Abstract
Parkinson disease (PD) is a progressive neurodegenerative disease that leads to a wide range of motor and non-motor deficits. Specifically, voice and swallow deficits manifest early, are devastating to quality of life, and are difficult to treat with standard medical therapies. The pathological hallmarks of PD include accumulation of the presynaptic protein alpha-synuclein as well as degeneration of substantia nigra dopaminergic neurons. However, there is no clear understanding of how or when this pathology contributes to voice and swallow dysfunction in PD. In the present study, we evaluated the effect of loss of function of the PTEN-induced putative kinase 1 gene in rats (PINK1 −/−), a model of autosomal recessive PD in humans, on vocalization, oromotor and limb function, and neurodegenerative pathologies. Behavioral measures included ultrasonic vocalizations, tongue force, biting, and gross motor performance that were assayed at 2, 4, 6, and 8 months of age. Aggregated alpha-synuclein and tyrosine hydroxylase immunoreactivity were measured at 8 months. We show that compared to wildtype controls PINK1 −/− rats develop (1) early and progressive vocalization and oromotor deficits; (2) reduced tyrosine hydroxylase immunoreactivity in the locus coeruleus that correlates with vocal loudness and tongue force; and (3) alpha-synuclein neuropathology in brain regions important for cranial sensorimotor control. This novel approach of characterizing a PINK1 −/− genetic model of PD provides the foundational work necessary to define behavioral biomarkers for the development of disease-modifying therapeutics for PD patients.
Keywords: Parkinson disease, cranial-sensorimotor, ultrasonic vocalization, alpha synuclein, rat, PINK1, RRID: AB_390204, AB_1977522
Graphical abstract
Introduction
Parkinson disease (PD) is a neurodegenerative condition that affects nearly 10 million people worldwide (de Lau et al. 2004). The pathological hallmarks of PD include the development of alpha-synuclein (aSyn) aggregates within Lewy bodies, and loss of nigrostriatal dopamine (DA) neurons. In addition to the cardinal motor symptoms, up to 90% of PD patients will develop dysarthria (disordered voice/speech) and/or dysphagia (disordered swallowing) that negatively impact quality of life and increase risk of aspiration pneumonia, the leading cause of death in PD (Ho et al. 1998; Marras et al. 2008; Michou and Hamdy 2010). While much is known about the pathology underlying the cardinal motor symptoms, the neural correlates and mechanisms underlying cranial sensorimotor dysfunction in PD are poorly understood, and consequently deficits remain undertreated.
Studies show unilateral degeneration of nigrostriatal DA neurons in rats induced by the neurotoxin 6-hydroxydopamine leads to impaired ultrasonic vocalization (Ciucci et al. 2007), swallowing (Russell et al. 2012), chewing (Plowman et al. 2013), and licking (Ciucci et al. 2013b) similar to dysarthria and dysphagia found in PD patients. In addition, unilateral injections of recombinant adeno-associated virus expressing human wild-type aSyn into the substantia nigra (SNpc) results in ultrasonic vocalization deficits in rats (Gombash et al. 2013). However, as in humans, vocalization deficits in the 6-hydroxydopamine rat model are resistant to standard DA replacement therapy (Ciucci et al. 2013a). Thus, while the nigrostriatal system clearly contributes to aspects of vocalization function, structures outside of the SNpc may be implicated in vocalization and swallowing impairments. Indeed, mice over-expressing human wildtype aSyn show early vocalization deficits in the absence of nigrostriatal DA cell loss (Grant et al. 2014). These mice also develop proteinase K-resistant aSyn aggregates in the periaqueductal gray (PAG) (Grant et al. 2014), locus coeruleus (LC), and SNpc (Fernagut et al. 2007) suggesting aSyn pathology may be a key contributor in PD vocalization deficits.
Genetic models of PD offer an opportunity to identify behavioral deficits and early pathology associated with the disorder. Mutations in the PTEN-induced putative kinase 1 gene (PINK1) are the second most common cause of autosomal recessive PD (Houlden and Singleton 2012) and are also implicated in sporadic cases (Valente et al. 2004). The function of PINK1 remains unclear, but studies implicate PINK1 in mitochondrial function and mitophagy (Geisler et al. 2010). In humans, PINK1 genetic variants cause progressive functional deficits and nigrostriatal DA cell loss (Bonifati 2012; Bonifati et al. 2002; Guo et al. 2011). It has been reported that PINK1 knock-out (−/−) rats develop metabolic and mitochondrial pathogenesis (Villeneuve et al. 2014) as well as sensorimotor deficits and nigrostriatal DA cell loss (Dave et al. 2014) in the early stages indicating they are an ideal model to investigate early vocalization and oromotor function related to PD. However, the impact of the loss of PINK1 function on cranial sensorimotor behaviors has not been characterized. To address this gap in knowledge, we measured vocalization and oromotor function in PINK1 −/− homozygous (PINK1 −/−), PINK1 heterozygous (PINK1 +/−), and wildtype (WT) rats at 2, 4, 6, and 8 months (mo) of age. Tyrosine hydroxylase immunoreactivity (TH-ir) in striatum (SR), SNpc, and LC were quantified. Additionally, proteinase K-resistant aSyn aggregations in the PAG, SR, LC and SNpc were assayed.
Materials & Methods
Animals
Male Long-Evans rats (PINK1 −/− n=16, PINK1 +/− n=16, WT n=16) were generated and obtained from SAGE® Laboratories (Dave et al. 2014) and housed in pairs (like genotypes were paired together) in standard polycarbonate cages, on a 12:12 hour (hr) reverse light cycle with testing occurring during the dark period in partial red light illumination. All rats arrived at 6 weeks of age, were handled and acclimated to the tasks for 2 weeks prior to the first testing cycle. Food and water were provided ad libitum, except for the tongue function testing days, which required overnight water restriction, and the pasta biting testing days, which required overnight food restriction. Rats were tested at 2, 4, 6, and 8 mo of age for vocalization and tongue function. Additionally, motor performance (tapered balance beam) and pasta biting (chewing), was assessed at 4 and 8 mo of age, and spontaneous activity assessment (cylinder) was performed at 8 mo of age. All rats in each of the genotypes underwent each of the behavioral assays. Due to the availability and nature of generating this genetic strain, rats were tested in two cohorts with all of the PINK1 +/− and half of the wild type in one cohort and all of the PINK1 −/− in the other with the remaining wild type rats. The wild type rats were evenly distributed across the two cohorts. A subset of rats from each genotype was randomly selected and euthanized after behavioral testing at 4 mo (n=4 per genotype, for pilot analysis not discussed here) and the remaining cohort was euthanized at 8 mo (n=12 per genotype). All procedures were approved by the University of Wisconsin-Madison Animal Care and Use Committee (IACUC-Protocol M02505) and were conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MA) as well as the Use of Animals and Humans in Research published by the Society for Neuroscience.
Behavioral Tests
Ultrasonic Vocalization
Ultrasonic vocalizations in rats and vocal communication in humans are controlled by similar physiological mechanisms (Johnson et al. 2010; Riede 2011; Riede 2013; Riede 2014; Van Daele and Cassell 2009) IN addition, rat ultrasonic vocalizations serve a communicative function as they are semiotic in nature and capable of eliciting a response out of the signal-recipient (Brudzynski 2009; McGinnis and Vakulenko 2003). Given this, rat vocalizations have been used previously to relate neural mechanisms to vocal parameters (Ciucci et al. 2009; Ciucci et al. 2007). Recordings were made using an ultrasonic microphone (CM16, Avisoft, Germany) with 16-bit depth and sampling rate of 250-kilohertz (kHz). The microphone was mounted 15 centimeters (cm) above a standard polycarbonate rat cage. Male rats were placed alone in the homecage with a sexually receptive female. When the male demonstrated interest in the female (sniffing, mounting, chasing), the female was removed and recording captured only male vocalizations for 90 seconds (sec). Offline acoustic analysis was performed with a customized automated program using SASLab Pro (Avisoft, Germany). Spectrograms were built from each waveform with the frequency resolution set to a Frequency Fourier Transformation (FFT) of 512 points, frame size of 100%, flat top window, and the temporal resolution set to display 75% overlap. Vocalization analysis was performed by experienced raters masked to experimental condition. Each call was visually and acoustically inspected, noise was removed, and calls were manually classified to determine an overall call profile (Wright et al. 2010). The following acoustic variables were analyzed: average bandwidth in Hertz (Hz), average peak frequency in kHz, average duration in sec, average intensity in decibels (dB), and percent complex calls (Ciucci et al. 2009; Johnson et al. 2011).
Tongue Function
Tongue protrusion, akin to licking, is intricately involved in swallowing, and assays of tongue function have previously been used to better understand changes with aging, disease, and pharmacological manipulations (Ciucci and Connor 2009; Connor et al. 2009). Tongue forces were acquired using a specialized device that allows for modification and measurement of tongue forces during complex protrusive tongue movements. This set-up involves a traditional learning paradigm in which access to water is gradually restricted, and rats are trained to press a disk with their tongue in exchange for a water reward (reference Connor et al., 2009 and Ciucci and Connor, 2009 for details). Measuring behavioral tongue forces involves pressing the tongue against the operandum on a variable ratio 5 reinforcement schedule with force requirements incremented up based on performance throughout the session. Prior to testing, water was restricted for 21 hr. On the day of testing, animals were placed individually into a polycarbonate enclosure equipped with a 1 × 1 cm aperture and force operandum that delivered aliquots of water based on tongue press behaviors. When the rat pressed with the required force, an aliquot of water was delivered. Testing was performed over 3 consecutive days and participation on each testing day was quantified. Data from the day with the highest participation values were analyzed (see Ciucci et al 2011), consistent with previous research. Tongue presses were recorded at 200 Hz using custom-designed computer data acquisition software (Matrix Product Development, Cottage Grove, WI) and analyzed with custom designed algorithms in MATLAB software (MathWorks®, Natick, MA). A waveform for each press waveform has an onset time, offset time, and peak force generated. From this waveform, we calculated maximal amount of force per session in milliNewtons (mN), the average of all forces per session in mN, and the standard deviation of forces in mN. Additionally, lick rate and licks per sec was calculated in 30 sec intervals, up to 120 sec. Body weight was used as a covariate.
Pasta Biting
Acoustic and temporal patterns of biting during consumption of pasta are an established method of assaying oromotor function related to eating and has been described previously (Allred et al. 2008). Pasta biting acoustic signals were recorded during consumption of a 7 cm piece of Vermicelli pasta. A condenser microphone (Digidesign, Burlington, MA) was suspended 10 cm above the home cage and ProTools LE software (Digidesign: Version 8.0.3) with a sampling rate of 44.1 kHz and 16-bit depth was used to capture biting acoustics. Files were noise-reduced, exported, and analyzed with a custom code written in house using MATLAB (MathWorks®, Natick, MA). Bite intensity in dB and interbite regularity in milliseconds (ms) were measured. Data was recorded for 5 trials at each timepoint, analyzed by a rater masked to genotype, and the values from 5th trial to capture effects from fatigue were used for statistical analysis.
Tapered Balance Beam
This task assesses motor performance and coordination as each rat traversed a ledged, tapered beam (Lafayette Instrument Company, Lafayette, IN) (Fleming et al. 2004). Briefly, the testing apparatus (overall length 165 cm) consisted of an elevated, tapered beam (135 cm; starting width: 6.35 cm, ending width: 1.27 cm) of High Density Polyethylene plastic with a safety walk non-slip material on the top surface. The beam was suspended 1 meter (m) above the ground, with the loading platform (15 cm × 6 cm) resting on a cart and the unloading platform (15 cm × 2 cm) resting on the homecage, to encourage the rat to move across the beam. After 3 days of 5 trials to acclimate to the task, each rat was placed on the loading platform and was allowed to traverse the beam toward the homecage for a total of 5 trials. All trials were recorded (Sony HDR-CX210, New York, NY), and recordings were viewed and rated in slow motion by a rater masked to genotype using Windows Media Player (Microsoft, Redmond, VA). The following dependent variables were assessed: time to traverse the beam (sec), time to traverse the final third of the beam (sec), and the number of foot faults along the length the beam. Foot faults were defined as times when the rat stepped off the upper surface and relied on the lower ledge. Foot faults were not counted if the rat was not moving forward or if the animal’s head was oriented to either side of the beam.
Spontaneous Activity
Spontaneous activity was measured in a transparent cylinder (20 × 30 cm) similar to Fleming et al. (2004). The cylinder was placed on a piece of glass and a camera (Sony HDR-CX210, New York, NY) was positioned below to allow a clear view of movements along the ground and walls of the cylinder. Recordings were viewed and rated in slow motion by a rater masked to genotype. The number of forelimb and hindlimb steps over a two-minute period was measured for each rat.
Immunohistochemistry and Quantification Analysis
Following the completion of testing, rats were deeply anesthetized with 5% isoflurane, transcardially perfused with 200 ml of cold saline followed by 500mL of cold 4% paraformaldehyde in 1% phosphate buffered saline (PBS). Fixed brains were excised, post-fixed for 24 hr in 4% paraformaldehyde at 4 °C, submerged in 0.02% sodium azide in 0.1M PBS solution and mounted on a freezing microtome where 60 micron coronal sections were harvested through the cortex and brainstem. Free-floating brain sections were stored in cryoprotectant at −20 °C until they were stained for immunoreactivity over every 6th section. For this study, sections were immunolabeled for (1) Tyrosine hydroxylase immunoreactivity (TH-ir) and (2) insoluble proteinase K-resistant aSyn immunoreactivity (aSyn-ir) (Table 1).
Table 1.
Antibody information.
| Antibody | Immunogen Target |
Manufacture, Catalog, Lot number, RRID |
Raised Species |
Type | Concentration |
|---|---|---|---|---|---|
| Anti-Tyrosine Hydroxylase | Tyrosine Hydroxylase (NCBI Gene ID: 25085) | EMD Millipore, Cat # AB152, RRID: AB_390204 | Rabbit | Polyclonal | 1:2000 |
| Anti-alpha-Synuclein | alpha-Synuclein (N-term) clone EP1646Y Rabbit | EMD Millipore, Cat #04-1053, RRID: AB_1977522 | Rabbit | Monoclonal | 1:250 |
Methodological information for the TH and aSyn antibodies used in this study.
Antibody Characterization
The immunohistochemical analysis was run in a single batch for each respective chemical assay to minimize differences in batch variability. Additionally, control sections incubated without primary antibody were devoid of immunoreactivity. Specificity of the antibody was also assessed by the manufacturer; western immunoblot detected bands at the expected molecular weight of 62 kDa for TH and 14–19kDa for aSyn (Millipore, Billerica, MA). The antibody stains the appropriate pattern of cellular morphology and distribution as demonstrated in previous publications (Ciucci et al. 2007; Grant et al. 2014)
TH-ir
Tissue sections containing the SNpc (at the approximate level of Bregma −4.56), SR(at the approximate level of Bregma 2.04), and LC (at the approximate level of Bregma −9.48) were blocked in 20% normal goat serum solution, incubated overnight in primary solution: polyclonal rabbit anti-TH at 1:2000 (RRID:AB_390204, AB152, Millipore, Billerica, MA) as described previously (Ciucci et al. 2007). Subsequently, samples were incubated in conjugated biotinylated secondary solution at 1:500 (Millipore, Billerica, MA) for 2 hr, incubated in an avidin-biotin solution (Vector Laboratories, Burlingame, CA) for 1 hr, and the complex was visualized using filtered 3, 3-diaminobenzidine (DAB, Sigma Aldrich, St. Louis, MO) with 0.02% hydrogen peroxide. All sections were float mounted onto gelatin-coated slides, dehydrated in a graded series of alcohols and xylenes, and coverslipped using Cytosol™ 60 mounting medium (Richard-Allen Scientific, Kalamazoo, MI).
For TH-ir analysis, a Leica DFC310 FX Camera (Leica Microsystems Inc., Buffalo Grove, IL) connected to a microscope (Nikon Eclipse E600, Melville, NY) and a computer were used to acquire images of TH-ir immunolabeled brain tissue using MetaMorph® Microscopy Automation and Image Analysis Software version 7.8.4 (Molecular Devices LLC, Sunnyvale, CA). Using bright-field microscopy, the optical density of TH-ir labeled terminals was quantified bilaterally, rostral-to-caudal, from 3 serial sections in SR at 20X magnification. Anatomically equivalent sections were used from each animal. In brief, standardized rectangle boxes were placed selectively on digital photomicrographs within the set boundaries of SR (0.48 mm × 0.39 mm) based on the histological criteria of the rat atlas (Paxinos and Watson 2005). This resulted in a total of 6 measurements per animal which were then averaged. Optical density measurement quantifies the opacity of the tissue when exposed to transmitted light, calculated as the inverse logarithm of the grayscale transmittance. The MetaMorph® autoscale function was used to calculate the correct exposure of the image as a percent of the total range of light as a method to reduce variation in background between individual animals. Then, a computer-generated inclusive threshold was used to select labeled material that two observers, masked to the genotype and behavioral outcomes, agreed to represent TH-ir immunolabeling. The threshold value for measurement inclusion was based on the grayscale values of the 8-bit photomicrograph. In cases of tissue damage, labeling was quantified on an additional fourth section, or the individual was dropped from analysis.
Cell counts in the SNpc and LC were performed using a total enumeration protocol that eliminates the possibility of neuron splitting and yields counts comparable to unbiased stereological methods (Tramontin et al., 1998; Gombash et al., 2014). Briefly, the number of discrete immunolabeled soma within a standardized rectangle counting area in SNpc (.29 mm × .42 mm) and LC (0.43 mm × 0.49 mm) was quantified using the MetaMorph® cell counter by placing digital marks onto the nucleolus in photomicrographs at 10X magnification. TH-ir positive digital markers were placed bilaterally, rostral-to-caudal, on 3 serial sections for SNpc (2 for LC) serial sections and averaged for each animal. Anatomically equivalent sections were used from each animal. Correlational analyses were performed on the cell counts in the SNpc and LC with behavioral outcomes.
Because a reduction in TH-ir can develop in the absence of actual cell loss TH-ir labeled tissue in the SNpc was counterstained with haematoxylin in order to study integrity of dopaminergic cells. Briefly, coverslips were removed, slices were quenched by rehydrating through alcohol dilutions, counterstained (SH30, Harris’ Modified Haematoxylin with Acetic Acid, Fisher, Waltham, MA, USA), dehydrated and re-coverslipped. Using the Image J Cell Counter Macro (National Institutes of Health), the number of TH-ir and TH-ir+HE counterstained labeled cells within the SNpc were qualified by an investigator, masked to genotype or behavior, on the digital images (box dimension: 1.13mm × 0.85mm). The number of TH-ir and TH-ir+HE cells were averaged across two sections for the SNpc (left and right hemispheres) for each animal. Anatomically equivalent sections were used from each animal.
Insoluble proteinase K resistant aSyn-ir
Free floating tissue sections were pretreated with proteinase K at 1:4000 (Invitrogen, Grand Island, NY) for 10 min. Sections were blocked in 20% normal goat serum and incubated overnight in primary solution: monoclonal rabbit anti-aSyn at 1:250 (RRID:AB_1977522, 04–1053, Millipore, Billerica, MA). Samples were incubated in conjugated biotinylated secondary solution at 1:500 for 90 min, incubated in an avidin biotin solution for 1 hr, visualized using filtered DAB with 0.02% hydrogen peroxide and coverslipped as described above.
The presence or absence of insoluble proteinase K-resistant aSyn-ir and/or the number of aggregates were counted on a single brain section containing PAG, SNpc, SR, LC, and nucleus ambiguus (AMB) respectively. Each region was assessed semi-quantitatively and received a score based on the number of visible aggregations: 0 (absent), 1–5 (slight), 6–10 (moderate), or >10 (severe) (Braak et al. 2003). A second investigator confirmed these findings.
Additionally, two images from the dorsal medial and lateral PAG (DMPAG, LPAG) in each animal bilaterally were acquired using MetaMorph® at 20X magnification across 4–5 sections totaling 16–20 images per animal (at the approximate level of Bregma −5.40). Particle analysis was performed with a fixed inclusive threshold within a standardized rectangle box (0.43 mm × 0.28 mm), to include all aggregates as described in Grant et al. (2014). The area occupied was measured using MetaMorph®’s Total Pixel Area; measuring the area covered by the total number of computer-generated thresholded pixels.
Statistical Analyses
Unless otherwise indicated, a mixed 3×4 or 3×2 repeated measures ANOVA design was employed to make comparisons for behavioral testing dependent variables (described below) with independent variables being genotype (3 levels between groups: PINK1 −/−, PINK1 +/−, WT) and time (4 levels within groups: 2, 4, 6, 8 mo, or 2 levels 4 and 8 mo). Main effects and interactions were examined. Pearson correlation analysis was performed for all behavioral variables, TH-ir and aSyn-ir. Critical level for significance was set a priori at 0.05. Post-hoc analysis was performed with Fisher’s LSD. Critical level for significance was set a priori at 0.05. Data were transformed (either rank or log transformed) if data failed to conform to assumptions for ANOVA.
Results
Ultrasonic vocalization
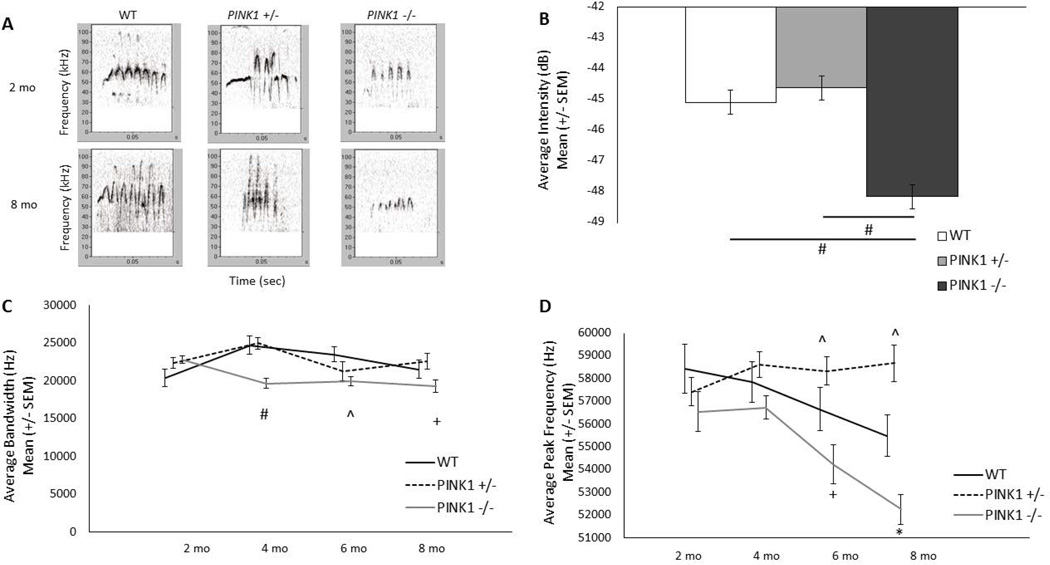
Representative calls from each genotype at 2 and 8 mo are displayed in Fig. 1A.
Figure 1. Ultrasonic vocalizations.
A) Representative frequency modulated calls from WT (left), PINK1 +/− (middle) and PINK1 −/− (right) rats at 2 mo (top panels) and 8 mo of age (bottom panels). Relative intensity is encoded by darkness of the signal; darker is louder. B) Average intensity of frequency modulated calls. Data are collapsed across time to show main effect for genotype. Note: a more negative value indicates a quieter call. C) Average bandwidth of frequency modulated calls. PINK1 −/− had significantly reduced bandwidth compared to WT at 4 and 6 mo. D) Average peak frequency of frequency modulated calls. PINK1 −/− had significantly reduced peak frequency compared to PINK1 +/− at 6 and 8 mo, and PINK1 +/− had significantly increased peak frequency compared to WT at 6 and 8 mo. * denotes p<0.0001, # denotes p<0.001, + denotes p<0.01, ^denotes p<0.05.
Average intensity of frequency modulated calls
There were significant main effects for genotype [F(2, 45)=24.80, p<0.0001; Fig. 1B; data collapsed for age to show main effects of genotype] and age [F(3, 110)=4.62, p=0.0044]. Intensity was reduced (quieter) for PINK1 −/− rats compared to WT (p<0.0001) and PINK1 +/− rats (p<0.0001), regardless of age (Fig. 1B). There was not a significant difference between WT and PINK1 +/− rats. Overall, rats at 4 mo of age had significantly louder calls compared to 2 mo of age (p=0.0003; data not shown).
Average bandwidth of frequency modulated calls
For bandwidth, there was a significant interaction for age × genotype [F(6, 110)=4.64, p<0.0003]. At 4 and 6 mo of age, PINK1 −/− rats demonstrated significantly decreased bandwidth as compared to WT rats (p=0.0004, p=0.026, respectively). In addition, the deficit was progressive where at 4, 6, and 8 mo of age, PINK1 −/− rats showed a significantly reduced bandwidth compared to 2 mo of age (p=0.005, p=0.027, p= 0.006, respectively; Fig. 1C).
Average peak frequency of frequency modulated calls
For peak frequency, there was a significant interaction for age × genotype [F(6, 110)=4.42, p<0.0005; Fig. 1D]. At 6 and 8 mo of age, PINK1 −/− rats demonstrated significantly decreased peak frequency as compared to PINK1 +/− but not WT rats (p=0.001, p<0.0001, respectively). At 6 and 8 mo of age, PINK1 −/− rats showed a significantly reduced peak frequency compared to 2 mo of age (p=0.01, p<0.0001, respectively) and 4 mo of age (p=0.008, p<0.0001, respectively). PINK1 +/− rats showed increased peak frequency as compared to WT at 6 and 8 mo (p=0.048 and p=0.01, respectively).
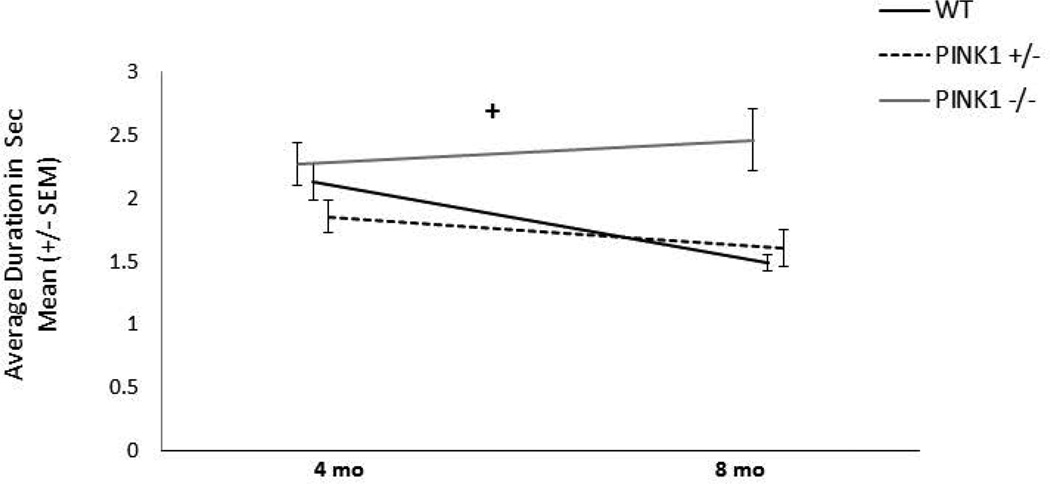
Average duration of frequency modulated calls
There were only main effects of age for duration of frequency modulated calls [F(3, 110)=9.06, p<0.0001; Table 2]. Duration was increased at 4, 6, and 8 mo compared to 2 mo of age, for all rats, regardless of genotype (p<0.0001 p<0.0001, p=0.0005, respectively).
Table 2.
Behavioral data not displayed in figures.
| Genotype | 2 months | 4 Months | 6 Months | 8 Months | |
|---|---|---|---|---|---|
| USV-Duration | WT | 0.036 (0.0014) | 0.045 (0.0017) | 0.043 (0.0019) | 0.043 (0.0021) |
| PINK1 +/− | 0.035 (0.0012) | 0.046 (0.0023) | 0.041 (0.0032) | 0.044 (0.0037) | |
| PINK1 −/− | 0.041 (0.0019) | 0.044 (0.0026) | 0.046 (0.0027) | 0.044(0.0018) | |
| USV-% Complex | WT | 46.68 (3.10) | 41.29 (4.32) | 63.03 (3.49) | 62.23 (3.65) |
| PINK1 +/− | 44.06 (4.06) | 46.16 (2.59) | 63.55 (3.96) | 67.86 (4.044) | |
| PINK1 −/− | 38.07 (3.39) | 50.87 (2.85) | 55.45 (4.19) | 60.54 (4.33) | |
| Pasta- Intensity | WT | Not Tested | 0.15 (0.013) | Not Tested | 0.20 (0.018) |
| PINK1 +/− | Not Tested | 0.16 (0.012) | Not Tested | 0.22 (0.016) | |
| PINK1 −/− | Not Tested | 0.14(0.0076) | Not Tested | 0.22 (0.017) | |
| Beam- Foot Faults | WT | Not Tested | 0.87 (0.039) | Not Tested | 0.46 (0.12) |
| PINK1 +/− | Not Tested | 0.85 (0.033) | Not Tested | 0.39 (0.087) | |
| PINK1 −/− | Not Tested | 0.67 (0.060) | Not Tested | 0.53 (0.14) | |
| Beam- Time to Traverse | WT | Not Tested | 2.95 (0.23) | Not Tested | 2.12 (0.14) |
| PINK1 +/− | Not Tested | 2.98 (0.20) | Not Tested | 2.55 (0.20) | |
| PINK1 −/− | Not Tested | 2.84 (0.17) | Not Tested | 2.97 (0.16) | |
| Spontaneous Activity- Forelimb | WT | Not Tested | Not Tested | Not Tested | 21.5 (1.73) |
| PINK1 +/− | Not Tested | Not Tested | Not Tested | 25.25 (1.66) | |
| PINK1 −/− | Not Tested | Not Tested | Not Tested | 32.75 (4.61) | |
| Spontaneous Activity- Hindlimb | WT | Not Tested | Not Tested | Not Tested | 18.92 (2.41) |
| PINK1 +/− | Not Tested | Not Tested | Not Tested | 16.5 (1.59) | |
| PINK1 −/− | Not Tested | Not Tested | Not Tested | 23.5 (1.64) |
Means (SEM) for USV duration (seconds) and % complex, Pasta Biting mean intensity (decibels), Challenging Beam foot faults (average over 5 trials) and time to traverse (seconds), and Spontaneous Activity forelimb and hindlimb steps (total number of steps).
Percent complex calls
There was a significant age × genotype interaction for percent of complex calls produced in a session [F(6, 110)=2.39, p=0.033; Table 2]. All rats produced more complex calls over time; PINK1 +/− and WT rats made significantly more complex calls at 6 and 8 months compared to 2 and 4 months (p<0.001 for all comparisons), while PINK1 −/− rats made significantly more complex calls at 4, 6, and 8 mo compared to 2 mo (p<0.001 for all comparisons), WT rats made more complex calls at 8 mo compared to 4 mo (p=0.01).
Tongue function
Average force
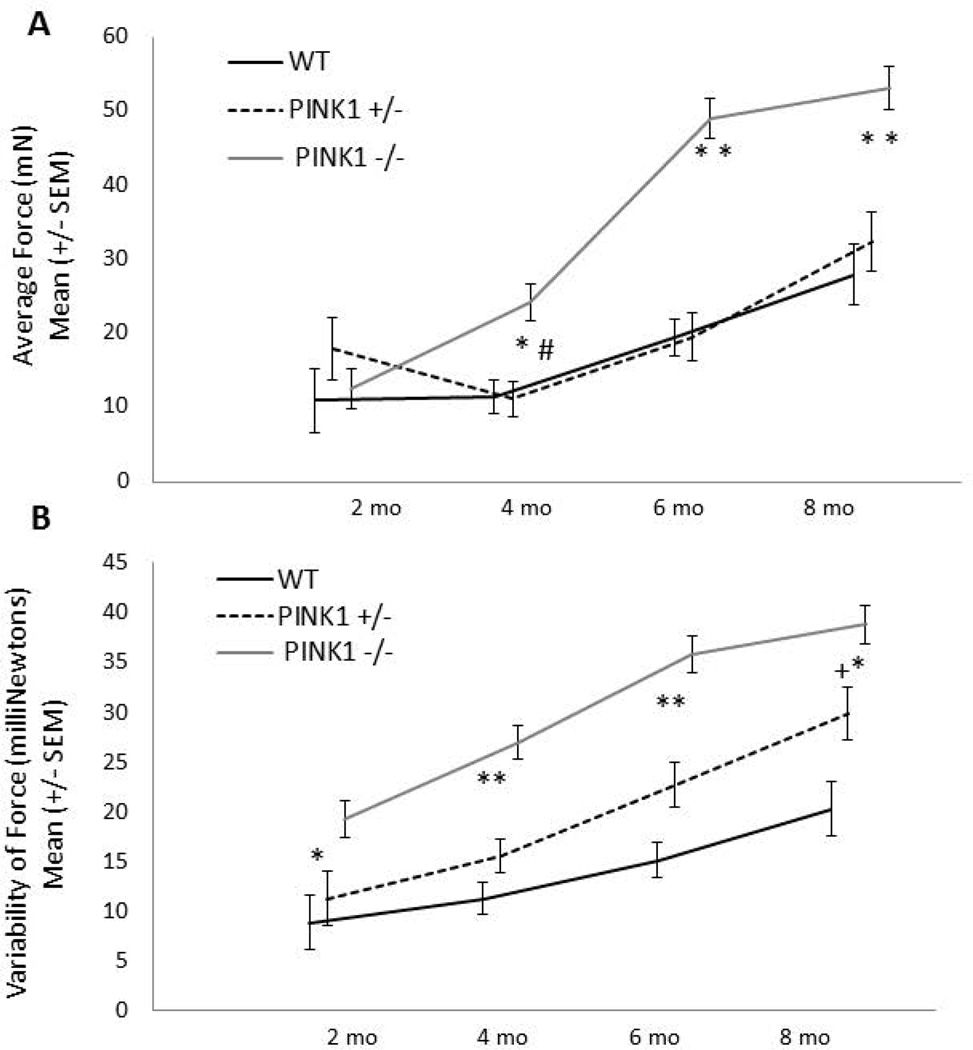
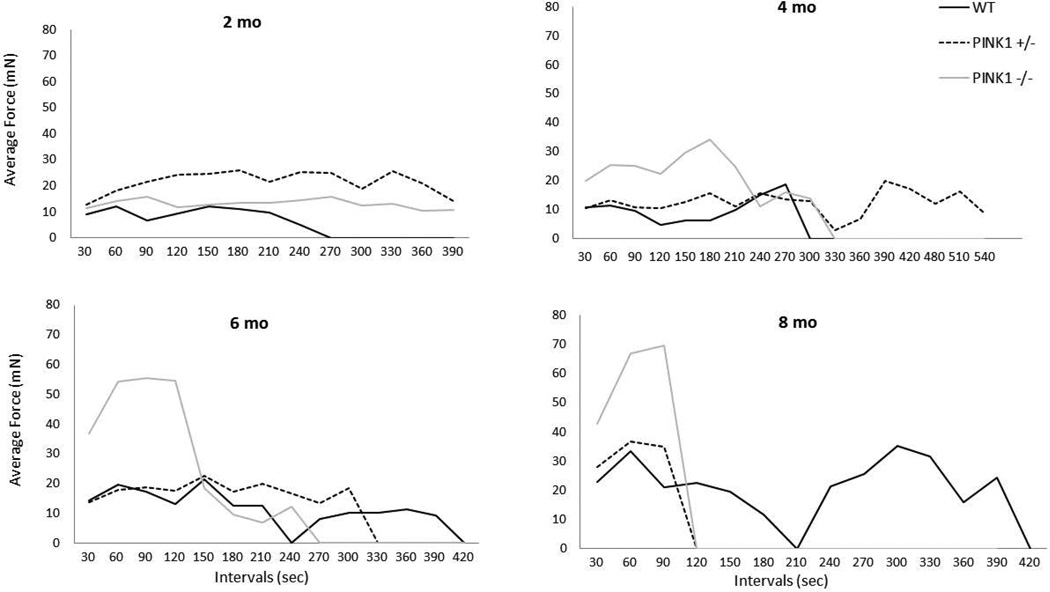
For all variables, body weight was not a significant co-variate. There was a significant interaction for age × genotype [F(6, 99)=12.14, p<0.0001; Fig. 2A]. At 2 mo of age, there were no significant differences in average press force among genotypes. At 4 mo of age, the PINK1 −/− rats pressed with greater force than the PINK1 +/− (p<0.0001) and WT rats (p=0.0005). This persisted through the 6 and 8 mo time points (p< 0.0001 for all comparisons). There were no significant differences between WT and PINK1 +/− rats at any time point. Over time, PINK1 −/− rats pressed with increased forces as compared to 2 mo (p<0.0001 for all comparisons). PINK1 +/− rats had increased forces over time from 4 to 6 (p=0.004), 4 to 8 (p<0.0001) and 6 to 8 (p<0.0001) mo. WT rats also increased their forces between 4 and 6 (p=0.0048) and 4 and 8 (p=0.0006) mo of age. This trend may reflect a larger body weight over time, and potential learning of the task. Qualitatively, licking strategies over the course of the testing session were different across groups. Average forces were higher earlier in the session for PINK1 −/− rats compared to PINK1 +/− and WT rats beginning at 4 months, but particularly at 6 and 8 mo (Fig. 3).
Figure 2. Lingual force and variability.
A) Average lingual force. PINK1 −/− rats had significantly greater forces than PINK1 +/− and WT and PINK1 +/− at 4, 6 and 8 mo of age; B) Variability of force per press. PINK1 −/− rats showed an increased variability in the amount of force per press during the session as compared to WT at 2 mo and compared to PINK1 +/− and WT rats at 4, 6 and 8 mo. * denotes p<0.0001, # denotes p<0.001, + denotes p<0.01. mN = milliNewtons
Figure 3. Lingual force across the session.
Average lingual forces across the testing session at 2, 4, 6, and 8 mo. PINK1 −/− had high lingual forces early in each testing session (higher than WT), but were unable to sustain this high force beginning at 4 mo of age. mN = milliNewtons
Variability of force
There was a significant interaction for age × genotype [F(6, 99)=4.38, p=0.0006; Fig. 2B]. PINK1 −/− rats showed an increased variability in the amount of force per press during the session as compared to WT at 2 mo (p<0.0001) and compared to WT and PINK1 +/− rats at 4 (p<0.0001, p<0.0001), 6 (p<0.0001, p<0.0001) and 8 mo (p<0.0001, p=0.0026). Rats tended to increase their variability over time. Notably, PINK1 −/− rats significantly increased their variability between 2 to 4 mo (p=0.0014) and 4 to 6 mo (p<0.0001) and this plateaued at 6 mo. PINK1 +/− rats also significantly increased their variability from 4 to 6 (p<0.0001) and 6 to 8 (p<0.0001) mo. WT significantly increased variability from 4 to 6 mo (p=0.026) and 6 to 8 mo (p=0.034).
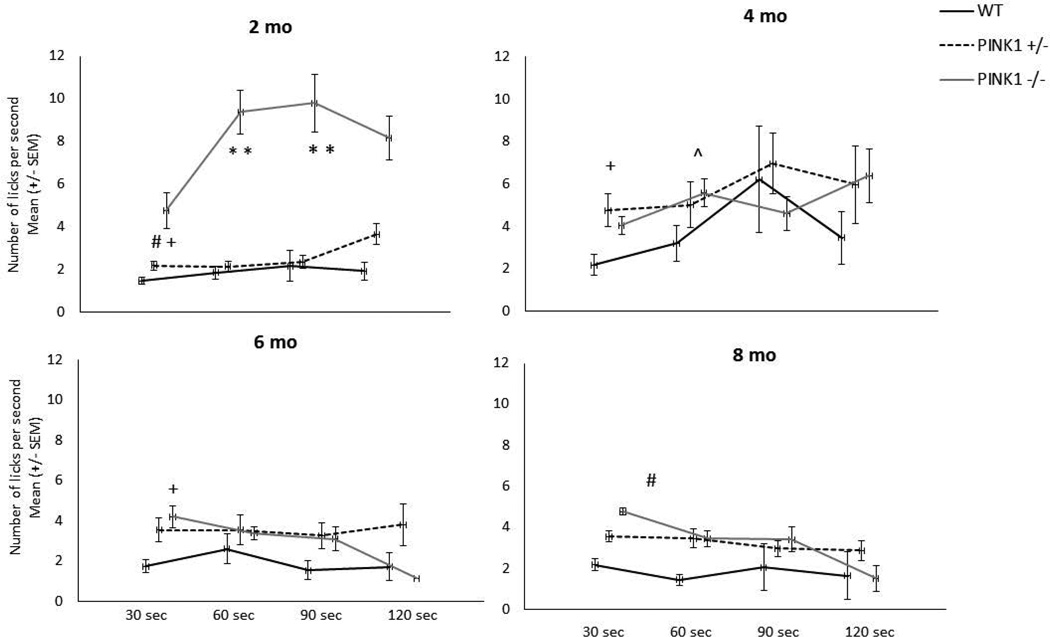
Lick rate at 30, 60, 90 and 120 sec
Lick rates across the testing sessions are displayed in separate graphs for each time point in Fig. 4. There was a significant interaction for age × genotype for lick rate at the 30 sec interval [F(6, 99)=2.43, p=0.031], the 60 sec interval, [F(6,94)=5.95, p<0.0001], and the 90 sec interval [F(6,61)=4.80, p<0.0005], and a main effect of age at the 120 sec time interval [F(3,37)=3.03, p=0.042]. At 2 mo, PINK1 −/− had significantly higher lick rates at the 30, 60, and 90 sec intervals compared to the PINK1 +/− (p=0.0089, p<0.0001, p<0.0001, respectively) and WT (p=0.0007, p<0.0001, p<0.0001, respectively). At 4 mo, PINK1 +/− had higher lick rates than WT (p=0.0043) at the 30 sec interval and at the 60 sec interval PINK1 −/− rats had significantly higher lick rates compared to WT at 4 mo (p=0.028). PINK1 −/− had significantly higher lick rates for the 30 sec interval compared to WT at 6 and 8 months, (p=0.0029 and p=0.006, respectively). For the 30 sec interval, PINK1 +/− had significantly higher lick rates at 6 and 8 months compared to 4 mo (p=0.026 and 0.035, respectively). For the 60 sec interval, lick rate was significantly decreased for PINK1 −/− rats at 4, 6 and 8 mo compared to 2 mo (p=0.025, 0.0004, and 0.0018, respectively) and at 6 mo compared to 4 mo (p=0.035). Lick rate at the 90 sec interval was significantly decreased for PINK1 −/− rats at 4 and 6 mo compared to 2 mo (p=0.027 and 0.0047, respectively) and lick rate decreased at the 90 sec interval for WT and PINK1 +/− rats between 4 and 6 mo (p=0.013 and 0.038, respectively).
Figure 4. Lick rate.
Data for each genotype are displayed at each time point at 30 sec intervals across the testing session. At 2 mo at the 30, 60 and 90 sec time intervals, PINK1 −/− rats had significantly higher lick rates compared to WT and PINK1 +/−. At 4 mo, PINK1 +/− rats had significantly higher lick rates at the 30 sec interval and PINK1 −/− rats had significantly higher lick rates at the 60 second time interval, compared to WT. At 6 and 8 mo, PINK1 −/− rats had significantly increased lick rates compared to WT at the 30 sec interval. * denotes p<0.0001, # denotes p<0.001. + p<0.01, ^ p<0.05.
Pasta Biting
There was a main effect of age for intensity [F(1, 74)=30.23, p<0.0001], with 8-mo-old rats having greater bite intensity than 4 mo-old rats (p<0.0001, Table 2). There was also a main effect of genotype for inter-bite interval [F(2, 74)=10.12, p<0.0001], with PINK1 −/− rats having more variable inter-bite intervals compared to WT (p=0.0001) and PINK1 +/− (p=0.0005).
Tapered Balance Beam
There was a significant age by genotype interaction [F(2, 33)=4.39, p=0.02). PINK1 −/− rats were significantly slower to traverse at 8 mo compared to WT (p=0.0018), but not PINK1 +/− (p=0.059) (Table 2). The last portion of the beam is the narrowest and thus most challenging. Therefore, we also measured the time to traverse this particular section for all genotypes. There was a significant interaction for age by genotype [F(2, 33)=4.64, p=0.017; Fig. 5]. PINK1 −/− rats were significantly slower than PINK1 +/− and WT rats at 8 mo (p=0.0029 and p=0.0005, respectively). There was only a main effect of age for the average foot faults across the entire beam [F(1, 33)=4.66, p=0.038; Table 2]. At 8 mo of age the rats made significantly more foot faults regardless of genotype (p=0.038).
Figure 5. Tapered balance beam.
Average time to cross the final third of the tapered balance beam. PINK1 −/− rats had significantly increased transit time at 8 mo compared to both PINK1 +/− and WT; + denotes p<0.01.
Spontaneous activity
At 8 mo, there was a significant effect of genotype [F(2, 33)= 3.81, p=0.032; Table 2]. Specifically, PINK1 −/− rats made significantly more hindlimb steps than PINK1 +/− (p=0.007), but not WT. There was no significant difference in the number of forelimb steps [F(2, 35)=2.64, p=0.086; Table 2].
Immunohistochemistry
At 8 mo, there were no significant differences between genotype and TH-ir for optical density in the SR [F(2, 29)=2.68, p=0.086; Table 3]. Additionally, there were no differences between genotypes in TH-ir positive cell counts in the SNpc [F(2,32) =0.39, p=0.68; Table 3]. There was also no age × genotype interaction [F(41,3)=0.27, p=0.74]. HE staining did not differ between any of the genotypes.
Table 3.
TH-ir data not displayed in figures.
| Genotype | 8 Months TH-ir Optical Density (SEM) SR |
8 Months TH-ir Soma Numbers (SEM) SNpc |
8 Months TH-ir Soma Numbers (SEM) LC |
|---|---|---|---|
| WT | 1.363 (0.0018) | 111.5 (11.01) | 18.66 (2.64) |
| PINK1 +/− | 1.38 (0.02) | 109.88 (9.15) | n/a |
| PINK1 −/− | 1.36 (0.022) | 116.2 (8.05) | 10.94 (2.41) |
Means (SEM) for TH-ir in SR, SN and LC at 8 mo.
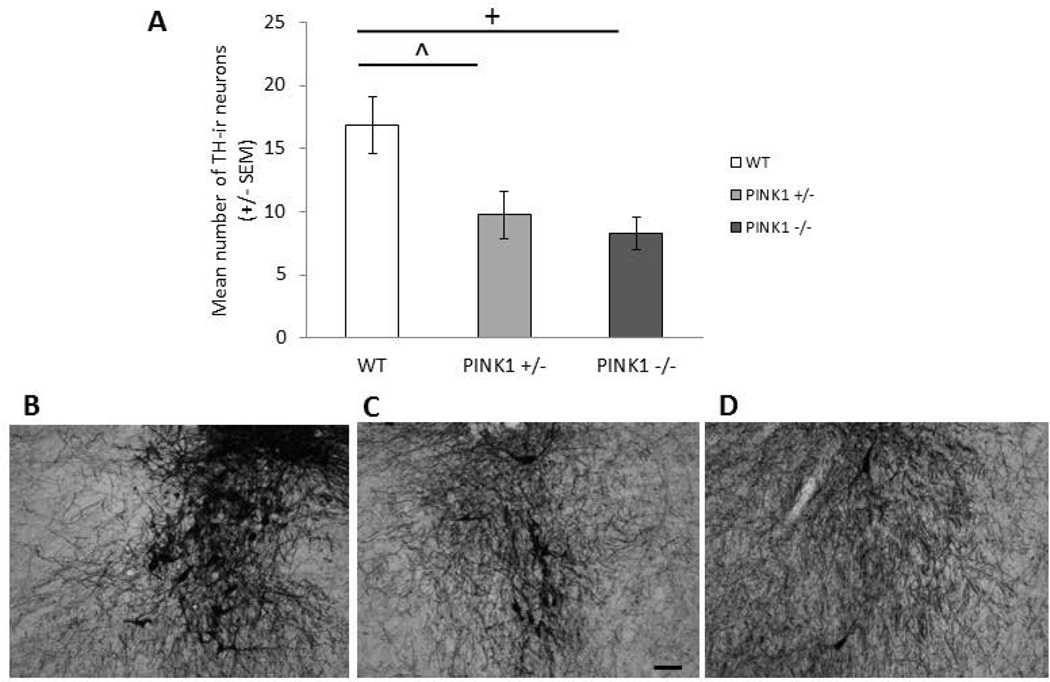
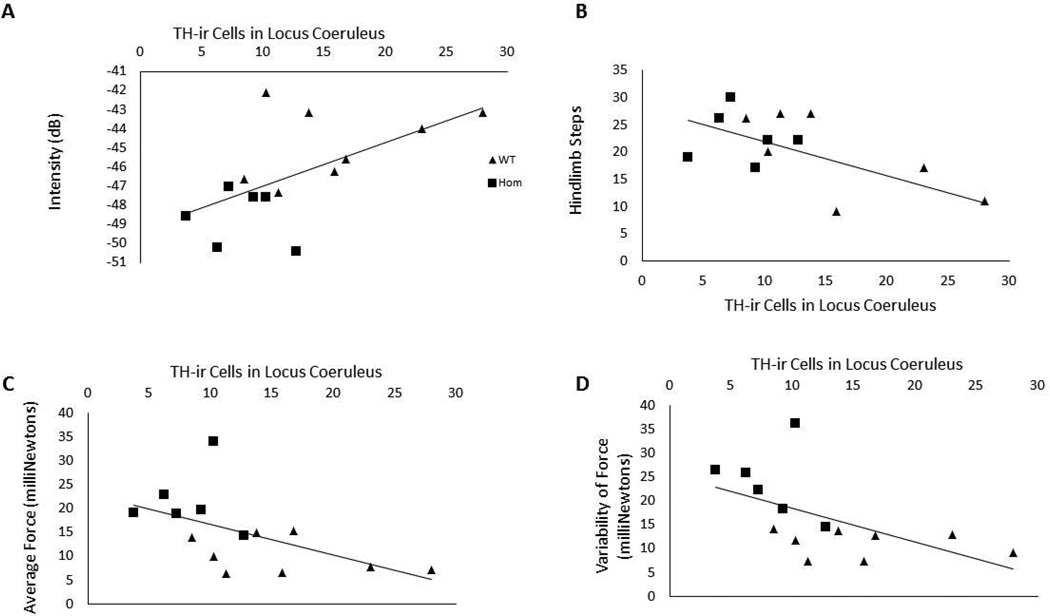
There were significant differences in TH-ir soma numbers in the LC [F(2, 19)= 5.46, p=0.015; Table 3]. Specifically, PINK1 −/− rats had fewer immunolabeled soma compared to WT (p=0.008) and PINK1 +/− had fewer soma compared to WT (p=0.03) (Fig. 6A). Pearson correlation analysis determined that there was an association between the behavior and LC TH-ir, but not SNpc TH-ir or PAG aSyn-ir. Specifically, LC TH-ir soma counts were significantly positively correlated with vocalization intensity (df=14; r=0.58, p=0.028; Fig. 7A), negatively correlated with number of hindlimb steps in the spontaneous movement test (df=14; r=−0.55, p=0.042; Fig. 7B) as well as the average of maximum tongue press force (df=14, r=−0.55, p=0.041; Fig. 7C) and the standard deviation of force (df=14, r=−0.56, p=0.04; Fig. 7D).
Figure 6. TH-ir in LC at 8 mo.
A) Mean number of TH immunoreactive cells with standard errors of the mean in WT, PINK1 +/− and PINK1 −/− at 8 mo. + denotes p<0.01, ^ denotes p<0.05. Photomicrographs of TH immunohistochemistry in the LC at 20X magnification B) WT, C) PINK1 +/−, D) PINK1 −/−. Scale bar in C is 0.1mm.
Figure 7. Correlation scatterplots.
A) There was a positive correlation between TH-ir cells in locus coeruleus and intensity of frequency modulated calls. B) There was a significant negative correlation between TH-ir cells in the locus coeruleus and number of hindlimb steps. C) There was a significant negative correlation between TH-ir cells in the locus coeruleus and the average force of the tongue press task. D) There was a significant negative correlation between TH-ir cells in the locus coeruleus and the variability of force of the tongue press task. Squares indicate PINK1 −/− and triangles indicate WT. dB = decibels
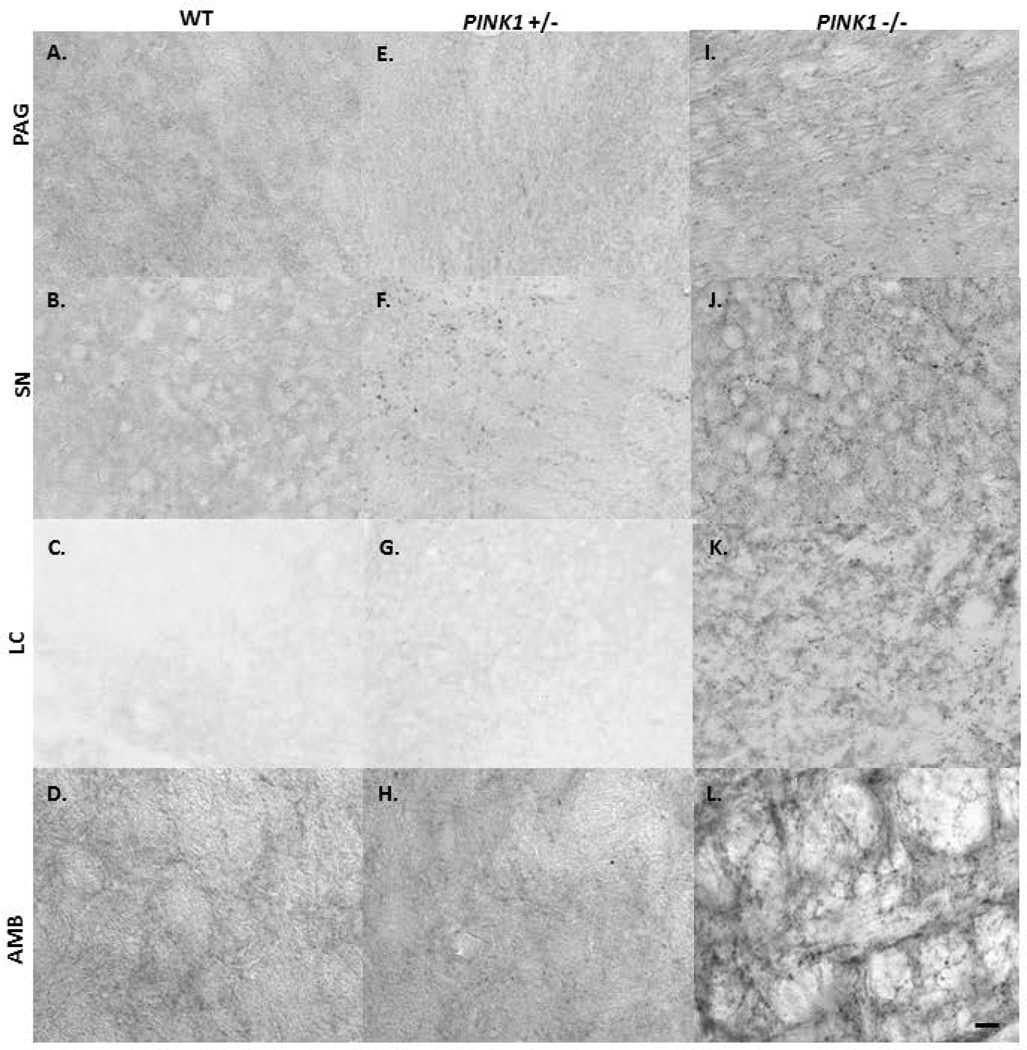
aSyn-ir Aggregation
Insoluble aSyn aggregates were defined as punctated immunolabel that appeared to be region specific. At the 8 mo timepoint, there were clear differences in immunolabeling for aSyn positive aggregates between genotypes (Fig. 8; Table 4), with the PINK1 −/− rats showing the greatest number of aSyn aggregates. The label was most dense in the PAG (Fig. 8A, E, I), SNpc (Fig. 8 B, F, J), and LC (Fig. 8 C, G, K) compared to the SR (not shown) in the PINK1 −/− rats. Immunolabeling in the AMB appeared as punctated label with highlighted fibers (Fig. 8 D, H, I). WT rats did not develop aSyn positive aggregates in the PAG, SR, SNpc, LC or AMB. Some aSyn positive aggregates were observed in PINK1 +/− rats, but they were fewer in number compared to PINK1 −/− rats. Specific to the PAG, WT animals did not demonstrate a high level of aSyn immunoreactivity; additionally, the PINK1 +/− and −/− had increased pixel area immunolabeling variability within genotypes.
Figure 8. Proteinase K resistant aggregated aSyn immunohistochemistry at 8 mo.
Photomicrographs of insoluble aSyn immunolabeling (anti-aSyn immunolabel (dark, aggregated protein)) in coronal brain sections containing PAG, SN, LC, and AMB in WT (A–D), PINK1 +/− (E–H) and PINK1 −/− (I–L). Scale bar in the lower right of panel A is 0.1mm, bright field magnification is 20X.
Table 4.
Alpha-synuclein Aggregates.
| Genotype | N | PAG | SNpc | SR | LC* | AMB* |
|---|---|---|---|---|---|---|
| WT | 11 | + | + | + | + | + |
| PINK1 +/− | 12 | +++ | ++++ | ++ | ++++ | +++ |
| PINK1 −/− | 12 | ++++ | ++++ | ++ | ++++ | ++++ |
Quantitative analysis for the average aSyn positive aggregate immunohistochemistry per genotype at 8 mo. Scale: +: 0; ++: 1–5; +++: 6–10; ++++ : >10.
Sample size was 2–4 animals per genotype.
Discussion
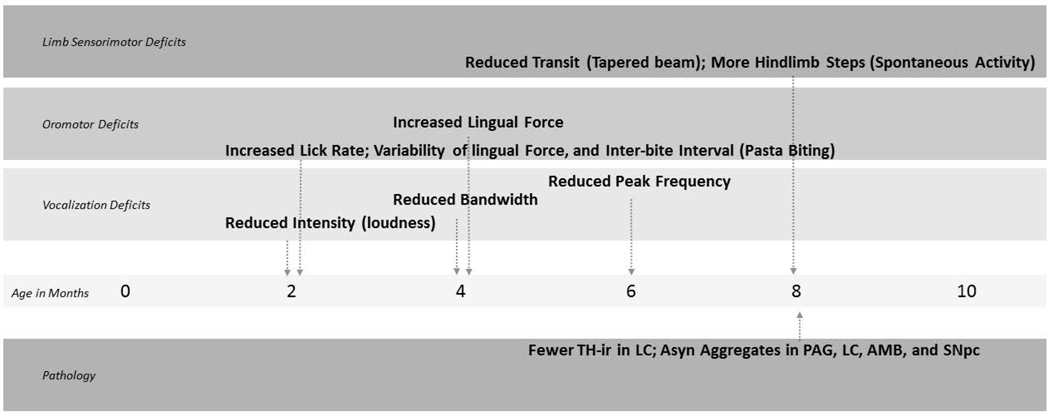
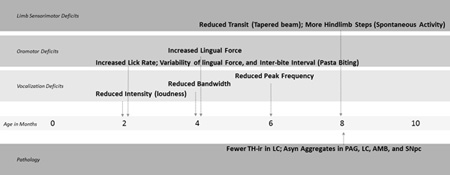
In the present study, we show that PINK1 −/− rats develop early and progressive vocalization and oromotor deficits reminiscent of those observed in PD. Importantly, these cranial sensorimotor deficits manifest prior to the onset of limb deficits and significant nigrostriatal DA cell loss (reviewed in Figure 9). Our immunohistochemical analyses indicate a moderate reduction of TH-ir in the LC and widespread accumulation of insoluble aSyn that likely contributes to behavioral deficits in PINK1 −/− rats.
Figure 9. Time line of deficits.
TH-ir = Tyrosine Hydryxlase-immunoreactivit, LC; Asyn Aggregates in PAG, LC, AMB and SNpc
Overall, PINK1 −/− rats demonstrate early vocalization deficits, with some features that are compromised early, but are stable (intensity, bandwidth) and others that are more progressive (peak frequency). Reduced loudness (intensity), is a hallmark feature of voice deficits in PD (Saxena et al. 2014), and was significantly reduced in PINK1 −/− rats as early as 2 mo of age. Evidence in humans indicates that vocal deficits emerge early in PD (Harel et al. 2004; Rusz et al. 2011; Stewart et al. 1995), possibly prior to the onset of cardinal motor signs such as bradykinesia, tremor, and muscle rigidity that are associated with nigrostriatal DA cell loss. Peak frequency in PINK1 -/- rats was significantly reduced at 6 and 8 months compared to PINK1 +/− rats, which was increased at those time points. However, the PINK1 −/− rats had significantly reduced peak frequency at 6 and 8 months compared to both 2 and 4 months. This was not true for the wild type rats, who also show declines in peak frequency over time. As peak frequency declines with age (Basken et al, 2012), it is likely that the mechanisms underlying the decline in peak frequency in wild type rats reflects changes with normal aging. Peterson et (2013) also found age related decreases in peak frequency as well as reductions in elastin, hylaluronic acid and collagen in the lamina propria of the vocal fold of aged rats (Peterson et al. 2013). Reductions in hyaluronic acid, in particular, were significantly correlated with peak frequency, and the authors suggest these compositional changes to the viscoelastic properties of the lamina propria might render the vocal folds more stiff and less elastic, ultimately limiting the range of frequency the rat can produce. Other features, such as call duration and percent of complex calls increased across time for all rats, indicating that either these acoustic features are not vulnerable to the PINK1 −/− phenotype, or are not affected before 8 mo of age. Interestingly, the PINK1 +/− rats did not show any robust vocalizations deficits up to 8 mo of age.
Oromotor function was also measured in PINK1 −/− and control rats using a licking task that measures tongue force (Ciucci and Connor 2009). Surprisingly, during the licking task, PINK1 −/− rats pressed with a greater amount of force compared to controls. In contrast, previous work in the unilateral 6-OHDA rat model of PD showed reductions in lick force in rats with loss of nigrostriatal DA neurons (Ciucci and Connor 2009). However, the increased lick force in PINK1 −/− rats does not appear to be a function of tongue strength or central drive to the tongue. The task is based on incrementing forces when the rat presses with a target force (minimal force needed to receive water reward) and the PINK1 −/− rats seemed to consistently overshoot the target force and then fatigue. Force overshoot has also been observed in grasping tests in PD patients (Nowak and Hermsdorfer 2006). In addition to overshooting, the target PINK1 −/− rats also had more variable lick forces compared to controls. As the PINK1 −/− rats aged, their lick force pattern changed. At 4 mo of age PINK1 −/− rats showed an increase in force that gradually declined by approximately 5 minutes. At 6 and 8 mo of age the overshoot was much higher reaching up to 70 mN and by 8 mo the PINK1 −/− rats could not maintain the high force and performance abruptly tapered at approximately 2 minutes. Inability to finely grade and sustain oral movements is a key feature of PD (Rosen et al. 2005). Overshooting could reflect a lack of fine motor control (which is supported by significantly increased variability of pressing over time) or a cognitive dysfunction, such as failure to learn the task. Given PINK1 −/− rats can learn the basic operant task, suggests impairments in sensory integration and motor control.
Biting was also impaired in PINK1 KO rats compared to controls. WT rats were able to quickly and efficiently consume the piece of pasta, almost in one fluid-like motion and pasta biting patterns (acoustic) reflected this, with regular, rhythmic bites (Allred et al. 2008; Kane et al. 2011). In contrast, PINK1 −/− rats had more irregular and inconsistent biting patterns leading to increased inter-bite intervals. This is similar to PINK1 −/− rat licking behaviors, which were more variable than PINK1 +/− and WT rats, and reflects a similar impairment in sensory motor integration and fine motor control. Interestingly, during this task, 20% of the PINK1 −/− rats ejected partially masticated pieces of pasta before continuing to consume the remaining pasta, which was not observed in controls. Irregular biting patterns indicate altered eating strategies and are reminiscent of oromotor dysfunction in humans with PD (Bakke et al. 2011).
In contrast to vocalization and licking deficits, which emerged early (2 and 4 mo of age), PINK1 −/− rats did not demonstrate sensorimotor deficits on the tapered balance beam until 8 mo of age. Time to traverse the beam did not differ between the genotypes at 4 mo but there was a significant increase in time to traverse in PINK1 −/− rats compared to controls at 8 mo of age. Spontaneous activity was measured at only the 8 mo time point, and PINK1 −/− rats made more hindlimb steps in the cylinder compared to controls, similar to a shuffling gait. This differs from a recent study that found significant sensorimotor deficits in PINK1 −/− rats at 4 mo of age (Dave et al. 2014). While it is unclear why the PINK1 −/− rats in the present study differed in sensorimotor function, it is likely that our methodological and handling factors contributed to this difference. All rats were tested during the dark cycle in the present study and it is well established that activity levels are higher during this time for rodents (Richter 1978). Additionally, the rats in the present study were handled on a regular basis, almost daily, during acclimation to vocalization and oromotor tasks. Daily handling can have a significant impact not only on behavior, but also on brain morphology (Bezard et al. 2003; Zigmond and Smeyne 2014). Specifically, the difference in handling alone could be considered an ‘enriched’ environment, which has been shown to affect neurotransmission, growth factors, and cell morphology. For example, an enriched environment promotes increased in brain-derived neurotrophic factor in the SR and a significant DA neuronal protection in a MPTP mouse model of PD (Bezard et al. 2003). As we strive to improve the translation of therapeutics from animal models to humans it will be essential to determine the reliability of the phenotype across different researchers and institutions. Despite the discrepancy between the two studies, it is clear that sensorimotor function is altered in PINK1 −/− rats. As was the case in Dave et al. (2014), a subset of the PINK1 -−/− rats experienced hindlimb paresis of unknown etiology at approximately 6 months, which was resolved by 8 months.
At 8 mo of age, we observed no significant differences in TH-ir in the SR between the genotypes. Because of the compensatory capacity of the nigrostriatal DA system it was still possible that DA cell loss occurred despite similar TH-ir in the SR. Therefore, we performed cell counts in the SNpc and LC. Surprisingly, we did not observe a significant reduction in TH-ir in the SNpc of the PINK1 −/− rats although previous reports show a moderate reduction in DA SNpc at the same age (Dave et al. 2014). Again, it is possible that our experimental methodology, daily handling and social enrichment, led to a protection of dopaminergic neurons. It is also important to note that the quantification method used in the present study, while comparable to unbiased stereology, is not as sensitive in detecting slight alterations in TH-ir. However, we did detect a moderate reduction in TH-ir in LC, and aggregated aSyn was detected in these regions, indicating the development of early PD-related pathology. We observed an approximately 50% reduction in TH-ir labeled soma within the LC in the PINK1 −/− rats compared to the WT at 8 mo of age. This suggests that noradrenergic dysfunction manifests early in the PINK1 −/− model and may contribute to both cranial and limb sensorimotor deficits. We show that TH-ir counts in the LC are significantly positively correlated with intensity of vocalization and negatively correlated with measures of lingual force and hindlimb function. Specifically, decreased immunoreactivity was associated with quieter ultrasonic vocalizations and abnormal lingual forces. These data suggest a relationship between noradrenergic dysfunction and early vocalization and oromotor deficits. This is particularly interesting, as intensity was affected at 2 mo, a finding consistent with early decreases in vocal loudness in humans with PD (Darley et al. 1969). Moreover, aSyn pathology in the LC is thought to precede significant SNpc pathology (Braak et al. 2003) and loss of noradrenergic neurons in the LC can be even greater than the loss of nigrostriatal DA neurons (Zarow et al. 2003) indicating a potentially underappreciated role for the LC in PD (Rommelfanger and Weinshenker 2007).
Loss of function of PINK1 also resulted in widespread aSyn pathology in the PAG and AMB, brainstem regions important for vocalization and swallowing behaviors, as well as the SNpc. The presynaptic protein aSyn is involved in both familial and sporadic forms of PD strongly indicating a role for aSyn in PD pathology (Polymeropoulos et al. 1997; Singleton et al. 2003; Spillantini et al. 1997). In vitro, loss of function of PINK1 leads to abnormal accumulation of aSyn toxicity and overexpression of PINK1 rescues aSyn toxicity (Liu et al. 2009). In vivo, mice with loss of function of PINK1 also develop aggregated aSyn in the absence of dopaminergic cell loss (Kitada et al. 2007). We recently showed that mice overexpressing human aSyn demonstrate vocalization deficits and aSyn aggregation in the PAG (Grant et al. 2014).
In summary, these findings are the first to demonstrate early, progressive vocalization and oromotor deficits in PINK1 −/− rats that accompany reduced TH-ir in the LC and abnormal aSyn accumulation in hindbrain and midbrain regions. The results of this study support the use of the PINK1 −/− rat model for investigation of cranial-sensorimotor deficits, and provide a robust research tool to explore pharmaceutical and behavioral therapies.
Significant Statement.
This work is the first to show that PINK1 knockout rats have early and progressive vocalization and oromotor deficits. These findings underscore and extend previous work suggesting that abnormal alpha-synuclein aggregation and catecholamine pathologies in brain regions critical for cranial sensorimotor control accompany behavioral deficits. Our results indicate that the PINK1 knockout rat has high construct validity for studying early cranial sensorimotor dysfunction. Importantly, voice and swallowing deficits have the potential to be powerful early behavioral biomarkers for diagnosis and for the development of disease-modifying therapeutics for Parkinson disease.
Acknowledgments
We would like to thank Glen Leverson, PhD for statistical consultation; Dr. Lauren V. Riters for microscopy facilities.
Funding Michael J Fox Foundation (Ciucci), Department of Surgery-Division of Otolaryngology (Ciucci), Gardner Family Center for Parkinson’s Disease and Movement Disorders (Fleming), T32 DC009401 (NIDCD, National Institutes of Health) (Grant), F32 DC014399 (NIDCD, National Institutes of Health) (Kelm-Nelson).
Footnotes
Conflict of Interest:
None.
Author Roles: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LG, CKN, SMF, MRC. Acquisition of data: LG, CKN, BLH, KVB, ESPR, JDP. Analysis and interpretation of data: LG, CKN, BLH, ESPR, SMF, MRC. Drafting of the manuscript: LG, CKN, BLH, KVB, ESPR, JDP, MRC. Critical revision of the manuscript for important intellectual content: LG, CKN, SMF, MRC. Statistical analysis: LG, CKN, SMF, MRC. Obtained funding: LG, CKN, MRC. Administrative, technical, and material support: : LG, CKN, BLH, KVB, ESPR, JDP. Study supervision: LG, CKN, MRC.
References
- Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, Jones TA. The Vermicelli Handling Test: A simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170(2):229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke M, Larsen SL, Lautrup C, Karlsborg M. Orofacial function and oral health in patients with Parkinson’s disease. Eur J Oral Sci. 2011;119(1):27–32. doi: 10.1111/j.1600-0722.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. Enriched Environment Confers Resistance to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine and Cocaine: Involvement of Dopamine Transporter and Trophic Factors. J Neurosci. 2003;23(35):10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V. Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18(Supplement 1)(0):S4–S6. doi: 10.1016/S1353-8020(11)70004-9. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Dekker MC, Vanacore N, Fabbrini G, Squitieri F, Marconi R, Antonini A, Brustenghi P, Dalla Libera A, De Mari M, Stocchi F, Montagna P, Gallai V, Rizzu P, van Swieten JC, Oostra B, van Duijn CM, Meco G, Heutink P. Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK7. Neurol Sci 23 Suppl. 2002;2:S59–S60. doi: 10.1007/s100720200069. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Communication of Adult Rats by Ultrasonic Vocalization: Biological, Sociobiological, and Neuroscience Approaches. ILAR J. 2009;50(1):43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: Degradation of 50-khz ultrasonic vocalization in rats. Behav Neurosci. 2009;123(2):328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Connor NP. Dopaminergic influence on rat tongue function and limb movement initiation. Exp Brain Res. 2009;194(4):587. doi: 10.1007/s00221-009-1736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Grant LM, Rajamanickam ESP, Hilby BL, Blue KV, Jones CA, Kelm-Nelson CA. Early Identification and Treatment of Communication and Swallowing Deficits in Parkinson Disease. Semin Speech Lang. 2013a;34(03):185–202. doi: 10.1055/s-0033-1358367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: A preliminary study. Behav Brain Res. 2007;182(2):284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Schaser AJ, Russell JA. Exercise-induced rescue of tongue function without striatal dopamine sparing in a rat neurotoxin model of Parkinson disease. Behav Brain Res. 2013b;252(0):239–245. doi: 10.1016/j.bbr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Differential Diagnostic Patterns of Dysarthria. J Speech Hear Res. 1969;12(2):246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer Iii RC, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MAS, Fiske BK, Sherer TB, Frasier MA. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis. 2014;70(0):190–203. doi: 10.1016/j.nbd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- de Lau LML, Giesbergen PCLM, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MMB. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology. 2004;63(7):1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and Histopathological Consequences of Paraquat Intoxication in Mice: Effects of α-Synuclein Over-Expression. Synapse (New York, NY) 2007;61(12):991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and Progressive Sensorimotor Anomalies in Mice Overexpressing Wild-Type Human α-Synuclein. The Journal of Neuroscience. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Gombash SE, Manfredsson FP, Kemp CJ, Kuhn NC, Fleming SM, Egan AE, Grant LM, Ciucci MR, MacKeigan JP, Sortwell CE. Morphological and Behavioral Impact of AAV2/5-Mediated Overexpression of Human Wildtype Alpha-Synuclein in the Rat Nigrostriatal System. PLoS One. 2013;8(11):e81426. doi: 10.1371/journal.pone.0081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant LM, Richter F, Miller JE, White SA, Fox CM, Zhu C, Chesselet MF, Ciucci MR. Vocalization deficits in mice over-expressing alpha-synuclein, a model of pre-manifest Parkinson’s disease. Behav Neurosci. 2014;128(2):110–121. doi: 10.1037/a0035965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JF, Wang L, He D, Yang QH, Duan ZX, Zhang XW, Nie LL, Yan XX, Tang BS. Clinical features and [11C]-CFT PET analysis of PARK2, PARK6, PARK7-linked autosomal recessive early onset Parkinsonism. Neurol Sci. 2011;32(1):35–40. doi: 10.1007/s10072-010-0360-z. [DOI] [PubMed] [Google Scholar]
- Harel B, Cannizzaro M, Snyder PJ. Variability in fundamental frequency during speech in prodromal and incipient Parkinson’s disease: a longitudinal case study. Brain and cognition. 2004;56(1):24. doi: 10.1016/j.bandc.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behav Neurol. 1998;11(3):131. [PubMed] [Google Scholar]
- Houlden H, Singleton A. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124(3):325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. J Acoust Soc Am. 2010;128(2):EL75–EL79. doi: 10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR. Targeted Training of Ultrasonic Vocalizations in Aged and Parkinsonian Rats. JOVE. 2011;(54):e2835. doi: 10.3791/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JR, Ciucci MR, Jacobs AN, Tews N, Russell JA, Ahrens AM, Ma ST, Britt JM, Cormack LK, Schallert T. Assessing the role of dopamine in limb and cranial-oromotor control in a rat model of Parkinson’s disease. J Commun Disord. 2011;44(5):529–537. doi: 10.1016/j.jcomdis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci. 2007;104(27):11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Vives-Bauza C, Acín-Peréz R, Yamamoto A, Tan Y, Li Y, Magrané J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang X-Y, Beal MF, Manfredi G, Li C. PINK1 Defect Causes Mitochondrial Dysfunction, Proteasomal Deficit and α-Synuclein Aggregation in Cell Culture Models of Parkinson’s Disease. PLoS One. 2009;4(2):e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: Results from the DATATOP trial. Mov Disord. 2008;23(5):653–659. doi: 10.1002/mds.21853. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol Behav. 2003;80(1):81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- Michou E, Hamdy S. Dysphagia in Parkinson’s disease: a therapeutic challenge? Expert Rev Neurother. 2010;10(6):875–878. doi: 10.1586/ern.10.60. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Predictive and reactive control of grasping forces: on the role of the basal ganglia and sensory feedback. Exp Brain Res. 2006;173(4):650–660. doi: 10.1007/s00221-006-0409-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson G. In: The Rat Brain in Stereotaxic Coordinates. Press EA, editor. Burlington, MA: Elsevier; 2005. [Google Scholar]
- Peterson JR, Watts CR, Morris JA, Shelton JM, Cooper BG. Laryngeal aging and acoustic changes in male rat ultrasonic vocalizations. Dev Psychobiol. 2013;55(8):818–828. doi: 10.1002/dev.21072. [DOI] [PubMed] [Google Scholar]
- Plowman EK, Maling N, Rivera BJ, Larson K, Thomas NJ, Fowler SC, Manfredsson FP, Shrivastav R, Kleim JA. Differential sensitivity of cranial and limb motor function to nigrostriatal dopamine depletion. Behav Brain Res. 2013;237(0):157–163. doi: 10.1016/j.bbr.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Richter CP. “Dark-Active” Rat Transformed into “Light-Active” Rat by Destruction of 24-Hr Clock: Function of 24-Hr Clock and Synchronizers. Proc Natl Acad Sci U S A. 1978;75(12):6276–6280. doi: 10.1073/pnas.75.12.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. 2011:2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Stereotypic laryngeal and respiratory motor patterns generate different call types in rat ultrasound vocalization. J Exp Zool A Ecol Genet Physiol. 2013;319(4):213–224. doi: 10.1002/jez.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Rat ultrasonic vocalization shows features of a modular behavior. J Neurosci. 2014;34(20):6874–6878. doi: 10.1523/JNEUROSCI.0262-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochem Pharmacol. 2007;74(2):177. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rosen KM, Kent RD, Duffy JR. Task-based profile of vocal intensity decline in Parkinson’s disease. Folia Phoniatr Logop. 2005;57(1):28–37. doi: 10.1159/000081959. [DOI] [PubMed] [Google Scholar]
- Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic Assessment of Deglutitive Behaviors in a Rat Model of Aging and Parkinson Disease. Dysphagia. 2012;28(1):95–104. doi: 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusz J, Cmejla R, Ruzickova H, Ruzicka E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson’s disease. The Journal of the Acoustical Society of America. 2011;129(1):350. doi: 10.1121/1.3514381. [DOI] [PubMed] [Google Scholar]
- Saxena M, Behari M, Kumaran SS, Goyal V, Narang V. Assessing speech dysfunction using BOLD and acoustic analysis in parkinsonism. Parkinsonism Relat Disord. 2014;20(8):855–861. doi: 10.1016/j.parkreldis.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. alpha-Synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stewart C, Winfield L, Hunt A, Bressman SB, Fahn S, Blitzer A, Brin MF. Speech dysfunction in early Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 1995;10(5):562. doi: 10.1002/mds.870100506. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Daele DJ, Cassell MD. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience. 2009;162(2):501–524. doi: 10.1016/j.neuroscience.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve L, Purnell P, Boska M, Fox H. Early Expression of Parkinson’s Disease-Related Mitochondrial Abnormalities in PINK1 Knockout Rats. Mol Neurobiol. 2014:1–16. doi: 10.1007/s12035-014-8927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 2010;211(1):1. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60(3):337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Smeyne RJ. Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord. 2014;20(Suppl 1):S123–S127. doi: 10.1016/S1353-8020(13)70030-0. [DOI] [PubMed] [Google Scholar]