HLA class I staining (Figure 5C). We reasoned that in vivo binding of DSA to circulating donor cells might result in reduced in vitro access of the anti-class I antibody to donor HLA molecules. To test this hypothesis, we incubated donor and recipient pre-transplant cells with pre-transplant recipient serum and stained with an anti-class I antibody (Figure 5D). Donor, but not recipient cells incubated with recipient serum displayed decreased detectable HLA class I levels. This finding suggests that DSA competed with anti-class I antibody for binding to donor HLA molecules. To demonstrate more directly that DSA were bound to circulating donor T cells, PBMCs collected at POD7 were stained with an anti-human IgG antibody (Figure 5E). The Donor/Recipient mean fluorescent intensity (MFI) ratio was 2.9 fold greater with the anti-human IgG staining compared to the fluorescence-minus-one control, demonstrating in vivo binding of donor cells by anti-donor antibody.
Figure 5. Early clearance of DSA-bound circulating donor cells.
(A,B): Post-transplant persistence of preformed high titer anti-class I DSA in a sensitized multiviceral transplant recipient (A), was associated with early disappearance of T-cell chimerism (B). (C): In this highly sensitized patient (Pt10), post-transplant, but not pre-transplant, donor cells display a lower level of detectable HLA class I, when compared to recipient cells. In contrast, post-transplant donor and recipient cells have comparable HLA class I expression in non-sensitized patients (representative staining from Pt7). (D): Incubation with pre-transplant Pt10 serum decreases the HLA-class I staining on donor, yet not on recipient, pre-transplant cells. (E): The positive anti-human IgG staining on the circulating donor cells showed that they had been bound in vivo by DSA.
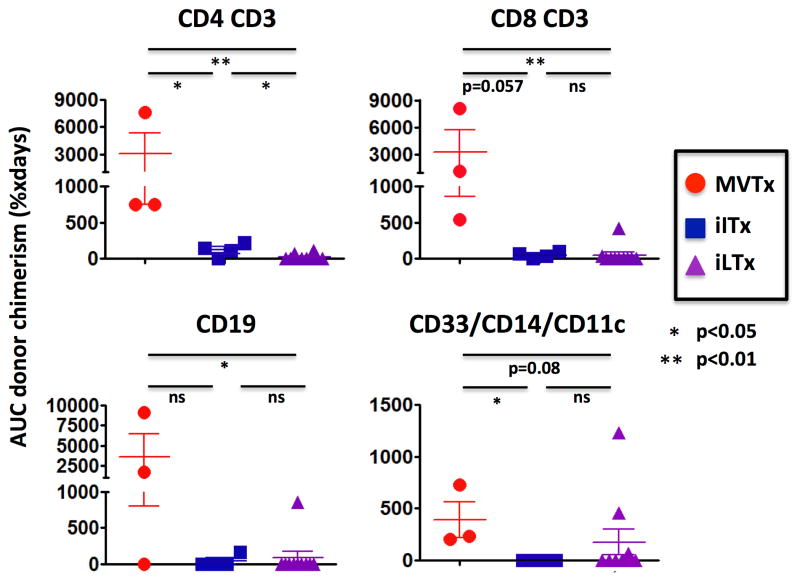
Figure 6.
Higher GVHD-free blood chimerism in non-sensitized MVTx compared to iLTx and iITx recipients.
Multivisceral transplantation is associated with multilineage and sustained chimerism
Multivisceral transplantation and combined liver-intestine transplantation have been associated with both a greater incidence of GVHD (11, 26) and a reduced rate of severe rejection compared with isolated intestinal transplantation (18–20). We hypothesized that the higher donor lymphoid load contained within a multivisceral allograft might induce greater donor chimerism. To avoid confounding factors in a size-limited cohort, we investigated a homogenous population that included only the 7 non pre-sensitized patients who were free of GVHD (Figure 6). The median duration [range] of T cell macrochimerism was 242 [127–378] and 35.5 [0–58] days in the MVTx and iITx recipients, respectively (p=0.057). B cell macrochimerism was also detected in the two MVTx recipients and in one of the 4 iITx recipients. Overall, CD3+ T (median [range]: 1073 [702–9098] vs 56.5 [0–101], p=0.057) and CD19+ B (median [range]: 1675 [0–9157] vs 0 [0–165], p=0.24) cell chimerism AUC tended to be greater in the MVTx than in the iITx recipients (Figure 6), reflecting mostly more sustained levels of mixed chimerism, but fell short of statistical significance in this limited population.
To further investigate the role of the liver allograft in the development of post-transplant chimerism, 10 isolated liver transplant recipients were also studied. Although 6/7 of the non-sensitized GVHD-free intestinal transplant recipients exhibited T cell macrochimerism, only 3 out of 10 iLTx recipients had T cell macrochimerism (Fischer’s exact test, p<0.05). T-(p<0.01) and B-cell (p<0.05) chimerism AUC were significantly lower in iLTx recipients than in the MVTx recipients (Figure 6). Finally, all three MVTx and three of 10 iLTx recipients had chimerism > 1% in at least one myeloid lineage, while myeloid chimerism was never detected in recipients of intestinal allografts without a liver (Table 1, Figure 6).
Discussion
Our study is the first to prospectively assess multilineage chimerism with multicolor flow-cytometry and to study its clinical significance among intestinal transplant recipients. Mixed chimerism after intestinal transplantation, mostly in T cell lineages, has been previously assessed in cross-sectional studies using molecular techniques (27, 28) or, less often, four-color flow-cytometry (26, 29), and was repeatedly correlated with the development of GVHD (26, 27). T-cell macrochimerism has also been reported sporadically in a few patients who are free of GVHD (26, 27).
One of the key findings of our study is that the occurrence of T-cell mixed chimerism (≥1%) after intestinal transplantation (8/9) was far more frequent than the occurrence of GVHD (1/9). This finding is in contrast to most previous studies, in which donor chimerism has been used as a biomarker for the development of GVHD. Some authors have gone as far as to propose that chimerism greater than 20% is highly specific for GVHD (28, 30). Nevertheless, we found that lymphoid lineage chimerism exceeding 40% occurred in a MVTx recipient without any signs of GVHD, providing the first documented evidence that chimerism of this magnitude can be achieved in multivisceral graft recipients without irradiation or bone marrow infusion and remain present for greater than one year even in the absence of pathology. Moreover, our data suggest that absolute donor T cell count, rather than percentage of T-cell chimerism, might be a more accurate predictor of GVHD.
Multivisceral transplantation is known to elicit a protective effect against intestinal graft rejection (18, 19, 24) but to increase the risk of GVHD following intestinal transplantation (11, 26). Adult liver and small intestine, each weighing approximately 1.5 to 2 kg, are estimated to contain 1×1010 and 1.2×1010 lymphocytes, respectively (31, 32), without taking into account organ-associated lymph nodes. We hypothesized that, as a consequence of the greater donor lymphoid mass carried by the allograft, a higher level of mixed chimerism would be observed in MVTx compared to iITx transplant recipients. We compared patients with and without moderate to severe rejection during the first 3 months post-transplant and found that the latter had higher and more sustained mixed chimerism. We considered the possibility that the lower chimerism in rejecting patients might simply reflect a less dramatic reduction of recipient lymphoid mass (Figure S1). However, there are two arguments that definitively support the existence of a true increase in chimerism in the patients who were free of rejection. First, absolute counts demonstrated a higher number of circulating donor T cells in the absence of significant rejection. Second, greater B-cell chimerism was also observed in this group, although ATG has no effect on B-cell number (33).
We believe that the presence of blood chimerism after intestinal transplantation is determined by the balance between Host-vs-Graft (HvG) and Graft-vs-Host (GvH) responses. At one extreme, a deficient immune system in the recipient, either related to young age, or intestinal atresia-associated immune deficiency or splenectomy (11, 26), has been associated with a higher risk of GVHD. Our study also suggests that the HvG response may influence the level of GVHD-free chimerism. Spleen removal reduces the recipient lymphoid compartment in MVTx recipients and may thereby further decrease the HvG response and promote the recirculation of donor cells. The occurrence of delayed peak chimerism in two subjects after the successful treatment of a moderate rejection episode suggests that control of HvG responses allowed mixed chimerism to develop. Interestingly, the sole patient who remained free of significant early rejection despite a lack of ATG-induced lymphodepletion (Figure S1) experienced GVHD in association with high-level donor chimerism. Our results are consistent with the possibility that a strong GvH response can also promote donor cell engraftment by counterbalancing the HvG response (16). The association between microchimerism and lack of rejection after SOT has been intensely controversial, reflecting the complexity of a chicken and egg situation in which it is unclear which leads to the other (34). However, our study focused on macrochimerism, which has been associated with the induction of both central (35) and peripheral (36) tolerance mechanisms. We thus propose that sustained macrochimerism in multivisceral transplant recipients may further reduce HvG T and B-cell responses. Combined with recipient spleen removal and the immunoprotective effect of the liver allograft (37), macrochimerism may contribute to lower rates of rejection in MVTx recipients.
The lack of recipient T-cell depletion may account for the low incidence of T-cell chimerism that we observed in iLTx recipients, none of whom underwent ATG induction therapy. In addition, the lower incidence of GVHD after iLTx (0.1 to 2%) (38) compared to intestinal transplantation (4.5 to 9%) (11, 26) may also reflect differences between mature lymphoid cell populations contained within intestinal versus liver allografts. Conventional T cells contained within gut-associated lymphoid tissue (GALT) and intestinal lamina propria (39), rather than the more numerous, unconventional intestinal IEL and liver lymphoid cells, known to poorly recirculate in the blood (31, 39), may account for the early wave of T-cell chimerism among intestinal allograft recipients, mainly driven by GvH reactivity. Lymphohematopoietic GvH Responses (LGVHR), which are GvH reactions confined to the lymphohematopoietic system that spare the epithelial GVHD target organs, make hematopoietic “space” that promotes multilineage chimerism in experimental models (40).
Human liver and small bowel also host hematopoietic stem cells and lineage-committed hematopoietic progenitors, which have been implicated in late and sustained mixed chimerism after liver transplantation (41). Strikingly, a few cases of donor-derived multilineage long-term hematopoiesis without GVHD have been reported after isolated liver (16, 17) and combined liver and intestinal (42) transplantation. Consistently, hematopoietic stem cells isolated from the liver successfully reconstitute hematopoiesis in lethally irradiated animals (43). However, liver and intestinal hematopoietic progenitors may differ in their multilineage differentiation potential. Although liver hematopoietic stem cells can give rise to multiple lineages, including lymphoid (44) and myeloid (45) cells, human gut-derived progenitors cells are almost exclusively lymphoid (CD7 positive) (46) and therefore only able to generate T cells (47). Consistent with this observation, in contrast to liver and multivisceral recipients, liver-free intestinal transplant recipients never displayed myeloid macrochimerism in our study. Likewise, chimerism among liver-free allograft recipients lasted for a shorter period of time post-operatively compared to subjects whose transplants included hepatic tissue.
Finally, the study of chimerism in a highly sensitized MVTx recipient demonstrated that preformed high titer DSA could bind in vivo to circulating donor cells (in vivo flow-crossmatch), possibly accelerating their clearance. This finding explains the protective effect of positive crossmatch against GVHD after intestinal transplantation (26) and is consistent with the growing recognition that DSA increases the risk of graft failure after bone marrow transplantation (48). In addition, this finding provides further evidence that anti-HLA sensitization may be a major hurdle for inducing tolerance through mixed chimerism, similar to observations in the mouse model (49).
Our study is limited by the small sample size, underscoring the need for a prospective multi-center study to confirm and verify our findings. Should larger studies reproduce our findings, routine monitoring of mixed chimerism may become a very useful tool for treatment tailoring after intestinal and multivisceral transplantation. These studies might also pave the way for pilot protocols combining bone marrow and intestinal transplantation, such as those established in rodents, to promote donor-specific tolerance (50).
In summary, GVHD-free mixed T-cell chimerism occurs as a general rule after intestinal transplantation in the absence of an overwhelming cellular and/or humoral HvG response. Conversely, its absence may indicate under-immunosuppression and an increased risk of moderate to severe rejection and development of de novo DSA. The association between macrochimerism and lack of significant rejection suggests that the greater chimerism observed after MVTx might contribute, at least in part, to the lower incidence of rejection among that population of transplant recipients.
Supplementary Material
Figure S1: Absolute lymphocyte count.
Early ATG-induced lymphodepletion and lymphocyte reconstitution until 1 year after transplantation were assessed for each group, according to the rejection status. Circles and triangles indicate MVTx (Pts 1, 3, 7 and 10) and iITx (Pts 2, 4, 5, 6, 9) recipients, respectively.
Table S1: HLA class I typing and anti-HLA antibodies used to distinguish donor from recipient cells in intestinal transplant recipients
HLA-A9 (*) and HLA-B12 (**) are broad antigen HLA serotypes that encompass the HLA-A23 / HLA-A24 serotypes, and the HLA-B44 / HLA-B45 serotypes, respectively.
Abbreviations: ITx, intestinal transplant recipient; LTx, liver transplant recipient
Table S2: Minimum number of donor cells that should be detected C(b), depending upon the number of interrogated events (b), to ensure the presence of a true macrochimerism (frequency of donor cells 1%) in a given lineage.
Acknowledgments
We thank Dr Adam Griesemer for helpful review of the manuscript and Ms. Susan Allen for assistance with the submission. The authors are also grateful to Samina Munir for her assistance in obtaining biological samples, to Monica Velasco for her outstanding care of intestinal/multivisceral transplant recipients, to Shana Coley for helpful advices on HLA typing, and to Jean-Bernard Zuber for his assistance in area-under-the-curve calculations. JZ was successively supported by French-speaking Society of Transplantation, Fulbright, Monahan foundation and Schaefer research scholarships. SR, B. Sprangers and SD received support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), AST/Astellas Clinical Science Fellowship Grant from the American Society of Transplantation and the Kidney Research Student Scholar Grant from the American Society of Nephrology, respectively. The study was funded by the Schaefer research scholar program and NIAID grant P01 AI106697. A flow cytometer used in this study was purchased with NIH award 1S10RR027050-01A1.
Abbreviations
- Graft-versusGVHD
graft-versus-host disease
- iITx
isolated intestinal transplantation
- iLTx
isolated liver transplantation
- MVTx
multivisceral transplantation
Footnotes
Disclosure section
The authors of this manuscript have no conflict of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992 Jun 27;339(8809):1579–82. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew JM, Leventhal JR, Miller J. Microchimerism in promoting graft acceptance in clinical transplantation. Curr Opin Organ Transplant. 2011 Aug;16(4):345–52. doi: 10.1097/MOT.0b013e3283489a42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nierhoff D, Horvath HC, Mytilineos J, Golling M, Bud O, Klar E, et al. Microchimerism in bone marrow-derived CD34(+) cells of patients after liver transplantation. Blood. 2000 Jul 15;96(2):763–7. [PubMed] [Google Scholar]
- 5.Hisanaga M, Hundrieser J, Boker K, Uthoff K, Raddatz G, Wahlers T, et al. Development, stability, and clinical correlations of allogeneic microchimerism after solid organ transplantation. Transplantation. 1996 Jan 15;61(1):40–5. doi: 10.1097/00007890-199601150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Cosimi AB, Sachs DH. Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism. Curr Opin Organ Transplant. 2011 Aug;16(4):366–71. doi: 10.1097/MOT.0b013e3283484b2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013 May 9;368(19):1850–2. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000 Apr;6(4):464–9. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 9.Orlando G, Hematti P, Stratta RJ, Burke GW, 3rd, Di Cocco P, Pisani F, et al. Clinical operational tolerance after renal transplantation: current status and future challenges. Ann Surg. 2010 Dec;252(6):915–28. doi: 10.1097/SLA.0b013e3181f3efb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollack MS, Speeg KV, Callander NS, Freytes CO, Espinoza AA, Esterl RM, et al. Severe, late-onset graft-versus-host disease in a liver transplant recipient documented by chimerism analysis. Hum Immunol. 2005 Jan;66(1):28–31. doi: 10.1016/j.humimm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Selvaggi G, Nishida S, Moon J, Island E, Ruiz P, et al. Graft-versus-host disease after intestinal and multivisceral transplantation. Transplantation. 2011 Jan 27;91(2):219–24. doi: 10.1097/TP.0b013e3181ff86ec. [DOI] [PubMed] [Google Scholar]
- 12.Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005 Dec;5(12):2968–73. doi: 10.1111/j.1600-6143.2005.01110.x. [DOI] [PubMed] [Google Scholar]
- 13.Hahn AB, Baliga P. Rapid method for the analysis of peripheral chimerism in suspected graft-versus-host disease after liver transplantation. Liver Transpl. 2000 Mar;6(2):180–4. doi: 10.1002/lt.500060214. [DOI] [PubMed] [Google Scholar]
- 14.Rogulj IM, Deeg J, Lee SJ. Acute graft versus host disease after orthotopic liver transplantation. J Hematol Oncol. 2012;5:50. doi: 10.1186/1756-8722-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, et al. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004 Feb 15;77(3):441–6. doi: 10.1097/01.TP.0000103721.29729.FE. [DOI] [PubMed] [Google Scholar]
- 16.Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, et al. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008 Jan 24;358(4):369–74. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 17.Collins RH, Jr, Anastasi J, Terstappen LW, Nikaein A, Feng J, Fay JW, et al. Brief report: donor-derived long-term multilineage hematopoiesis in a liver-transplant recipient. N Engl J Med. 1993 Mar 18;328(11):762–5. doi: 10.1056/NEJM199303183281104. [DOI] [PubMed] [Google Scholar]
- 18.Kato T, Tzakis AG, Selvaggi G, Gaynor JJ, Takahashi H, Mathew J, et al. Transplantation of the spleen: effect of splenic allograft in human multivisceral transplantation. Ann Surg. 2007 Sep;246(3):436–44. doi: 10.1097/SLA.0b013e3181485124. discussion 45–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzakis AG, Kato T, Levi DM, Defaria W, Selvaggi G, Weppler D, et al. 100 multivisceral transplants at a single center. Ann Surg. 2005 Oct;242(4):480–90. doi: 10.1097/01.sla.0000183347.61361.7a. discussion 91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Elmagd KM, Kosmach-Park B, Costa G, Zenati M, Martin L, Koritsky DA, et al. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg. 2012 Sep;256(3):494–508. doi: 10.1097/SLA.0b013e318265f310. [DOI] [PubMed] [Google Scholar]
- 21.Sykes M. Mechanisms of transplantation tolerance in animals and humans. Transplantation. 2009 May 15;87(9 Suppl):S67–9. doi: 10.1097/TP.0b013e3181a2a6b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remotti H, Subramanian S, Martinez M, Kato T, Magid MS. Small-bowel allograft biopsies in the management of small-intestinal and multivisceral transplant recipients: histopathologic review and clinical correlations. Arch Pathol Lab Med. 2012 Jul;136(7):761–71. doi: 10.5858/arpa.2011-0596-RA. [DOI] [PubMed] [Google Scholar]
- 23.Selvaggi G, Gaynor JJ, Moon J, Kato T, Thompson J, Nishida S, et al. Analysis of acute cellular rejection episodes in recipients of primary intestinal transplantation: a single center, 11-year experience. Am J Transplant. 2007 May;7(5):1249–57. doi: 10.1111/j.1600-6143.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Elmagd KM, Wu G, Costa G, Lunz J, Martin L, Koritsky DA, et al. Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant. 2012 Nov;12(11):3047–60. doi: 10.1111/j.1600-6143.2012.04237.x. [DOI] [PubMed] [Google Scholar]
- 25.Olausson M, Mjornstedt L, Norden G, Rydberg L, Molne J, Backman L, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007 Jan;7(1):130–6. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 26.Mazariegos GV, Abu-Elmagd K, Jaffe R, Bond G, Sindhi R, Martin L, et al. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004 Sep;4(9):1459–65. doi: 10.1111/j.1600-6143.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 27.Shin CR, Nathan J, Alonso M, Yazigi N, Kocoshis S, Tiao G, et al. Incidence of acute and chronic graft-versus-host disease and donor T-cell chimerism after small bowel or combined organ transplantation. J Pediatr Surg. 2011 Sep;46(9):1732–8. doi: 10.1016/j.jpedsurg.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Ruiz P. Solid organ transplant-associated acute graft-versus-host disease. Arch Pathol Lab Med. 2010 Aug;134(8):1220–4. doi: 10.5858/2008-0679-RS.1. [DOI] [PubMed] [Google Scholar]
- 29.Metes D, Logar A, Rudert WA, Zeevi A, Woodward J, Demetris AJ, et al. Four-color flow cytometric analysis of peripheral blood donor cell chimerism. Hum Immunol. 2003 Aug;64(8):787–95. doi: 10.1016/s0198-8859(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi AP, Bone BA, Edwards AR, Parker MK, Delos Santos RB, Hagopian J, et al. Graft-Versus-Host Disease After Simultaneous Pancreas-Kidney Transplantation: A Case Report and Review of the Literature. Am J Transplant. 2014 Sep 12;14(11):2651–56. doi: 10.1111/ajt.12862. [DOI] [PubMed] [Google Scholar]
- 31.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000 Apr;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 32.Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010 Sep;10(9):2132–41. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood K, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol Today. 1996 Dec;17(12):584–7. doi: 10.1016/s0167-5699(96)10069-4. discussion 8. [DOI] [PubMed] [Google Scholar]
- 35.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996 Aug 15;62(3):380–7. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 36.Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011 Jun;11(6):1236–47. doi: 10.1111/j.1600-6143.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006 Oct;213:101–18. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 38.Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: a comprehensive literature review. World J Gastroenterol. 2012 Oct 7;18(37):5240–8. doi: 10.3748/wjg.v18.i37.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki S, Sugahara S, Shimizu T, Tada T, Minagawa M, Maruyama S, et al. Low level of mixing of partner cells seen in extrathymic T cells in the liver and intestine of parabiotic mice: its biological implication. Eur J Immunol. 1998 Nov;28(11):3719–29. doi: 10.1002/(SICI)1521-4141(199811)28:11<3719::AID-IMMU3719>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 40.Sykes M, Sheard MA, Sachs DH. Graft-versus-host-related immunosuppression is induced in mixed chimeras by alloresponses against either host or donor lymphohematopoietic cells. J Exp Med. 1988 Dec 1;168(6):2391–6. doi: 10.1084/jem.168.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang XQ, Lo CM, Chen L, Cheung CK, Yang ZF, Chen YX, et al. Hematopoietic chimerism in liver transplantation patients and hematopoietic stem/progenitor cells in adult human liver. Hepatology. 2012 Oct;56(4):1557–66. doi: 10.1002/hep.25820. [DOI] [PubMed] [Google Scholar]
- 42.Gilroy RK, Coccia PF, Talmadge JE, Hatcher LI, Pirruccello SJ, Shaw BW, Jr, et al. Donor immune reconstitution after liver-small bowel transplantation for multiple intestinal atresia with immunodeficiency. Blood. 2004 Feb 1;103(3):1171–4. doi: 10.1182/blood-2003-04-1187. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996 Feb;2(2):198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 44.Golden-Mason L, Curry MP, Nolan N, Traynor O, McEntee G, Kelly J, et al. Differential expression of lymphoid and myeloid markers on differentiating hematopoietic stem cells in normal and tumor-bearing adult human liver. Hepatology. 2000 Jun;31(6):1251–6. doi: 10.1053/jhep.2000.7713. [DOI] [PubMed] [Google Scholar]
- 45.Crosbie OM, Reynolds M, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. In vitro evidence for the presence of hematopoietic stem cells in the adult human liver. Hepatology. 1999 Apr;29(4):1193–8. doi: 10.1002/hep.510290402. [DOI] [PubMed] [Google Scholar]
- 46.Lynch L, O’Donoghue D, Dean J, O’Sullivan J, O’Farrelly C, Golden-Mason L. Detection and characterization of hemopoietic stem cells in the adult human small intestine. J Immunol. 2006 May 1;176(9):5199–204. doi: 10.4049/jimmunol.176.9.5199. [DOI] [PubMed] [Google Scholar]
- 47.Gunther U, Holloway JA, Gordon JN, Knight A, Chance V, Hanley NA, et al. Phenotypic characterization of CD3-7+ cells in developing human intestine and an analysis of their ability to differentiate into T cells. J Immunol. 2005 May 1;174(9):5414–22. doi: 10.4049/jimmunol.174.9.5414. [DOI] [PubMed] [Google Scholar]
- 48.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010 Apr 1;115(13):2704–8. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levesque V, Bardwell PD, Shimizu I, Haspot F, Benichou G, Yeap BY, et al. B-cell-dependent memory T cells impede nonmyeloablative mixed chimerism induction in presensitized mice. Am J Transplant. 2011 Nov;11(11):2322–31. doi: 10.1111/j.1600-6143.2011.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakao A, Toyokawa H, Kimizuka K, Nalesnik MA, Nozaki I, Bailey RJ, et al. Simultaneous bone marrow and intestine transplantation promotes marrow-derived hematopoietic stem cell engraftment and chimerism. Blood. 2006 Aug 15;108(4):1413–20. doi: 10.1182/blood-2006-02-004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Absolute lymphocyte count.
Early ATG-induced lymphodepletion and lymphocyte reconstitution until 1 year after transplantation were assessed for each group, according to the rejection status. Circles and triangles indicate MVTx (Pts 1, 3, 7 and 10) and iITx (Pts 2, 4, 5, 6, 9) recipients, respectively.
Table S1: HLA class I typing and anti-HLA antibodies used to distinguish donor from recipient cells in intestinal transplant recipients
HLA-A9 (*) and HLA-B12 (**) are broad antigen HLA serotypes that encompass the HLA-A23 / HLA-A24 serotypes, and the HLA-B44 / HLA-B45 serotypes, respectively.
Abbreviations: ITx, intestinal transplant recipient; LTx, liver transplant recipient
Table S2: Minimum number of donor cells that should be detected C(b), depending upon the number of interrogated events (b), to ensure the presence of a true macrochimerism (frequency of donor cells 1%) in a given lineage.