Abstract
Hematopoietic stem cell transplantation is the treatment of choice for many hematologic malignancies and genetic diseases. However, viral infections continue to account for substantial post-transplant morbidity and mortality. While antiviral drugs are available against some viruses, they are associated with significant side effects and are frequently ineffective. This review focuses on the immunotherapeutic strategies that have been used to prevent and treat infections over the past 20 years and outlines different refinements that have been introduced with the goal of moving this therapy beyond specialized academic centers.
Keywords: adoptive immunotherapy, hematopoietic stem cell transplantation, T cells, viral infections
Background
Hematopoietic stem cell transplantation (HSCT) can be curative for a variety of malignant and nonmalignant hematologic conditions and congenital diseases [1–5]. However, serious viral infections remain a major cause of morbidity and are responsible for mortality in up to 39% [6–9]. An increasing array of viruses have been implicated in post-transplant infectious complications, due in part to more comprehensive patient screening using improved detection methods, but also to the extension of this therapeutic modality to higher-risk patients (i.e., individuals without a human leukocyte antigen [HLA]-matched sibling donor) who receive more extensively manipulated products and/or prolonged immunosuppression. Thus, reactivation of latent viruses including cytomegalovirus (CMV; 20–68%) [10,11], Epstein-Barr virus (EBV; 0.5–29%) [12], herpes simplex virus (HSV 1/2; 80%) [12], human herpes-virus 6 (HHV-6; 33–48%) [11,13], varicella zoster virus (10–68%) [12], human herpes-virus 7 (HHV-7; 10–57%) [11,14] and BK virus (BKV; 10–25%) [15,16] are frequent, while infections associated with an array of community-acquired respiratory viruses, including influenza (1.3–44%) [17], para-influenza (2–7%) [18,19], metapneumovirus (2.5–9%) [20], Adenovirus (AdV; 6–28%) [21] and respiratory syncytial virus (RSV; 0.3– 12) [18,19] are increasingly reported [22–25]. T-cell therapies that restore virus-specific immunity in the HSCT setting are a viable alternative to the traditional and often toxic antiviral drugs. Here, we explore the immunotherapeutic strategies currently used to provide immediate and long-term antiviral protection to adult- and pediatric-HSCT patients.
Donor lymphocyte infusion
The first adoptive T-cell transfer approach utilized in the allogeneic HSCT setting involved the adoptive transfer of unmanipulated donor lymphocytes – termed donor lymphocyte infusions (DLI). DLI therapy was based on the assumption that unmanipulated lymphocytes isolated from seropositive donors should contain populations of virus-specific T cells that were able to expand in vivo and provide antiviral protection. DLIs have proven effective in treating EBV-associated post-transplant lymphoproliferative disease (EBV-PTLD) [26], an AdV urinary tract infection [27], CMV reactivation [28], HHV-6 encephalopathy [29] and persistent RSV pneumonia [30]. However, the efficacy of this approach is limited by the low circulating frequency of T cells directed against many acute viruses, while the substantially higher frequency of alloreactive T cells within the infused product significantly increases the risk of causing graft versus host disease (GvHD). Thus, in order to preserve the benefits and minimize the risks associated with DLI infusions, techniques to selectively deplete alloreactive T cells or to induce anergy have been investigated.
Selective ex vivo allodepletion
Ex vivo allodepletion involves the selective removal of T cells with alloreactive potential prior to adoptive transfer. In order to identify this particular T-cell subset, donor T cells are first exposed to recipient-derived antigen-presenting cells (APCs) including peripheral blood mononuclear cells (PBMCs), activated T cells, EBV-transformed lymphoblastoid cell lines (EBV-LCL), dendritic cells (DCs) and/or fibroblasts [31–35]. Subsequently, cells that are alloactivated upregulate markers such as CD25, CD69, CD71, CD134, CD137 and HLA-DR, and proliferate, allowing their physical removal with magnetic beads, apoptosis-inducing chemotherapy, immunotoxins or photodynamic purging [33,35–42]. To date, only anti-CD25-conjugated immunotoxins and photodynamic purging have been used clinically.
Montagna and colleagues depleted alloreactive T cells using RFT5-SMPT-dgA – an anti-CD25 murine monoclonal antibody (RFT5 IgG1) coupled to the deglycosylated ricin A chain (dgA) via the cross-linker 4-succinimidyloxycarbonyl-α-methyl-α-(2-pyridyldithio-toluene) (SMPT). In preclinical studies, Montagna et al. demonstrated that the co-culture of patient PBMCs with irradiated haploidentical (Haplo) donor PBMCs followed by RFT5-SMPT-dgA exposure resulted in cell populations that retained virus-specific precursors but exhibited diminished cytolytic activity against patient bone marrow [31]. Based on these results, Andre-Schmutz and colleagues performed a Phase I/II clinical study in which 1–8 × 105 allodepleted T cells/ kg were infused to 15 pediatric Haplo or matched unrelated donor (MUD) HSCT recipients between days 15–47 post-transplant. Despite the absence of GvHD prophylaxis, no cases of grade III/IV GvHD were reported, comparing favorably with a rate of 40% grade ≥ II acute (a) GvHD in patients who were infused with just 1 × 105 unmanipulated DLI cells/kg [43,44]. Furthermore, the infused cells appeared to provide antiviral protection. In patients with active infections, immune reconstitution was accelerated (absolute T-cell numbers of >1.0 × 109/l within 4 weeks) and three patients with active CMV, a patient with persistent AdV and one with refractory EBV-PTLD were able to clear their infections post-infusion [33]. Allodepletion with RFT5-SMPT-dgA has also been used in the adult HSCT setting by Solomon and colleagues, who infused 16 recipients of matched-related donor (MRD) transplants (following reduced intensity conditioning) with a median of 1 × 108 allodepleted donor T cells/kg (range 0.2–1.5 × 108/ kg). aGvHD (grade I–IV) occurred in 46 ± 13%, but the rate of grade III/IV GvHD (12 ± 8%) was reduced when compared with a rate of 34% reported in previous studies [45]. Finally, our group has also investigated the safety and antiviral activity of allodepleted T cells and has demonstrated that a dose of 1 × 105 cells/kg was required to promote antiviral immune reconstitution in vivo [37].
An alternative method of allodepletion is photodynamic purging, which involves the exposure of alloactivated cells to a phototoxic dye [4, 5-dibromorhodamine 123 (TH9402)]. While the dye permeates both activated and nonactivated cells, it is selectively retained in the activated subset due to inactivation of the multidrug-resistance pump p-glycoprotein (MDR1). This confers cells with sensitivity to visible light (514 nm), which induces mitochondrial oxidation and cell death [46]. To assess the potency of this approach clinically, Mielke and colleagues infused 24 HLA-identical sibling HSCT recipients (17–74 years) with 5 × 106 photodepleted-donor T cells/kg on the day of transplantation. Engraftment was rapid for all patients, but unfortunately the incidence of both acute and chronic (c) GvHD was high (38 ± 10% probability of developing aGvHD [grade II-IV] and 65 ± 11% cGvHD). Furthermore, complications associated with viral (20/24 patients reactivated CMV, two patients developed BK-associated hemorrhagic cystitis, AdV [n = 2] and BK + AdV [n = 1] and a patient died of RSV pneumonitis), bacterial and invasive fungal infections were both unexpectedly frequent and severe resulting in early termination of the trial [47]. Further investigation indicated that the high GvHD rates were likely due to the poor alloactivation achieved in the matched-sibling setting, while the photodepletion process preferentially depleted CD4+ and CD8+ memory T cells, including populations responsible for providing protection from infection [48]. Thus in ongoing studies, photodepletion is being utilized only in the Haplo setting and preliminary results are encouraging with only 2 of 12 patients developing aGVHD (grade I) [49,50].
Overall, these studies demonstrate that adoptive transfer of allodepleted T cells is a feasible means of hastening immune reconstitution and preventing/ treating viral infections. However, the efficiency of allodepletion varies, impacting in vivo safety, antiviral control and the incidence of GvHD.
Induction of anergy
An alternate strategy to neutralize alloantigen-specific T cells is to render them anergic. This approach relies on the requirement of T cells for both an HLA-restricted, antigen-specific signal and a second costimulatory signal to become activated and proliferate. Thus anergy can be induced by blocking the interaction between CD28 (on T cells) and B7–1 (CD80) and B7–2 (CD86) on APCs. The first clinical Phase I studies to exploit this method were performed by Davies et al. in 24 pediatric and adult patients with high-risk hematologic malignancies or bone marrow failure who received a Haplo HSCT [51]. Patients received bone marrow grafts containing a median of 29 × 106 CD3+ T cells/kg (range 7–129 × 106/kg) following ex vivo treatment with CTLA4-Ig (n = 19) or anti-B7–1 and B7–2 antibodies (n = 5). Only 5 of 21 evaluable patients developed grade III (n = 4) or IV (n = 1) aGvHD and a patient developed cGvHD, which was substantially lower than that of historical controls [52]. In addition, the majority of infused patients had CD4+ and CD8+ counts >200/µl by 4 months. These reconstituting cells included virus-specific precursors, as shown by the detection of CMV and EBV pentamer-positive populations. Indeed, of five patients who reactivated CMV, four cleared the virus within 3 days of receiving antiviral medication [51,53].
Suicide genes
Administering donor T cells that have been transgenically modified to express a ‘suicide gene’ that can be triggered in vivo in the event of GvHD is an alternative to ex vivo allodepletion. Although a variety of suicide systems have been tested preclinically, only HSV thymidine kinase (HSV-tk) and inducible caspase 9 (iC9) have been utilized in the clinic.
HSV-tk modified T cells are able to phosphorylate the nontoxic prodrug ganciclovir into ganciclovir monophosphate, which is converted to a triphosphate form by cellular kinases and incorporated into DNA resulting in chain termination and cell death. The HSV-tk/ganciclovir system also causes cell death by inducing expression of the CD95 receptor, which self-aggregates, leading to the formation of a death-inducing signaling complex [54].
In a prospective nonrandomized multicenter Phase I/II trial, 50 high-risk leukemia patients aged 17–66 years received a Haplo HSCT, followed, in 28 subjects, by 1–4 monthly infusions of HSV-tk cells (range 0.9–40 × 106/kg) from day 28 post-transplant. The HSV-tk cell infusions were well tolerated and administration of cell doses of ≥0.9 × 106/kg supported post-transplant immune reconstitution (defined as circulating CD3+ T-cell numbers of greater than or equal to 100 cells/µL detected on two consecutive occasions), which occurred in a median of 23 days post-T-cell administration. Indeed, CMV- and EBV-specific immunity in infused patients could be detected within 3 months post-transplant. Interestingly, after a first wave of circulating tk+ cells, the majority of T cells supporting long-term immune reconstitution did not carry the suicide gene and displayed a naive phenotype suggesting that the HSV-tk infusions were able to drive the recovery of thymic activity in adults. Of the 11 individuals who developed aGvHD (grade I– IV) and cGvHD and received ganciclovir, all exhibited a significant reduction (p = 0·005) in the circulating frequency of tk-directed T cells with resolution of GvHD, showing the transgene was functional. Notably, in these patients, the frequency of CD3+ HSV-tk negative lymphocytes was unaffected (p = 0.14) and virus-specific T-cell activity was not diminished. These data suggest that in patients with GvHD but without a viral infection, activation of the suicide switch results in the selective depletion of alloreactive cells but leaves the virus-reactive population untouched [55,56].
Despite these results, there are some concerns with the HSV-tk platform. Cell killing is restricted to dividing cells and induction of cell death may require several days, both factors that limit the clinical benefit of this approach. Furthermore, dependence on ganciclovir as a prodrug precludes its use as treatment of post-HSCT viral infections. Finally, the virus-derived HSV-tk gene can be immunogenic, leading to the unintentional elimination of engineered T cells especially in relatively immunocompetent recipients. Indeed, in the aforementioned trial, all seven of the patients infused late (range 24–278 weeks) after HSCT developed a tk-directed CD8+ T-cell response [57].
Our group has utilized the iC9 suicide gene system. In this platform, iC9 is activated by administration of a chemical inducer of dimerization (CID) – a bio-inert small molecule - which leads to rapid cell death (92.9 ± 3.8% cell death, 24 h post-CID) [58]. Ten Haplo HSCT recipients (5–18 years) were infused with iC9 T cells (range 0.1–1 × 107 cells/kg) 30–90 days post-transplant. Four patients developed grade I/ II GvHD, but following a single infusion of CID, the circulating frequency of transgenic cells decreased by >90% within 30 min of drug administration. GvHD rapidly resolved without recurrence, despite subsequent re-expansion of residual transgenic T cells [59]. Remarkably, the residual transgenic cells were capable of providing antiviral benefit as demonstrated in three patients whose endogenous virus-specific T cells controlled CMV reactivation (n = 2) and an AdV infection (n = 1). Furthermore, like HSV-tk infusions, iC9 T cells appeared to promote endogenous immune reconstitution as evidenced by the detection of naive CD4+ T cells of thymic origin with no evidence of an immune response against the transgenic cells [60].
Regulatory T-cell (Treg) infusion
Infusion of Tregs has also been proposed as an immunotherapeutic approach to suppress alloactivity in HSCT patients. This work was founded on the observation that patients who received a graft from donors with low absolute Treg numbers (median 8 cells/µl, range 3–19) were at greater risk of developing GvHD than those receiving grafts from donors with a higher number of Tregs (median 13 cells/µl, range 8–39). Subsequently, Rezvani and colleagues postulated that the selective expansion and infusion of donor Tregs at the time of transplant could reduce the risk of GvHD, without affecting virus-specific T-cell activity [61].
Brunstein et al. established a method for positively selecting Tregs from cryopreserved third party partially HLA matched (4–6/6 HLA match) umbilical cord blood (UCB) using clinical grade anti-CD25-conjugated magnetic microbeads. Following selection, cells were expanded (18 ± 1 days) ex vivo in the presence of anti-CD3/CD28 antibody-coated beads and IL2, which resulted in a median 211-fold increase in cells (range 13–1796) containing a median of 1.058 × 109 (range 7.4 × 107 – 1.26 × 1010) CD4+/CD25+ cells. The suppressive effects of the resultant products was demonstrated in a mixed lymphocyte reaction with a 1:4 ratio (Treg: DC/T cell) resulting in 86% suppression of proliferation (range 39–95%). Subsequently, this approach was tested clinically in a Phase I clinical trial where 23 recipients (median age 52 years) of double UCB transplants received either one (day +1 – n = 5) or two (days +1 and +15 – n = 18) infusions of third party-derived UCB Tregs at doses ranging from 1–30 × 105/kg. In historical controls who did not receive Tregs, the incidence of grade II-IV aGVHD was 61%. Here, the rate of GvHD was reduced to 43% and despite the potential for indiscriminate Treg-associated immunosuppressive effects, the risk of viral infections did not increase. On the contrary, there was a reduced risk of infection (35 vs 45% in the control group) [62]. Di Ianni and colleagues also evaluated the activity of adoptively transferred Tregs. In their initial study, 28 Haplo HSCT recipients received both a DLI product (range 0.5–2 × 106 cells/kg) and fresh Tregs (2–4 × 106 cells/kg), which were isolated from a donor apheresis product by first depleting CD8+ and CD19+ cells followed by selection of CD25+ cells. The Tregs appeared to protect against GvHD, since only 2 of 26 evaluable patients developed aGVHD (grade II) and both were among the five that received the highest DLI dose. Moreover, the cell infusions were associated with accelerated-immune reconstitution with pathogen-specific CD4+ and CD8+ T cells detected as early as 2 months post-transplant compared with the previously reported 9–12 month timeframe. The infusions also correlated with a reduced incidence of CMV reactivation/disease relative to conventional Haplo HSCT recipients (n = 150) [63,64]. These studies suggest that adoptive immunotherapy with Tregs can limit allore-activity without compromising immune reconstitution and antigen-specific T-cell responses.
Direct infusion of virus-specific T cells
An alternative strategy to provide post-HSCT antiviral protection is with the infusion of virus-specific T cells (VST) that have been either isolated directly from peripheral blood or selectively expanded ex vivo to enrich for virus-directed populations with a consequent reduction in residual alloreactive T cells.
Direct isolation of virus-specific T cells
To date, two direct selection methods have been tested clinically – multimer selection and IFN-γ capture. Multimer selection relies on the ability of T cells to bind to a complex of peptide-loaded HLA molecules via their T-cell receptor, while the IFN-γ capture technique isolates T cells based on their ability to secrete IFN-γ following antigen stimulation (Table 1).
Table 1.
Clinical trials using directly isolated virus-specific T cells.
| Target virus(es) | Stimulus | Cell dose (cells/kg) |
Timing of infusion (days post-HSCT) |
Prophylaxis or treatment (number of patients) |
Clinical outcomes | |
|---|---|---|---|---|---|---|
| Multimer selection |
CMV | pp65, IE1 | 1.2 × 103–3.3 × 104 |
18–247 | Treatment (9) | 8CR 1 PR [65] |
| EBV | LMP2, BMLF1 | 0.8–24.6 × 104 | −7–305 | Treatment (1) | 1/1 CR of EBV/PTLD | |
| ADV | Hexon | Treatment (1) | NR of AdV infection | |||
| CMV | pp65 | Treatment (6) | 4/6 CR, 1/6 PR and 1/6 NR of CMV infection [66] |
|||
| CMV | pp65 | 0.37–2.2 × 105 | Unavailable | Treatment (2) | 2 CR [71] | |
| CMV | pp65 | 3750–5130 | 15–150 | Treatment (2) | 2 CR [73] | |
| IFN-γ capture | AdV | Adenoviral antigen type C | 1.2–50 × 103 | 40–378 | Treatment (9) | 3 CR 2 PR 1 NR [74] |
| CMV | pp65 | 1.2–166 × 103 | 19–308 | Treatment (18) | 9 CR 6 PR 3 NR [75] |
|
| EBV | LMP2, EBNA1, EBNA3A, EBNA3B, EBNA3C, BZLF1, BRLF1, BMLF1, BHRF1, BLLF1, BNRF1 |
0.4–9.7 × 104 | 47–93 | Treatment (6) | 3 CR 3 NR [77] |
|
| CMV | pp65 | 1 × 104 | 18–63 | Prophylaxis (7) | 0/7 required antiviral medication |
|
| Pre-emptive treatment (11) |
11/11 CR[76] | |||||
| EBV | EBNA1 | 148–53796 | 59–413 | Treatment (10) | 6 CR 1 PR 3 NR [78] |
|
Adv: Adenovirus; CR: Complete response; NR: No response; PR: Partial response; PTLD: Post-transplant lymphoproliterative disease.
Multimer selection
Multimers are complexes of peptide-loaded HLA molecules labeled with a fluorescent tag or magnetic bead that can be used to specifically select T cells reactive against the presented peptide. Cobbold and colleagues were the first to use this approach clinically to treat CMV reactivations post-transplant. Using a panel of CD8+ tetramers (complexes of four peptide-loaded HLA molecules) directed against pp65 (HLA A1, A2, A35 and B7) and IE-1 (HLA B8) peptides, they selected CMV-directed T-cell precursors from donor peripheral blood. These cells were infused to nine recipients of MRD (n = 6) or MUD (n = 3) transplants with CMV viremia within 4 h of selection. Despite the low number of exclusively CD8+ T cells infused (median of 8.6 × 103 tetramer-selected T cells/kg; range 1.2 × 103–3.3 × 104/kg), CMV-specific T cells became detectable in all patients within 10 days of transfer, expanded by up to 250-fold in vivo, and cleared infection in eight of nine cases [65].
Uhlin and colleagues subsequently used higher avidity pentamers (complexes of five HLA molecules) directed to a panel of HLA-A1, A2, B7 and B35 peptides from EBV, CMV and AdV to select VSTs from frozen donor grafts (n = 2) or haploidentical third party peripheral blood (n = 6). The selected cells (range 0.8–24.6 × 104 cells/kg) were safely infused to eight HSCT patients (age 0.5–59 years) with EBV-PTLD (n = 1), CMV reactivation (n = 6) or AdV infection (n = 1). Post-transfer the infused cells were detected as early as day +1 and persisted for up to 76 days in five of six evaluated patients. Furthermore, the infusions were associated with clinical benefit; within 2 weeks, there was a decrease of viral titers in both patients who received donor-derived cells and in four of the six patients treated with third party cells [66].
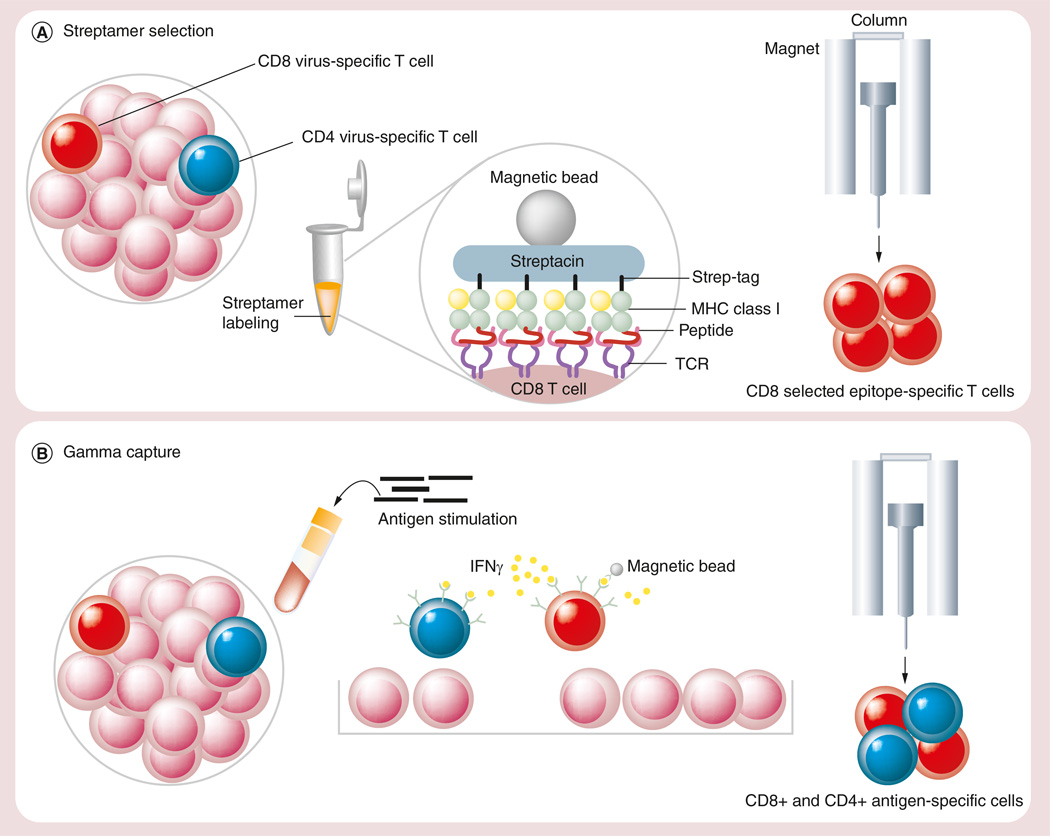
Tetramers/pentamers bind to T cells irreversibly, giving rise to multiple concerns about their clinical use, including their potential for inducing immune responses or in vivo toxicity. Furthermore, a number of groups have reported changes in T-cell phenotype following multimer engagement, with the induction of tolerance or even apoptosis [67–69]. To address these concerns, Knabel and colleagues developed a ‘next generation’ multimeric complex called the streptamer [69]. Streptamers can be easily dissociated from the T-cell receptor by addition of D-biotin, which causes the multimers to monomerize and detach from T cells within seconds, leaving the selected population pheno-typically and functionally identical to unmanipulated cells [70] (Figure 1A).
Figure 1. Direct isolation of virus-specific T cells.
(A) Streptamer selection. (B) IFN-γ capture.
MHC: Major histocompatibility complex; TCR: T-cell receptor; VST: Virus-specific T cell.
Clinically, donor-derived streptamer-selected T cells have been used to treat two adult MUD HSCT recipients with refractory CMV infections. Patient 1 received a total of 2.2 × 105 HLA B7+/CMV pp65-specific CD8+ T-cells/kg and patient 2 was infused with 0.37 × 105 HLA-A24+/CMVpp65-specific CD8+ T-cells/kg. After a single infusion, the frequency of CMV-specific CD8+CD45RA+CCR7-effector T cells increased from 0% to a maximum of 27.1% of all T cells in patient I and from 0.03 to 0.48% in patient 2. These T cells were donor-derived and did not result from endogenous reconstitution, as demonstrated by analysis of donor chimerism, T-cell receptor excision circles and Vβ-spectratyping by PCR. The infusions were safe and resulted in a persistent clearance of CMV antigenemia [71].
To preclinically investigate the minimum number of cells required for in vivo benefit Stemberger and colleagues used a murine Listeria monocytogenes infection model to demonstrate that even a single epitope-specific CD8+ T-cell was able to proliferate and differentiate into effector and central-memory T-cell subsets that could provide pathogen-specific effects [72,73]. Subsequently, the same group administered 3750 and 5130 cells per kilogram, respectively to two pediatric Haplo HSCT recipients with refractory CMV. The infused cells became detectable in the periphery 32 and 7 days post-infusion, respectively and transitioned from a less differentiated (CCR7+/CD45RA− = 14.5%) to a mature differentiated effector population (CCR7-/CD45RA+ = 71.4%) in vivo, with a concurrent decrease in both patients’ viral load [73].
These studies demonstrate the feasibility, efficacy and safety of multimer-selected cells in vivo. However, this approach can only be applied to immunologically well-characterized viruses, and to date has been restricted to CD8+ T cells.
IFN-γ capture
The IFN-γ capture approach selects T cells (both CD4+ and CD8+) based on their ability to secrete effector cytokines in response to antigen stimulation, and thus is an approach that is neither peptide nor HLA restricted. (Figure 1B) This strategy has been successfully used to both prevent and treat AdV, CMV and EBV infections in HSCT recipients. Feuchtinger and colleagues first used IFN-γ-captured AdV-directed T cells to treat nine pediatric (age 1–11 years) recipients of MUD (n = 3), mismatched (MM)UD (n = 4), MRD (n = 1) and Haplo (n = 1) transplants, all of whom had systemic AdV infections (n = 2) or disease (n = 7). The isolated cells, which represented a mix of CD4+ (63 ± 10%) and CD8+ (29 ± 8%) T cells, were infused between days 40–378 post-transplant at doses ranging from 1.2 to 50 × 103 cells/kg. The infusions were well tolerated with a single report of skin cGvHD aggravation and expanded in vivo, controlling infections/disease in five of six evaluable patients [74]. The same group also treated 18 patients (age 0.4 months to 59 years) with refractory CMV infections (n = 10) or disease (n = 8) after MRD, Haplo, MUD or MMUD HSCT using the same method. T cells reactive against pp65 were infused (mean 21.3 ± 38.8 × 103 CD3/kg) at 19–308 days post-transplant with no side effects or GvHD. The infused cells expanded in vivo and overall 15 of 18 patients cleared or had a significant reduction (>1log) in their viral loads post-infusion, including two patients with encephalitis [75]. Peggs and colleagues reported similar success when using CMV-captured T cells prophylactically (n = 8) or pre-emptively (n = 11) in a Phase I/II clinical trial. In this study, despite the infusion of small cell numbers (median 2840 and 630 CMV-specific CD4+ and CD8+ cells/kg, respectively), the infused cells provided antiviral protection; only one of the prophylactically-infused patients required antiviral drugs within the next 6 months. Furthermore, 9 of 11 pre-emptively infused patients required brief antiviral drug administration (11–33 days), with no instances of reactivation [76].
EBV reactivations were also sensitive to this therapy, as shown by Moosmann et al. who used EBV-peptide pools containing 23 epitope peptides from 11 EBV antigens (five latent, four early lytic and two late lytic) to stimulate EBV-specific T cells from donor-peripheral blood leukaphereses. Six adults with biopsy-proven EBV-PTLD that was unresponsive to conventional treatment received a single dose of selected cells (0.4–9.7 × 104 cells/kg). In three patients with early-stage disease, the transferred cells expanded in vivo as detected by pentamer staining and IFN-γ ELISPOT, and produced complete remissions. However, the profile in three patients with late-stage PTLD and multi-organ failure was different, with no T-cell expansion detected and no clinical response [77]. Finally, Icheva and colleagues targeted the universally-expressed latent protein EBNA-1 and infused reactive T cells (mean 5794 CD3+ cells/kg) to ten pediatric and adult patients (age 2–51 years) with refractory EBV viremia or PTLD 59–413 days post-HSCT. They observed in vivo expansion of transferred cells in eight of ten patients and of these, seven had clinical and virologic responses, defined as decrease of viral load greater than one log and resolution of PTLD [78].
These studies demonstrate that IFN-γ capture can successfully select polyclonal T cells used to prevent or treat even refractory AdV, CMV and EBV infections post-HSCT. However, similar to multimer selection, this method can only be applied to viruses with a high frequency of circulating specific T cells and to viruses for which immunogenic/protective antigens have been identified.
Ex vivo expanded virus-specific T cells
An alternative strategy to prepare a virus-enriched T-cell fraction is to specifically expand this population ex vivo by repetitive stimulation with antigen-loaded APCs, resulting in the loss of cells with alloreactive potential (Table 2).
Table 2.
Clinical trials using ex vivo expanded virus-specific T cells.
| Target virus(es) | Stimulus | Cell dose | Timing of Infusion (days post HSCT) |
Prophylaxis or treatment (number of patients) |
Clinical outcomes |
|---|---|---|---|---|---|
| CMV | AD169 strain of CMV |
33 × 106–1 × 109/m2 | 30–40 | Prophylaxis (14) | No CMV infections [79] |
| CMV | CMV lysate | 1 × 107/m2 | 79–479 | Treatment (8) | 6 CR 1 PR 1 NR [80] |
| CMV | CMV lysate | 0.6–1 × 105/kg | 21–34 | Prophylaxis (10) Treatment (20) |
23 responded to VSTs with antivirals [81] |
| CMV | pp65 | 2× 107/m2 | 29–115 | Prophylaxis (50) | 26 patients developed CMV infections 9 required antivirals 1 CMV-related death [82] |
| EBV | EBV B95–8 strain | 2× 107–1 × 108/m2 | Unavailable | Prophylaxis (101) Treatment (13) |
No new EBV infections 11 CR 2 deaths [85,87,109,110] |
| EBV | EBV B95–8 strain | 2–4 × 107/m2 | 31–148 | Treatment (6) | 5 displayed a decrease in viral load 1 EBV related death [89] |
| EBV | EBV B95–8 strain | 1 dose of 5 × 106/kg and 1–4 doses of 1–2 × 106/kg |
unavailable | Treatment (4) | 3 CR 1 death of multiorgan deficiency [90] |
| EBV | EBV B95–8 strain | 0.2–9 × 106/kg | 64–247 | Treatment (19) | 13 CR 1 EBV-related death [88] |
| EBV | EBV B95–8 strain | 5× 106–1 × 108/m2 | 35–150 | Prophylaxis (10) | 3/3 CR of EBV infection/PTLD |
| AdV | Ad5f35pp65 vector | AdV treatment (1) | 3/3 CR of CMV infection | ||
| CMV | 6/6 CR of AdV infection/disease [92] | ||||
| EBV | EBV B95–8 strain | 5× 106–1.35 × 108/m2 | 40–150 | Prophylaxis (9) | No new EBV or AdV infections |
| AdV | Ad5f35null vector | EBV treatment (3) AdV treatment (2) |
3 CR 2 CR [93] |
||
| EBV | BZLF1 | 5× 106–2 × 107/m2 | 28 days– 4 years |
EBV treatment (4) | 3 CR |
| AdV | Hexon, Penton | AdV treatment (5) | 5CR | ||
| CMV | IE1, pp65 | CMV treatment (5) | 4 CR, 1 patient with persistent colitis proceeded with colectomy [95] |
||
| EBV | EBNA1, LMP2, BZLF1 | 5× 106–2 × 107/m2 | 38–139 | Prophylaxis (3) | No viral infections in the prophylaxis arm |
| AdV | Hexon, Penton | EBV treatment (5) | 5 CR | ||
| CMV | IE1, pp65 | AdV treatment (1) | 1 CR | ||
| BKV | LT, VP1 | CMV treatment (3) | 2 CR, 1 PR | ||
| HHV-6 | U11, U14, U90 | BKV treatment (7) | 5 CR, 1 PR, 1 NR | ||
| HHV-6 treatment (2) | 2 CR [99] |
BKV: BK virus; CR: Complete response: HHV 6: Human herpesvirus 6; NR: No response; PR: Partial response; VST: Virus-specific T cells.
Cytomegalovirus
CMV was the first virus to be specifically targeted using ex vivo expanded T cells. Walter and colleagues used fibroblasts infected with the AD169 strain of CMV to stimulate CD8-enriched PBMCs, which were further expanded with either anti-CD3 antibody or CMV-infected fibroblasts and autologous-feeder cells. The resulting VSTs were administered prophylactically to 14 HLA-identical HSCT recipients in four weekly escalating doses (3.3 × 107 to 1 × 109/m2) starting 30–40 days post-transplant with no infusion-related toxicity noted. Only mild GvHD (grade I/II) was reported in just three patients, but remarkably none of the infused patients developed CMV viremia or disease. All 14 patients also demonstrated an increase in the circulating frequency of CMV-specific T cells post-infusion. However, in patients without endogenous CMV-specific CD4+ T cells, CD8+ T cell numbers rapidly declined, highlighting the importance of the helper T-cell subset in vivo [79]. Subsequently, Einsele and colleagues used an alternative method to prepare polyclonal CMV-directed VSTs. They incubated donor-derived PBMCs with CMV lysate for 10 days, followed by restimulation with CMV antigen and irradiated autologous feeder cells. After four stimulations, the final product contained both virus-specific IFN-γ-producing CD4+ (mean 77 ± 10%) and CD8+ (mean 6 ± 3%) T cells, and lacked alloreactivity even when donor and recipient were mismatched on three HLA alleles. Eight recipients of MRD, MMRD, MUD or MMUD transplants with ganciclovir-resistant CMV were treated with a single dose of 107 CMV-specific T cells/m2 a median of 120 days post-HSCT (range 79–429 days). The infusions were well tolerated with no reported GvHD. All seven evaluable patients had a significant reduction (greater than one log) in CMV-DNA, which was durable in five. In two patients with the highest viral loads, the decrease in CMV-DNA was transient. However, a second infusion was sufficient to produce sustained viral clearance in a patient while the other patient refused a second infusion and eventually succumbed to his/her infection [80].
In a Phase II/III study, Peggs and colleagues administered CMV-STs prophylactically or pre-emptively to high risk HSCT recipients. In those infused prophylactically the cells appeared to be protective since the incidence of CMV was reduced compared with historical controls. Furthermore, in patients who received pre-emptive T-cell therapy the infused T cells expanded substantially in vivo producing viral clearance in all [81]. Finally, Blyth and colleagues reported the results of a Phase II clinical trial where 50 allogeneic transplant recipients (age 4–68 years) were pro-phylactically infused with 2 × 107 cells/m2 CMV-ST lines generated with DCs pulsed with the HLA-A2-restricted CMV pp65 peptide NLV (n = 10) or transfected with an adenoviral vector genetically modified to express pp65 (n = 40). Although infusions did not reduce the frequency of CMV reactivations both the percentage of patients requiring antiviral drugs and the duration of treatment was decreased in the study population (17 vs 36% and 3.4 vs 8.9 days, respectively) [82]. The data from these studies suggest that CMV-specific T cells (STs) mediate direct antiviral effects but also reduce the requirement for antiviral therapy with a corresponding reduction in drug and disease-associated morbidity and in transplant costs.
EBV
EBV was first targeted immunotherapeutically by our group using donor-derived T cells expanded using autologous EBV-LCLs as APCs. EBV-LCLs are particularly good APCs in this setting, since the transformed B cells express the same antigen profile as the malignant cells, thereby maximizing the therapeutic benefit of the expanded T-cell lines [83]. To date, 114 transplant recipients (age 5 months to 38 years, mean 8.4 years) have been infused with EBV-STs to prevent (n = 101–n = 90 T-cell depleted HSCT; n = 11 high risk of lymphoprolipherative disease) or treat biopsy-proven or probable EBV-PTLD (n = 13). None of the patients infused prophylactically developed EBV-PTLD, compared with an incidence of 11% in a historical control cohort. Furthermore, the first 26 patients received VSTs that were genetically marked with the neomycin-resistance gene, which allowed us to track the cells for up to 9 years post-infusion and demonstrated that adoptively transferred cells can acquire a memory phenotype and, like natural memory EBV T cells, are able to survive long-term and expand upon antigen stimulation. Finally, of 13 patients with proven or probable disease at the time of EBV-ST treatment, 11 had complete and sustained clinical responses [84–87].
These results have been recapitulated at numerous other centers including Memorial Sloan Kettering [88], the Karolinska Institute [89] and University of Pavia [90]. However, the rare treatment failures have also taught important lessons. For example, Dubrovina and colleagues reported on three non-responding patients who were infused with T-cell lines that recognized donor-derived LCLs transformed with the EBV B-95 laboratory-strain virus but not the strain of EBV expressed by the patients’ tumor [88]. Our group reported a similar phenomenon in a patient who failed to respond to EBV-STs and was later found to harbor a tumor virus with a deletion in the EBNA-3B gene that removed the immunodominant HLA-A11 epitopes exclusively targeted by the infused line [91]. These cases highlight the importance of infusing polyclonal T cells reactive against multiple antigens/epitopes expressed by the endogenous tumor to ensure clinical benefit.
Multivirus-specific T cells
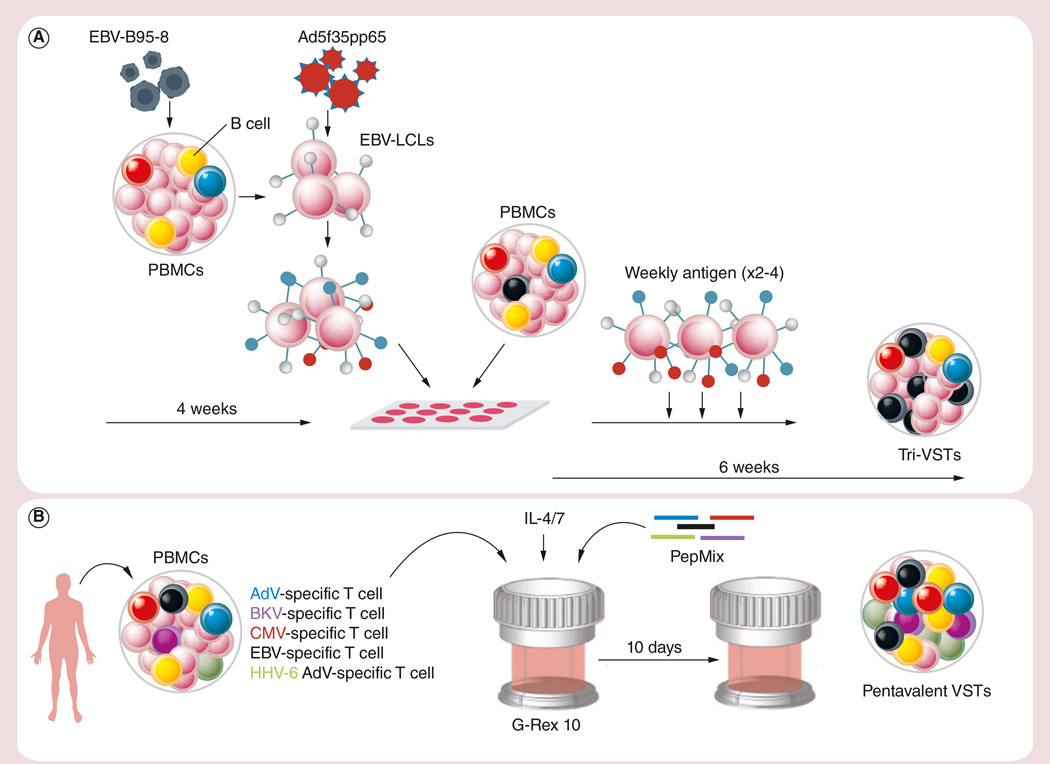
In an attempt to target not one but multiple clinically problematic viruses simultaneously, our group developed a strategy to generate bi- and trivirus-directed VSTs with specificity for Adv+EBV (from CMV sero-negative donors) or AdV+EBV+CMV. Multispecific T cells were activated using monocytes transduced with a chimeric Ad5f35-null vector (for bivirus) or Ad5f35pp65 followed by weekly stimulation with autologous EBV-LCLs transduced with the same vector. (Figure 2A) The bi- and trivirus lines were polyclonal and showed activity against all the target viruses based on IFN-γ ELISPOT and cytotoxicity assays, though in the trivirus products the CMV-specific fraction was dominant [92,93].
Figure 2. Generation of ex vivo expanded virus-specific T cells.
(A) Our original manufacturing procedure for generating AdV, EBV and CMV-specific VSTs. (B) Our current method for generating multivirus-directed VSTs.
Ad5f35pp65: Adeniviral vector encoding for the CMV antigen pp65; AdV: Adenovirus; BKV: BK virus; EBV-LCLs: EBV-transformed lymphoblastoid cell lines; HHV-6: Human herpesvirus 6; PBMC: Peripheral blood mononuclear cell; VST: Virus-specific T cell.
In total, 24 patients (age 1–63 years) were safely infused with 5 × 106 to 1.35 × 108 multivirus-directed T cells/m2 between days 35 and 150 post MRD, MMRD, MUD or Haplo transplant. Monitoring the impact that infusions had on virus-specific immune reconstitution, we saw two patterns emerging. For the latent EBV and CMV viruses, there was an increase in the precursor frequency of reactive cells irrespective of whether patients were infused prophylactivally or therapeutically. However, for AdV, we detected an increase in specific cells only in patients with active infections, highlighting the importance of antigenic stimulation for in vivo expansion. Of note, none of the infused patients developed de novo AdV infections, compared with an incidence of 68% in similar pediatric transplant recipients in the absence of VST therapy [94], suggesting that the infused cells can persist, expand following stimulation and provide long-term protection against adenoviral disease. These multispecific cells were also associated with clinical benefit, inducing partial or complete responses in three patients with CMV reactivation (including one refractory case), six patients with EBV (including a patient with EBV-PTLD) and five patients with AdV infections [92,93].
To reduce manufacturing time and avoid the use of live virus or viral vectors, we next investigated whether viral antigen-encoding DNA plasmids could be used to expand antigen-specific T cells. Using the AMAXA nucleofection system, plasmid-nucleofected DCs were used to activate antigen-specific T-cell populations with trivirus specificity. In a total of 17 days we were consistently able to generate VSTs with similar phenotypic, specificity and functional profiles as traditionally generated trivirus-directed T cells [95]. These cells were safe when infused in ten patients with CMV (n = 5), EBV (n = 4) and AdV (n = 5) infections and produced complete responses in 80%, including in all four patients with dual infections [96].
More recently, we extended the spectrum of antigens targeted using a single T-cell line to include not only EBV, CMV, AdV but also BKV and HHV6. In this study, the manufacturing process was further simplified by substituting clinical grade pepmixes (15-mer peptides overlapping by 11aa) for plasmids, which allowed the direct stimulation of PBMCs for antigen activation followed by 10 days in a G-Rex device [97,98] (Figure 2B). The clinical multivirus (m) VSTs produced were a mix of CD4+ (57 ± 2%) and CD8+ (35 ± 2%) cells with specificity that reflected donor serostatus – for example, donors that were CMV seronegative lacked a CMV-reactive T-cell component, etc. Nevertheless, the majority of the lines generated recognized at least three viruses. Eleven lines were administered to recipients of MRD (n = 5), MUD (n = 3), MMUD (n = 2) or Haplo (n = 1) transplants at doses ranging from 0.5 × 107 to 2 × 107 cells/m2 either as prophylaxis (n = 3) or to treat one to four viral infections (n = 8). Despite receiving just a single in vitro stimulation, the infused mVSTs exhibited a safety profile similar to more extensively expanded T-cell products. Furthermore, the cells produced clinical responses in all patients with EBV (n = 5), CMV (n = 3), HHV-6 (n = 2) and AdV (n = 1) infections/reactivations, while six of seven patients with BKV infections had a complete or partial response (defined as a reduction of >50% in the baseline-viral load with improvement of clinical signs/symptoms). These responses included all four patients with symptomatic tissue involvement (EBV-PTLD [n = 1] and BKV-cystitis [n = 3]) [99]. These data demonstrate that production of broad spectrum VSTs is feasible and can provide safe and effective antiviral protection.
VST generation from seronegative donors
The above sections illustrate the relative simplicity associated with preparing virus-specific T cells from seropositive donors. However, the isolation of such cells from either seronegative donors or UCB is substantially more challenging given the low circulating precursor frequency of virus-reactive cells and their naïve phenotype. Hence, to activate and expand these naive cells in vitro, one requires professional APCs as well as potent, Th1-polarizing cytokines, such as IL-7, IL-15 and IL-12 [100,101]. Nevertheless, to test whether such VSTs can prevent/treat post-transplant infections, Hanley and colleagues generated trivirus VSTs (CMV, EBV and AdV) from the 20% fraction of the cord blood unit, and infused these into seven cord blood transplant recipients at doses ranging from 0.5–2.5 × 107 cells/m2. The infusions were well tolerated, with no early or subsequent GvHD, and protective in all five patients who were treated prophylactically. The two remaining patients had a CMV and AdV infection, respectively, and both were able to clear the viruses, even though a second VST infusion was required for the patient with CMV [102,103]. Thus, early reports suggest that these UCB-derived VSTs can promote virus-directed immune reconstitution in vivo.
Third-party banks
Despite the safety profile and clear clinical benefit associated with adoptively transferred VSTs, this therapy is still restricted to select academic centers with specialized good manufacturing practice infrastructure and expertise. The requirement for individualized products is problematic when the transplant donor is seronegative or in cases of urgent need. To address these issues, the utility of banks of ‘off the shelf’ VSTs generated from transplant-eligible donors has been investigated. However, while this source is immediately available, one must also consider the potentially greater risk of GvHD associated with the infusion of lines that are mismatched at one or more HLA loci. Nevertheless, Haque and colleagues generated and tested the activity of third-party EBV-STs in 31 solid organ transplant and two HSCT recipients with refractory biopsy-proven EBV-PTLD. The lines for infusion were chosen on the basis of HLA matching and in vitro cytotoxicity of EBV. Patients received products that were matched at two to five HLA alleles in four weekly doses of 2 × 106 cells/kg. The infusions were well tolerated in these refractory patients and resulted in clinical responses in 64% at 5 weeks and 52% at 6 months. Subsequent analysis demonstrated that the level of HLA matching correlated with outcomes at 6 months and overall better response rates (p = 0.048) [104]. In a follow-up study, Vickers and colleagues infused lines matched at 3/10 to 9/10 alleles to 11 patients with EBV-PTLD post solid organ transplant (n = 5) or HSCT (n = 8). Eight of ten evaluable patients achieved a complete remission, including four patients with CNS disease. O’Reilly and colleagues also reported results from five patients with EBV-PTLD who were treated with third-party lines, four of whom were complete responders [105].
Finally, our group has utilized banks of T cells with specificity for CMV, EBV, and AdV to treat patients with refractory CMV, AdV and/or EBV infections post-HSCT. Eighty two HSCT transplant recipients were screened for participation on the study and a suitable line - based on overall level of HLA match and the presence of T-cell activity against the infecting virus through a shared HLA allele - was identified for 74 individuals from a bank of just 32 products. Of these, a total of 18 lines were infused to 50 patients with severe, or refractory infections (CMV [n = 23], AdV [n = 18], and EBV-PTLD [n = 9]) after marrow (n = 14), peripheral blood (n = 21) or cord blood (n = 15) transplant. Despite the low level of HLA matching (range 1/6 to 4/6 matched alleles), de novo GVHD occurred in only two patients and was grade I in both cases. We achieved responses for all three viruses targeted with a cumulative response rate at 6 weeks postinfusion of 74%; 74%, 78% and 67% for CMV, AdV and EBV, respectively, the majority of which were durable [106]. Overall, these studies demonstrate that despite initial concerns, infusions of third-party VSTs are safe and clinically beneficial, justifying broader implementation of this approach (Table 3) [107].
Table 3.
Clinical trials using third party virus-specific T cells.
| Target virus | Stimulus | Patients infused |
Cell dose | Timing of infusion (days post HSCT) |
Clinical outcome | |
|---|---|---|---|---|---|---|
| Multimer selection | EBV | pp65 | 1 | 0.8–24.6 × 104 | −7–305 | 1 CR |
| AdV | Hexon | 1 | 1 NR | |||
| CMV | LMP2, BMLF1 | 4 | 2/4 CR, 1/4 PR, 1/4NR [66] | |||
| Ex vivo expansion | EBV | EBV B95–8 strain | 2 | 2 × 106/kg × 4 doses | Unavailable | 2 CR of EBV-PTLD [104] |
| EBV | EBV B95–8 strain | 2 | 5–9 × 106/kg | 277–347 | 2 CR of EBV- PTLD [107] | |
| EBV | EBV B95–8 strain | 19 | 0.2–9 × 106/kg | 64–247 (median 91) | 13 CR 6 NR [88] |
|
| EBV | EBV B95–8 strain | 9 | 2–× 107–1 × 108/m2 | Unavailable | 2/9 CR, 4/9 PR, 3/9 NR of EBV infection/ PTLD |
|
| AdV | Ad5f35pp65 vector | 23 | 9/23 CR, 8/23 PR, 6/23 NR of CMV Infection |
|||
| CMV | 18 | 7/18 CR, 7/18 PR, 4/18 NR of AdV infection/disease [106] |
||||
| EBV | EBV B95–8 strain | 11 | 1–2 × 106/kg ×4 doses |
Unavailable | 8 CR 1 PR 2 NR [105] |
|
AdV: Adenovirus; CR: Complete response: NR: No response; PR: Partial response; PTLD: Post-transplant lymphoproliferative disease.
VSTs as a platform for genetic modification
The major causes of post-transplant morbidity and mortality are relapse and infection. Since donor-derived VSTs are able to expand and persist long-term without inducing GvHD, our group has recently also explored the possibility of providing dual antiviral and antitumor activity by genetically modifying VSTs to express a tumor-targeted chimeric antigen receptor (CAR). Indeed, Cruz and colleagues modified trivirus-specific T cells with a CAR directed against CD19 and administered these to eight patients with CD19 positive B-cell malignancies (age 9–59 years) post MRD or MUD transplant. There were no infusion-related toxicities and no GvHD. Since the cells were gene-modified, quantitative PCR was used to track the transgenic populations, which persisted for a median of 8 weeks in blood and up to 9 weeks at disease sites, and in two evaluable patients correlated with objective clinical responses. Moreover, CD19. CAR-VSTs retained antiviral activity in vivo, since two patients who reactivated EBV had a concomitant increase in the frequency of circulating EBV-specific precursors by IFN-γ ELISPOT and of the CD19.CAR Q-PCR signal, suggesting virus-induced expansion. Thus, this pilot study demonstrates the feasibility of conferring VSTs with the ability to target tumor-expressed antigens and provides preliminary evidence supporting the use of a single product to prevent both relapse and infection [108].
Conclusion & future perspective
Viral infections are an increasing cause of morbidity and mortality following HSCT. T-cell therapy to reconstitute antiviral immunity is beyond doubt a clinically safe and effective treatment strategy. Using rapid isolation techniques, it is possible to select virus-specific populations that can be infused within hours, while expansion protocols can be utilized to prepare cell banks for infusion in the donor-specific and third-party setting. Finally, since current mVST manufacturing platforms can accommodate additional specificities, one can now consider extending immunotherapeutic protection to other clinically problematic viruses including JC, HHV7, influenza, parainfluenza, human metapneumovirus, coronavirus, RSV and bocavirus to provide broad spectrum antiviral protection.
Executive summary.
Virus-specific T cells are crucial for providing protection against viral infections post allogeneic hematopoietic stem cell transplantation.
Infusion of donor lymphocyte infusion can provide antiviral benefit, but with a coincident risk of graft versus host disease (GvHD) due to the presence of alloreactive T cells.
The risk of GvHD can be mitigated by inactivating alloreactive T cells or infusing selected virus-specific T cells.
Broad spectrum antiviral protection can be provided by a single T-cell line with simultaneous specificity for multiple viruses.
T cells can be banked and administered as an ‘off the shelf product to partially human leukocyte antigen-matched recipients to provide clinical benefit.
Acknowledgments
The current work represents a comprehensive literature review and as such did not receive any specific grant funding. However, the Center for Cell and Gene Therapy, of which I Tzannou and AM Leen are members, receives funding from a variety of sources including NIH grants P50CA126752, PO1 CA94237, U54 HL081007, N01-HB-10-03, and the Production Assistance for Cellular Therapies (PACT) program (NHL-BI contract #HHSN268201000007C) and shared resources provided by the Dan L Duncan Cancer Center support grant P30CA125123.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•of interest; •• of significant interest
- 1.Gaziev J, Lucarelli G. Stem cell transplantation and gene therapy for hemoglobinopathies. Curr. Hematol. Rep. 2005;4(2):126–131. [PubMed] [Google Scholar]
- 2.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117(8):2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 3.Peffault de Latour R, Porcher R, Dalle JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122(26):4279–4286. doi: 10.1182/blood-2013-01-479733. [DOI] [PubMed] [Google Scholar]
- 4.Samarasinghe S, Steward C, Hiwarkar P, et al. Excellent outcome of matched unrelated donor transplantation in paediatric aplastic anaemia following failure with immunosuppressive therapy: a United Kingdom multicentre retrospective experience. Br. J. Haematol. 2012;157(3):339–346. doi: 10.1111/j.1365-2141.2012.09066.x. [DOI] [PubMed] [Google Scholar]
- 5.Saussele S, Hehlmann R, Gratwohl A, Hochhaus A. Outcome of patients with CML after SCT in the era of tyrosine kinase inhibitors. Bone Marrow Transplant. 2012;47(2):304. doi: 10.1038/bmt.2011.70. [DOI] [PubMed] [Google Scholar]
- 6.Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36(9):757–769. doi: 10.1038/sj.bmt.1705140. [DOI] [PubMed] [Google Scholar]
- 7.Bacigalupo A, Soraru M, Dominietto A, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45(3):458–463. doi: 10.1038/bmt.2009.188. [DOI] [PubMed] [Google Scholar]
- 8.Locatelli F, Crotta A, Ruggeri A, et al. Analysis of risk factors influencing outcomes after cord blood transplantation in children with juvenile myelomonocytic leukemia: a EUROCORD, EBMT, EWOG-MDS, CIBMTR study. Blood. 2013;122(12):2135–2141. doi: 10.1182/blood-2013-03-491589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangiolo D, Storb R, Deeg HJ, et al. Outcome of allogeneic hematopoietic cell transplantation from HLA-identical siblings for severe aplastic anemia in patients over 40 years of age. Biol. Blood Marrow Transplant. 2010;16(10):1411–1418. doi: 10.1016/j.bbmt.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91(1):78–83. [PubMed] [Google Scholar]
- 11.Ljungman P, de la Camara R, Cordonnier C, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42(4):227–240. doi: 10.1038/bmt.2008.162. [DOI] [PubMed] [Google Scholar]
- 12.Styczynski J, Reusser P, Einsele H, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43(10):757–770. doi: 10.1038/bmt.2008.386. [DOI] [PubMed] [Google Scholar]
- 13.de Pagter PJ, Schuurman R, Meijer E, van BD, Sanders EA, Boelens JJ. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J. Clin. Virol. 2008;43(4):361–366. doi: 10.1016/j.jcv.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Hubacek P, Sedlacek P, Keslova P, et al. Incidence of HHV7 in donors and recipients of allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer. 2008;50(4):935. doi: 10.1002/pbc.21436. [DOI] [PubMed] [Google Scholar]
- 15.Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr. Opin. Organ Transplant. 2008;13(6):569–574. doi: 10.1097/MOT.0b013e3283186b93. [DOI] [PubMed] [Google Scholar]
- 16.Koskenvuo M, Dumoulin A, Lautenschlager I, et al. BK polyomavirus-associated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: treatment response and evidence for nosocomial transmission. J. Clin. Virol. 2013;56(1):77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Engelhard D, Mohty B, de la Camara R, Cordonnier C, Ljungman P. European guidelines for prevention and management of influenza in hematopoietic stem cell transplantation and leukemia patients: summary of ECIL-4 (2011), on behalf of ECIL, a joint venture of EBMT, EORTC, ICHS, and ELN. Transpl. Infect. Dis. 2013;15(3):219–232. doi: 10.1111/tid.12054. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin. Infect. Dis. 2013;56(2):258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo MS, Lee GM, Gunawardane N, Burchett SK, Lachenauer CS, Lehmann LE. The impact of RSV, adenovirus, influenza, and parainfluenza infection in pediatric patients receiving stem cell transplant, solid organ transplant, or cancer chemotherapy. Pediatr. Transplant. 2013;17(2):133–143. doi: 10.1111/petr.12022. [DOI] [PubMed] [Google Scholar]
- 20.Renaud C, Xie H, Seo S, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol. Blood Marrow Transplant. 2013;19(8):1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011) Transpl. Infect. Dis. 2012;14(6):555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 22.Marr KA. Delayed opportunistic infections in hematopoietic stem cell transplantation patients: a surmountable challenge. Hematology. Am. Soc. Hematol. Educ. Program. 2012;2012:265–270. doi: 10.1182/asheducation-2012.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeckh M, Erard V, Zerr D, Englund J. Emerging viral infections after hematopoietic cell transplantation. Pediatr. Transplant. 2005;9(Suppl. 7):48–54. doi: 10.1111/j.1399-3046.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 24.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol. Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaia J, Baden L, Boeckh MJ, et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44(8):471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 1994;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 27.Hromas R, Cornetta K, Srour E, Blanke C, Broun ER. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood. 1994;84(5):1689–1690. [PubMed] [Google Scholar]
- 28.Couriel D, Canosa J, Engler H, Collins A, Dunbar C, Barrett AJ. Early reactivation of cytomegalovirus and high risk of interstitial pneumonitis following T depleted BMT for adults with hematological malignancies. Bone Marrow Transplant. 1996;18(2):347–353. [PubMed] [Google Scholar]
- 29.Yoshihara S, Kato R, Inoue T, et al. Successful treatment of life-threatening human herpesvirus-6 encephalitis with donor lymphocyte infusion in a patient who had undergone human leukocyte antigen-haploidentical nonmyeloablative stem cell transplantation. Transplantation. 2004;77(6):835–838. doi: 10.1097/01.tp.0000119603.59880.47. [DOI] [PubMed] [Google Scholar]
- 30.Kishi Y, Kami M, Oki Y, et al. Donor lymphocyte infusion for treatment of life-threatening respiratory syncytial virus infection following bone marrow transplantation. Bone Marrow Transplant. 2000;26(5):573–576. doi: 10.1038/sj.bmt.1702559. [DOI] [PubMed] [Google Scholar]
- 31.Montagna D, Yvon E, Calcaterra V, et al. Depletion of alloreactive T cells by a specific anti-interleukin-2 receptor p55 chain immunotoxin does not impair in vitro antileukemia and antiviral activity. Blood. 1999;93(10):3550–3557. [PubMed] [Google Scholar]
- 32.van Dijk AM, Kessler FL, Stadhouders-Keet SA, Verdonck LF, de Gast GC, Otten HG. Selective depletion of major and minor histocompatibility antigen reactive T cells: towards prevention of acute graft-versus-host disease. Br. J. Haematol. 1999;107(1):169–175. doi: 10.1046/j.1365-2141.1999.01675.x. [DOI] [PubMed] [Google Scholar]
- 33.Andre-Schmutz I, Le DF, Hacein-Bey-Abina S, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a Phase 1/2 study. Lancet. 2002;360(9327):130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 34.Amrolia PJ, Muccioli-Casadei G, Yvon E, et al. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003;102(6):2292–2299. doi: 10.1182/blood-2002-11-3516. [DOI] [PubMed] [Google Scholar]
- 35.Albon SJ, Mancao C, Gilmour K, et al. Optimization of methodology for production of CD25/CD71 allodepleted donor T cells for clinical use. Cytotherapy. 2013;15(1):109–121. doi: 10.1016/j.jcyt.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Wehler TC, Nonn M, Brandt B, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109(1):365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 37.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge X, Brown J, Sykes M, Boussiotis VA. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol. Blood Marrow Transplant. 2008;14(5):518–530. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartwig UF, Nonn M, Khan S, Meyer RG, Huber C, Herr W. Depletion of alloreactive T cells via CD69: implications on antiviral, antileukemic and immunoregulatory T lymphocytes. Bone Marrow Transplant. 2006;37(3):297–305. doi: 10.1038/sj.bmt.1705238. [DOI] [PubMed] [Google Scholar]
- 40.Samarasinghe S, Mancao C, Pule M, et al. Functional characterization of alloreactive T cells identifies CD25 and CD71 as optimal targets for a clinically applicable allodepletion strategy. Blood. 2010;115(2):396–407. doi: 10.1182/blood-2009-08-235895. [DOI] [PubMed] [Google Scholar]
- 41.Nonn M, Herr W, Khan S, et al. Selective depletion of alloreactive T lymphocytes using patient-derived nonhematopoietic stimulator cells in allograft engineering. Transplantation. 2008;86(10):1427–1435. doi: 10.1097/TP.0b013e31818810d6. [DOI] [PubMed] [Google Scholar]
- 42.Solomon SR, Tran T, Carter CS, et al. Optimized clinical-scale culture conditions for ex vivo selective depletion of host-reactive donor lymphocytes: a strategy for GvHD prophylaxis in allogeneic PBSC transplantation. Cytotherapy. 2002;4(5):395–406. doi: 10.1080/146532402320775982. [DOI] [PubMed] [Google Scholar]
- 43. Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, O’Reilly RJ. Adoptive immunotherapy using donor leukocytes following bone marrow transplantation for chronic myeloid leukemia: is T cell dose important in determining biological response? Bone Marrow Transplant. 1995;15(4):591–594. • First study to report a suicide-gene safety switch is clinically effective in controlling alloreactivity.
- 44.de Vries E, van Tol MJ, van den Bergh RL, et al. Reconstitution of lymphocyte subpopulations after paediatric bone marrow transplantation. Bone Marrow Transplant. 2000;25(3):267–275. doi: 10.1038/sj.bmt.1702141. [DOI] [PubMed] [Google Scholar]
- 45.Solomon SR, Mielke S, Savani BN, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106(3):1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mielke S, Nunes R, Rezvani K, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008;111(8):4392–4402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mielke S, McIver ZA, Shenoy A, et al. Selectively T cell-depleted allografts from HLA-matched sibling donors followed by low-dose posttransplantation immunosuppression to improve transplantation outcome in patients with hematologic malignancies. Biol. Blood Marrow Transplant. 2011;17(12):1855–1861. doi: 10.1016/j.bbmt.2011.05.019. • Clinical study demonstrating that iC9 suicide gene controls GvHD, while sparing T-cell antiviral immunity.
- 48.McIver ZA, Melenhorst JJ, Grim A, et al. Immune reconstitution in recipients of photodepleted HLA-identical sibling donor stem cell transplantations: T cell subset frequencies predict outcome. Biol. Blood Marrow Transplant. 2011;17(12):1846–1854. doi: 10.1016/j.bbmt.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy DC, Maertens J, Walker I, et al. Selective photodepletion of recipient-alloreactive T-cells enables safe and efficatious haploidentical HSCT: initial results from a Phase 2 trial in patients with AML, ALL and MDS. Blood. 2014;124:314. Abstract. [Google Scholar]
- 50.Perruccio K, Topini F, Tosti A, et al. Optimizing a photoallodepletion protocol for adoptive immunotherapy after haploidentical SCT. Bone Marrow Transplant. 2012;47(9):1196–1200. doi: 10.1038/bmt.2011.237. [DOI] [PubMed] [Google Scholar]
- 51.Davies JK, Gribben JG, Brennan LL, Yuk D, Nadler LM, Guinan EC. Outcome of alloanergized haploidentical bone marrow transplantation after ex vivo costimulatory blockade: results of 2 Phase 1 studies. Blood. 2008;112(6):2232–2241. doi: 10.1182/blood-2008-03-143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a Phase II study in patients with acute leukemia at high risk of relapse. J. Clin. Oncol. 2005;23(15):3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 53.Davies JK, Barbon CM, Voskertchian AR, Nadler LM, Guinan EC. Induction of alloantigen-specific anergy in human peripheral blood mononuclear cells by alloantigen stimulation with co-stimulatory signal blockade. J. Vis. Exp. 2011;49:2673. doi: 10.3791/2673. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beltinger C, Fulda S, Kammertoens T, Meyer E, Uckert W, Debatin KM. Herpes simplex virus thymidine kinase/ ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc. Natl. Acad. Sci. USA. 1999;96(15):8699–8704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised Phase I-II study. Lancet Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 56.Vago L, Oliveira G, Bondanza A, et al. T-cell suicide gene therapy prompts thymic renewal in adults after hematopoietic stem cell transplantation. Blood. 2012;120(9):1820–1830. doi: 10.1182/blood-2012-01-405670. [DOI] [PubMed] [Google Scholar]
- 57.Traversari C, Marktel S, Magnani Z, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109(11):4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 58.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol. Blood Marrow Transplant. 2007;13(8):913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Di SA, Tey SK, et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood. 2014;123(25):3895–3905. doi: 10.1182/blood-2014-01-551671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. • First clinical trial using the IFN-γ capture approach and the first trial to target adenovirus.
- 63.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 64.Martelli MF, Di IM, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124(4):638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 65.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J. Exp. Med. 2005;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uhlin M, Gertow J, Uzunel M, et al. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin. Infect. Dis. 2012;55(8):1064–1073. doi: 10.1093/cid/cis625. [DOI] [PubMed] [Google Scholar]
- 67. Maile R, Wang B, Schooler W, Meyer A, Collins EJ, Frelinger JA. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J. Immunol. 2001;167(7):3708–3714. doi: 10.4049/jimmunol.167.7.3708. •• First ever adoptive virus-specific T-cell transfer study.
- 68.O’Herrin SM, Slansky JE, Tang Q, et al. Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J. Immunol. 2001;167(5):2555–2560. doi: 10.4049/jimmunol.167.5.2555. [DOI] [PubMed] [Google Scholar]
- 69.Knabel M, Franz TJ, Schiemann M, et al. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat. Med. 2002;8(6):631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 70.Neudorfer J, Schmidt B, Huster KM, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J. Immunol. Methods. 2007;320(1–2):119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Schmitt A, Tonn T, Busch DH, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51(3):591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 72.Buchholz VR, Flossdorf M, Hensel I, et al. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340(6132):630–635. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- 73.Stemberger C, Graef P, Odendahl M, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood. 2014;124(4):628–637. doi: 10.1182/blood-2013-12-547349. [DOI] [PubMed] [Google Scholar]
- 74.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br. J. Haematol. 2006;134(1):64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 75. Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. •• Clinical trial demonstrating long-term safety and efficacy of Epstein-Barr (EBV) virus-specific T cells (EBV-STs)
- 76.Peggs KS, Thomson K, Samuel E, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin. Infect. Dis. 2011;52(1):49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 77.Moosmann A, Bigalke I, Tischer J, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115(14):2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 78.Icheva V, Kayser S, Wolff D, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J. Clin. Oncol. 2013;31(1):39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 79.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 80. Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. • First clinical trial that proved the feasibility and efficacy of a single VSTs line simultaneously targeting three viruses.
- 81.Peggs KS, Verfuerth S, Pizzey A, Chow SL, Thomson K, Mackinnon S. Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin. Infect. Dis. 2009;49(12):1851–1860. doi: 10.1086/648422. [DOI] [PubMed] [Google Scholar]
- 82.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 83.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 84.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 85.Rooney CM, Heslop HE, Brenner MK. EBV specific CTL: a model for immune therapy. Vox Sang. 1998;74(Suppl. 2):497–498. doi: 10.1111/j.1423-0410.1998.tb05463.x. [DOI] [PubMed] [Google Scholar]
- 86.Heslop H, Rooney C, Brenner M, et al. Administration of neomycin resistance gene-marked EBV-specific cytotoxic T-lymphocytes as therapy for patients receiving a bone marrow transplant for relapsed EBV-positive Hodgkin disease. Hum. Gene Ther. 2000;11(10):1465–1475. doi: 10.1089/10430340050057530. [DOI] [PubMed] [Google Scholar]
- 87.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doubrovina E, Ofaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95(3):807–814. [PubMed] [Google Scholar]
- 90.Comoli P, Basso S, Zecca M, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am. J. Transplant. 2007;7(6):1648–1655. doi: 10.1111/j.1600-6143.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 91.Gottschalk S, Ng CY, Perez M, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97(4):835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 92. Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat. Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. •• First clinical trial showing the safety and efficacy of third party VSTs in hematopoietic stem cell transplantation patients.
- 93.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Myers GD, Bollard CM, Wu MF, et al. Reconstitution of adenovirus-specific cell-mediated immunity in pediatric patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39(11):677–686. doi: 10.1038/sj.bmt.1705645. • Large clinical trial where third-party VSTs were successfully used to treat EBV, cytomegalovirus and adenovirus.
- 95.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol. Ther. 2009;17(9):1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol. Ther. 2013;21(11):2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol. Ther. 2012;20(8):1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vera JF, Brenner LJ, Gerdemann U, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J. Immunother. 2010;33(3):305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci. Transl. Med. 2014;6(242) doi: 10.1126/scitranslmed.3008825. 242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quintarelli C, Dotti G, De AB, et al. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112(5):1876–1885. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared with adult peripheral blood. Exp. Hematol. 2003;31(8):708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 102.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hanley PJ, Leen AM, Gee A, et al. Multivirus-specific-T-cell therapy for patients after stem cell and cord blood hematopoietic stem cell transplantation. Blood. 2013;122:140. Absract. [Google Scholar]
- 104.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a Phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 105.Vickers MA, Wilkie GM, Robinson N, et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic epstein-barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br. J. Haematol. 2014;167(3):402–410. doi: 10.1111/bjh.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116(23):5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a Phase 1 study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heslop HE, Perez M, Benaim E, Rochester R, Brenner MK, Rooney CM. Transfer of EBV-specific CTL to prevent EBV lymphoma post bone marrow transplant. J. Clin. Apher. 1999;14(3):154–156. doi: 10.1002/(sici)1098-1101(1999)14:3<154::aid-jca9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 110.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]