Abstract
BACKGROUND
Preclinical evidence suggests that sustained adrenergic activation can promote ovarian cancer growth and metastasis. We examined the impact of beta-adrenergic blockade on clinical outcome of women with epithelial ovarian, primary peritoneal or fallopian tube cancers (collectively, EOC).
METHODS
A multicenter review of 1,425 women with histopathologically confirmed EOC was performed. Comparisons were made between patients with documented beta blocker use during chemotherapy and those without beta blocker use.
RESULTS
The median age of patients in this study was 63 years (range, 21–93 years). The sample included 269 patients who received beta blockers. Of those, 193 (71.7%) were receiving beta-1 adrenergic receptor (ADRB1) selective agents, and the remaining patients were receiving non-selective beta antagonists. The primary indication for beta blocker use was hypertension but also included arrhythmia and post-myocardial infarction management. For patients receiving any beta blocker, the median overall survival (OS) was 47.8 months versus42 months (P = 0.04) for non-users. The median OS based on beta blocker receptor selectivity was 94.9 months for those receiving non-selective beta blockers versus 38 months for those receiving ADRB1 selective agents (P < 0.001). Hypertension was associated with decreased OS compared to no hypertension across all groups. However, even in patients with hypertension, users of a non-selective beta blocker had a longer median OS than non-users observed (38.2 vs 90 months, P < 0.001).
CONCLUSION
Use of non-selective beta blockers in epithelial ovarian cancer patients was associated with longer OS. These findings may have implications for new therapeutic approaches.
Keywords: ovarian cancer, beta-blockers, survival
INTRODUCTION
The role of the adrenergic system in epithelial ovarian cancer carcinogenesis makes it an attractive target for the treatment of ovarian cancer. Reverse transcriptase-PCR studies have demonstrated constitutive expression of adrenergic receptors in the cell lines studied1. Extensive preclinical data have firmly established that the activation of the receptors results in the growth and progression of ovarian cancer1–4. In one study, norepinephrine and isoproterenol (an adrenergic agonist) significantly enhanced the production of vascular endothelial growth factor, which plays a crucial role in angiogenesis1. Propranolol, a non-specific beta blocker (NSBB), blocked the production of vascular endothelial growth factor.
In another in vitro study, norepinephrine and epinephrine (beta adrenergic receptor agonists) were found to increase the invasive potential of ovarian cancer cells, but this effect was abrogated by propranolol. Norepinephrine also increased tumor cells’ expression of matrix metalloproteinases (MMP)-2 and -9, and pharmacological blockade of MMPs inhibited the effects of norepinephrine on tumor cells invasive potential2. In an orthotopic mouse model, daily restraint stress resulted in higher tissue catecholamine levels, greater tumor burden, and a more infiltrative pattern of ovarian cancer. These effects were mediated primarily through adrenergic receptor-β2 (ADRB2) activation of the protein kinase A signaling pathway. Tumors in these stressed animals showed increased vascularization and enhanced expression of vascular endothelial growth factor, MMP-2, and MMP-9, and these effects could be reversed by propranolol3.
This extensive preclinical evidence that adrenergic signaling promotes the growth of ovarian cancer, combined with similar clinical evidence for cancers in other organs such as breast, pancreas, and colon, suggests that there could be clinical benefit found in evaluating the use of beta blockers on survival in ovarian cancer patients4. There are a number of studies that have investigated the impact of beta blocker use. These studies have had conflicting conclusions, which may be due, in part, to small patient numbers. The lack of attention to beta blocker selectivity must also be considered as an explanation for varying results5–7. At an in vitro level, the positive effects of beta blockade on ovarian cancer rely on ADRB2 inhibition. However, ADRB1-selective beta blockers (SBBs) are more commonly prescribed than NSBBs, and populations with greater SBB use are unlikely to show a benefit from their beta blocker use8. To examine the impact of selective versus non-selective ADRB blockade on patient survival, we conducted a multi-institutional retrospective cohort study of women with newly diagnosed epithelial ovarian, fallopian tube or primary peritoneal cancer (collectively referred to as EOC).
MATERIALS AND METHODS
Study Design
A multi-institution retrospective chart review was conducted on all EOC patients diagnosed and treated with at least one cycle of platinum based doublet chemotherapy from 2000 to 2010 at four institutions (The University of Texas MD Anderson Cancer Center, Washington University School of Medicine, Mayo Clinic, and Mercy Medical Center). Institutional review board approval was obtained at all participating institutions. Patient charts, both electronic and paper, were reviewed for demographic information, the presence of hypertension or diabetes mellitus, tumor characteristics, cancer treatments, surgical outcome (optimal cytoreduction < 1 cm residual disease), usage of beta blockers, and survival data. Usage of beta blockers was defined as any documentation of beta blocker use in the medical record during neoadjuvant (NACT) or adjuvant chemotherapy (ACT). Overall survival (OS) was measured from date of diagnosis to date of death from any cause which was confirmed by patient chart or social security death index. The OS of patients with different prognostic factors was determined in addition to the OS effect of beta blocker usage. Progression free survival calculations were not conducted due.
Statistical Analysis
Patients were first evaluated using descriptive statistics to summarize the demographic and clinical characteristics of the two groups: those who used beta blockers and those who did not. Fisher’s exact tests were used to compare groups with respect to distribution of categorical data, and a two-sample t-test was used to compare groups with respect to the means for continuous data. If normality assumptions for the t-test were not met, the non-parametric Mann-Whitney test was used to compare groups. Using the Kaplan-Meier method, OS was estimated for groups by beta blocker use and type of beta blocker used (SBB vs NSBB)9. Log-rank tests were conducted to examine differences by beta blocker use and type10. P values < 0.05 were considered to be statistically significant; P values were not adjusted for multiple comparisons.
RESULTS
Demographics and Disease Characteristics
From the four participating institutions, 1,425 EOC patients were identified as eligible for inclusion in this study. Demographic, disease, and treatment characteristics are shown in Table 1. Beta blocker users were older, had higher BMIs, and were more likely to have hypertension compared to non-users. Over 90% of patients received upfront surgery followed by adjuvant chemotherapy (ACT). Patients receiving NACT were more likely to be on beta blockers than non-users (P = 0.005).
Table 1.
Demographic and Disease Characteristics
| Characteristic | Beta blocker non-users (N = 1156) | Beta blocker users (N = 269) | P value | Non-selective beta blocker users (N = 75) | P value |
|---|---|---|---|---|---|
| Age in years, median (range) | 61.6 (21.9–93.0) | 68 (39–87.6) | < 0.001 | 65 (42–84) | < 0.001 |
| Race | |||||
| White | 583 (86.4%) | 135 (89.4%) | 0.352 | 38 (86.4%) | 0.521 |
| Non-white | 92 (13.6%) | 16 (10.6%) | 6 (13.6) | ||
| Unknown | 481 | 118 | 31 | ||
| Stage | |||||
| I/II | 112 (9.7%) | 28 (10.4%) | 0.733 | 9 (12%) | 0.755 |
| III/IV | 1044 (90.3%) | 241 (89.6%) | 66 (88%) | ||
| Histology | |||||
| Serous | 932 (80.6%) | 223 (82.9%) | 0.437 | 63 (84%) | 0.723 |
| Non-serous | 224 (19.4%) | 46 (17.1%) | 12 (16%) | ||
| Body mass index (kg/m2) | |||||
| Mean (SD) | 27.8 (6.4) | 29.7 (7.0) | < 0.001 | 29.4 (6.9) | < 0.001 |
| Neoadjuvant therapy | 96 (8.3%) | 38 (14.1%) | 0.005 | 10 (13.3%) | 0.013 |
| Cytoreduction | |||||
| Optimal (<1 cm residual) | 789 (69.5) | 181 (69.9%) | 0.940 | 59 (79.7%) | 0.100 |
| Suboptimal | 347 (30.5%) | 78 (30.1%) | 15 (20.3%) | ||
| Missing | 20 | 10 | 1 | ||
| Comorbidities | |||||
| Hypertension | 350 (30.3%) | 250 (92.9%) | < 0.001 | 68 (90.7%) | < 0.001 |
| Diabetes | 63 (5.4%) | 22 (8.2%) | 0.114 | 8 (10.7%) | 0.118 |
Data are number of patients (%) unless otherwise specified.
Prognostic Factors
Age, stage, sequence of therapy, surgical outcome, histology, BMI, tumor grade, and race were evaluated for effect on OS for all patients. Older patients (>65 years) had a decreased OS rate (P < 0.001). Patients with stage III or IV disease at presentation had shorter median OS than those presenting with stage I or II disease (P < 0.001). Those receiving NACT had decreased survival when compared to those who had upfront surgery followed by chemotherapy (28.7 vs 45.6 months, P < 0.001). Optimal interval cytoreduction (<1 cm residual disease) was associated with an increased median OS for NACT patients when compared to NACT patients who had a suboptimal surgery (37.4 vs22.6 months, P = 0.002). Patients who received ACT with serous histology had a shorter median OS (44.5 months) compared to those with non-serous histology who received ACT (55.9 months, P = 0.035). However, histology made no difference for those who had NACT (30.5 vs 28.4 months, P = 0.51). BMI had no effect on OS except for NACT patients (P = 0.024). Race and tumor grade had no effect on OS.
The presence of comorbidities was also evaluated for effect on survival in the overall group. Hypertension was associated with decreased survival compared to those with normal blood pressure (40.1 vs 47.4 months, P < 0.001). Diabetes mellitus had no significant effect on OS (39.8 vs43.4 months, P = 0.503).
Overall Survival by Beta Blocker Use
The influence of beta blocker use on OS in all patients was examined alone and in relation to the presence of comorbidities, and the results are outlined in Table 2. Beta blocker use of any kind was associated with a longer median OS than non-use (47.8 vs42 months, P = 0.036). When further classifying patients based upon beta blocker selectivity (SBB vs NSBB), no difference in median OS was observed between SBB users and non-users (38 vs42 months, P = 0.196). However, patients receiving NSBB had a longer median OS than non-users (94.9 vs42 months, P < 0.001). Additional comparisons were made based on beta blocker use and sequence of chemotherapy (NACT vs ACT). Beta blockers users had an overall survival benefit compared to non-users, regardless of whether they underwent upfront cytoreductive surgery followed by ACT (49.9 vs 44.5 months, P = 0.042) or they received NACT (37.9 vs26.3 months, P = 0.048).
Table 2.
Analyses of Beta Blocker Use and Comorbidities for OS in Patients with EOC*
| Patient Population | Beta blocker usage | Median time to event, months | Log-rank P-values | No beta blockers v. SBB | No beta blockers v. NSBB | SBB v. NSBB | |
|---|---|---|---|---|---|---|---|
| No beta blocker v. beta blockers | No beta blockers, SBB, NSBB | ||||||
| All patients | No beta blockers (n = 1156) | 42 | 0.036 | < 0.001 | 0.196 | < 0.001 | < 0.001 |
| Any beta blocker (n = 268) | 47.8 | ||||||
| Selective beta blockers (n = 193) | 38.0 | ||||||
| Non-selective beta blockers (n = 75) | 94.9 | ||||||
| No diabetes | No beta-blockers (n = 1093) | 42.4 | 0.029 | < 0.001 | 0.328 | < 0.001 | < 0.001 |
| Any beta blockers (n = 246) | 48.5 | ||||||
| Selective beta blockers (n = 179) | 38.2 | ||||||
| Non-selective beta blockers (n = 67) | 94.9 | ||||||
| Diabetes | No beta blockers (n = 63) | 38.4 | 0.987 | 0.079 | 0.183 | 0.183 | 0.001 |
| Any beta blockers (n = 22) | 47.4 | ||||||
| Selective beta blockers (n = 14) | 31.2 | ||||||
| Non-selective beta blockers (n = 8) | 67.7 | ||||||
| No hypertension | No beta blockers (n = 806) | 47.9 | 0.715 | 0.002 | 0.003 | 0.057 | 0.001 |
| Any beta blockers (n = 18) | 42.8 | ||||||
| Selective beta blockers (n = 11) | 33.4 | ||||||
| Non-selective beta blockers (n = 7) | 112 | ||||||
| Hypertension | No beta blockers (n = 350) | 34.2 | < 0.001 | < 0.001 | 0.007 | < 0.001 | < 0.001 |
| Any beta blockers (n = 250) | 49.0 | ||||||
| Selective beta blockers (n = 182) | 38.2 | ||||||
| Non-selective beta blockers (n = 68) | 90.0 | ||||||
One beta blocker user was excluded from OS analysis owing to missing information.
Overall Survival by Beta Blocker Use and Comorbidities
Patients without diabetes had a significantly longer median OS if they received a NSBB compared to beta blocker non-users (94.9 vs42.4 months, P < 0.001) and a non-significant decrease in median OS if an SBB (38.2 months) was used (Table 2). Among patients with diabetes, NSBB users had a significant increase in median OS compared to SBB users (Table 2).
For beta blocker users the presence of hypertension had no significant effect on median OS compared to those normal blood pressure (49 vs42.8 months, P = 0.54). Among patients without hypertension, those who received a SBB had a shorter median OS (33.4 months) than beta blocker non-users (47.9 months, P = 0.003). Normotensive NSBB users’ numerically greater median OS (112 months, P = 0.057) was not statistically significant compared to non-users (Table 2), but when compared to SBB users with normal blood pressure significant improvement was observed (33.4 vs112 months; P = 0.001). The OS improvement for normotensive NSBB users over non-users represented the largest numerical difference in median OS (64.1 months).
For hypertensive patients, any beta blocker usage was associated with a longer median OS compared to non-users (49 vs 34.2 months, P < 0.001). Hypertensive patients receiving SBBs had a longer OS (38.2 months) than non-users (34.2 months, P = 0.007). NSBB users, however, were observed to have a longer median OS (90 months; P < 0.001) than either SBB or non-user patients with elevated blood pressure (Fig. 1). Hypertension had no statistically significant effect on OS in ACT patients using beta blockers (39.6 vs 50.4 months, P = 0.517). Among NACT patients who did not receive beta blockers, those with hypertension had a shorter median OS than normotensive patients (19.7 vs30.5 months, P < 0.001). This negative OS effect of hypertension was not seen in the NACT patients taking beta-blockers (37.9 vs42.8 months, P = 0.80).
Figure 1. Kaplan-Meier overall survival curves of patients with hypertension based on beta blocker use (non-users, selective beta blocker users, and non-selective beta blocker users).
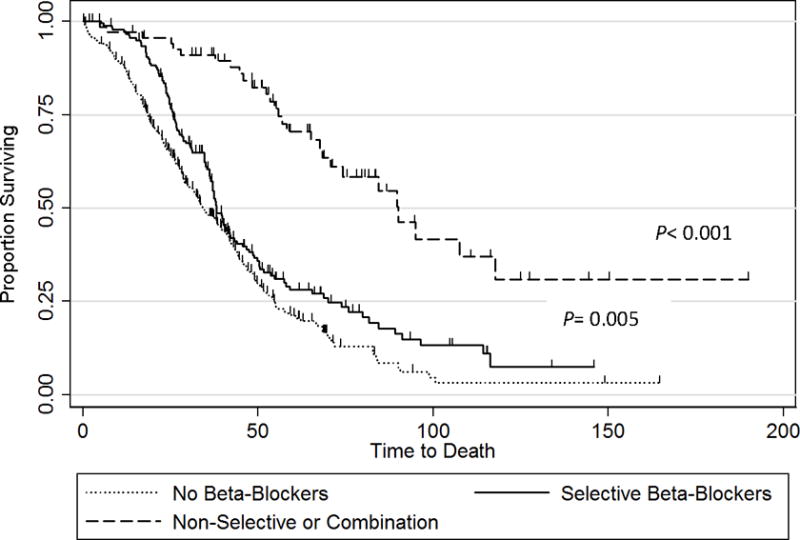
Median overall survival was 34.2 months for non-users, 38.2 months (P = 0.005) for selective beta blocker users, and 90 months (P < 0.001) for non-selective beta blocker users.
DISCUSSION
The prolonged OS of EOC patients receiving beta blockers, especially NSBB, is an important finding, and to our knowledge, our study is the first to demonstrate an OS benefit in relation to beta blocker selectivity in these patients. The ability to improve EOC patients’ survival via ADRB2 blockade using beta blockers would be the culmination of years of research into the biology and pathogenesis of EOC. Particularly interesting is the fact that beta blocker users in this study presented at a higher stage, had increased average BMI and were more likely to be hypertensive. All of these factors were associated with decreased survival, yet those who received beta blockers had either equivalent or improved OS. Further examination revealed that NSBB users had improved OS regardless of the presence of prognostic factors or comorbidities shown to decrease OS. This was not true for those who took SBBs, in some cases decreased OS was observed. While further study is needed these results highlight the importance of ADRB2 in ovarian carcinogenesis and the utility of NSBB.
Our study is limited by the retrospective design and the resulting inability to document the duration of beta blocker use and dosages used by EOC patients. While it would be ideal to have better documentation of beta blocker use in our study population, the fact that improvement was seen in patients that used beta blockers for any duration at any dose during their chemotherapy is promising. The validity of our findings is improved due to the study being multi-institutional with a large cohort of EOC patients. Most importantly, the stratification of patients by beta blocker usage and selectivity makes it unique amongst all other studies examining the impact of beta blocker use in ovarian cancer patients5–7,11.
In contrast to the current study’s findings, Eskander and colleagues found no difference in progression-free survival or OS for EOC patients who did or did not use beta blockers6. Similarly, when Johannesdottir and colleagues stratified the Danish Cancer Registry of over 6000 ovarian cancer patients by current (≤90 days), past (>90 days), and never use of beta blockers, the authors found no difference in all-cause mortality based on beta blocker use7. None of these studies reported on the selectivity of the beta blockers used. A multi-institutional European study that evaluated the impact of beta blocker usage in patients with platinum-sensitive recurrent EOC did report the selectivity of beta blockers used—approximately 10% of their population were on beta blockers, and of those, only 1.5% were on an NSBB—but did not stratify survival outcomes by beta blocker selectivity11. Without stratification for beta blocker selectivity, direct comparison of these conflicting results is difficult. In vitro studies have shown that it is specifically ADRB2 stimulation that contributes to ovarian cancer development and metastasis2–4,12. This is supported by the improvements in OS seen in patients taking NSBB compared to patients taking any beta blocker. More telling is the fact that in some cases, those taking SBB had worsened survival. It is unclear why those taking SBB did worse than those not taking beta blockers but it could be related to the increased age, higher median BMI and presence of comorbidities in that group. Whether or not SBB independently result in decreased OS will require further investigation. These results showcase the importance of ADB-β2 in EOC pathogenesis and potential for NSBB to improve outcomes in all EOC patients. Thus, it is necessary to stratify patients based on beta blocker selectivity in future studies so that we may best understand how to incorporate NSBB into treatment to improve individual patients’ outcomes.
In studies examining the effects of beta blockers on survival in patients with breast, lung, ovarian cancer or melanoma, patients were taking beta blockers for cardiac or other clinical indications and not for cancer therapy5–7,11,13,15. However, with the mounting evidence of the potential impact of beta blockers on cancer outcomes, a prospective clinical trial is warranted to identify patients who would benefit most from beta blocker use and to identify the best beta blocker for a specific tumor type based on adrenergic receptor expression. Tumor cell expression of ADRB could be used as a biomarker for selecting the patients who would benefit from a specific beta blocker. Beta blockers could then be used as an adjuvant therapy during surgical recovery and chemotherapy to decrease tumor angiogenesis, tumor growth, delays in wound healing, and metastasis14,15. Beta blockers may also reduce cancer-related psychological distress in newly diagnosed cancer patients16. Therefore, beta blockers have the potential to impact not only cancer biology and immunology but also the psychological well-being of cancer patients.
Because the biological effects and recommended dosing schedules of beta blockers for hypertension are well known, adding these drugs to ACT should be relatively easy. However, beta blockers are degraded by the enzyme cytochrome P450 2D6, and understanding the activity of this enzyme may play a key role in identifying doses that are likely to have maximal clinical benefit. Several genetic polymorphisms in this gene exist, and variations in drug sensitivity that result from these polymorphisms may determine the individual pharmacokinetics for each patient to allow for dose optimization15.
Currently, two clinical trials are evaluating the combination of chemotherapy and variable doses of propranolol on cancer biology as well as the NSBB’s effect on stress modulators in newly diagnosed EOC patients17,18. The preliminary data from these feasibility trials will help us to design adequately powered prospective randomized clinical trials to determine whether NSBBs can improve outcomes for patients with EOC.
Acknowledgments
FUNDING SOURCES: Portions of the work in this paper were supported by NIH grants (CA 140933, CA104825, CA109298, P50CA083639, U54CA151668, U54CA96300, and U54CA96297, CA016672), an Ovarian Cancer Research Fund program Project Development Grant, Department of Defense (OC073399, W81XWJ-10-0158, and OC100237), the Betty Ann Asche Murray Distinguished Professorship, the RGK Foundation, the Gilder Foundation, the Blanton-Davis Ovarian Cancer Research Program, and the Gynecologic Cancer Foundation-St. Louis Ovarian Cancer Awareness grant.
Footnotes
Conflict of Interest: Dr. Thaker has consulted for Incyte Pharmaceuticals and received research funding from Egen Pharmaceuticals.
Non-selective beta-blockers are associated with longer overall survival in a retrospective cohort study of ovarian cancer patients.
References
- 1.Lutgendorf S, Cole S, Costanzo ES, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;12:4514–4521. [PubMed] [Google Scholar]
- 2.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 4.Tang J, Li Z, Cho CH. β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol. 2013;23(6):533–542. doi: 10.1016/j.semcancer.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2012;127(2):375–378. doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 6.Eskander R, Bessonova L, Chiu C, et al. Beta blocker use and ovarian cancer survival: a retrospective cohort. Gynecol Oncol. 2012;127(1):S21. [Google Scholar]
- 7.Johannesdottir SA, Schmidt M, Phillips G, et al. Use of β-blockers and mortality following ovarian cancer diagnosis: a population-based cohort study. BMC Cancer. 2013;13:85. doi: 10.1186/1471-2407-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A review of the use of medicines in the United States in 2012. IMS Institute for Healthcare Informatics; Declining medicine use and costs: for better or worse? Available from URL: www.imshealth.com/deployedfiles/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/2012%20U.S.%20Medicines%20Report/2012_U.S.Medicines_Report.pdf. Accessed April 1, 2014. [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 10.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 11.Heitz F, duBois A, Harter P, et al. Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer- a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC-CTG and EORT-GCG. Gynecol Oncol. 2013;129(3):463–466. doi: 10.1016/j.ygyno.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Sood AK, Armaiz-Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120(5):1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagaraja AS, Sadaoui NC, Lutgendorf SK, Ramondetta LM, Sood AK. β-blockers: a new role in cancer chemotherapy? Expert Opin Investig Drugs. 2013;22(11):1359–1363. doi: 10.1517/13543784.2013.825250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Shahzad MM, Lin YG, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindgren ME, Fagundes CP, Alfano CM, et al. Beta-blockers may reduce intrusive thoughts in newly diagnosed cancer patients. Psychooncology. 2013;22(8):1889–1894. doi: 10.1002/pon.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therapeutic targeting of stress factors in ovarian cancer patients. Available from URL: http://clinicaltrials.gov/ct2/show/NCT01308944. Accessed April 1, 2014.
- 18.Beta-blocker/ovarian. Available from URL: http://clinicaltrials.gov/ct2/show/NCT01504126. Accessed April 1, 2014.


