Abstract
Objectives
This study was performed to determine whether a 4-tiered classification of left ventricular hypertrophy (LVH) defines subgroups in the general population which are at variable risk of adverse cardiovascular outcomes.
Background
We recently proposed a 4-tiered classification of LVH where eccentric LVH is subdivided into “indeterminate hypertrophy” and “dilated hypertrophy” and concentric LVH into “thick hypertrophy” and “both thick and dilated hypertrophy,” based on the presence of increased left ventricular end-diastolic volume.
Methods
Participants from the Dallas Heart study who underwent cardiac magnetic resonance imaging and did not have LV dysfunction or history of heart failure (HF) (n = 2,458) were followed for a median of 9 years for the primary outcome of HF or cardiovascular (CV) death. Multivariable Cox proportional hazard models were used to adjust for age, sex, African-American race, hypertension, diabetes, and history of cardiovascular disease (CVD).
Results
In the cohort, 70% had no LVH, 404 (16%) had indeterminate hypertrophy, 30 (1%) had dilated hypertrophy, 289 (12%) had thick hypertrophy, and 7 (0.2%) had both thick and dilated hypertrophy. The cumulative incidence of HF or CV death was 2% with no LVH, 1.7% with indeterminate, 16.7% with dilated, 11.1% with thick, and 42.9% with both thick and dilated hypertrophy (log rank p< 0.0001). Compared with participants without LVH, those with dilated (HR 7.3, 95% CI 2.8–18.8), thick (HR 2.4, 95% CI 1.4–4.0), and both thick and dilated (HR 5.8, 95% CI 1.7–19.5) hypertrophy remained at increased risk for HF or CV death after multivariable adjustment, whereas the group with indeterminate hypertrophy was not (HR 0.9, 95% CI 0.4–2.2).
Conclusion
In the general population, the 4-tiered classification system for LVH stratified LVH into subgroups with differential risk of adverse CV outcomes.
Unstructured Abstract:
Participants from the Dallas Heart Study were stratified using a 4-tiered classification of left ventricular hypertrophy (LVH) where eccentric LVH is subdivided into “indeterminate hypertrophy” and “dilated hypertrophy” and concentric LVH into “thick hypertrophy” and “both thick and dilated hypertrophy.” Compared with participants without LVH, those with dilated, thick, and both thick and dilated hypertrophy were at increased risk for heart failure or CV death after multivariable adjustment, whereas the group with indeterminate hypertrophy was not. In the general population, the 4-tiered classification system for LVH stratified LVH into subgroups with differential risk of adverse CV outcomes.
Keywords: left ventricular geometry, hypertrophy, troponin, heart failure, cardiac MRI
Introduction
Left ventricular (LV) hypertrophy (LVH), as defined by increased LV mass, is associated with significant cardiovascular (CV) morbidity and mortality.(1–4) LVH assessed by echocardiography is often categorized into 2 patterns based on the relative wall thickness (RWT), a ratio derived from left ventricular (LV) wall thickness and LV chamber dimension. LVH with increased RWT is classified as concentric, and when the RWT is not increased, LVH is classified as eccentric.(5) Though widely used, this classification system has important limitations, including relying on a ratio of linear dimensions for wall thickness and chamber size, and not accounting for LV dilation in isolation, an important aspect of geometric remodeling.
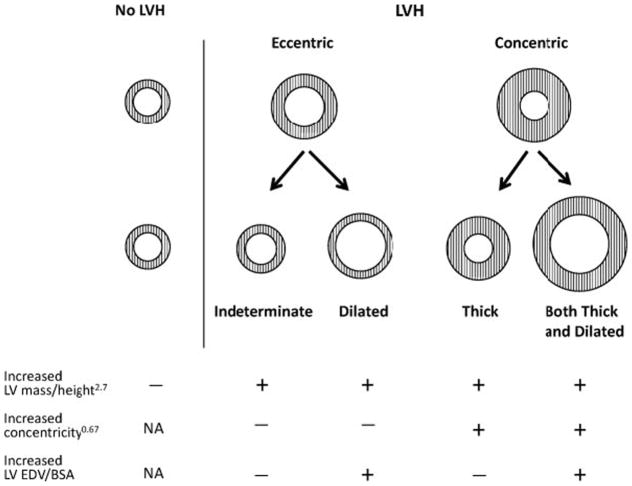
We have previously proposed a 4-tiered classification of LVH based on LV end-diastolic volume (EDV) and concentricity index (a marker of wall thickness) as assessed by cardiac magnetic resonance imaging.(6) In this classification, eccentric hypertrophy was further divided into dilated hypertrophy and indeterminate hypertrophy based on whether the LV volume was increased. Similarly, concentric hypertrophy was divided into thick hypertrophy and both thick and dilated hypertrophy. (Figure 1)(6)
Figure 1. Schematic of standard 2-tier and 4-tier classification for LVH.
Both of the standard 2-tiered subgroups are sub-classified based on the presence of increased indexed LV volume. Cited with permission from Khouri et al.(6)
In our initial cross-sectional description of this classification system, we demonstrated clear phenotypic differences between the 2 newly proposed subcategories of both eccentric and concentric hypertrophy. Those with dilated hypertrophy were more likely to have elevated levels of natriuretic peptides and lower LV ejection fraction (EF) then those with indeterminate hypertrophy. Similarly those with both thick and dilated hypertrophy had a higher prevalence of reduced LV EF than those with isolated thick hypertrophy. Subsequent studies have related the 4-tiered classification system to clinical outcomes in patients with hypertension and coronary artery disease.(7,8) However these studies used echocardiography, which has known limitations in assessing LV mass and LV volume when compared to cardiac magnetic resonance imaging (MRI).(5,9) Moreover, this classification system has not yet been related to clinical outcomes in an unselected general population sample.
We therefore classified participants from the Dallas Heart Study with the 4-tiered classification system for LVH, utilizing cardiac MRI and determined associations of the 4-tiered subgroups with incident heart failure (HF) and cardiovascular (CV) death. In addition, we compared levels of high sensitive cardiac troponin T (hs-cTnT), a marker of cardiac injury shown to be associated with HF and death (10–12), across the subgroups.
Methods
Study Population
The Dallas Heart Study (DHS) is a multi-ethnic, population-based, cohort study of Dallas County adults in which deliberate over-sampling of African-Americans was performed. The design and detailed methods of the DHS have been previously described.(13) In brief, the study was conducted in 3 visits. Visit 1 was an initial in-home visit (n=6,101) in which demographics, medical history and blood pressure were obtained. This was followed by collection of fasting blood and urine samples at a second in-home visit (n=3,557). Visit 3 was conducted on the campus of University of Texas Southwestern (UTSW) Medical Center during which detailed imaging studies including cardiac MRI were performed (n=2,803). Participants were subsequently followed for pre-defined clinical events and death. For this study we excluded participants with a LV EF <40% and those with a clinical history of HF at baseline, resulting in a final cohort of 2,458 participants. Written informed consent was provided by all participants, and the UTSW Institutional Review Board approved the study.
Cardiac MRI
Cardiac MRI was performed using 2 comparable 1.5-Tesla systems (Phillips Medical Systems, Best, The Netherlands). As previously described, mass and volume measurements were calculated from short-axis breath-hold ECG gated cine MRI and MASS software (Medis Medical Imaging Systems, Leiden, The Netherlands) was used to analyze data.(14) The papillary muscles were included in the mass of the left ventricle. The mean wall thickness of the left ventricle was determined by averaging the wall thickness of each slice, excluding the apical slice. Further details of the cardiac MRI protocol have been previously published including intraobserver, interobserver, and interscan variability.(14,15)
Definitions
For the primary analysis, LVH was classified as increased LV mass when indexed to height2.7 using thresholds of ≥48 g/m2.7 for men and ≥39 g/m2.7 for women.(14) A sensitivity analysis was performed indexing LV mass to body surface area (BSA) using thresholds of ≥112 g/m2for men and ≥89 g/m2 for women. LV concentricity0.67 was defined as LV mass/LV end diastolic volume (EDV)0.67 as previously described.(6) Previously defined thresholds for elevated LV EDV indexed to BSA (≥74 mL/m2 for men and ≥68 mL/m2 for women) and LV concentricity0.67 (≥9.1 g/mL0.67 for men and ≥8.9 g/mL0.67 for women) were used.(6) The 4 categories of LVH were: 1. Indeterminate hypertrophy (neither increased concentricity0.67 nor increased LVEDV/BSA) 2. Dilated hypertrophy (increased LVEDV/BSA without increased concentricity0.67) 3. Thick hypertrophy (increased concentricity0.67 without increased LVEDV/BSA) and 4. Both thick and dilated hypertrophy (increased concentricity0.67 and LVEDV/BSA). In order to contrast the 4-tiered classification with the prior 2 tiered classification, we defined LVH in the 2-tiered classification as concentric when concentricity0.67 was increased, and eccentric when concentricity0.67 was not increased.(6)
Hs-cTnT (Elecys-2010 Troponin T hs STAT, Roche Diagnostics) was measured from baseline samples as previously described.(10) Elevated hs-cTnT was defined as equal to or greater than the limit of blank of the assay (>3ng/L).
Outcomes
The primary outcome was the composite of incident HF or CV death. Incident HF was defined as first hospitalization for HF with reduced EF or preserved EF. A blinded endpoint committee adjudicated nonfatal CV events (including HF). The secondary endpoint was CV death alone and incident HF alone. Death events were determined through December 31, 2010 from the National Death Index (NDI) and classified as cardiovascular based on the International Classification of Diseases 10 codes I00 – I99.(16)
Statistical Analysis
Statistical comparisons of variables among the four groups, indeterminate, dilated, thick, and both thick and dilated, were done using the Chi-Square test for dichotomous variables and Wilcoxon-rank sum test for continuous variables. No adjustments were made for multiple comparisons. The incidence of the primary outcome among each group was estimated using Kaplan-Meier analysis. Multivariable Cox proportional hazard models were used to adjust for age, sex, African-American race, hypertension, diabetes, and history of cardiovascular disease (CVD). Due to the limited number of events, secondary endpoints of CV death alone and incident HF alone were not adjusted. Four sensitivity analyses were performed including repeating the primary analyses using LVH defined by LV mass indexed to BSA; using the continuous parameter of systolic blood pressure (SBP) instead of hypertension as a covariate; including body mass index (BMI) as a covariate; and repeating the primary analysis after further excluding all patients with history of CVD. All statistical analyses were performed with SAS version 9.1 (SAS Institute, Inc, Cary, NC) statistical software and all p-values are two-sided with an alpha of 0.05.
Results
The baseline characteristics of the study cohort are shown in Table 1, stratified by the 4-tiered classification system for LVH. Among the 2,458 participants meeting study criteria (mean age 44, 56% women, 48% African-American), 730 (30%) had LVH, of whom, 404 were classified as indeterminate, 30 as dilated hypertrophy, 289 as isolated thick hypertrophy, and 7 as having both thick and dilated hypertrophy. In the study group, 773 (31%) of the participants had hypertension and 245 (10%) had diabetes, with a higher proportion of hypertension and diabetes seen in the participants with isolated thick and both thick and dilated LVH.
Table 1.
Baseline characteristics of the study population.
| LVH | |||||
|---|---|---|---|---|---|
| Eccentric | Concentric | ||||
| Variable | No LVH N=1728 |
Indeterminate N=404 |
Dilated N=30 |
Thick N = 289 |
Both N=7 |
| Age (yrs) | 43 [36, 52] | 43 [36, 51] | 42.5 [35, 46] | 49 [42, 56]* | 53 [46, 58] |
| Men (%) | 51.8 | 13.6* | 43.3 | 40.8* | 85.7 |
| Black (%) | 40.8 | 54.9* | 73.3* | 78.9* | 71.4 |
| BMI | 27.5 [24.1, 31] | 35.1 [31, 40.3]* | 27.0 [24.3, 34.6] | 34.4 [29.8, 39.7]* | 32.7 [28.7, 44.6]† |
| Hypertension (%) | 23.4 | 37.1 | 36.7 | 73.8* | 85.7* |
| SBP | 122 [112, 131] | 125 [115, 138]* | 131 [122, 141]† | 141 [128, 155]* | 145 [128, 159]† |
| DBP | 76 [71, 82] | 79 [73, 85]* | 76 [74, 83] | 85 [78, 92]* | 86 [76, 89] |
| Diabetes (%) | 7.3 | 10.9 | 6.7 | 23.9* | 57.1* |
| CVD (%) | 2.7 | 2.7 | 3.3 | 10.4* | 14.3 |
| eGFR (ml/min per 1.73 m2) | 95.8 [84.0, 109.3] | 102.9 [90.8, 116.3]* | 111.4 [94.9, 135.7]* | 95.4 [81.6, 110.2] | 97.3 [81.1, 111.4] |
| LV EF | 73 [68, 77] | 75 [71, 80]* | 72 [59, 76] | 72 [67, 78] | 62 [45, 76] |
| LV Mass/Height2.7 | 36.0 [32.1, 39.5] | 44 [41.2, 48.8]* | 52.9 [48.1, 59.9]* | 51.9 [46.7, 57.4]* | 65.8 [63.7, 69]* |
| LV Mass/BSA | 76.9 [66.9, 88.9] | 78.0 [73.4, 85.6]* | 108.7 [92.5, 126.4]* | 99.8 [85.7, 115.9]* | 143.5 [124.7, 145.7]* |
| LV EDV/BSA | 50.7 [44.7, 57.3] | 53.0 [47.5, 57.8]* | 81.1 [70.6, 91.7]* | 47.8 [41.0, 55.9]* | 79.6 [74.4, 87.5]* |
| Concentricity0.67 | 7.0 [6.2, 8] | 7.2 [6.7, 7.7] | 7.4 [6.4, 7.8] | 9.5 [9, 10.6]* | 9.7 [9.2, 9.8]* |
| LV Wall Thickness | 11.0 [10, 12.2] | 11.3 [10.8, 12.1]* | 12.1 [10.7, 12.8]† | 14.0 [13.1, 15.1]* | 15.4 [14.5, 16.1]* |
Values are reported as median [25%, 75% percentile] or proportion (%) where indicated.
p < 0.001 versus no LVH group.
p < 0.01 versus no LVH group.
LVH = left ventricular hypertrophy. BMI = body mass index. SBP = systolic blood pressure. DBP = diastolic blood pressure. CVD = cardiovascular disease. eGFR = estimated glomerular filtration rate. LV = left ventricle. EF = ejection fraction. EDV = end diastolic volume. BSA = body surface area.
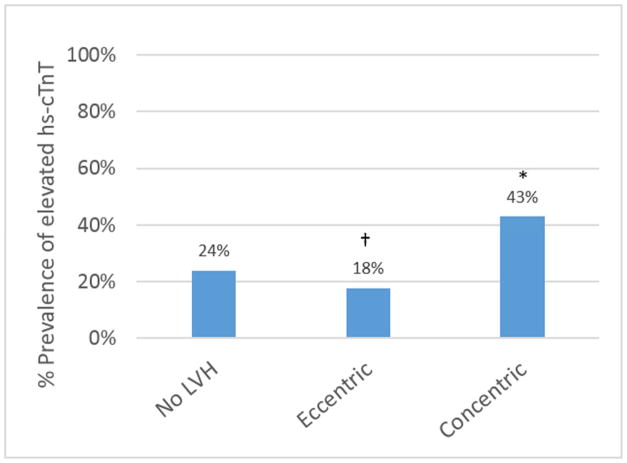
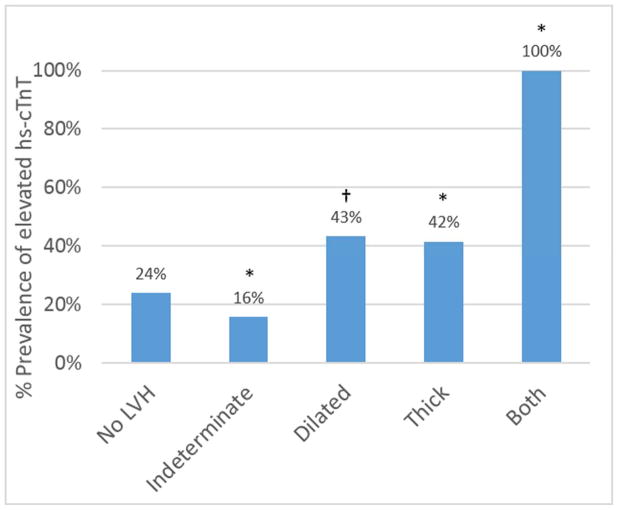
When using the standard 2-tiered LVH classification, the prevalence of detectable hs-cTnT in participants with eccentric LVH was lower compared with those without LVH (18% versus 24%, p=0.005) (Figure 2A). In contrast, when eccentric hypertrophy was subdivided in the 4-tiered classification (Figure 2B), the dilated subgroup had a higher prevalence of detectable cTnT (43%) as compared to those without LVH (24%, p=0.02) or those with indeterminate hypertrophy (16%, p=0.0004). Similarly, subjects with both thick and dilated hypertrophy were more likely to have elevated hs-cTnT than those with isolated thick hypertrophy (100% versus 42%, p=0.002).
Figure 2. Prevalence of elevated hs-cTnT in the 2-tier (A) and 4-tier (B) classification for LVH.
*p<0.001 versus no LVH group.
†p<0.02 versus no LVH group. LVH = left ventricular hypertrophy
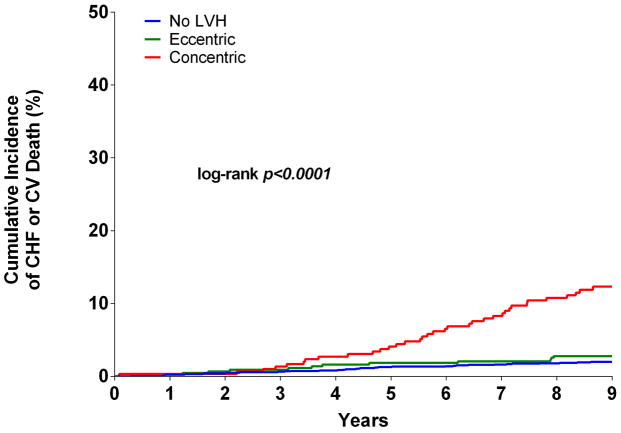
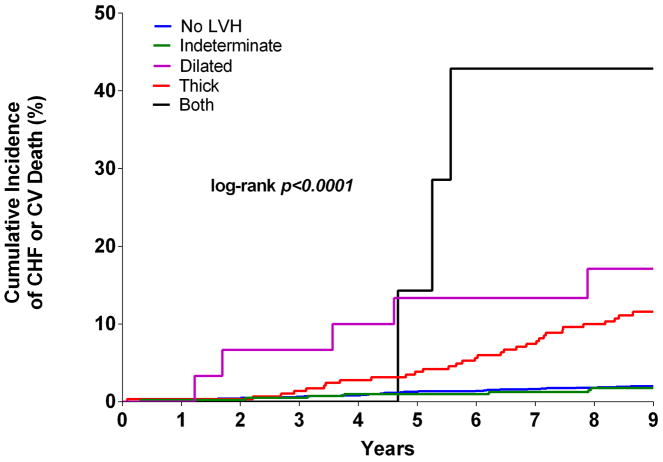
Over a median follow up period of 9.1 (interquartile range 8.6 to 9.6) years, the primary composite outcome of incident HF or CV death occurred in 81 (3.3%, [95% confidence intervals 2.6 to 4.1]) participants, including 35 HF events and 47 CV deaths. Based on the 2-tiered classification system, the cumulative incidence of HF or CV death in participants with concentric LVH was 11.8% [95% CI 8.1 to 15.6] compared with 2.8% [95% CI 1.2 to 4.3] in the eccentric LVH group and 2.0% [95% CI 1.3 to 2.7] in the group with no LVH (log rank p< 0.0001). No significant difference in the primary endpoint was seen between the eccentric LVH and no LVH groups (p=0.31) (Figure 3A). In the 4-tiered classification system, the cumulative incidence of HF or CV death was 2.0% [95% CI 1.3 to 2.7] with no hypertrophy, 1.7% [95% CI 0.47 to 3] with indeterminate hypertrophy, 16.7% [95% CI 3.0 to 30.4] with dilated hypertrophy, 11.1% [95% CI 7.4 to 14.8] with isolated thick hypertrophy, and 42.9% [95% CI 6.2 to 79.5] in those with both thick and dilated hypertrophy (log rank p< 0.0001) (Figure 3B). There was no significant difference in the risk of HF or CV death between those with indeterminate hypertrophy and those without LVH (p=0.74).
Figure 3. Kaplan-Meier curves for Incident HF or CV Death.
Unadjusted Kaplan-Meier curves for cardiovascular (CV) death or incident heart failure (HF) stratified by 2-tier (A) and 4-tier (B) classification for LVH. CHF = congestive heart failure. CV = cardiovascular death. LVH = left ventricular hypetrophy.
In multivariable analyses, using the 2-tiered classification, those with concentric LVH but not eccentric were at increased risk of HF or CV death (Table 2). When applying the 4-tiered classification of LVH, eccentric LVH was stratified into lower risk (indeterminate hypertrophy) and higher risk (dilated hypertrophy) subgroups. Specifically, as compared to those without LVH, participants with indeterminate hypertrophy were not at increased risk, while those with dilated hypertrophy were at significantly increased risk of HF or CV death (Table 2). Similarly, compared with participants with no LVH, isolated thick hypertrophy and both thick and dilated hypertrophy remained associated with increased HF and CV death (Table 2). Dilated hypertrophy was associated with increased risk compared with isolated thick hypertrophy (HR 3.1 [95% CI 1.2 to 8.0]). Dilated, thick, and both thick and dilated hypertrophy remained independently associated with adverse CV outcomes when hs-cTnT was added to the multivariable adjustment (Table 2). Finally, the continuous parameters of LV EDV (HR 1.5 [95% CI 1.2 to 1.9]), LV wall thickness (HR 1.4 [95% CI 1.2 to 1.7]), and hs-cTnT (HR 1.2 [95% CI 1.02 to 1.5]) were independently associated with increased risk of HF or CV death in multivariable analysis adjusted for age, sex, African-American race, hypertension, diabetes, and history of CVD.
Table 2.
Unadjusted and multivariable adjusted associations of 2-tiered and 4-tiered classification of LVH with incident HF or CV death
| LVH Classification | N | E | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR† (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| No LVH* | 1728 | 34 | 1.0 | 1.0 | 1.0 |
| Eccentric LVH | 434 | 12 | 1.4 (0.7, 2.7) | 1.5 (0.8, 3.1) | 1.4 (0.7, 2.9) |
| Concentric LVH | 296 | 35 | 6.3 (3.9, 10.1) | 2.5 (1.5, 4.2) | 2.2 (1.3, 3.8) |
|
| |||||
| No LVH* | 1728 | 34 | 1.0 | 1.0 | 1.0 |
| Indeterminate | 404 | 7 | 0.9 (0.4, 2.0) | 0.9 (0.4, 2.2) | 0.9 (0.4, 2.1) |
| Dilated | 30 | 5 | 9.5 (3.7, 24.2) | 7.3 (2.8, 18.8) | 5.5 (2.0, 14.9) |
| Thick | 289 | 32 | 5.9 (3.6, 9.5) | 2.4 (1.4, 4.0) | 2.2 (1.3, 3.7) |
| Both | 7 | 3 | 26.8 (8.2, 87.3) | 5.8 (1.7, 19.5) | 4.6 (1.4, 15.7) |
LVH is indexed to height2.7.
Referent group. Adjusted hazard ratio includes adjustments for age, sex, race, diabetes, hypertension, and CVD.
Adjusted hazard ratio also includes added adjustment for hs-cTnT.
N = number of participants. E = number of events. HR = hazard ratio. CI = confidence interval. NA = Not applicable given there were no events in this subgroup, which precluded calculation of a hazard ratio.
Secondary endpoints of incident HF alone and CV death alone are shown in Table 3. In unadjusted analysis, indeterminate hypertrophy was not associated with increased risk of either HF alone or CV death alone, when compared with no LVH. Thick, dilated, and both thick and dilated hypertrophy were associated with increased risk of incident HF compared with no LVH. Thick hypertrophy and both thick and dilated hypertrophy were associated with increased risk of CV death alone.
Table 3.
Unadjusted associations of 2-tiered and 4-tiered classification of LVH with incident HF (A) and CV death (B)
| A.
| |||
|---|---|---|---|
| Unadjusted Incident HF
| |||
| LVH Classification | N | E | HR (95% CI) |
|
| |||
| No LVH* | 1728 | 7 | 1.0 |
| Eccentric LVH | 434 | 7 | 4.0 (1.4, 11.3) |
| Concentric LVH | 296 | 21 | 18.6 (7.9, 43.7) |
|
| |||
| No LVH* | 1728 | 7 | 1.0 |
| Indeterminate | 404 | 3 | 1.8 (0.5, 7.0) |
| Dilated | 30 | 4 | 37.2 (10.9, 127.0) |
| Thick | 289 | 18 | 16.2 (6.8, 38.9) |
| Both | 7 | 3 | 135.4 (34.9, 524) |
| B.
| |||
|---|---|---|---|
| Unadjusted CV Death
| |||
| LVH Classification | N | E | HR (95% CI) |
|
| |||
| No LVH* | 1728 | 27 | 1.0 |
| Eccentric LVH | 434 | 5 | 0.7 (0.3, 1.9) |
| Concentric LVH | 296 | 15 | 3.3 (1.8, 6.2) |
|
| |||
| No LVH* | 1728 | 27 | 1.0 |
| Indeterminate | 404 | 4 | 0.6 (0.2, 1.8) |
| Dilated | 30 | 1 | 2.2 (0.3, 16.1) |
| Thick | 289 | 14 | 3.2 (1.7, 6.0) |
| Both | 7 | 1 | 9.6 (1.3, 70.3) |
LVH is indexed to height2.7.
Referent group.
N = number of participants. E = number of events. HR = hazard ratio. CI = confidence interval.
In a sensitivity analysis in which LVH was defined based on LV mass indexed to BSA (Supplemental Table 1), there were no events among the 35 subjects with indeterminate LVH while dilated LVH remained associated with the outcome in both unadjusted (HR 11.9 [95% CI 4.7 to 30.1]) and adjusted models (HR 8.3 [95% CI 3.2 to 21.0]). Similarly, thick and both thick and dilated hypertrophy remained associated with adverse CV outcomes (Supplemental Table 1). Additional sensitivity analyses excluding participants with a history of CVD, using SBP as a covariate in place of hypertension, and adjusting for BMI revealed similar associations (data not shown).
Discussion
The present study demonstrates that individuals from the general population with concentric or eccentric LVH, as defined by the standard 2-tiered classification system, can be sub-classified based on the presence or absence of LV dilation into 2 further subgroups with distinct longitudinal trajectories. Participants with eccentric hypertrophy can be sub-classified into a low risk group (indeterminate hypertrophy) and a high risk group (dilated hypertrophy). Similarly, concentric hypertrophy can be divided into two at risk groups, thick hypertrophy and both thick and dilated hypertrophy.
In our initial description of the 4-tiered classification system, we demonstrated that participants with indeterminate hypertrophy did not have a reduced LVEF, elevated natriuretic peptides, or higher frequency of detectable cTnT by conventional assay when compared with those without LVH.(6) The hs-cTnT assay is approximately 10-fold more sensitive than the conventional assay in the assessment of cardiac injury and has been strongly associated with abnormalities in cardiac structure and function and subsequent mortality.(10,11) In our present study we found that participants with indeterminate hypertrophy had a lower prevalence of detectable hs-cTnT and no increased risk of HF or CV death compared with participants without hypertrophy.
Prior studies by others looking at the 4-tiered classification system assessed by echocardiography have also shown that indeterminate hypertrophy was not associated with adverse outcomes in individuals with pre-existing hypertension or coronary artery disease.(7,8) Cardiac MRI has improved accuracy and interstudy reproducibility in the assessment of LV mass.(17,18) As such, our results provide further evidence suggesting that indeterminate hypertrophy is a benign phenotype, a finding that has important implications given the strong association of indeterminate hypertrophy with obesity, which is increasingly prevalent in the population.
Recently, we showed that low circulating concentrations of hs-cTnT identify a malignant phenotype of LVH.(19) Here we show that although dilated hypertrophy, thick hypertrophy, and both thick and dilated hypertrophy are each associated with detectable hs-cTnT, the association of these LV geometric subtypes with adverse CV outcome persists despite adjustment for hs-cTnT. This finding suggests that both LV geometry and chronic subclinical myocardial injury are important contributors to HF risk in the population.
The risk of heart failure or CV death was increased in participants with either dilated hypertrophy or both thick and dilated hypertrophy. In addition, the continuous parameter of LV EDV was independently associated with increased risk of adverse CV outcomes in multivariable analysis. These data, along with previous reports of LV dilation alone being associated with incident HF (20), confirm the value of refining the phenotypic characterization of subjects with increased LV mass based on the presence or absence of LV dilation. Further, the presence of LV dilation may identify a sub-population of patients with LVH that may benefit from aggressive prevention and treatment to improve CV outcomes.
Limitations
The number of HF and CV death events is relatively few due to the low-risk general population cohort that was studied. The associations of increased risk seen in the participants with both thick and dilated hypertrophy should be considered preliminary given the low number of participants and events in this subgroup. The NDI was used to ascertain CV death, which may be inaccurate.(21) Finally, our study was limited to assessment of the role of LV dilation among subgroups with LVH and does not consider the role of LV dilation in the absence of LVH.
Conclusions
Compared with the standard 2-tiered classification system for LVH, the 4-tiered classification system for LVH identifies sub-phenotypes of LVH in the general population that are at variable risk of HF and CV death. In particular, eccentric LVH can be stratified based on the absence or presence of ventricular dilation into a group at low risk (indeterminate hypertrophy) or high risk (dilated hypertrophy) for these outcomes.
Supplementary Material
Supplemental Table 1: Association of incident HF or CV death with LVH when indexed to BSA in unadjusted and multivariable adjusted analysis
Perspectives.
Competency in Medical Knowledge
A 4-tiered classification for LVH, which accounts for increased LV wall thickness and end-diastolic volume, can identify sub-groups of patients at differential risk of adverse CV outcomes.
Translational Outlook
Additional studies are needed to determine why individuals develop different patterns of LV remodeling (i.e., thick hypertrophy, dilated hypertrophy, or both thick and dilated hypertrophy). Further work is also needed to determine the pathophysiological links between subclinical myocardial injury, LV geometry, and subsequent adverse clinical events including the development of heart failure.
Abbreviations List
- HF
heart failure
- LVH
left ventricular hypertrophy
- CV
cardiovascular
- LV
left ventricle
- EDV
end diastolic volume
- MRI
magnetic resonance imaging
- BSA
body surface area
- hs-cTnT
high sensitive cardiac troponin T
Footnotes
Disclosures:
The Dallas Heart Study was funded by the Donald W. Reynolds Foundation and was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105. Dr. Drazner has received support from the James M. Wooten Chair in Cardiology, University of Texas Southwestern Medical Center. Drs. de Lemos, Berry and Drazner are funded by the American Heart Association Strategically Focused Research Grant (14SFRN20740000). Dr. de Lemos has received grant support from Roche Diagnostics and from Abbott Diagnostics. All other authors have reported that they have no disclosures relevant to the content of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 2.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Velagaleti RS, Gona P, Pencina MJ, et al. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117–22. doi: 10.1016/j.amjcard.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3:164–71. doi: 10.1161/CIRCIMAGING.109.883652. [DOI] [PubMed] [Google Scholar]
- 7.Bang CN, Gerdts E, Aurigemma GP, et al. Four-group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low-risk subset of eccentric hypertrophy in hypertensive patients. Circ Cardiovasc Imaging. 2014;7:422–9. doi: 10.1161/CIRCIMAGING.113.001275. [DOI] [PubMed] [Google Scholar]
- 8.Huang BT, Peng Y, Liu W, et al. Subclassification of left ventricular hypertrophy based on dilation stratifies coronary artery disease patients with distinct risk. Eur J Clin Invest. 2014;44:893–901. doi: 10.1111/eci.12320. [DOI] [PubMed] [Google Scholar]
- 9.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 14.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 15.Chung AK, Das SR, Leonard D, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113:1597–604. doi: 10.1161/CIRCULATIONAHA.105.574400. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–8. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 18.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 19.Neeland IJ, Drazner MH, Berry JD, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–95. doi: 10.1016/j.jacc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse Left Ventricular Remodeling in Community-Dwelling Older Adults Predicts Incident Heart Failure and Mortality. J Am Coll Cardiol HF. 2014 doi: 10.1016/j.jchf.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Association of incident HF or CV death with LVH when indexed to BSA in unadjusted and multivariable adjusted analysis