Abstract
Indications for cochlear implantation have expanded today to include very young children and those with syndromes/multiple handicaps. Programming the implant based on behavioral responses may be tedious for audiologists in such cases, wherein matching an effective MAP and appropriate MAP becomes the key issue in the habilitation program. In ‘Difficult to MAP’ scenarios, objective measures become paramount to predict optimal current levels to be set in the MAP. We aimed, (a) to study the trends in multi-modal electrophysiological tests and behavioral responses sequentially over the first year of implant use, (b) to generate normative data from the above, (c) to correlate the multi-modal electrophysiological thresholds levels with behavioral comfort levels, and (d) to create predictive formulae for deriving optimal comfort levels (if unknown), using linear and multiple regression analysis. This prospective study included ten profoundly hearing impaired children aged between 2 and 7 years with normal inner ear anatomy and no additional handicaps. They received the Advanced Bionics HiRes 90K Implant with Harmony Speech processor and used HiRes-P with Fidelity 120 strategy. They underwent, Impedance Telemetry, Neural Response Imaging, Electrically Evoked Stapedial Response Telemetry and Electrically Evoked Auditory Brainstem Response tests at 1, 4, 8 and 12 months of implant use, in conjunction with behavioral Mapping. Trends in electrophysiological and behavioral responses were analyzed using paired t test. By Karl Pearson’s correlation method, electrode-wise correlations were derived for NRI thresholds versus Most Comfortable Levels (M-Levels) and offset based (apical, mid-array and basal array) correlations for EABR and ESRT thresholds versus M-Levels were calculated over time. These were used to derive predictive formulae by linear and multiple regression analysis. Such statistically predicted M-Levels were compared with the behaviorally recorded M-Levels among the cohort, using Cronbach’s Alpha Reliability test method for confirming the efficacy of this method. NRI, ESRT and EABR thresholds showed statistically significant positive correlations with behavioral M-Levels, which improved with implant use over time. These correlations were used to derive predicted M-Levels using regression analysis. Such predicted M-Levels were found to be in proximity to the actual behavioral M-Levels recorded among this cohort and proved to be statistically reliable. When clinically applied, this method was found to be successful among subjects of our study group. Although there existed disparities of a few clinical units, between the actual and predicted comfort levels among the subjects, this statistical method was able to provide a working MAP, close to the behavioral MAP used by these children. The results help to infer that behavioral measurements are mandatory to program cochlear implantees, but in cases where they are difficult to obtain, this study method may be used as reference for obtaining additional inputs, in order to set an optimal MAP. The study explores the trends and correlations between electrophysiological tests and behavioral responses, recorded over time among a cohort of cochlear implantees and provides a statistical method which may be used as a guideline to predict optimal behavioral levels in difficult situations among future implantees. In ‘Difficult to MAP’ scenarios, following a protocol of sequential behavioral programming, in conjunction with electrophysiological correlates will provide the best outcomes.
Keywords: Cochlear Implant (CI), Impedance Telemetry (IT), Evoked Compound Action Potential (ECAP), Neural Response Imaging (NRI), Electrically Evoked Stapedial Response Telemetry (ESRT), Electrically Evoked Auditory Brainstem Response (EABR), Measurable Auditory Percept (MAP), Most Comfortable Level (M-Level), Clinical Unit (CU)
Introduction
Cochlear implantation (CI) has been established as a successful time-tested technology for restoration of hearing in individuals with bilateral severe to profound hearing loss. The inclusion criteria for CI, has expanded today in various aspects, to include candidates ranging from post-lingual adults with partial high frequency hearing loss to children with congenital profound hearing loss, as young as 6 months of age. As many young children and those with multiple disabilities/syndromic associations are being implanted today, even experienced audiologists may face the daunting task of programming ‘Difficult to MAP’ children using conventional methods. Children with multiple handicaps may have cognitive problems, developmental delay, attention deficit etc., and it is often difficult to elicit consistent responses from them. Behavioral responses may be inconsistent in such cases, since they vary depending upon their age, listening experience and cognitive abilities [1, 2]. In such scenarios objective electrophysiological tests pave the way forward to program an initial MAP for them. Studies have shown that post-operative objective electrophysiological tests like ECAP, ESRT and EABR thresholds correlate well with behavioral levels and these measurements may be used to ascertain an optimal behavioral Map for the implantee (Spivak and Chute [1], Hodges et al. [2]).
In clinical practice, when a ‘Difficult to MAP’ scenario is anticipated or encountered, audiologists may perform an intra-operative or post-operative electrophysiological test like ECAP measurement (NRI/NRT/ART) or ESRT, in order to get an idea of the optimal current level required for stimulation via the implant. They incorporate these current levels into the programming software to set a baseline MAP at ‘Switch-On’, and further redefine the levels thenceforth using psychophysical behavioral responses of the child. This method is quite successful for providing a working MAP for the child at ‘Switch-On’ and later-on fine-tuning the MAP is based on the child’s habilitation performance and psychoacoustical feedback [3]. Sometimes in clinical practice, there have been situations where a child’s behavioral Mapping levels were found to be inappropriate or erroneous and hence the habilitation outcomes were sub-optimal [2]. Such children may return to the audiologist for trouble-shooting and Re-Mapping.
Recent Mapping software, like the Soundwave 2.0.33, has provision for incorporating the electrophysiological current levels (tested intra-operatively or post-operatively) into the programming module for setting an ideal MAP. Sometimes in clinical practice, such a method has not been very successful, due to inherent disparity between the electrophysiological current levels and the actual behavioral current levels which need to be set in the MAP. While ECAP thresholds help to identify the current levels required to stimulate the auditory nerve, they may not evoke an optimal behavioral response from the child when set in the MAP. This disparity has been implicated to the variation in parameters like the stimulation rate and pulse duration, while measuring an ECAP and while programming a MAP [4]. A higher stimulation rate is used in Mapping for optimal processing of stimuli, while a lower stimulation rate is preferred while performing ECAP measurements, since accurate electrophysiological thresholds can thus be identified [4]. Literature states that ECAP thresholds may be successfully recorded in 80–83 % of cases, but are not sensitive to identify accurate Mapping levels. ESRT is known to over-predict the optimal behavioral comfort levels during the initial period of habilitation and EABR though reliable, is found to be cumbersome, time-consuming and impractical to be done electrode-wise, in order to comprehensively program a cochlear implantee [2, 5–7]. Hence, no single electrophysiological test has been found to have high sensitivity and reliability for setting an ideal MAP [8].
In literature, especially in the Indian context, there is lack of normative data and reference values, for correlation of electrophysiological thresholds and behavioral responses, which may be used as a guideline for programming cochlear implantees. This practical fact, triggered the need for this study, which was conceptualized with the hypothesis that, multi-modal correlations of various electrophysiological tests with behavioral levels recorded in a cohort, would help to statistically predict reliable and optimal behavioral levels (when unknown) using the linear and multiple regression models, rather than using a single electrophysiological threshold for direct incorporation into the MAP, which has been the conventional method clinically followed.
Materials and Methods
Study Design
This prospective multi-centric clinical study was performed at the Cochlear Implant Electrophysiology Lab and Habilitation Clinic, Madras ENT Research Foundation (MERF), Chennai and at the Cochlear Implant Program Centre of Sri Ramachandra University (CLIPS), Chennai over 2 years from May 2010 till May 2012. The study included ten non-syndromic, pre-lingual, profoundly hearing impaired children aged between 2 and 7 years with normal inner ear anatomy and no additional handicaps. Clinical data of the study group has been shown in Table 1. These children received the Advanced Bionics HiRes 90K Implant with Harmony Speech processor and used HiRes-P with Fidelity 120 strategy. After counseling regarding the test protocol, a written and informed consent was obtained from the parents of these children, prior to their inclusion. This research work was approved by the Institutional Ethical Committees of both centers of study.
Table 1.
Clinical data of study group
| Subject | Age at present/sex | Lingual status | Significant aetiology | Hearing aid usage prior to implantation | Age at implantation |
|---|---|---|---|---|---|
| 1 | 5/M | Pre-lingual | Birth asphyxia | 2 years | 4 years 3 months |
| 2 | 4/M | Pre-lingual | None | 1 year | 3 years 1 month |
| 3 | 3/M | Pre-lingual | Pre-term birth | Nil | 2 years 5 months |
| 4 | 3/M | Pre-lingual | Consanguinity | 8 months | 2 years |
| 5 | 8/F | Peri-lingual | Kemicterus | 3 years (irregular use) | 6 years 10 months |
| 6 | 5/M | Pre-lingual | Consanguinity | 3 years | 4 years |
| 7 | 7/M | Pre-lingual | Familial hearing loss | 4 years (irregular use) | 5 years 7 months |
| 8 | 4/F | Pre-lingual | Pre-term birth | Nil | 2 years 2 months |
| 9 | 5/M | Pre-lingual | None | 1 year (irregular use) | 4 years 9 months |
| 10 | 6/F | Pre-lingual | Consanguinity | 2 years | 5 years |
Objectives
The study aimed to develop a statistical method, which may be used to program ‘Difficult to MAP’ cochlear implantees. The objectives were, (a) to study the trends in multi-modal electrophysiological tests and behavioral responses, sequentially from the time of ‘Switch-On’, till the completion of Habilitation program, over a period of 1 year, (b) to generate normative data for electrophysiological tests and behavioral responses based on the trends, (c) to correlate the multi-modal electrophysiological thresholds levels with behavioral comfort levels, and (d) to create predictive formulae for deriving optimal behavioral comfort levels, based on their electrophysiological correlations, using linear and multiple regression statistical methods.
Methods
All children underwent multi-modal electrophysiological tests—Impedance Telemetry (IT), Neural Response Imaging (NRI), Electrically Evoked Stapedial Response Telemetry (ESRT) and Electrically Evoked Auditory Brainstem Responses (EABR), at periodic intervals after ‘Switch-On’ at 1, 4, 8 and 12 months respectively, in conjunction with sequential Behavioral Mapping, as per standard habilitation protocols. At each schedule, conventional psychophysical behavioral Mapping was performed, prior to conducting electrophysiological tests, in order to record the actual comfort levels, while the children were fully alert and cooperative. Electrophysiological tests were performed on the same day or on the subsequent day, when the child was cooperative or sedated and sleeping. The testing sequence was staged as follows—IT, EABR (for 3 offsets across the array—El 1 in apical array, El 8 in mid-array and El 16 in basal array), NRI (electrode-wise) and ESRT (for 3 offsets across the array—El 1 in apical array, El 8 in mid-array and El 16 in basal array). EABR was tested first when the child was asleep or sedated since, it required a tedious set up, was time consuming and EEG disturbances/muscle artifacts needed to be minimal during the test. NRI was performed following EABR and ESRT was tested last since most children were averse to the loudness of stimuli and would otherwise not cooperate for further testing. In between tests, adequate rest time was allowed, in order to obtain maximum cooperation from the child and to avoid the fatigue factor. Most children (7 out of 10) needed to be sedated for EABR testing, while a few children (3 out of 10) needed sedation for ESRT testing, since they were not cooperative. No child required sedation more than once at each schedule and with experience these tests could be performed faster, while the children were asleep after an afternoon meal. In cases where satisfactory recordings were not obtained, due to technical issues or patient incompliance, tests were repeated on the next day. Thus, the authors could successfully acquire all required data within 2 days for each child.
Techniques
All electrophysiological and Mapping current levels were recorded in Clinical Units (CU), which represents the basic unit of stimulus intensity used in the testing and programming Software—Soundwave (Version 2.0.33). The Advanced Bionics HiRes 90K Cochlear Implant system was connected to the Soundwave software via a Platinum Speech Processor (PSP), during the various tests. Default stimulus parameters for pulse duration and stimulation rate were maintained during electrophysiological measurements, since any change in them would influence a bias in the values measured between various electrodes across the array and at subsequent test schedules [4]. If a representative electrode showed no response during testing, the test was repeated on the subsequent day and in three cases extrapolated data from the adjacent electrodes were used for statistical analysis in the study. The threshold for all objective measures, was defined as the lowest stimulation level at which a response was identified as present. Visual inspection of characteristic peaks was performed by the experienced audiologist for each objective measure, in order to identify and confirm the thresholds of stimulation. The learning curve was difficult in the initial period of the study, due to various issues like technical and software snags, stimulus artifacts and electrical interferences (especially with EABR), patient compliance (especially with ESRT) and other logistic reasons.
Mapping Protocol
The Advanced Bionics Cochlear Implant, uses a comfort level based fitting technique. Programming is based on Most Comfortable Levels (M-Levels), while the Threshold Level (T-Level) for each electrode is auto-set by the software at 10 % of the M-Level values in CUs. This helps to maintain an optimal dynamic range throughout the period of habilitation. Using this conventional Mapping technique at each schedule of programming, psychophysical behavioral comfort levels (M-Level) were sequentially obtained across the array, and these were incorporated into the speech processor as the most stable and preferred MAP for the child. M-Levels were determined by increasing stimulus intensity until the child indicated that the sound was loud but tolerable. Younger children, whose ability to judge the loudness was limited, were monitored for eye blinking, crying and changes in facial expression or activity level during and shortly after stimulus presentation, in order to identify their M-Levels. An in-house child psychologist and the mothers of these young children were also part of the tests, in order to help identify the behavioral responses appropriately.
EABR Testing
EABR stimulus was delivered by the SCLIN2000 software (Version 1.08) with electrical pulses of 25 μs of alternating polarity presented at a repetition rate of 11–31 Hz. This pulse was carried by the trigger cable via the PSP processor onto the implant and the response was received by a pre-amplifier and sent to the Intelligent Hearing Systems (IHS) SmartEP (evoked potential) software (Version 3.91USBez), in a paired computer to synchronize the recording window with the stimulus presentation. Recordings on the IHS-SmartEP module were made between 5 and 80 ms, relative to stimulus onset and a time window of 10 ms, was used for visual inspection of the EABR waveforms. Three representative electrodes from the three offsets created across the array (apical array El 1, mid-array El 8 and basal array El 16) were used for EABR testing. Larger intensity level steps of 10 CU were used for EABR to minimize test time, in an effort to complete testing for all three electrodes across the array at one sitting, while the children were asleep or sedated. In a few cases, EABR waveforms were interspersed with non-auditory waveforms or artifacts and in these cases, a polarity reversal with adjustments in high/low pass filter settings needed to be done, in order to overcome any ambiguity and clearly identify EABR responses. While recording EABR, it was observed that waves, eIII and eV were clearly recordable between 2 and 7 ms, with their amplitudes being more prominent at higher intensity levels. The EABR T-Level, was identified as the lowest intensity of stimulus which evoked a consistent, clearly recognizable wave eV and this was considered as the confirmation of a brainstem response to electrical stimulation via the implant.
ECAP Testing
NRI thresholds were serially obtained using the in-built ECAP module of the Soundwave 2.0.33 software, with automated settings for all electrodes across the array. The default stimulation range was between 100 and 350 CU with a cathodic-first stimulation sequence, gain of 300 and averages per data point of 128. EP Growth Function was sequentially monitored at various stimulation levels, by the appearance of typical N1–P2 waveforms and the NRI threshold was identified as the lowest intensity of stimulus which evoked a recognizable ECAP response on the Soundwave software.
ESRT Testing
Stapedius reflex measurement was performed in the implanted ear, after confirming normal middle ear function with tympanometry. A tone-burst pulse train stimulus from the Soundwave software at 500 ms interval with 18 μs pulse width and 3,712 pps channel rate in Automated Pulse Width mode, was used to trigger ESRT responses which were recorded on the Reflexometer of the Interacoustics AZ 26 Impedance Bridge. We used three representative electrodes from three offsets created across the array for measuring ESRT responses—namely El 1 in apical array, El 8 in mid-array and El 16 in basal array. ESRT thresholds were identified to be that minimal stimulus level, which evoked a recognizable deflection on the reflexometer. ESRT thresholds were accepted as present, if three clear immittance deflections were observed on the reflexometry for a particular stimulus level.
Statistical Analysis
Data was analyzed by the Bio-statistician using the SPSS 17.0 software. Trends in electrophysiological and behavioral responses of the auditory nerve recorded during the study period were analyzed using the paired t test and normatives were obtained for this cohort. Using the Karl Pearson’s correlation method, electrode-wise correlations were derived for NRI versus M-Level and offset based correlations for ESRT and EABR versus M-Level were calculated sequentially over time. The following reference range was used for correlations wherein; ‘r’ <0.001 = no significant correlation, 0.001–0.300 = poor correlation, 0.301–0.700 = moderate correlation and 0.701–0.999 = good correlation. These correlations were used to derive predictive formulae by linear and multiple regression analysis. By this method NRI, ESRT and EABR values recorded for a representative electrode across the array, could be applied into the regression formulae, for deriving an optimal M-Level for that electrode (if unknown). This predicted value may be used as reference to program that electrode. Such statistically predicted M-Levels were compared with the actual (behaviorally recorded) M-Levels among the study group, using Cronbach’s Alpha Reliability test method. The following reference range was used for reliability testing wherein; ‘R’ <0.001 = no significant reliability, 0.001–0.400 = poor reliability, 0.401–0.700 = moderate reliability and 0.701–0.999 = good reliability.
Observations and Results
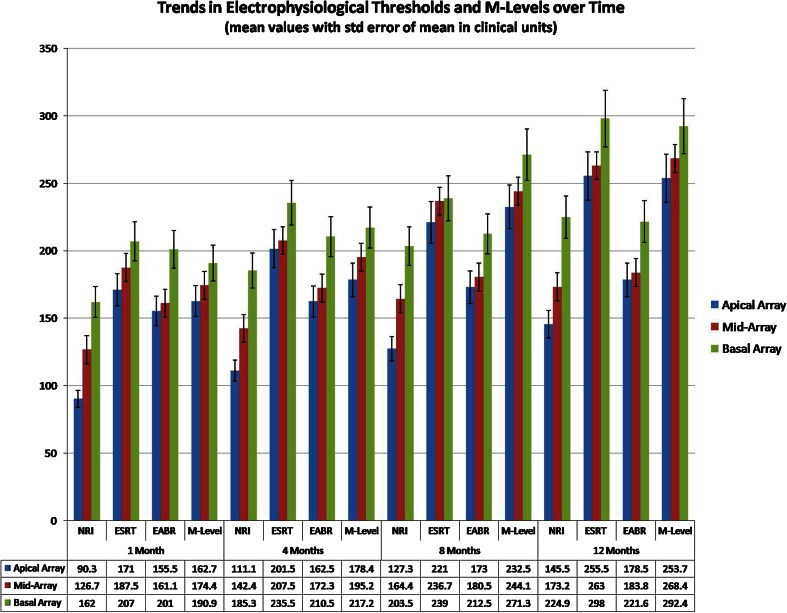
The longitudinal trends observed in the electrophysiological tests and comfort levels at the four test schedules, have been displayed in Fig. 1. The mean average current levels have been represented in CUs with error of mean across electrodes shown as error bars.
Fig. 1.
Trends in electrophysiological thresholds and M-levels over time
Trends in IT
The impedance changes were monitored through each schedule of electrophysiological testing. The mean average Impedance levels ranged between 4.8 and 7.9 kΩ across the array. The initial impedance measurements, when checked at first month of implant use were found to be higher than subsequent measurements and a trend of higher impedance levels in the apical and basal array was observed in the study group. The overall Impedance values in the mid-array electrodes were lesser by a mean average of 1.58 kΩ (±0.30 SD), than the apical and basal electrodes at 1 year of follow up. This was statistically significant with a ‘p’ value of 0.034. The Impedance levels across the array stabilized over time with implant use.
Trends in Behavioral M-Levels
Electrode-wise trends in psychophysical behavioral comfort levels when observed over time, showed a sequential gradual ascent in the M-Levels across the array, starting from a mean average of 155 CU at first month of implant use to 272 CU, by the end of 1 year of implant use. This signified the expansion in the dynamic range of implant aided hearing, as the children’s auditory perception skills and capacity to tolerate higher intensity sounds through the implant improved over time of implant use. M-Levels gradually rose from the apical electrodes towards the basal array. M-Levels were higher by a mean average of 36.3 (±7 SD) CU, between the apical and basal arrays at completion of 1 year of implant use. This was statistically significant with a ‘p’ value of 0.017. The higher M-Levels noted in the basal array, implied that louder impulses were required to address the basal region of the cochlea, which has higher density of spiral ganglions and codes for higher frequencies of auditory stimulation.
Trends in ECAP Thresholds
NRI thresholds were typically lower than M-Levels and were stable across the electrode array at all schedules of testing. NRI thresholds ranged between a mean average of 113 (±11 SD across the mean) CU noted at first schedule to 202 (±18 SD across the mean) CU at 1 year of implant use. A pattern of gradual ascent was observed in NRI thresholds from the apical array towards the basal array in the study group.
Trends in ESRT Thresholds
An offset-wise analysis of electrodes, for trends in ESRT thresholds, showed a gradual ascent from the apical array towards the basal array. The Initial ESRT thresholds, recorded at first month of implant use, showed a mean average value of 173 CU across the array, while the corresponding mean average value for M-Levels across the array was 155 CU. This supports the fact documented in literature [1, 2], that in the initial period of implant use, ESRT thresholds may over-estimate the comfort levels and they may be a good indicator of maximum comfort levels, rather than M-Levels. Hence, audiologists setting an ESRT based initial MAP for an uncooperative child, must be cautious in order to avoid any Mapping level above the ESRT thresholds, which may induce an uncomfortable response to acoustic stimulation in the child and aversion to further implant use. At later stages of implant use, it was observed that ESRT levels gradually increased with time and fell close to the M-Levels, with the mean average ESRT value across the array at 1 year being 275 CU as compared to the corresponding M-Level value of 272 CU. The overall ESRT thresholds increased over time with a mean average rise of 82.5 (±16 SD) CU, between the first and fourth schedules of testing. This was statistically significant with a ‘p’ value of 0.028.
Trends in EABR Thresholds
EABR thresholds were higher than ECAP thresholds, but lower than ESRT thresholds in the study. The authors infer that EABR thresholds were higher due to the need for a higher energy of stimulation via CI that is required to elicit a recordable action potential from the brainstem. EABR thresholds gradually rose from the apical electrodes towards the basal array. EABR thresholds were higher by a mean average of 46.8 (±7 SD) CU and 54.5 (±11 SD) CU, in the basal array when compared to the apical array, at 1 month and 1 year of implant use respectively. This was statistically significant with a ‘p’ value of 0.050. EABR responses were quite stable among all the three offsets along the electrode array, through all schedules of testing. There was not much of a change in EABR thresholds, except for a few CUs, when recorded in the same electrode over time. This supports the fact noted in literature [6, 7] that EABR threshold patterns remain unchanged during the first year of implant use and EABR is useful for objective programming of implants, through the period of habilitation.
Correlation and Regression Analysis
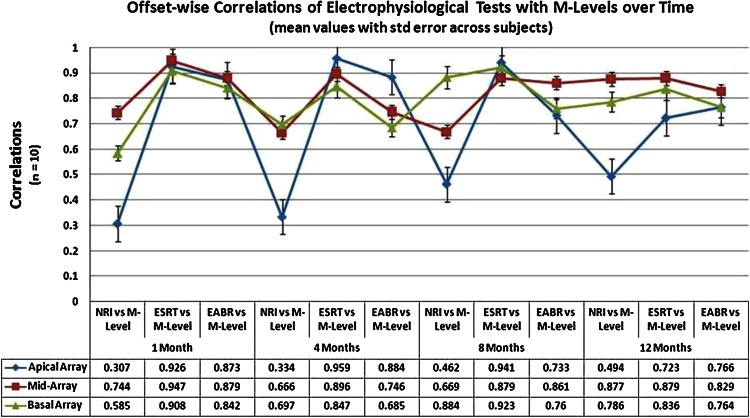
The longitudinal correlations of NRI, ESRT and EABR versus M-Levels measured over time have been shown in Fig. 2. All correlations recorded between the various electrophysiological tests and behavioral comfort levels, were found to be positive throughout the study period, ranging from moderate to good and statistically significant at the level of p = 0.01–0.05 (two-tailed). There were significant correlations between the objective measures and behavioral responses, right from the time of Switch-On of the device, and they had a tendency to gradually improve over time with implant use. NRI and ESRT correlations with M-Levels were statistically significant at the level of p < 0.05 (two-tailed); while EABR correlations with M-Levels were significant at the level of p = 0.01 (two-tailed). This finding suggests that EABR correlations are more statistically significant than NRI and ESRT. Hence, EABR may be a more sensitive tool than ECAP or ESRT for objective programming in ‘Difficult to Map’ conditions. The initial correlation for NRI versus M-Level was modest at r = 0.416, but over time it improved to r = 0.704. ESRT correlation with M-Level proved to be good from the first schedule at r = 0.794 and it improved gradually to r = 0.927 by the last schedule. EABR correlation with M-Level remained stable through the period of study, ranging from r = 0.871 to 0.824 at the first and fourth schedules respectively.
Fig. 2.
Correlations of electrophysiological tests with M-levels over time
Cross-correlations between the three electrophysiological tests were found to be moderate, ranging from r = 0.487 at first schedule to r = 0.493 at the last schedule. This helped to infer statistically whether there was any undue influence of one on another, while combining them into a multiple regression model. These cross-correlations were significantly lower than the individual longitudinal correlations of the three tests with comfort levels measured in our cohort. Hence, the three correlations could be combined together in a multi-modal regression model for predicting offset-based comfort levels across the array. ESRT and EABR thresholds correlated well with M-Levels across the array ranging from r = 0.697–0.984 at all schedules of testing, with ‘p’ values ranging between 0.01 and 0.05 (two-tailed). The NRI thresholds showed poor to moderate correlations with M-Levels over time in the apical array, ranging from r = 0.287–0.524 (p value 0.05), while in the mid array and basal array they had moderate to good correlations ranging from r = 0.589–0.891 (p value <0.03). Based on the electrophysiological correlations, prediction formulae for unknown comfort levels were generated for the first and last schedules, using linear and multiple regression analysis. Electrode-wise prediction formulae were created by linear regression of NRI thresholds, while offset-wise (apical, mid-array and basal) prediction formulae were obtained by linear regression of ESRT and EABR thresholds (as shown in Tables 2, 3, 4). Offset-wise prediction formulae were also generated by incorporating all the three thresholds into a multiple regression model (as shown in Table 5).
Table 2.
NRI threshold based electrode-wise linear regression formulae
| NRI based linear regression formulae | ||
|---|---|---|
| N = 10 | 1 month | 12 months |
| Overall (mean avg) | M-Level = 128.743 + 0.382 × NRI | M-Level = 167.004 + 0.522 × NRI |
| Electrode 1 | M-Level = 158.448 + 0.047 × NRI | M-Level = 200.790 + 0.330 × NRI |
| Electrode 2 | M-Level = 151.003 + 0.126 × NRI | M-Level = 169.300 + 0.521 × NRI |
| Electrode 3 | M-Level = 127.783 + 0.399 × NRI | M-Level = 203.736 + 0.362 × NRI |
| Electrode 4 | M-Level = 151.463 + 0.119 × NRI | M-Level = 176.271 + 0.453 × NRI |
| Electrode 5 | M-Level = 99.999 + 0.697 × NRI | M-Level = 193.702 + 0.411 × NRI |
| Electrode 6 | M-Level = 32.612 + 1.172 × NRI | M-Level = 181.862 + 0.438 × NRI |
| Electrode 7 | M Level = 95.631 + 0.653 × NRI | M-Level = 107.588 + 0.797 × NRI |
| Electrode 8 | M-Level = 83.028 + 0.721 × NRI | M-Level = 115.912 + 0.764 × NRI |
| Electrode 9 | M-Level = 112.175 + 0.457 × NRI | M-Level = 140.257 + 0.612 × NRI |
| Electrode 10 | M-Level = 136.794 + 0.304 × NRI | M-Level = 162.118 + 0.532 × NRI |
| Electrode 11 | M-Level = 149.247 + 0.235 × NRI | M-Level = 147.838 + 0.609 × NRI |
| Electrode 12 | M-Level = 174.052 + 0.068 × NRI | M-Level = 184.322 + 0.443 × NRI |
| Electrode 13 | M-Level = 135.955 + 0.378 × NRI | M-Level = 156.155 + 0.590 × NRI |
| Electrode 14 | M-Level = 134.603 + 0.374 × NRI | M-Level = 90.912 + 0.863 × NRI |
| Electrode 15 | M-Level = 134.788 + 0.365 × NRI | M-Level = 136.086 + 0.630 × NRI |
| Electrode 16 | M-Level = 123.957 + 0.440 × NRI | M-Level = 118.190 + 0.711 × NRI |
Table 3.
ESRT threshold based offset-wise linear regression formulae
| ESRT based linear regression formulae | ||
|---|---|---|
| N = 10 | 1 month | 12 months |
| Overall (mean avg) | M-Level = 43.504 + 0.713 × ESRT | M-Level = 88.262 + 0.671 × ESRT |
| Electrode 1 | M-Level = 49.439 + 0.662 × ESRT | M-Level = 103.148 + 0.589 × ESRT |
| Electrode 6 | M-Level = 42.252 + 0.724 × ESRT | M-Level = 71.844 + 0.711 × ESRT |
| Electrode 11 | M-Level = 35.300 + 0.795 × ESRT | M-Level = 94.218 + 0.653 × ESRT |
| Electrode 16 | M-Level = 39.813 + 0.695 × ESRT | M-Level = 104.152 + 0.632 × ESRT |
Table 4.
EABR threshold based offset-wise linear regression formulae
| EABR based linear regression formulae | ||
|---|---|---|
| N = 10 | 1 month | 12 months |
| Overall (mean avg) | M-Level = 43.765 + 0.703 × EABR | M-Level = 76.739 + 0.704 × EABR |
| Apical array (Ell) | M-Level = 40.994 + 0.735 × EABR | M-Level = 70.976 + 0.729 × EABR |
| Mid array (El 8) | M-Level = 19.575 + 0.819 × EABR | M-Level = 68.618 + 0.734 × EABR |
| Basal array (El 16) | M-Level = 71.064 + 0.572 × EABR | M-Level = 88.200 + 0.665 × EABR |
Table 5.
Multi-modal thresholds based offset-wise multiple regression formulae
| NRI + ESRT + EABR based multiple regression formulae | ||
|---|---|---|
| N = 10 | 1 month | 12 months |
| Overall | M Level = 44.147 − 0.011 × NRI + 0.635 × ESRT + 0.083 × EABR | M-Level = 64.048 + 0.159 × NRI + 0.387 × ESRT + 0.248 × EABR |
| Apical array (El 1) | M-Level = 66.366 − 0.186 × NRI + 0.762 × ESRT − 0.104 × EABR | M-Level = 35.891 + 0.146 × NRI + 0.395 × ESRT + 0.373 × EABR |
| Mid array (El 8) | M-Level = 51.877 + 0.098 × NRI + 0.783 × ESRT − 0.153 × EABR | M-Level = 75.512 + 0.396 × NRI + 0.352 × ESRT + 0.065 × EABR |
| Basal array (El 16) | M-Level = 36.739 − 0.073 × NRI + 0.772 × ESRT + 0.026 × EABR | M-Level = 64.106 + 0.192 × NRI + 0.351 × ESRT + 0.250 × EABR |
Reliability Analysis
The predicted M-Levels were analyzed for their statistical reliability with actual (behavioral) M-Levels recorded among the study group over time. The Cronbach’s Alpha Reliability test showed that all objective measures had good reliability while predicting M-Levels independently (Reliability Value ranging from 0.546 to 0.964). EABR and ESRT showed better reliability values than NRI at the first month, but all the three parameters had comparable reliability values at 1 year of implant use. The multi-modal prediction method showed significantly higher reliability values at both 1 month (0.968) and 1 year (0.949) of implant use respectively, which suggests that this method may be a better way for comfort level prediction at any point of time.
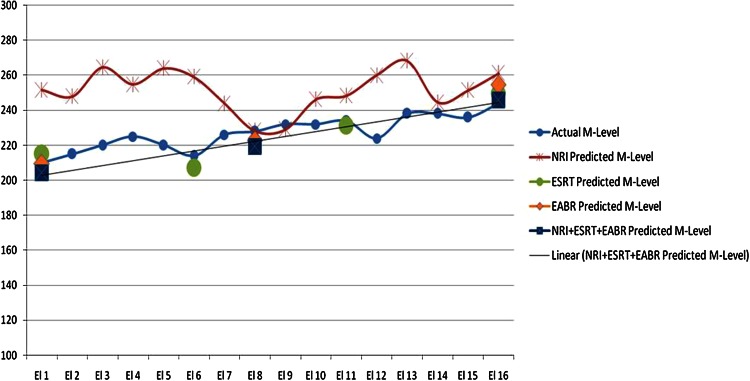
On clinical application of this statistical method, in a subject of our study group (Subject A), the authors found proximity of the predicted M-Levels to the actual behavioral M-Levels (with differences ranging from 3 to 10 CUs), as represented in Fig. 3 (at 1 month) and Fig. 4 (at 12 months). When this statistically predicted MAP was incorporated into Subject A’s speech processor, it was found to be as useful as the behavioral MAP used by him previously. Such observations were also noted in other members of the study group, wherein the statistical method produced M-Levels within the range of 3–17 CUs, from the actual behavioral M-Levels. These results highlight the efficacy of this statistical method, as it was able to provide a working MAP, close to the behavioral MAP used by these children.
Fig. 3.
Comparison of behavioral versus predicted M-levels in subject A at 1 month
Fig. 4.
Comparison of behavioral versus predicted M-levels in subject A at 12 months
Discussion
Behavioral responses are sufficient to obtain optimal threshold and comfort levels for programming majority of the post-lingual older children and adult cochlear implantees. Although these levels are reasonably accurate at the time of programming, the threshold and comfort levels tend to change over time and hence sequential re-programming of the Maps based on behavioral responses is necessary, as and when required [6]. On the contrary, establishing accurate behavioral thresholds and comfort levels is extremely challenging for very young children and those with syndromic associations/multiple disabilities. The behavioral observation technique used in infants and toddlers for implant programming is likely to over-estimate threshold measures, when compared with procedures used in older children that use conditioned responses [6, 7]. Programming very young children is clinically challenging even for experienced audiologists at times. Hence, in the present day various electrophysiological tests have taken precedence in the programming of such ‘Difficult to MAP’ individuals.
Suspicion of a disparity between the electrophysiological thresholds and the behavioral parameters, needs to be thought of when a cochlear implantees’ performance is not up to expectations, as diagnosed by their poor auditory-verbal skills and general behavior to implant usage. A multitude of electrophysiological tests are clinically available today, to provide a helping hand for confirming the integrity of the implant in such cases. Intra-operatively and post-operatively EABR, ESRT and ECAP measurements can be used to assess the device integrity and to measure amplitude growth function of the nerve response (Mason [5]). Such objective data help in sequentially programming the device and can also be used as possible predictor of implant performance over time (Brown and Carolyn [7]).
Although all implant manufacturers provide commercially available standardized testing modules for performing electrophysiological tests like ESRT, EABR and ECAP along with their programming software, they do not stress the necessity for performing these tests as a routine in order to program the implant. By and large, these tests have been used for trouble-shooting and for research purposes. In newer software, there is an option of importing ECAP thresholds into the Mapping module, for optimal setting of current levels. But many a times, a single objective measurement like the ECAP may not necessarily correlate and predict the behavioral levels accurately [8]. This may be due to the inherent differences in the pulse width and rate of stimulation, which exist between the electrical response of the auditory nerve recorded as an ECAP and the actual behavioral response used to program the implantee [4].
Gordon et al. [8] stated that electrophysiological thresholds might be useful (when behavioral results are questionable) to provide young children using cochlear implants with audible and comfortable auditory inputs, from which they can learn to detect sounds. These authors emphasized that behavioral measures of threshold remain the gold standard of setting minimum stimulation levels. This is the principle being followed in the Nucleus Cochlear Implant system, which uses a T-Level based programming technique.
However, Gordon et al. [8] concluded that current clinical techniques may not be the best methods for determining maximum stimulation levels. This aspect of their observation, induced interest in the present study, since identifying the M-Levels seems to be the pivotal factor, in order to program a ‘Difficult to MAP’ child, using the MedEl or Advanced Bionics Implant systems (which use a comfort level based programming technique). Hence, the present study focused on utilizing objective measures to predict optimal comfort levels for a cohort of comparable cochlear implantees.
Literature has documented comparisons between the intra-operative and post-operative electrophysiological responses of the auditory nerve [5, 6] and these papers conclude that there is a definite variation in the current levels, which may be attributed to factors like wound healing with reduction in the neural tissue–electrode interface, alteration of the electro-chemical gradient within the cochlea, neural re-organization within the cochlea and adaptation of the auditory nerve to become more conducive for electrical stimulation over time [3, 6, 7]. It is believed that impedance to current passage reduces in due course of time and synchronous firing for electrical stimuli via the implant sets in. The higher auditory centers also become more receptive and fine-tuned for stimulation through the cochlear implant, with implant usage over time. Hence, post-operative electrophysiological tests are more efficient in predicting the Mapping levels, rather than relying upon intra-operative measures [9]. The present study explored this relationship from the time of ‘Switch-On’, through the period of habilitation for 1 year.
Today, it is accepted that ECAP thresholds significantly correlate with both threshold and comfort levels (more so with T-Levels), but raw ECAP data is not adequate for estimation of absolute Mapping levels in implantees and correction factors are suggested for ECAPs to be of any predictive value. A study by Thai-Van et al. [9] suggests that the correlation between the neural response threshold and behavioral T-Levels may improve from the base towards the apex of the cochlea. However, a significant correlation can be demonstrated for all tested electrodes at 12 months post-implantation. During the first months, care must be exercised when interpreting neural response telemetry measurements, as a positive test does not necessarily mean that the stimulus delivered to the acoustic nerve will be centrally processed with the result of an auditory perception. Abbas et al. [14] revealed chronological changes in NRT over time from the day of surgery. Statistically significant changes in the NRT thresholds of children were observed until 3–8 months following initial stimulation. Measures of NRT slope in children did not stabilize until 12-months post-implantation and longitudinal trends in NRT measures mirrored the T-Levels more closely than comfort levels.
Investigators have also assessed the efficacy of ESRT in predicting comfort levels for optimal programming and they found ESRT to be of greater predictive value than ECAP for estimation of behavioral comfort levels [8, 10, 11]. Postoperative ESRT thresholds show high correlations with behaviorally obtained comfort levels and help predict the maximum comfort level pattern across electrodes [8]. Spivak and Chute [1] found that comfort levels and ESRT thresholds rose over the first year of implant use and the increased tolerance to higher levels of stimulation as shown by increasing M-Levels and ESRT over time, was possibly due to changes in the conditioning of the auditory nerve and lower brain stem. Thus, an expanded dynamic range emerges over time and this may suggest a change in neural response with increasing stimulus level, with on-going implant use. Hence, accurate estimation of comfort levels and loudness balancing are of greater value than setting behavioral T-Levels, while programming young children.
Studies have shown that EABR thresholds correlate well with behavioral thresholds similar to ECAPs and they provide a sensitive and effective technique to comprehensively test implant function by assessing neural survival along the cochlea and integrity of the auditory pathway up to brainstem level [6–8]. EABR has been the gold-standard tool for meticulous analysis of individual electrodes along the array, to identify non-auditory electrodes and confirm device failures. In a poor CI user, EABR helps to identify and redefine erroneous maps which may exist undiagnosed even by ECAP or ESRT measurements [6, 8]. The possible reasons for EABRs not being widely used in clinical practice today, is that it requires a cumbersome set up, is time-consuming, needs expertise and a fully cooperative patient.
A number of investigators have described various correction factors and yet there does not seem to be a universal approach for calculation of the predicted MAP values from the ECAPs alone [6, 8, 9, 12–15]. In the past, correction factors proposed to predict threshold and comfort levels from objective measures, were based on the difference between objective thresholds and at least one behavioral measure (Abbas et al. [6], Zimmerling and Hochmair [15], Brown and Carolyn [7]). Subsequent literature suggests that correction factors based on ECAP, ESRT and EABR thresholds are needed to predict behavioral levels required for programming in difficult situations (Di Nardo et al. [13], Han et al. [10], Caner et al. [11]). These studies suggested extrapolation of the averaged value calculated by these correction factors, across the array in order to set behavioral levels. But, this technique was not foolproof, since there were variations in behavioral levels between the apical and basal array electrodes. Changes in objective and behavioral responses with respect to the electrode location and over time with ongoing implant use, imply that such correction factors do not remain static at all times.
All electrodes along the array may not respond to stimulation in the same way, since, apical electrodes may have significantly lower thresholds when measured by ESRT, ECAP, EABR and behavioral measures than the basal electrodes [8, 9]. Gordon et al. [8], studied this interesting phenomenon by dividing the electrode array into three offsets—apical array, mid-array and basal array, wherein a correction factor was created based on the difference between the objective threshold and behavioral level for a representative electrode in each offset across the array. She found a tendency toward increased differences over time in the apical electrode and mid-array, while less significant increases in the basal electrode differences over time. She also proposed that ECAP and ESRT can be used independently to predict minimum and maximum stimulation levels, respectively, and thus optimize the dynamic range along the electrode array.
In order to overcome any inherent difference in current levels, observed while using a single measurement like ECAP or ESRT for predicting Mapping levels, the present study hypothesized the use of three objective measures (ESRT, EABR and ECAP), which together may correlate and predict behavioral levels better. The authors have followed a model of three offsets across the array, similar to Gordon’s method [8], for predicting M-Levels based on the linear and multiple regression models, since this would provide at least three optimal predicted M-Levels across the array, which will be of vital use to begin programming if behavioral levels are unknown.
Results from the present study have helped the authors to infer that, behavioral measurements, even if minimally recordable are mandatory to program cochlear implantees. Electrophysiological measurements help to predict behavioral levels, but these alone cannot substitute or replace a behavioral MAP accurately. There existed disparities of few programming units between the behaviorally measured and statistically predicted comfort levels at the various schedules of testing, due to various factors as described below. Electrophysiological measurements are performed at default stimulation parameters that are different from the stimulation rates eventually used during cochlear implant programming. Sensitivity and neural reactions recorded to electrophysiological stimuli are different from the behavioral reactions recorded at higher rates of stimulation, used while programming [9, 10, 13].
Behavioral response elicited by electrical stimulation with a cochlear implant electrode is the result of a combination and superposition of the following phenomena at three different levels: (1) electrode–tissue impedance and positioning of the electrode contact towards the neural tissue. The higher thresholds for electrophysiological responses at the basal electrodes are due to the physical current distribution; (2) neural preservation and excitability of the nerve fibers and (3) cortical and behavioral reactions to the excitation patterns in the higher auditory pathways as influenced by the age at onset of deafness, cognition, intellect, hearing aid usage and duration of hearing deprivation prior to implantation. All electrophysiological measures like ECAP, EABR and ESRT are the measures of the phenomena occurring at levels 1 and 2, yet take no account of the variability present at level 3 [9, 10, 13].
The present study has shown that multi-modal electrophysiological testing by recording a minimum of three offset-based electrophysiological thresholds is helpful in predicting optimal M-Levels across the array and in providing a working MAP, when behavioral levels are unknown or minimally available. The study has also shown that construction of a multi-modal metric, combining the results of three electrophysiological tests decreased the variability of the electrophysiological measures and increased the accuracy of prediction, to a reliable extent. Such data may be of reference for performing similar studies among complex, difficult to Map implantees in future. At present the authors are pursuing an on-going study, to look into the application of this method, for programming in a spectrum of ‘Difficult to MAP’ situations.
Conclusion
The study explores the trends and correlations between electrophysiological thresholds and behavioral comfort levels recorded over time, among a cohort of comparable cochlear implantees. Although, inter-patient and inter-electrode variables were bound to be an integral part of this study, an overall trend was observed in the electrical and behavioral responses of the auditory nerve over time, which provided a way for correlating the various parameters and to derive predictive formulae for calculating optimal behavioral comfort levels using regression analysis. Such predicted comfort levels were found to be in proximity to the behaviorally measured levels in the study group. Hence, the study provides a statistical method which may be used as a guideline to predict optimal behavioral levels in difficult situations among future implantees. Observation of behavioral reactions to stimulation remains an indispensable element of the cochlear implant fitting, but objective measures of implant function become vital, especially while programming very young cochlear implantees and those with special needs. In such cases, following a protocol of sequential behavioral Mapping in conjunction with multi-modal electrophysiological correlates as described in the study will provide the best outcomes.
References
- 1.Spivak LG, Chute PM. The relationship between electrical acoustic reflex thresholds and behavioral comfort levels in children and adult cochlear implant patients. Ear Hear. 1994;15(2):184–192. doi: 10.1097/00003446-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Hodges AV, Butts SL, King JE. Electrically evoked stapedial reflexes: utility in cochlear implant patients. Cochlear implants—objective measures. London: Whurr Publishers; 2003. pp. 81–93. [Google Scholar]
- 3.Shallop JK, Ash KR. Relationships among comfort levels determined by cochlear implant patient’s self-programming, audiologist’s programming, and electrical stapedius reflex thresholds. Ann Otol Rhinol Laryngol Suppl. 1995;166:175–176. [PubMed] [Google Scholar]
- 4.Davids T, Valero J, Papsin BC, Harrison RV, Gordon KA. Effects of stimulus manipulation on electrophysiological responses of pediatric cochlear implant users—Part I: duration effects and Part II: rate effects. Hear Res. 2008;244:7–24. doi: 10.1016/j.heares.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Mason S. Electrophysiologic and objective monitoring of the cochlear implant during surgery: implementation, audit and outcomes. Int J Audiol. 2004;43:33–38. [PubMed] [Google Scholar]
- 6.Abbas PJ, Hughes ML, Brown CJ, Luk B, Wolaver A, Gervais J. The relationship between EAP and EABR thresholds and levels used to program the nucleus 24 speech processor: data from adults. Ear Hear. 2000;21:151–163. doi: 10.1097/00003446-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Brown CJ. Clinical uses of electrically evoked auditory nerve and brainstem responses. Curr Opin Otolaryngol Head Neck Surg. 2003;11(5):383–387. doi: 10.1097/00020840-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Gordon KA, Papsin BC, Harrison RV. Toward a battery of behavioral and objective measures to achieve optimal cochlear implant stimulation levels in children. Ear Hear. 2004;25:447–463. doi: 10.1097/01.aud.0000146178.84065.b3. [DOI] [PubMed] [Google Scholar]
- 9.Thai-Van H, Chanal JM, Coudert C, Veuillet E, Truy E, Collet L. Relationship between NRT measurements and behavioral levels in children with the nucleus 24 cochlear implant may change over time: preliminary report. Int J Pediatr Otorhinolaryngol. 2001;58:153–162. doi: 10.1016/S0165-5876(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 10.Han D-M, Chen X-Q, Zhao X-T, Kong Y, Li Y-M, et al. Comparisons between Neural Response Imaging thresholds; electrically evoked auditory reflex thresholds and most comfortable loudness levels in CII Bionic Ear users with HiRes sound processing strategies. Acta Oto-Laryngol. 2005;125(7):732–735. doi: 10.1080/00016480510026890. [DOI] [PubMed] [Google Scholar]
- 11.Caner G, Olgun L, Gultekin G, Balaban Optimizing fitting in children using objective measures such as neural response imaging and electrically evoked stapedius reflex threshold. Otol Neurotol. 2007;28(5):637–640. doi: 10.1097/mao.0b013e3180577919. [DOI] [PubMed] [Google Scholar]
- 12.Smoorenburg GF, Willeboer C, Van Dijk JE. Speech perception in Nucleus CI24M cochlear implant users with processor settings based on electrically evoked compound action potential thresholds. Audiol Neurootol. 2002;7:335–347. doi: 10.1159/000066154. [DOI] [PubMed] [Google Scholar]
- 13.Di Nardo W, Ippolito S, Quaranta N, Cadoni G, Galli J. Correlation between NRT measurement and behavioural levels in patients with the nucleus 24 cochlear implant. Acta Otorhinolaryngol Ital. 2003;23:352–355. [PubMed] [Google Scholar]
- 14.Abbas PJ, Hughes ML, Van der Werff KR, Brown CJ, Kelsay DM, Teagle HF, Lowder MW. A longitudinal study of electrode impedance, the electrically evoked compound action potential and behavioral measures in nucleus 24 cochlear implant users. Ear Hear. 2001;22(6):471–486. doi: 10.1097/00003446-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerling MJ, Hochmair ES. EAP recordings in CI patients—correlations with psychophysical measures and possible implications for patient fitting. Ear Hear. 2002;23:81–91. doi: 10.1097/00003446-200204000-00001. [DOI] [PubMed] [Google Scholar]