Abstract
In the present study, we evaluated the diameter of internal acoustic canal in patients with Bells palsy to investigate the role of anatomical differences of the temporal bone in etiology of Bell’s palsy. Sixty-four patients who were diagnosed as Bells Palsy and temporal bone computed tomography imagings of them were included into the study group (Group 1). The control group (Group 2) was consisted of 35 healthy subjects without Bell’s Palsy. All patients had temporal bone computed tomography imaging. The internal auditory canal inlet, mid-canal, outlet and canal lengths were measured at the most distinctive cross-section of the seventh and eighth cranial nerves bifurcation. In the study group, Bells palsy was on the right side in 26 patients (40.6 %) and on the left side in 38 patients (59.4 %). Initial House–Brackmann (HB) score was HB-2 in 29 patients (45.3 %), HB-3 in 18 patients (28.1 %), HB-4 in 13 patients (20.3 %) and HB-5 in 4 patients (6.2 %). At 6-month evaluation, HB-score of the patients were HB-1 in 37 patients (57.8 %), HB-2 in 25 patients (39.1 %) and HB-3 in 2 patients (3.1 %). Internal auditory canal (IAC) measurements of the groups showed that there were no significant differences between the measurements of right-mid canal, right canal length; and left canal outlet and left canal length of the study and control groups. Right inlet and outlet; and left inlet and mid-canal values of the study group (Bell’s palsy) were significantly lower than those of the control group. In Bell’s palsy group, left inlet, outlet and canal length values were significantly higher than those of the right ones. Correlation analysis showed that there were no significant correlation between paralysis side; initial HB stage; and IAC measurement results. In patients with higher initial HB score, their 6-month later HB-score was also higher. In patients with higher 6-month HB score; R canal inlet, R mid-canal, L-canal inlet, and L-mid canal values were lower. Lower IAC inlet and mid-canal values were detected in patients with Bell’s palsy. Therefore narrow IAC inlet and mid-canal values may be one of the risk factors for Bell’s palsy.
Keywords: Bell’s Palsy, Internal auditory canal (IAC), Canal length, House–Brackmann score
Introduction
Bell’s palsy (idiopathic facial paralysis) is caused by the acute onset of lower motor neurone weakness of the facial nerve with no detectable cause. With a lifetime risk of 1 in 60 and an annual incidence of 11–40/100,000 population, the condition resolves completely in around 71 % of untreated cases [1].
It is characterised by sudden onset, and unilateral, lower motor neuron weakness of the facial nerve with no readily identifiable cause. Other features may include retroauricular pain which may extend into the neck and occiput, impaired tolerance of noise, and ipsilateral disturbance of taste [2]. Inflammation and edema of the facial nerve are considered fundamental parts of the pathogenesis in Bell’s palsy [3]. Clinical studies have shown that corticosteroids significantly improve outcome compared with placebo, probably through their effects against inflammation and edema [4, 5].
Kefalidis, et al. [3] performed a high-resolution computerized tomography of the temporal bone with 1-mm thick contiguous axial sections in 25 patients with unilateral Bell’s palsy. The width of the fallopian tube was measured at the meatal foramen and the middle part of its labyrinthine segment. The measured width of the affected ear was found significantly smaller than that of the unaffected side, both at the meatal foramen (P = 0.007) and at the middle part of the labyrinthine segment (P = 0.03). They concluded that Bell’s palsy seems to usually coincide with the narrower fallopian tube of the patient.
Electrical stimulation of exposed facial nerves in Bell’s palsy has revealed a blocking of nerve impulses in the labyrinthine portion of the facial canal and in the internal auditory meatus [6, 7]. In the present study, we evaluated the diameter of internal acoustic canal in patients with Bell’s palsy to investigate the role of anatomical differences of the temporal bone in etiology of Bells palsy.
Materials and Methods
This retrospective study was conducted in Otolaryngology Clinic, Dr.Lutfi Kirdar Education and Research Hospital, Istanbul, Turkey, between 2013 and 2014 years. All steps of the study was performed according to the rules outlined in declaration of Helsinki [8]. The study was approved by the Ethical Committee of Dr.Lutfi Kirdar Education and Research Hospital.
Subjects
Sixty-four patients who were diagnosed as Bell’s Palsy and temporal bone computed tomography imagings of them were included into the study group (Group 1). Their mean age was 41.3 ± 1.4 years (ranged from 18.0 to 71.0). There were 38 males (59.4 %) and 26 females (40.6 %). In the physical examination, they had normal external ear canal and tympanic membrane.
Patients who were admitted to our outpatient clinic other than ear disease with the temporal bone computed tomography results; and published in our previous study [9] were taken as control group (Group 2). The control group was consisted of 35 healthy subjects without Bell’s Palsy. Their mean age was 41.3 ± 1.17 years (ranged from 20.0 to 65.0). There were 9 males (25.7 %) and 26 females (74.3 %).
Exclusion Criteria
The patients with any ear complaints such as chronic serous otitis media, chronic otitis media and external ear problems; the patients with malign diseases, head and neck trauma, epilepsy and known neurological diseases were excluded from the study.
Methods
1-All patients had temporal bone computed tomography imaging. The internal auditory canal inlet, mid-canal, outlet and canal lengths were measured at the most distinctive cross-section of the seventh and eighth cranial nerves bifurcation (Fig. 1). The measurements were performed by the same radiologist who did not know the patients group (study or control) and also did not know the side of the Bells palsy in the study group.
Fig. 1.
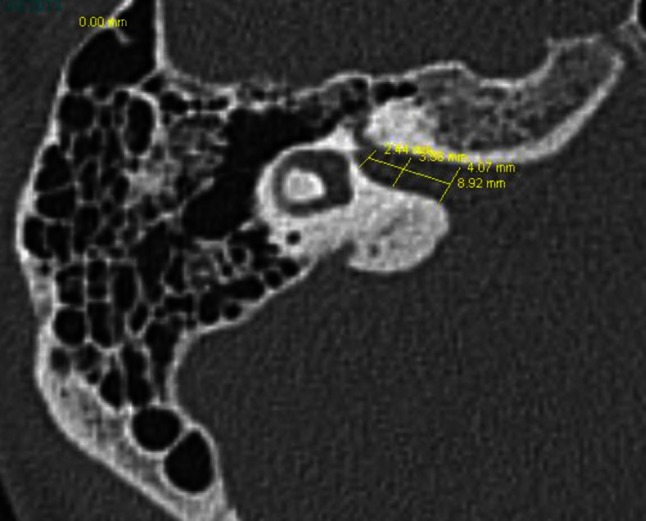
The internal auditory canal inlet, mid-canal, and outlet canal lengths were measured at the most distinctive cross-section of the seventh and eight cranial nerves bifurcation
2-In this retrospective study, hospital data was evaluated for the study group; and House–Brackmann (HB) scores and side of the Bells palsy were also evaluated.
Statistical Analysis
SPSS 16.0 (SPSS Inc., Chicago, 2007) was used. Independant Samples t test and paired t test were used.
P < 0.05 was defined as significant.
Results
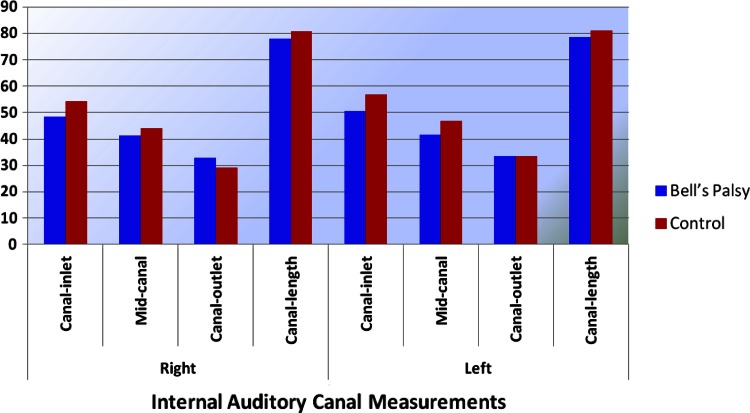
In the study group, Bell’s palsy was on the right side in 26 patients (40.6 %) and on the left side in 38 patients (59.4 %). Initial HB score was HB-2 in 29 patients (45.3 %), HB-3 in 18 patients (28.1 %), HB-4 in 13 patients (20.3 %) and HB-5 in 4 patients (6.2 %). At 6-month evaluation, HB-score of the patients were HB-1 in 37 patients (57.8 %), HB-2 in 25 patients (39.1 %) and HB-3 in 2 patients (3.1 %) (Fig. 2).
Fig. 2.
Internal auditory canal (IAC) measurements of the groups
Internal auditory canal (IAC) measurements of the groups were shown on Table 1. Regarding the right and left internal acoustic canal measurements (inlet, mid-canal, outlet and canal length), there were no significant differences between the measurements of right-mid canal, right canal length; and left canal outlet and left canal length of the study and control groups (p > 0.05) (Table 1) by independant samples t test. Right inlet and outlet; and left inlet and mid-canal values of the study group was significantly lower than those of the control group (p < 0.05).
Table 1.
Internal auditory canal (IAC) measurements of the groups
| Measurements | Group 1 (Bell’s Palsy) (n = 64) | Group 2 (Control (n = 35) | t | P* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Dev. | Min | Max | Mean | SD | Min | Max | ||||
| Right | Canal-inlet | 48.48 | 6.50 | 36.00 | 66.00 | 54.22 | 11.45 | 33.00 | 89.00 | 3.189 | 0.002 |
| Mid-canal | 41.23 | 5.79 | 30.00 | 56.00 | 43.91 | 8.32 | 28.00 | 63.00 | 1.878 | 0.063 | |
| Canal-outlet | 32.85 | 5.00 | 24.00 | 46.00 | 29.20 | 9.83 | 17.00 | 71.00 | −2.458 | 0.016 | |
| Canal-length | 77.81 | 8.84 | 61.00 | 98.00 | 80.80 | 13.25 | 50.00 | 103.00 | 1.341 | 0.183 | |
| Left | Canal-inlet | 50.43 | 7.48 | 36.00 | 69.00 | 56.74 | 16.34 | 19.00 | 98.00 | 2.630 | 0.010 |
| Mid-canal | 41.71 | 5.89 | 30.00 | 57.00 | 46.77 | 8.27 | 35.00 | 72.00 | 3.522 | 0.001 | |
| Canal-outlet | 33.39 | 5.25 | 24.00 | 46.00 | 33.48 | 10.74 | 23.00 | 87.00 | 0.059 | 0.953 | |
| Canal-length | 78.60 | 8.77 | 62.00 | 98.00 | 81.02 | 14.60 | 61.00 | 116.00 | 1.030 | 0.306 | |
* p value shows the results of Independant samples t-test
In each of the study and control groups separetely, the difference between right and left sides were analyzed by Paired samples t-test. In Bell’s palsy group, left inlet, outlet and canal length values were significantly higher than those of the right ones (p < 0.05). In the control group, left mid-canal and outlet values were significantly higher than those of the right ones (p < 0.05) (Table 2).
Table 2.
The difference between measurements of the right and left internal acoustic canal
| Group 1 (Bell’s Palsy) (n = 64) | Group 2 (Control (n = 35) | |||
|---|---|---|---|---|
| t | P* | t | P* | |
| Right-left canal-inlet | −3.748 | 0.000 | −1.379 | 0.177 |
| Right-left mid-canal | −1.612 | 0.112 | −3.181 | 0.003 |
| Right-left canal-outlet | −2.244 | 0.028 | −5.505 | 0.000 |
| Right-left canal-length | −2.119 | 0.038 | −0.114 | 0.910 |
* p value shows the results of paired samples t-test
Pearson Correlation Test Results
In the study group, the relationship between side of Bell’s palsy, initial and 6-month later House–Brackmann scores; and IAC measurements (right and left internal auditory canal inlet, mid-canal, and outlet canal lengths) was analyzed by Pearson Correlation test:
There was no significant correlation between paralysis side and IAC measurement results (p < 0.05)
There was no significant correlation between initial HB score and IAC measurement results (p > 0.05)
In patients with higher initial HB score, their 6-month later HB-stage was also higher (p = 0.005, r = 0.346)
In patients with higher 6-month HB score; R canal inlet (p = 0.048, r = −0.248), R mid-canal (p = 0.048, r = −0.248), L-canal inlet (p = 0.020, r = −0.290), and L-mid canal (p = 0.048, r = −0.249), values were lower.
Discussion
Swelling of the facial nevre is the principal finding in patients with Bell’s palsy [10]. Herpes simplex virus type 1 is considered one of the causes of Bell’s palsy. Several experimental animal studies showed that inoculation of herpes simplex virus type 1 induced facial nerve palsy in mice. In animals with facial paralysis, histopathologic examination revealed massive infiltration of inflammatory cells in the facial nerve. In addition, vacuolar degeneration, demyelination, and edematous swelling of the facial nerve were also observed [11]. MRI enhancement of the facial nerve in Bell’s palsy after administration of gadopentetate dimeglumine has been explained by hypervascularity of the perineural structures and formation of an increased vascular pool of contrast material, and/or disruption of the blood-nerve barrier by acute inflammation [12].
The strangulation of the nerve is more likely to take place at its labyrinthine portion and probably at the meatal foramen (the canalicular entrance to the labyrinthine segment of the facial nerve canal), which is the narrowest site of the bony canal where the loosely arranged intrameatal fibers of the facial nerve are constricted by a fibrous ligament [13, 14].
Vestibulocochlear nerve and the facial nerve enter the temporal bone through the internal auditory canal. The width of the internal acoustic canal varies from person to person [9]. In the present study, we measured the internal auditory canal diameters (inlet, mid-canal, and outlet canal lengths) in patients experienced Bell’s palsy compared to the control group.
In the study group, Bells palsy was on the right side in 26 patients (40.6 %) and on the left side in 38 patients (59.4 %). Initial HB score was HB-2 (45.3 %) and HB-3 (28.1 %). At 6-month evaluation, HB-scoreof the patients were HB-1 (57.8 %) and HB-2 (39.1 %). Internal auditory canal (IAC) measurements of the groups showed that there were no significant differences between the measurements of right-mid canal, right canal length; and left canal outlet and left canal length of the study and control groups. Right inlet and outlet; and left inlet and mid-canal values of the study group (Bells palsy) were significantly lower than those of the control group. In Bells palsy group, left inlet, outlet and canal length values were significantly higher than those of the right ones.
May et al. [15] reported that the narrowest part of the facial nerve canal was at the internal auditory canal entrance with an average diameter of 0.68 mm. However, the cross section of the bony canal might vary in shape, including round, ellipsoid, kidney-shaped, and a triangular contour [3]. These findings suggest that the cross-sectional area, not the diameter, of the facial nerve canal in the temporal bone may be an important factor for mechanical compression and circulatory disturbance of the swollen facial nerve in Bell’s palsy. This study clearly showed that the mean cross-sectional area of the labyrinthine segment of the facial nerve canal was significantly smaller on the affected side than on the unaffected side in patients with Bell’s palsy.
Correlation analysis showed that there were no significant correlation between paralysis side; initial HB score; and IAC measurement results. In patients with higher initial HB stage, their 6-month later HB-stage was also higher. In patients with higher 6-month HB score; R canal inlet, R mid-canal, L-canal inlet, and L-mid canal values were lower.
The exact cause of the smaller bony canals for the facial nerve in the affected side of patients with Bell’s palsy is unclear. The developing bony canal is speculated to acquire stimulus for its growth by the respective nerve, and bone does not begin to enclose the nerve until near the end of the fifth month, with the ossification not being completed even at birth [15]. Our results supported this data; and presence of smaller canal inlet and mid-canal values were related to higher HB scores in the future (6-month evaluation).
As a conclusion, lower IAC inlet and mid-canal values were detected in patients with Bell’s palsy. Therefore narrow IAC inlet and mid-canal values may be one of the risk factors for Bell’s palsy.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.McCaul JA, Cascarini L, Godden D, Coombes D, Brennan PA, Kerawala CJ. Evidence based management of Bell’s palsy. Br J Oral Maxillofac Surg. 2014;52(5):387–391. doi: 10.1016/j.bjoms.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Burgess LP, Yim DW, Lepore ML. Bell’s palsy: the steroid controversy revisited. Laryngoscope. 1984;94:1472–1476. doi: 10.1288/00005537-198411000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Kefalidis G, Riga M, Argyropoulou P, Katotomichelakis M, Gouveris C, Prassopoulos P, et al. Is the width of the labyrinthine portion of the fallopian tube implicated in the pathophysiology of Bell’s palsy? A prospective clinical study using computed tomography. Laryngoscope. 2010;120:1203–1207. doi: 10.1002/lary.20896. [DOI] [PubMed] [Google Scholar]
- 4.Gilden DH. Bell’s palsy. N Engl J Med. 2004;351:1323–1331. doi: 10.1056/NEJMcp041120. [DOI] [PubMed] [Google Scholar]
- 5.Engström M, Berg T, Stjernquist-Desatnik A, Axelsson S, Pitkäranta A, Hultcrantz M, et al. Prednisolone and valaciclovir in Bell’s palsy: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2008;7:993–1000. doi: 10.1016/S1474-4422(08)70221-7. [DOI] [PubMed] [Google Scholar]
- 6.Gantz BJ, Gmur A, Fisch U. Intraoperative evoked electromyography in Bell’s palsy. Am J Otolaryngol. 1982;3:273–278. doi: 10.1016/S0196-0709(82)80066-5. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins H, Herzog J, Coker N. Bell’s palsy in children. Cases of progressive facial degeneration. Ann Otol Rhinol Laryngol. 1985;94(4 pt 1):331–336. [PubMed] [Google Scholar]
- 8.WMA, Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects, in 59th WMA General Assembly, W.M. Association, Editor: Seoul, 2008
- 9.Kumral TL, Yıldırım G, Yılmaz HB, Ulusoy S, Berkiten G, Onol SD, et al. Is it necessary to do temporal bone computed tomography of the internal auditory canal in tinnitus with normal hearing? Sci World J Volume 2013 (2013) [DOI] [PMC free article] [PubMed]
- 10.Yanagihara N, Honda N, Hato N, Murakami S. Edematous swelling of the facial nerve in Bell’s palsy. Acta Otolaryngol. 2000;120:667–671. doi: 10.1080/000164800750000522. [DOI] [PubMed] [Google Scholar]
- 11.Sugita T, Murakami S, Yanagihara N, Fujiwara Y, Hirata Y, Kurata T. Facial nerve paralysis induced by herpes simplex virus in mice: an animal model of acute and transient facial paralysis. Ann Otol Rhinol Laryngol. 1995;104:574–581. doi: 10.1177/000348949510400713. [DOI] [PubMed] [Google Scholar]
- 12.Tien R, Dillon WP, Jackler RK. Contrast-enhanced MR imaging of the facial nerve in 11 patients with Bell’s palsy. AJR Am J Roentgenol. 1990;155:573–579. doi: 10.2214/ajr.155.3.2117359. [DOI] [PubMed] [Google Scholar]
- 13.Fisch U. Surgery for Bell’s palsy. Arch Otolaryngol. 1981;107:1–11. doi: 10.1001/archotol.1981.00790370003001. [DOI] [PubMed] [Google Scholar]
- 14.Fisch U, Esslen E. Total intratemporal exposure of the facial nerve. Arch Otolaryngol. 1972;95:335–341. doi: 10.1001/archotol.1972.00770080529008. [DOI] [PubMed] [Google Scholar]
- 15.May M, Schaitkin B. The facial nerve. 2. New York: Thieme Medical; 2000. [Google Scholar]