Abstract
Background
Perinatal hypoxic ischemic encephalopathy (HIE) is a major cause of neurodevelopmental impairment including cerebral palsy and intellectual disability. Brain magnetic resonance imaging is the gold standard for acute assessment of cerebral injury in HIE. Limited data are available regarding the significance of clinically manifested neurobehavioral impairments in the neonatal period.
Aim
To evaluate brain structure-function relationships in newborns with HIE using diffusion tensor imaging (DTI) and the NICU Network Neurobehavioral Scale (NNNS).
Study Design
Prospective observational study with secondary longitudinal component.
Subjects
Forty-five newborns (62% male) with HIE referred for therapeutic hypothermia who underwent MRI and neurobehavioral assessment prior to discharge.
Outcome Measures
DTI was performed at median age of 8 days (range 5–16) and NNNS at median 12 days of life (range 5–20, postmenstrual age 40±2 weeks). Developmental assessment with the Bayley Scales of Infant Development-II was performed at median age of 21.6 months (range 20.8–30.6).
Results
Significant associations were observed between DTI corticospinal tract integrity and NNNS neuromotor performance in HIE newborns. Neonatal neuromotor performance was also related to later early childhood motor outcomes.
Conclusions
NNNS performed after therapeutic hypothermia in newborns with HIE can identify neuromotor abnormalities that are related to microstructural brain injury in the corticospinal tract and later motor outcomes in early childhood. These data support the NNNS as a valid early functional assessment of perinatal brain injury.
Keywords: newborn, magnetic resonance imaging, diffusion tensor imaging, neurobehavior, hypoxic ischemic encephalopathy
Introduction
Perinatal hypoxic ischemic encephalopathy (HIE) is a leading cause of infant mortality and long term neurologic disability (1, 2). Therapeutic hypothermia is the current standard of care for newborns with moderate to severe HIE (3). However, investigations to establish adjuvant neurotherapies are ongoing, as nearly half of affected infants continue to have significant brain injury despite treatment with hypothermia (4–6). Methods to assess and quantify areas of deficit in the neonatal period are needed to gauge efficacy of hypothermia and other neuroprotective interventions, and to guide rehabilitative therapies for infants with perinatal brain injury.
The NICU Network Neurobehavioral Scale (NNNS) is a comprehensive standardized assessment of neurological integrity and behavioral functioning in the high-risk newborn (7). We previously reported the use of the NNNS for quantitatively documenting neurological status in newborns with HIE after therapeutic hypothermia (8). While we observed a significant relationship between NNNS motor abnormalities and severe brain injury by conventional MRI, we seek to extend this work by further examining brain structural-anatomical correlations using more robust quantitative MRI methods.
Diffusion tensor imaging (DTI) is a quantitative MRI method that can provide reproducible measures of brain microstructural integrity (9). The relationship between DTI and NNNS has not been previously evaluated in the newborn. Evaluating the association between DTI measures of brain injury and quantitative measures of neurobehavioral performance in the neonatal period may enable the detection of subtle relationships between regional brain structure and functional phenotype, providing pathobiological context to clinical abnormalities detected at the bedside. Similarly, establishing a link between NNNS neonatal neurobehavioral performance and later neurodevelopmental outcomes will further establish the significance of neurobehavioral abnormalities identified in the newborn period, and whether they can provide an early bedside observable indicator of later developmental risk. While the NNNS has been linked to later developmental outcome in other high-risk neonatal populations (10–12), neonatal neurobehavioral abnormalities detected by the NNNS have not been previously related to later outcomes in survivors of HIE.
This goal of this work is to establish the clinical significance of neonatal neurobehavioral abnormalities in newborns with HIE. To that end, this study aims to examine the relationship between brain microstructural organization measured by quantitative DTI and neonatal neurobehavioral performance in infants with HIE. Specifically, we hypothesized that impaired brain microstructure in the corticospinal tract (CST) and corpus callosum (CC) would be associated with poorer neurobehavioral performance in motor and non-motor domains, respectively, in newborn survivors of HIE. Secondarily, we hypothesized that neonatal neurobehavioral performance would be associated with later neurodevelopmental outcome in patients followed through early childhood.
Methods
Participants
Newborns with HIE admitted for therapeutic hypothermia to a level 4 neonatal intensive care unit (NICU) in a free-standing children’s hospital were approached for enrollment in this prospective observational study. Participants were treated with whole body hypothermia according to established criteria (4) (i.e. infants were greater than 36 weeks gestational age and 1800 grams at birth, had no known or suspected chromosomal abnormality or major congenital anomaly, had metabolic acidosis and/or low Apgar scores, and exhibited signs of moderate to severe clinical encephalopathy). The study was approved by the Institutional Review Board and written informed consent was obtained from the parents of eligible participants. This study group represents a subset (who had available DTI data) of a larger cohort for whom neurobehavioral and conventional MRI data have been previously reported (8).
Magnetic Resonance Imaging
Image Acquisition
MRI was performed at target 7–10 days of life on a 1.5T scanner (Signa HDx, GE Healthcare, Milwaukee, WI, USA) using an 8-channel receive head coil (InVivo Corp., Gainesville, FL, USA). Infants were scanned without the use of sedation when possible. Standard sequences included sagittal and axial spin echo (SE) T1-W, axial dual echo SE proton density (PD) and T2-W images, and coronal fast spin echo (FSE) T2-W. A single shot SE echoplanar imaging (EPI) axial DTI sequence was used with the following parameters: TR 10,000 ms, TE 100 ms, acquisition matrix 128×128, slice thickness 4 mm, 1 volume with no diffusion weighting (b=0) and 25 volumes in non-colinear gradient directions with diffusion weighting (b=1000 s/mm2).
Preprocessing of Diffusion Tensor Images
A semi-automated MRI pipeline optimized for neonatal data was used for DTI preprocessing, culminating in the calculation of parametric maps for fractional anisotropy (FA), mean (MD), axial (AD) and radial (RD) diffusivity (13). These DTI-derived metrics characterize the underlying diffusion of water molecules within tissues. RD has been proposed as a measure of myelination (14, 15), while AD may represent fiber coherence and axonal membrane structure and integrity (14). FA reflects these and other factors including fiber diameter and density, as well as extracellular and interaxonal spacing (14–16). MD quantifies the average magnitude of water diffusion along the 3 primary axes, providing a general measure of tissue density.
Corticospinal Tract and Corpus Callosum Fiber Tracking
We selected CST and CC because of their known association with motor and cognitive function respectively (17). DTI tractography of these major white matter pathways was performed using Fiber Assignment by Continuous Tracking in DTI Studio software (Johns Hopkins University, Baltimore, MD) (18). For the CST, regions of interest were manually placed at the level of the cerebral peduncles, posterior limb of the internal capsule and centrum semiovale anterior to the central sulcus on axial images (Figure 1a–c) (19). Left and right CST measures were averaged for analysis. The CC was manually delineated on a mid-sagittal image, and right and left para-sagittal images (Figure 1d–e). Mean FA, MD, AD and RD were calculated for each tract within DTI Studio. Tractography was performed by a single investigator (A.N.M.) who was masked to the clinical and NNNS data of the subjects.
Figure 1.
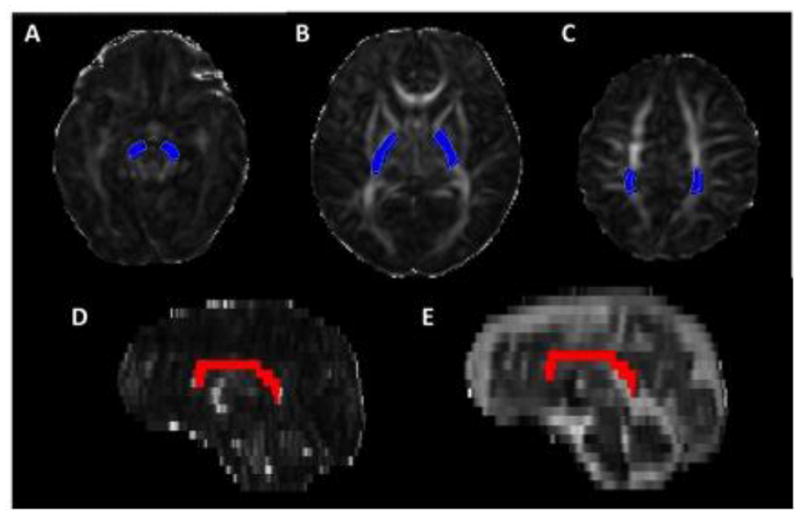
Visualization of white matter fiber tracts by diffusion tensor tractrography. Corticospinal tract was delineated by region of interest constraints at the level of the (A) cerebral peduncle, (B) posterior limb of the internal capsule (PLIC) and (C) centrum semiovale (CSO). Selected fibers are shown in blue on 2D axial images. Selected corpus callosum fibers are shown in red on (D) fractional anisotropy map and (E) T2-weighted trace images in the sagittal plane.
Neurobehavioral Assessment
The NNNS is a quantitative standardized assessment of biobehavioral organization in the neonate that can be easily and safely performed at the bedside (7). The examination consists of a series of administration and observation items that are individually scored, leading to an output of 13 summary scores that reflect performance in different motor and non-motor domains (20). The instrument has strong psychometric properties with reported Cronbach’s alpha ranging from .87–.90 for the summary scores (21, 22) and normative data are available from a large cohort of healthy term newborns (23). Certification to administer the NNNS is required after formalized instruction and reliability testing. The NNNS was performed in study participants at a target age of 14 days (or prior to discharge if earlier than 14 days) by a certified examiner who was blinded to clinical history and MRI findings of the subject. NNNS summary scores were conceptually grouped as previously described to calculate overall measures of motor and non-motor performance (8). Briefly, the sum of Hypertonia, Hypotonia, Asymmetry, Non-optimal Reflexes, Excitability, and Lethargy summary scores (reflecting counts of abnormal responses) comprised a Total Motor Abnormality Score, where a higher score represents increasing degree of motor abnormality. Conversely, z-scores (normalized to published values for healthy term newborns) for Habituation, Handling, Attention, Arousal, Regulation, and Stress/Abstinence were summated to provide a Total Non-motor Z-Score, where positive scores reflect better performance on given non-motor domains compared to norms. Quality of movement z-score was assessed separately as a measure of motor maturity.
Neurodevelopmental Assessment
Infants were assessed with the Bayley Scales of Infant Development (BSID-II) by a formally-trained and experienced developmental psychologist who was blinded to NNNS and MRI data (24). The BSID-II is a standardized assessment that includes a Mental Developmental Index (MDI) that assesses early cognitive, language and social skills, as well as a Psychomotor Developmental Index (PDI) that evaluates fine and gross motor development. BSID-II scores of 100 ± 15 represent the normative mean ± standard deviation. Children who were not testable at the time of evaluation (due to severe motor or cognitive impairment precluding administration of requisite basal items) were assigned a MDI or PDI score of 49 for analysis, as a score of 50 represents the floor of the assessment. Infants were assessed at 21 months of age as the earliest time point that coincided with our clinical follow-up schedule that would allow for more reliable assessment of language development. Although the third edition of the BSID was released in 2006 (25), the BSID-II continued to be used as the assessment tool in our developmental clinic during the study period and thus was the outcome measure used in this study.
Statistical Analysis
Descriptive statistics included standard measures of central tendency and variability for continuous data and frequencies for categorical variables. Linear regression models were developed to evaluate the association of NNNS score (dependent variable) with the corresponding DTI-derived metric (i.e. NNNS motor score with DTI measures in the CST and NNNS non-motor z-score with DTI measures in the CC). Separate models were developed for each DTI metric (FA, AD, RD, MD). Data were transformed when normality assumptions were not met. Multiple regression models were used to control for baseline characteristics including birthweight, gestational age, race, and gender, as well as clinical factors that could influence the relationship between NNNS and DTI (i.e. age at NNNS and age at MRI). If a significant relationship was observed between a DTI metric and composite NNNS score, exploratory models were also developed to evaluate the association between individual NNNS summary score components and the given DTI metric. In secondary analyses, composite scores that did not demonstrate significant associations with DTI measures were evaluated for cohesiveness by calculating Cronbach’s Alpha statistics. In the subset of patients for whom developmental outcome data were available, the associations between NNNS and Bayley scores were evaluated with linear regression models adjusting for similar demographic and clinical covariates. These models also included indicators of socioeconomic status (i.e. need for medical assistance and maternal education level) as these are known to influence developmental outcome. Statistical analysis was performed with STATA 11.0 software (StataCorp LP, College Station, TX, USA).
Results
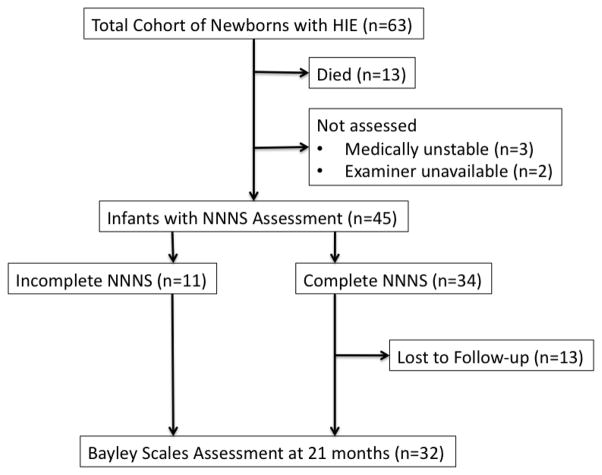
Of the 63 infants enrolled (Figure 2), 13 (20%) died prior to target age for NNNS examination. NNNS was not performed in another 5 eligible infants either due to medical instability precluding examination (n=3) or unavailability of an examiner at target age/discharge (n=2). NNNS was therefore performed in 45 infants at a median age of 12 days of life (range 5–20). Infants who did not undergo NNNS were more likely to have severe encephalopathy (p<0.001), have lower 1-minute Apgar Score (p=0.032) and lower presenting pH (p=0.003) compared to those who underwent NNNS. Of the 45 participants who underwent NNNS examination, 34 infants had complete assessment with requisite number of items for calculation of Total Non-motor Z-score. Infants who were unable to tolerate complete assessment were more likely to have severe encephalopathy at presentation (p=0.028) and history of electrographic seizure (p=0.001). MRI was performed in study patients at a median age of 8 days (range 5–16). A total of 32/45 (71%) infants were assessed with the BSID-II at 21 months. There were no significant differences between infants assessed and those lost to follow-up except for indicators of SES. Infants lost to follow-up were more likely to be recipients of medical assistance (38.5% vs 6% assessed, p=0.007) and had lower proportion of college-level maternal education level (23% vs 56%, p=0.043). Characteristics of the study population are presented in Table 1.
Figure 2.
Study population flow chart depicts attrition and available data used for analyses.
Table 1.
Characteristics of the Study Population
| Overall Cohort (n=63) |
Underwent NNNS (n=45) |
Complete NNNS (n=34) |
21 month BSID-II (n=32) |
|
|---|---|---|---|---|
| Birthweight (mean ± SD Kg) | 3.36 ± 0.7 | 3.36 ± 0.7 | 3.26 ± 0.7 | 3.45 ± 0.8 |
| Gestational Age (mean ± SD weeks) | 38.8 ± 1.9 | 38.7 ± 1.9 | 38.7 ± 2 | 39 ± 1.9 |
| Gender (n, %male) | 39 (62) | 29 (64) | 23 (68) | 20 (62) |
| Apgar Score | ||||
| 1 minute | 1 (0–6)a | 1 (0–6)* | 2 (0–5) | 1 (0–6) |
| 5 minute | 3 (0–7)a | 3 (0–7) | 4 (0–7) | 3 (0–7) |
| 10 minute | 5 (0–9)b | 5 (0–9)c | 5 (0–7) | 5 (0–9)d |
| Presenting pH | 6.87 (6.5-7-4)e | 6.94 (6.5–7.35)f* | 7.0 (6.69–7.34) | 6.9 (6.5–7.35)g |
| Base Deficit | 19 (8–36)h | 17 (8–36)i | 17 (8–36) | 18 (9–36) |
| Electrographic Seizure, n (%) | 22 (35) | 15 (33) | 7 (21)** | 12 (37) |
| Encephalopathy Grade (n, %) | ||||
| Moderate | 43 (68) | 38 (84) | 31 (91) | 26 (81) |
| Severe | 20 (32) | 7 (16)* | 3 (9)** | 6 (19) |
| Age at NNNS (days) | 12 (5–20) | 12 (5–20) | 12 (5–16) | 12 (5–20) |
| Age at MRI (days) | 8 (5–16) | 8 (5–16) | 8 (5–16) | 8 (5–16) |
Data presented as median (range) unless otherwise indicated
Data available for a62/63, b55/63, c38/45, d28/32, e61/63, f44/45, g31/32, h54/63, i39/45 infants
Significant difference between infants who did vs did not undergo NNNS (p>0.05)
Significant difference between infants with complete vs incomplete NNNS (p>0.05)
Relationship between Neurobehavioral Performance Across Motor Domains and Corticospinal Tract Microstructural Integrity
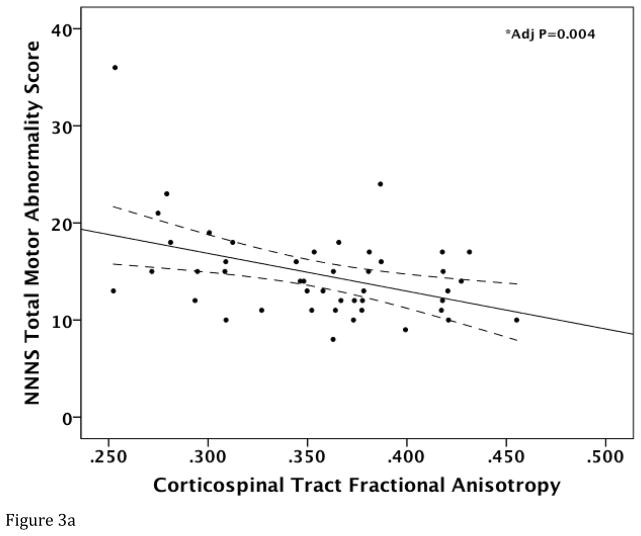
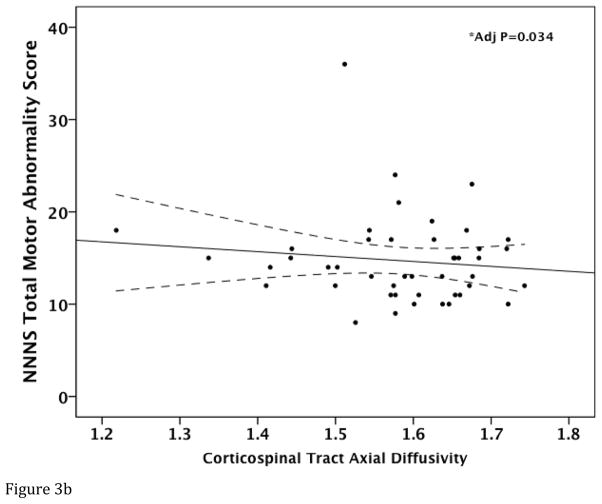
Corticospinal Tract FA (regression coefficient (B)=0.222; standard error (SE)=0.072; 95% confidence interval (CI)= 0.076, 0.368; p=0.004) and AD (B=0.054; SE=0.004; 95% CI= 0.004–0.103; p=0.034) were lower in infants with more motor abnormality (i.e. higher NNNS Total Motor Score) after adjusting for covariates (Figure 3A–B). While there was a trend towards a positive relationship between CST RD and NNNS Total Motor Score (p=0.085), this was not significant (p=0.216) after demographic and clinical variables were controlled. CST MD was not significantly associated with NNNS Total Motor Score (p>0.05). NNNS Quality of Movement Score was not significantly associated with any of the CST DTI metrics (p>0.05).
Figure 3.
Relationship between Total NNNS Motor Abnormality Score and corticospinal tract DTI measures. Raw data scatter plots are presented with unadjusted regression line and 95% confidence intervals (dotted line) for (A) fractional anisotropy and (B) axial diffusivity. *Denotes significant P value after adjusting for covariates.
In evaluating the individual NNNS summary scores, the summary scores for Hypertonia (p=0.036) and Non-optimal Reflexes (p=0.076), demonstrated significant or trend towards significant individual relationships with CST FA respectively. Thus, these components of the NNNS Total Motor Score were the most important contributors to the relationship between CST FA and NNNS Motor performance. CST AD was not individually associated with any of the component NNNS motor summary scores (p>0.05).
Relationship between Neurobehavioral Performance Across Non-Motor Domains and Corpus Callosum Microstructural Integrity
Total Non-motor Z-score was not significantly related to DTI metrics in the CC (p>0.05), thus individual component summary scores were not further explored. In secondary analysis, Total Non-motor Z-score was recalculated after removal of Habituation and Stress summary scores, as these subscales demonstrated low item-scale correlation (Habituation r=0.392 and Stress r=0.358) in our dataset. The reduced composite Total Non-motor Z-score trended towards a positive association with CC MD (B=6.08; SE =3.25; 95% CI= −0.6 – 12.76; p=0.073), but was not associated with CC RD (p = 0.118), CC AD (p = 0.128) or CC FA (p = 0.591).
Relationship between Neonatal Neurobehavioral Performance and Neurodevelopmental Outcome at 21 months
Developmental outcomes were assessed in 32/45 (71%) of study participants at a median age of 21.6 months (range 20.8–30.6). NNNS Total Motor Score was negatively associated with Bayley PDI after adjusting for covariates (p=0.040). NNNS Non-motor Z-score was not significantly related to Bayley MDI (p=0.521), although of note this model included a limited number of observations as only 21/32 (66%)of the infants assessed at 21 months had complete NNNS with requisite items for calculation of Total Z-score. The reduced NNNS Non-motor Z-score was likewise not statistically significantly associated with Bayley MDI (p=0.194).
Discussion
In this study, we sought to establish the clinical significance of neonatal neurobehavioral performance by examining early structural (MRI-DTI)-functional (NNNS) relationships in newborns with HIE following therapeutic hypothermia. We demonstrate that early neuromotor abnormalities detected by the NNNS in HIE newborns is associated with impaired CST microstructure and poorer psychomotor outcome in early childhood. Conversely, NNNS performance in non-motor domains was not significantly related to CC microstructural integrity or later outcomes. These data support the use of the NNNS as a valid assessment of neuromotor function, while the utility of the NNNS as a measure early neurobehavioral integrity across non-motor domains requires further evaluation in this population.
Assessing the presence, severity and significance of perinatal brain injury in the acute neonatal period remains a diagnostic challenge in clinical neuroscience. Standard neurological exam and severity classification scales according to Sarnat (26) and others (27, 28) have been demonstrated to have variable predictive ability for identifying later neurodevelopmental impairment in newborns with HIE. The NNNS is an attractive alternative to this traditional approach, as it provides quantitative measures rather than broad normal versus abnormal classifications. Additionally, it allows for more detailed evaluation of individual neurobehavioral domains. For example, we demonstrated that hypertonia and non-optimal responses to primitive reflex pathways are particularly important signs that distinguish newborns with altered CST integrity. This aspect of the NNNS is particularly important in newborns with HIE, as it has been well described that selective vulnerability to different types and durations of cerebral insults can lead to variable patterns of injury and outcome phenotypes (29, 30).
The strengths of the current study include the incorporation of advanced quantitative MRI techniques to gauge microstructural brain injury. While conventional brain MRI has been established as a reliable predictor of neurodevelopmental outcome after HIE (31, 32), it depends on interpretation by an experienced pediatric neuroradiologist and can therefore be subjective. Additionally, similar to clinical exam, conventional MRI interpretation allows for discrete injury classifications (e.g. mild, moderate, severe), rather than reproducible continuous measures that can provide more robust granularity to quantifying severity of injury. Quantitative DTI, particularly when used to assess regional brain injury, provides a powerful method to interrogate specific brain structural-functional relationships.
Despite the advantage of using quantitative measures of neurobehavioral function and anatomical injury, we were not able to demonstrate a significant relationship between NNNS performance in non-motor domains and callosal injury or later cognitive outcome. These findings are consistent with our prior report, where the association between Total Non-motor Z-score and severe conventional MRI injury did not remain significant after adjusting for covariates (8). However, we caution against the conclusion that NNNS cannot identify significant abnormalities in the non-motor domains based on these data, given the limited sample size that was included in these analyses. Even at 2 weeks of age, several infants were unable to undergo the requisite number of items to calculate total z-scores, which may be considered an inherent limitation of the exam in a critically-ill population. We purposely standardized the NNNS assessment time at 14 days of life to limit age-dependency of performance. Despite this it is recognized that nearly half (n=23) of infants were discharged prior to 14 days and thus assessed earlier, leading to the inclusion of age at NNNS assessment as a covariate in our regression models. It is possible that more complete data would be acquired if the exam were deferred until full clinical stability was achieved. It is also possible that alternative rubrics to evaluate NNNS performance across varied non-motor domains may yield different results, as the Total Non-motor Z-score proposed has not been widely validated. While the conceptual grouping of these items allows for an overall functional measure to evaluate structure-function relationships, we acknowledge that relationships between individual summary scores or alternative groups of summary scores and structural brain injury may be established in future studies. While we explored an alternative non-motor composite score by excluding items with low item-scale correlation in our secondary analyses, the Cronbach Alpha of the reduced NNNS Z-score remained low (α=0.57). Our limited sample size precluded robust factor analyses that could establish a more reliable composite score to reflect performance across non-motor domains, although this will be a focus of future work. Thus, the significance of NNNS abnormalities identified in the non-motor domains will require further investigation.
This study has additional limitations apart from the acknowledged limited sample size. Although a rare occurrence, that some eligible infants were not assessed due to unavailability of an examiner at target age may introduce selection bias. Also, for practical purposes, reliability across examiners was not assessed although all assessments were performed by examiners certified for reliability in administration and scoring. Likewise, follow-up attrition, as with any longitudinal study, was another potential source of bias. Although it is reassuring that there were no significant differences between infants lost to follow-up and those assessed at 21 months with regards to baseline clinical characteristics, that those lost to follow-up represented a higher SES risk profile remains a concern. Further study is needed to better define the relationship between NNNS abnormalities in the newborn period and later neurodevelopmental outcome. Finally, although the current edition of the Bayley Scales (BSID-III) was available during the study period, our clinical follow-up program continued to use the BSID-II in order to have comparable data to previously evaluated babies with HIE (4, 5), and because of initial concerns that the BSID-III was reported to oeverestimate developmental progress (33, 34). Further study may be needed to evaluate the relationship with neonatal NNNS performance and developmental outcome assessed by the BSID-III.
Conclusions
In newborns with HIE, neuromotor abnormalities assessed by the NNNS following therapeutic hypothermia are related to microstructural brain injury in the corticospinal tract and later motor outcomes in early childhood. Further study is warranted to evaluate the utility the NNNS for the acute assessment of significant functional deficits across neurobehavioral domains after perinatal brain injury.
HIGHLIGHTS.
Brain structure-function relationships were evaluated in newborns with HIE
Neonatal neurobehavior was evaluated with the NICU Network Neurobehavioral Scale
White matter microstructural integrity was evaluated with diffusion tensor imaging
Relationships were seen between neonatal neurobehavior and microstructural injury
Acknowledgments
The authors acknowledge Yao (Iris) Cheng, M.S. for her data management and statistical support, and Christen Meisel, M.S. and Maya B. Coleman Ph.D. for their efforts coordinating neurobehavioral assessment and developmental follow-up visits for study participants. This publication was supported by the Clinical and Translational Science Institute at Children’s National (NIH UL1TR000075, KL2TR000076), the Intellectual and Developmental Disabilities Research Consortium (NIH P30HD040677), and The Advanced Pediatric Brain Imaging Research and Training Program (DoD W81XWH-11-2-0198).
Footnotes
Conflict of interest statement
The authors have no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dilenge ME, Majnemer A, Shevell MI. Long-term developmental outcome of asphyxiated term neonates. J Child Neurol. 2001 Nov;16(11):781–92. doi: 10.1177/08830738010160110201. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012 Jun 9;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011 Nov;159(5):851–8. e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009 Oct 1;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011 Aug;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 7.Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004 Mar;113(3 Pt 2):634–40. [PubMed] [Google Scholar]
- 8.Coleman MB, Glass P, Brown J, Kadom N, Tsuchida T, Scafidi J, et al. Neonatal neurobehavioral abnormalities and MRI brain injury in encephalopathic newborns treated with hypothermia. Early Hum Dev. 2013 Sep;89(9):733–7. doi: 10.1016/j.earlhumdev.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995 Nov-Dec;8(7–8):333–44. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Bann C, Lester B, Tronick E, Das A, Lagasse L, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010 Jan;125(1):e90–8. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephens BE, Liu J, Lester B, Lagasse L, Shankaran S, Bada H, et al. Neurobehavioral assessment predicts motor outcome in preterm infants. J Pediatr. 2010 Mar;156(3):366–71. doi: 10.1016/j.jpeds.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester BM, Bagner DM, Liu J, LaGasse LL, Seifer R, Bauer CR, et al. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics. 2009 Nov;124(5):1355–62. doi: 10.1542/peds.2008-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelou I, Serag A, Bouyssi-Kobar M, Du Plessis A, Limperopoulos C. Optimized methodology for neonatal diffusion tensor imaging processing and study-specific template construction. Conf Proc IEEE Eng Med Biol Soc; 2014; pp. 2372–5. [DOI] [PubMed] [Google Scholar]
- 14.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002 Nov;17(3):1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, An H, Zhu H, Jewells V, Armao D, Shen D, et al. Longitudinal regression analysis of spatial-temporal growth patterns of geometrical diffusion measures in early postnatal brain development with diffusion tensor imaging. Neuroimage. 2011 Oct 15;58(4):993–1005. doi: 10.1016/j.neuroimage.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002 Dec;12(12):1237–43. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 17.Aralasmak A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Yousem DM. Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr. 2006 Sep-Oct;30(5):695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]
- 18.Melhem ER, Mori S, Mukundan G, Kraut MA, Pomper MG, van Zijl PC. Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol. 2002 Jan;178(1):3–16. doi: 10.2214/ajr.178.1.1780003. [DOI] [PubMed] [Google Scholar]
- 19.Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr. 2010 Jun;156(6):882–8. 8 e1. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004 Mar;113(3 Pt 2):641–67. [PubMed] [Google Scholar]
- 21.Fink NS, Tronick E, Olson K, Lester B. Healthy newborns’ neurobehavior: norms and relations to medical and demographic factors. J Pediatr. 2012 Dec;161(6):1073–9. doi: 10.1016/j.jpeds.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics. 2004 Mar;113(3 Pt 2):668–75. [PubMed] [Google Scholar]
- 23.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004 Mar;113(3 Pt 2):676–8. [PubMed] [Google Scholar]
- 24.Bayley N. Manual for the Bayley scales of infant development. New York: Psychological Corporation; 1969. [Google Scholar]
- 25.Bayley N. Bayley Scales of Infant Development. 3. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 26.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976 Oct;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 27.Miller SP, Latal B, Clark H, Barnwell A, Glidden D, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004 Jan;190(1):93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran S, Laptook AR, Tyson JE, Ehrenkranz RA, Bann CM, Das A, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2012 Apr;160(4):567–72. e3. doi: 10.1016/j.jpeds.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005 Apr;146(4):453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 30.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004 Apr;30(4):227–35. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010 Jan;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012 Nov;97(6):F398–404. doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010 Apr;164(4):352–6. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 34.Msall ME. Measuring outcomes after extreme prematurity with the Bayley-III Scales of infant and toddler development: a cautionary tale from Australia. Arch Pediatr Adolesc Med. 2010 Apr;164(4):391–3. doi: 10.1001/archpediatrics.2010.25. [DOI] [PubMed] [Google Scholar]