Abstract
Diabetes mellitus (DM) is a metabolic disorder caused by hyperglycemia which leads to dysfunction of various organs. Hearing acuity is equally hindered by this disorder. Among individuals with DM audiological characteristics of DM type 1 are of great concern in the literature. This study aims at establishing high frequency audiometry (HFA) as a useful tool in identifying early onset of hearing loss in individuals with DM type 2. 20 non-diabetic participants and 20 individuals with DM type 2 in the age range of 20–40 years were considered for the study. Subjects in both groups underwent otoscopic examination, PTA at 0.25, 0.5, 1, 2, 4 and 8 kHz and HFA at 9, 10, 11.2, 12.5, 14 and 16 kHz. Results revealed statistically significant difference in thresholds of both PTA and HFA at all frequencies across the group, but the mean threshold difference between the diabetic and non-diabetic group was marked in HFA than in PTA. In the diabetic subjects the thresholds of PTA was within 25 dBHL at all frequencies when compared to the thresholds of HFA. Individuals with DM type 2 showed bilateral symmetrical mild hearing loss in HFA and the hearing loss increased with ascending test frequencies from 9,000 to 16,000 Hz. Mild hearing loss in HFA is an indicator for early onset of hearing loss in DM type 2. Hence this present study emphasis the clinical utility of HFA in young adults with DM type 2.
Keywords: Diabetes mellitus type 2, Pure tone audiometry, High frequency audiometry, Minimal hearing loss
Introduction
Diabetes mellitus (DM) is a group of metabolic disorder characterized by hyperglycemia resulting from insulin secretion, insulin action or both. DM fall into two major categories, type 1 and type 2. Type 1 results from cellular mediated autoimmune destruction of β cells of the pancreas. Type 1 is also called as insulin dependent diabetes mellitus. Type 2 results from insulin resistance rather than deficiency. Of the two types, type 2 accounts for 90–95 % and type 1 for 0–5 % [1]. The chronic diabetes mellitus is associated with long term damage, dysfunction and failure of different organs especially the eyes, kidneys, nerves, heart and blood vessels.
Diabetes mellitus also affects hearing sensitivity [2]. Hearing thresholds were reported to be worse especially at high frequencies [5] and at low and middle frequencies [9]. Research studies on audiological evaluation on patients with DM, using pure tone audiometry at conventional frequencies showed variable results with frequency. The hearing loss was reported to be bilateral [4, 7, 8] progressive [4, 7] mild to moderate [8] sensorineural [6] affecting high frequencies in subjects with type 2 diabetes mellitus.
The reduction in hearing thresholds were correlated to microangiopathic involvement of endolymphatic sac and basilar membrane [10]. The changes observed in cochlear structures in type 2 DM were loss of outer hair cells in the lower and basal turns of the cochlea. In type 2 DM, walls of blood vessels of stria vascularis were thicker in basal turns in subjects who took oral hypoglycemic agents, walls of blood vessels of stria vascularis and basilar membrane were thicker in basal turns in subjects who were on insulin therapy. Atrophy of stria vascularis of most turns in subjects who were on insulin therapy and the atrophy involved the lower turns in oral hypoglycemic agent subjects [3].
There are no studies currently available in the literature regarding the extended high frequency sensitivity in individuals with type 2 DM. Extended High frequency audiometry has been used in early detection of hearing loss in various conditions like noise induced hearing loss and ototoxicity. So the present study aimed at establishing high frequency audiometry as a useful tool in identifying early onset hearing loss in individuals with type 2 DM.
Methods
Subjects
Two groups were taken for this present study. 20 non-diabetic subjects (7 males and 13 females) in the age range of 20–40 years and 20 subjects with type 2 Diabetes mellitus (3 males and 17 females) in the age range 20–40 years. Healthy nondiabetic subjects were volunteer students with no history of external and middle ear diseases or at any secondary risk of hearing damage such as noise exposure, ototoxicity or any other inner ear diseases. Type 2 diabetic subjects with minimum of 3 years duration of the disorder were considered for the present study and none of them had and otologic complaints and diseases. All the subjects from the diabetic group were referred from Department of Diabetology. They were subjected to ear, nose and throat examination to rule out external and middle ear diseases. Written consent was taken from all the subjects for audiological evaluation in the study. The study was carried out with ethical clearance from the local ethical body.
Instrumentation and Procedure
Pure Tone Audiometry (PTA) for both air conduction and bone conduction was performed using Inventis-piano dual channel digital Audiometer with TDH-39 and Radioear B-71 transducers for air conduction and bone conduction respectively in a sound treated room. Pure tone air conduction thresholds were established for the octave frequencies using modified Hughson–Westlake Method from 250, 500, 1,000, 2,000, 4,000 and 8,000 Hz. Bone conduction thresholds were also established for the octave frequencies of 250, 500, 1,000, 2,000 and 4,000 Hz using the same procedure.
High frequency Audiometry (HFA) air conduction thresholds were obtained for frequencies 9,000, 10,000, 11,200, 12,500, 14,000 and 16,000 Hz using modified Hughson–Westlake Method. HD-200 transducer was used in Inventis-piano Audiometer to evaluate high frequency thresholds.
Results
Statistical analysis was carried out for the PTA thresholds and HFA thresholds between the non-diabetic group and diabetic group using SPSS software (version 15.0). The mean and standard deviation of the thresholds at each frequency were computed at both conventional frequencies (250, 500, 1,000, 2,000, 4,000 and 8,000 Hz) and high frequencies (9,000, 10,000, 11,200, 12,500, 14,000 and 16,000 Hz) are shown in Tables 1 and 2 respectively.
Table 1.
The mean and standard deviation of the thresholds for non-diabetic group and diabetic group in conventional PTA
| Groups | Thresholds (dBHL) | Test frequencies | |||||
|---|---|---|---|---|---|---|---|
| 250 Hz | 500 Hz | 1,000 Hz | 2,000 Hz | 4,000 Hz | 8,000 Hz | ||
| NDG (N = 40 ears) | Mean | 9.00 | 11.75 | 10.25 | 9.25 | 10.12 | 10.00 |
| SD | 3.61 | 4.60 | 3.91 | 4.31 | 3.29 | 4.23 | |
| DG (N = 40 ears) | Mean | 14.12 | 16.50 | 22.62 | 16.25 | 17.50 | 18.00 |
| SD | 7.15 | 7.26 | 8.98 | 9.04 | 10.86 | 13.57 | |
NDG non diabetic group, DG diabetic group
Table 2.
The mean and standard deviation of the thresholds for non-diabetic group and diabetic group in HFA
| Groups | Thresholds (dBHL) | Test frequencies | |||||
|---|---|---|---|---|---|---|---|
| 9,000 Hz | 10,000 Hz | 11,200 Hz | 12,500 Hz | 14,000 Hz | 16,000 Hz | ||
| NDG (N = 40) | Mean | 19.50 | 15.87 | 15.00 | 13.94 | 18.97 | 34.28 |
| SD | 7.82 | 9.46 | 5.547 | 5.83 | 8.05 | 4.35 | |
| DG (N = 40) | Mean | 27.87 | 27.37 | 34.87 | 38.42* | 41.02** | 36.19*** |
| SD | 14.75 | 16.21 | 16.77 | 20.53 | 21.73 | 15.48 | |
NDG non diabetic group, DG diabetic group
* 2 ears had no response at 12,500 Hz for 70 dBHL
** 6 ears had no responses at 14,000 Hz for 60 dBHL
*** 19 ears had no response at 16,000 Hz or 50 dBHL
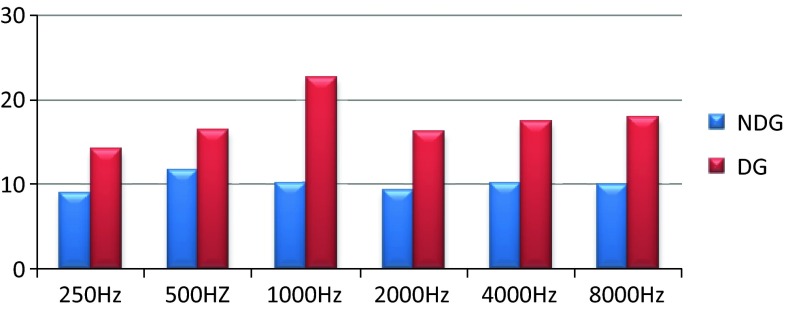
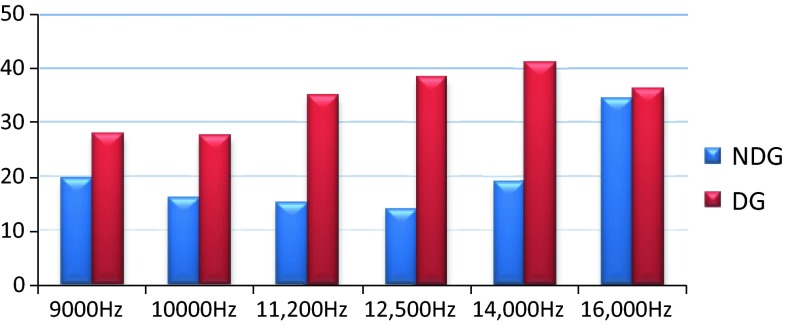
We define hearing impairment if pure tone thresholds were above 25 dBHL. The mean thresholds of the non-diabetic group were within 25 dBHL at all conventional test frequencies and high frequencies except 16,000 Hz.
In the diabetic group the mean thresholds at conventional test frequencies were slightly higher when compared to Non diabetic group but they were within 25 dBHL. The mean thresholds for high frequencies increased from 9,000 and 14,000 Hz as shown in Table 2. The mean thresholds were elevated for both the non-diabetic and diabetic group at 16,000 Hz. Among the 40 ears in diabetic group 2 ears, 6 and 19 ears had no responses at 12,500 Hz–70 dBHL, 14,000 Hz–60 dBHL and 16,000 Hz–50 dBHL respectively.
T test was used to statistically compare the thresholds of diabetic and non diabetic groups at conventional test frequencies and high frequencies. The t values, degree of freedom and the level of significance for PTA and HFA between diabetic and non diabetic group are shown in Tables 3 and 4 respectively. The difference between the thresholds of non-diabetic and diabetic group was significant at all conventional frequencies and high frequencies (p < 0.005) except 16,000 Hz.
Table 3.
t values, degree of freedom and the level of significance for PTA between Diabetic and Non diabetic group
| Values NDG–DG | 250 Hz | 500 Hz | 1,000 Hz | 2,000 Hz | 4,000 Hz | 8,000 Hz |
|---|---|---|---|---|---|---|
| t values | −3.914 | −3.344 | −7.827 | −4.895 | −4.040 | −3.676 |
| df | 39 | 39 | 39 | 39 | 39 | 39 |
| Significance | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.001 |
NDG non diabetic group, DG diabetic group
Table 4.
t values, degree of freedom and the level of significance for HFA
| Values NDG–DG | 9,000 Hz | 11,200 Hz | 10,000 Hz | 12,500 Hz | 14,000 Hz | 16,000 Hz |
|---|---|---|---|---|---|---|
| t values | −3.241 | −4.037 | −7.100 | −7.471 | −5.609 | −0.580 |
| df | 39 | 39 | 39 | 37 | 33 | 20 |
| Significance | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.568 |
NDG non diabetic group, DG diabetic group
The comparison of mean thresholds for the non-diabetic and diabetic individuals in PTA and HFA are shown in Figs. 1 and 2.
Fig. 1.
Mean thresholds of diabetic and non-diabetic groups in PTA
Fig. 2.
Mean thresholds of diabetic and non-diabetic groups in HFA
Discussion
As shown in Figs. 1 and 2 the control non diabetic subjects showed thresholds that are within 25 dBHL at conventional frequencies and high frequency. Within the group there are slight differences in between PTA and HFA thresholds which could be possibly due to variability of high frequency thresholds in normal hearing individuals.
In the type 2 diabetic group the thresholds were slightly elevated at conventional frequencies but were within 25 dBHL and variability within the group are minimal. But in HFA the type 2 diabetic individuals showed a significant bilateral mild or moderate degree symmetrical sloping hearing loss with high variability in thresholds. This can be accounted to the fact that atrophy and thickening of blood vessels of stria vascularis in basal turns of cochlea occurs in individuals with type 2 diabetic mellitus [3].
This study demonstrates a significant difference in the thresholds between the diabetic and non-diabetic subjects at all test frequencies and threshold difference was marked in HFA for subjects with type 2 diabetic mellitus at younger age group.
A significant difference between the thresholds of the diabetic and non-diabetic group was not found at 16,000 Hz which could be due to the facts that (1) many nondiabetic subjects had elevated thresholds at 16,000 Hz, (2) very few diabetic subjects had thresholds at 16,000 Hz and (3) the maximum audiometric limit at 16,000 Hz is 50 dBHL. Thus we suggest a high frequency audiometry as an effective tool in early identification of hearing loss in young type 2 diabetic subjects. This tool can also be effective in monitoring of progression of hearing loss since no other test is available to evaluate regions in cochlea beyond 8,000 Hz.
Conclusion
Type 2 DM is one of most common metabolic disorder which leads to dysfunction of various organs. The present study attempted to identify onset of hearing loss in young subjects with type 2 DM using conventional PTA and HFA. These findings reveal that a bilateral mild to moderate symmetrical sloping hearing loss in the HFA is an indicator for early onset of hearing loss. This study further emphasis that HFA is an essential tool to identify early onset of hearing loss if when compared to conventional pure tone Audiometry. Thus, we emphasis the use of HFA in the audiological test battery for the assessment of type 2 DM. This will also enable us for early identification and monitoring the progression of hearing loss in individuals with type 2 DM.
Contributor Information
S. S. Vignesh, Email: vigneshaslp@gmail.com
V. Jaya, Email: vjayashree70@gmail.com
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:62–69. doi: 10.2337/dc10-S062. [DOI] [Google Scholar]
- 2.Dalton DS, Cruickshanks KJ, Klein R, Klein BEK, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care. 1998;21(9):1540–1544. doi: 10.2337/diacare.21.9.1540. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima H, Cureoglu S, Schechter PA, Paparella MM, Harada T, Oktay MF. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132(9):934–938. doi: 10.1001/archotol.132.9.934. [DOI] [PubMed] [Google Scholar]
- 4.Kudelska MR, Chodynicki S, Kinalska I, Kowalska I. Hearing loss in patients with diabetes mellitus type II. Otolaryngol Pol. 2002;56(5):607–610. [PubMed] [Google Scholar]
- 5.Kuriena M, Thomasa K, Bhanua TS. Hearing threshold in patients with diabetes mellitus. J Laryngol Otol. 1989;103(2):164–168. doi: 10.1017/S0022215100108345. [DOI] [PubMed] [Google Scholar]
- 6.Panchu P. Auditory acuity in type 2 Diabetes mellitus. Int J Diab Dev Ctries. 2008;28(4):114–120. doi: 10.4103/0973-3930.45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pemmaiah KD, Srinivas DR. Hearing loss in diabetes mellitus. Int J Collab Res Intern Med Public Health. 2011;3(10):725–731. [Google Scholar]
- 8.Rajendran S, Anandhalakshmi MB, Rao V. Evaluation of the incidence of sensorineural hearing loss in patients with type 2 diabetes mellitus. Int J Biol Med Res. 2011;2(4):982–987. [Google Scholar]
- 9.Tay HL, Ray N, Ohri R, Frootko NJ. Diabetes mellitus and hearing loss. Clin Otolaryngol Allied Sci. 1995;20(2):130–134. doi: 10.1111/j.1365-2273.1995.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 10.Wackym PA, Linthicum FH. Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol. 1986;7(3):176–182. [PubMed] [Google Scholar]