Abstract
Objective
A recent investigation at Barnes-Jewish Hospital located in St. Louis, Missouri, found that an estimated 22% of adults presenting for inpatient surgery screened as high risk for obstructive sleep apnea (OSA). Surgical patients with OSA have multiple comorbidities and are at increased risk for perioperative complications. Our objective was to determine whether a prior diagnosis of OSA, or a positive screen for OSA is associated with increased risk for 30 days and one year mortality.
Methods
B-J APNEAS (Barnes-Jewish Apnea Prevalence in Every Admission Study) was a prospective cohort study. Unselected adult surgical patients at Barnes Jewish Hospital were prospectively enrolled between February 2006 and April 2010. All patients completed preoperative OSA screening and those who were at risk for OSA according to a combination of the Berlin and Flemons screening tools received targeted postoperative interventions. STOP and STOP-BANG scores were also obtained.
Results
Overall, the sample included 14,962 patients, of whom 1,939 (12.9%) reported a history of OSA. All four screening tools identified a high prevalence of undiagnosed patients at risk for OSA (9.5% to 41.6%), but agreement among screens was not strong with Kappa ranging from 0.225 to 0.611. There was no significant difference in 30 day postoperative mortality between patients with possible OSA (based on their history or on a positive OSA screen with any of the four instruments) and the rest of the surgical population. Significant differences in one-year mortality were noted between the low and high-risk groups as identified by the Flemons’ (4.96% versus 6.91%; p <0.0001), STOP (5.28% versus 7.57%; p <0.0001) and STOP-BANG (4.13% versus 7.45%; p <0.0001) screens. After adjusting for risk factors, none of the OSA screening tools independently predicted mortality rate up to one year postoperatively.
Conclusion
Neither a prior diagnosis of OSA, or a positive screen for OSA risk was associated with increased 30 day or one year postoperative mortality. It is possible that incorporating OSA screening and patient safety measures into routine care mitigated the early postoperative complications in this patient cohort. The results of this study highlight uncertainties and research priorities for the medical community.
Keywords: obstructive sleep apnea, perioperative screening, perioperative mortality, OSA screening tools, STOP-BANG, STOP, Berlin, Flemons
OSA is associated with cardiovascular manifestations such as hypertension [1], heart failure [2], stroke [3, 4], coronary artery disease [5], atrial fibrillation [6], and deep venous thrombosis [7]. It is also associated with sleepiness-related motor vehicle accidents [8]. Recent reports also demonstrate a high prevalence of chronic renal disease in patients with severe OSA without hypertension or diabetes [9, 10]. Furthermore, OSA has been associated with increased all-cause mortality [6]. Apart from the concerns relating to OSA itself, the frequent comorbidities associated with this condition are important considerations for all health care providers caring for patients with OSA [11].
There is a growing body of evidence suggesting that OSA may be an independent risk factor for perioperative morbidity. Patients with diagnosed OSA have an increased incidence of postoperative oxygen desaturation [12], cardiac ischemia, hemodynamic instability and arrhythmias [13]. Furthermore, there are specific concerns that people with OSA might have increased sensitivity to the respiratory depressant effects of opioid analgesics, general anesthetics and sedative medications [13, 14, 15]. Patients with OSA have been estimated to have twice the rate of hospitalization in the three years before OSA is diagnosed [16]. A recent investigation at Barnes-Jewish Hospital located in St. Louis Missouri found that an estimated 22% of adults presenting for inpatient surgery screened as high risk for OSA. Three quarters of these patients had not previously been diagnosed with OSA [17]. Therefore, the addition of a sleep apnea risk assessment to other screens commonly performed upon hospital admission might be an appropriate public health initiative.
Many OSA screening tools have been utilized in surgical patients, including the STOP [18] STOP-BANG [18, 19], and ASA Checklist [20], have been validated in this population against apneahypopnea index values from in-laboratory polysomnography. The Flemons [17] Berlin [20, 21], and the Epworth Sleepiness Scale have also been used in this population [22]. As a whole, these screening tools are easy to use and vary in length, focus, and method of scoring. Presently, there is no convincing evidence to suggesting that any of these screening tools is superior at identifying patients at risk for perioperative morbidity or mortality. Because of the hypothesized potential for increased perioperative complications and treatment costs [23] associated with undiagnosed OSA, the American Society of Anesthesiologists (ASA) recommends preoperative evaluation for OSA prior to surgery [23]. The ASA further recommends that specific perioperative safety measures be implemented for patients with probable OSA.
In accordance with these ASA guidelines, routine preoperative screening for OSA was implemented and perioperative precautions were introduced at Barnes-Jewish Hospital for patients who screened as high risk. This initiative was followed by the B-J APNEAS (Barnes-Jewish Apnea Prevalence in Every Admission Study) investigation which was designed as a prospective cohort study to test the hypothesis that a either a prior diagnosis of OSA, or a positive screen for OSA would be associated with increased risk for early or intermediate postoperative morbidity and mortality from all causes. The primary purpose of this investigation was to compare the prognostic performance of the Berlin, Flemons, STOP (loud Snoring, daytime Tiredness, Observed apneas and high blood Pressure), and STOP-BANG (STOP plus Body mass index >35 kg/m2, Age >50, Neck circumference >40 cm, male Gender) screening tools for sleep apnea among the B-J APNEAS patient population. The study also examined the association of the performance of these four screening tools with key risk factors for postoperative morbidity and mortality. Specifically, we asked whether screening positive on any of the four OSA screening tools was independently associated with an increased mortality rate up to one year post-operatively.
1. Materials and Methods
Study Population
Unselected adult surgical patients at Barnes Jewish Hospital between Feb 2006 and Apr 2010 who underwent preoperative OSA screening were prospectively enrolled. The Human Research Protection Office at Washington University School of Medicine (St. Louis, MO) approved the study. Because screening for OSA and the perioperative safety interventions were adopted as standard of care quality improvement initiatives, informed consent was not required. Obstetric patients and those under the age of 18 were excluded.
Protocol and Design
Patients were asked to fill out the OSA Risk evaluation form (Appendix 1) during pre-operative assessment. Licensed healthcare professionals completed the form by scoring it, measuring neck circumference, and assigning the patient to an OSA risk category. The evaluation form comprises three sections and assesses the presence of nighttime symptoms (section 1), daytime symptoms (section 2), and comorbidities such as hypertension, heart disease, and diabetes (section 3). For the purposes of clinical management, patients were deemed to be at high risk for OSA if they screened positive on a combination of the Berlin questionnaire and the Flemons’ Index, which are both well established clinical screening tools for OSA. Data were also prospectively collected on all patients allowing for scoring on the STOP (loud Snoring, daytime Tiredness, Observed apneas and high blood Pressure) and STOP-BANG (STOP plus Body mass index >35 kg/m2, Age >50, Neck circumference >40 cm, male Gender) screening tools [18, 20]. The screening form became a part of the medical record and patients who either screened high risk or reported a prior diagnosis of OSA received additional targeted interventions. These interventions consisted of an “OSA RISK” alert bracelet, an “OSA RISK” sign affixed to the patient’s bed, elevation at the head of the bed, and continuous pulse oximetry with an alarm at the central nursing station (Appendix 2). There are also options for additional postoperative monitoring, use of oxygen on the post-surgical unit, and use of home CPAP or BiPAP devices while in the hospital.
Mortality Data
All-cause mortality data were obtained from the Social Security Administration’s Death Master File (SSA DMF) by using a third party application (“Blinds” by CareEvolution, Inc, Ann Arbor, Michigan). Several reports have validated online versions of the SSA DMF for clinical research purposes [24, 25]. This service returns the date of death using an algorithm that incorporates Social Security Number, patient name, date of birth, and last date the patient was known to be alive. For the date known to be alive, the date of surgery was used in this study. The generation and handling of SSA DMF data was in compliance with the Health Insurance Portability and Accountability Act (HIPAA) and stringent efforts were undertaken to ensure the protection of all patient information.
Data Collection
Data for this investigation were entered into an Access™ (Microsoft Corporation, Redmond, WA) database. The needed information was obtained from preoperative, intraoperative, and postoperative records. The Barnes-Jewish Hospital Information Technology Section provided data on postoperative complications and intensive care admissions. Beginning in May 2009, electronic preoperative and intraoperative data were acquired from the anesthesia information management system (MetaVision MVOR, iMDsoft, Tel-Aviv, Israel).
Data Preparation and Statistical Analyses
All data were prepared and analyzed using SAS® proprietary software (SAS version 9.2, SAS Institute NC, USA). Commonly used risk factors for morbidity and mortality found in the literature were analyzed in this study, including patient’s gender, age, race, body mass index (BMI), physical status as defined by the ASA index, co-morbid conditions at intake (e.g., whether the patient had experienced hypertension, diabetes, coronary disease, arrhythmias, COPD, stroke, or congestive heart failure), and other risk behaviors such a lifetime smoking and alcohol consumption.
Continuous variables (i.e., age, BMI, and ASA index) were categorized to have an easier interpretation of the data and/or to reduce the influence of the few univariate outliers found in the data on the investigated associations. Specifically, age was categorized into three categories (Younger 18 to 44 years old, Old 45 to 65, and Older 65 and older) based on the interquartile range of the age distribution. The BMI score, simply defined as a ratio of weight to height (kg/m2), was categorized into four categories using standard clinical cutpoints corresponding to the World Health Organization (WHO) classification of the degree of obesity (Underweight <18.5 kg/m2, Normal weight =18.5 to 24.9 kg/m2, Overweight =25.0 to 29.9 kg/m2, and Obese ≥ 30 kg/m2). The ASA score was grouped into three categories to reflect the severity of the patient’s health condition (Normal healthy patient =1, healthy patient with emergencies or with mild systemic disease =1E to 2, and patient with other status, including severe systemic disease to not expected to survive ≥ 2E).
Descriptive statistics are presented as frequency and percentage for categorical variables and mean ± standard deviation for continuous variables. T-test and chi-square proportional test were used for between group comparisons, as appropriate. To examine the agreement among the OSA screening tools under investigation, four commonly used measures of concordance were calculated. These included the positive, negative and overall agreement as well as Cohen’s kappa as a general agreement measure. The predictive performances of the four OSA screening tools for 30 days and one-year all-cause mortality were also investigated using chi-square tests for significance. Kaplan-Meier graphs with log rank tests were used to investigate the bivariate associations between OSA risk on different OSA screens and intermediate (i.e., one year post-surgery) survival. Multivariate Cox proportional hazards regression was applied to model the rate of mortality over one year post-surgery, with the ultimate goal of assessing the effect of being screened positive across the four OSA screening tools, adjusting for patient’s preoperative characteristics as well as medical conditions. Performance of each screening tool was assessed in terms of the estimated hazard (or risk) of patient’s death, using chi-square proportional tests. All statistical tests were two tail-sided and only p values <0.05 were considered significant.
Logistic and linear regressions with backward elimination were performed to evaluate the relative contributions of high-risk scores on the various OSA screens toward hospital length of stay and postoperative intensive care unit (ICU) admission (versus post-anesthesia care unit [PACU]). Covariates included gender, age, BMI, American Society of Anesthesiologists Physical Status score, history of OSA, high risk categorization from Berlin, STOP, Flemons, and STOP-BANG screens, comorbidities, and risk behaviors. Age, BMI, and ASA score were categorized to account for the potential bimodal association of adverse outcomes with each predictor. Comorbidities included history of diabetes, hypertension, arrhythmias, coronary artery disease, cerebrovascular accident, congestive heart failure, and chronic obstructive pulmonary disease. Risk behaviors included a history of smoking or alcohol consumption.
2. Results
Table 1 shows patient demographics and other health related information for the overall sample and by OSA risk status as defined by the four investigated screening tools. Overall, the sample included 14,962 patients. The mean age of the sample was 54.9 ± 15.7 years, the mean BMI was 30.4 ± 7.8 kg/m2, and 54.9% of the sample was female. The majority of patients were either white, n=11928 (80.2%) or African American, n=2599 (17.4%). By all indicators, comorbidities were prevalent among these patients. The most prevalent condition was hypertension (48.6%) and the least common was congestive heart failure (3.1%). Twenty six percent of the sample had two or more comorbid conditions. Although not shown in Table 1, the largest surgical groups among these patients were scheduled for orthopedic surgery (n= 3154, 21.1%), general/colorectal surgery (n=1731, 11.6%), urologic surgery (n=1597, 10.7%), gynecologic surgery (n=1367, 9.1%), otolaryngologic surgery (n=1239, 8.2%), cardiothoracic surgery (n=953, 6.4%), neurosurgery (n=903, 6.1%), ophthalmologic surgery (n=866, 5.8%), plastic surgery 834 (5.6%), and vascular surgery (n=471, 3.1%). Information on surgical services was missing for 97 patients. There were 12,321 general anesthetics (82.3%), 1,572 (10.4%) monitored anesthesia care (sedation only) cases, 761 (5%) spinal/epidural anesthetics, and 167 (1.1%) other types of regional anesthesia cases. Documentation on anesthetic technique was missing for 127 patients (0.8%).
Table 1.
Patients demographic charactersitics and comorbidy by OSA screeners
| Overall Sample (n = 14,962) |
Berlin | STOP | STOP-BANG | Flemons | History of OSA (n = 1,939) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Risk (n = 9,422) |
High Risk (n = 3,601) |
Low Risk (n = 10,527) |
High Risk (n = 2,496) |
Low Risk (n = 6,797) |
High Risk (n = 6,226) |
Low Risk (n = 7,423) |
Intermediate Risk (n = 3,753) |
High Risk (n = 1,418) |
|||
| Male | 45.1% | 42.2% | 46%‡ | 42.5% | 46.7%‡ | 21.9% | 66.6%‡ | 27.1% | 62.3% | 77.1%‡ | 57%‡ |
| Age (years) | |||||||||||
| < 45 | 24.9% | 28.7% | 21.3% | 29.5% | 14.3% | 40.8% | 11.2% | 33.1% | 17.1% | 17.1% | 13.1% |
| 45 to 65 | 46.3% | 42.8% | 50.1%‡ | 44.1% | 48.0% | 39.5% | 50.7% | 42.2% | 47.9% | 51.3%‡ | 56.1%‡ |
| > 65 | 28.8% | 28.5% | 28.7% | 26.4% | 37.7%‡ | 19.8% | 38.1%‡ | 24.7% | 35.0% | 31.5% | 30.8% |
| Mean ± SD | 54.9 ± 15.7 | 53.9 ± 16.7 | 56.0 ± 14.3‡ | 53.3 ± 16.4 | 59.4 ± 13.9‡ | 49.2 ± 16.9 | 60.2 ± 13.1‡ | 51.9 ± 16.9 | 58.3 ± 14.5‡ | 57.6 ± 13.4 | 58.1 ± 12.3‡ |
| White | 80.2% | 79.3% | 81.1%† | 79.9% | 79.4% | 79.4% | 80.3% | 79.6% | 78.7% | 82.6%‡ | 82.8% |
| BMI (kg/m2) | |||||||||||
| < 18.5 | 1.7% | 2.3% | 0.9% | 2.0% | 1.6% | 2.8% | 1.0% | 2.9% | 0.3% | 0.4% | 0.2% |
| 18.5 to 24.9 | 22.2% | 29.8% | 11.6% | 27.0% | 15.5% | 35.4% | 13.1% | 36.1% | 9.8% | 2.6% | 4.6% |
| 25.0 to 29.9 | 31.4% | 37.4% | 23.5% | 34.4% | 30.1% | 35.3% | 31.8% | 36.2% | 35.2% | 15.7% | 17.0% |
| > 30.0 | 44.7% | 30.5% | 64.1%‡ | 36.7% | 52.8%‡ | 26.5% | 54.2%‡ | 24.8% | 54.8% | 81.2%‡ | 78.2%‡ |
| Mean ± SD | 30.4 ± 7.8 | 28.2± 6.5 | 32.5 ± 7.5‡ | 28.9 ± 6.8 | 31.4 ± 7.5‡ | 27.1 ± 5.8 | 31.8 ± 7.4‡ | 27.0 ± 5.8 | 31.7 ± 6.7 | 35.7 ± 7.8‡ | 37.1 ± 9.4‡ |
| ASA | |||||||||||
| = 1 | 6.1% | 8.7% | 2.1% | 8.3% | 1.0% | 11.3% | 2.2% | 9.9% | 3.0% | 1.3% | 0.4% |
| 1E to 2 | 50.1% | 53.9% | 48.7% | 55.3% | 40.7% | 59.5% | 44.7% | 57.2% | 48.4% | 40.9% | 34.0% |
| ≥2E | 43.9% | 37.4% | 49.2%‡ | 36.4% | 58.4%‡ | 29.2% | 53.1%‡ | 32.9% | 48.7% | 57.8%‡ | 65.6%‡ |
| Comorbid Conditions | |||||||||||
| Hypertension | 48.6% | 38.1% | 64.9%‡ | 38.4% | 75.5%‡ | 27.0% | 65.7%‡ | 27.2% | 67.0% | 86.0%‡ | 69.5%‡ |
| Diabetes | 16.9% | 12.4% | 19.7%‡ | 12.2% | 23.6%‡ | 7.5% | 22%‡ | 8.8% | 20.1% | 28.5%‡ | 33.7%‡ |
| Coronary Disease | 10.9% | 8.5% | 12.6%‡ | 8.2% | 16%‡ | 4.3% | 15.5%‡ | 5.7% | 14.0% | 18.4%‡ | 19.3%‡ |
| Arrhythmias | 6.5% | 5.8% | 6.0% | 5.4% | 7.7%‡ | 4.6% | 7.3%‡ | 4.8% | 6.7% | 8%‡ | 10.5%‡ |
| COPD | 5.8% | 4.6% | 6.2%‡ | 4.4% | 8.1%‡ | 3.5% | 6.8%‡ | 4.4% | 5.8% | 6.3%‡ | 10.9%‡ |
| CVA/Stroke | 4.6% | 3.8% | 5.9%‡ | 3.5% | 7.9%‡ | 2.8% | 6%‡ | 3.5% | 5.6% | 5.3%‡ | 6.3%‡ |
| Congestive Heart Failure | 3.1% | 2.1% | 3.1%‡ | 1.9% | 4.6%‡ | 1.3% | 3.6%‡ | 1.6% | 3.1% | 4.0%‡ | 7.7%‡ |
| Having 2+Conditions | 26.0% | 19.5% | 31.7%‡ | 18.7% | 40.7%‡ | 11.3% | 35.6%‡ | 13.1% | 33.7% | 44.9%‡ | 46.6%‡ |
| Risk Behaviors | |||||||||||
| Past/Current Smoking | 48.4% | 46.1% | 52.6%‡ | 46.0% | 56.1%‡ | 42.2% | 54.2%‡ | 44.2% | 52.0% | 57%‡ | 51.8%‡ |
| Alcohol Drinking | 56.9% | 55.6% | 57.9%† | 55.3% | 60.1%‡ | 56.8% | 55.6% | 56.0% | 55.6% | 52.5%† | 61%‡ |
Notes: † and ‡ indicate statistically significant difference between risk groups at the 5% and 1% level, respectively.
There was a high prevalence of OSA in the sample with 1,939 (12.9%) patients reporting a history of diagnosed OSA. In addition, the Berlin questionnaire identified 3,601 (24.1%) as high risk, STOP identified 2,496 (16.7%) as high risk, STOP-BANG identified 6,226 (41.6%) as high risk, and the Flemons’ Index identified 3,753 (25.1%) as intermediate and 1,418 (9.5%) as high risk for OSA.
As detailed also in Table 1, patients in the high-risk categories, identified by any of the four OSA screening tools, tended to be compared to those who screened at low-risk for OSA, predominantly male, older, had a higher BMI, and more severe health status with higher prevalence of hypertension, diabetes, coronary artery disease, arrhythmias, chronic obstructive pulmonary disease, and congestive heart failure, as well as a more extensive smoking history. All of these risk factor differences were highly significant (p <0.01) contrasting the groups of OSA risk status within each screener. Compared to one another, the four screener profiles did not differ significantly in terms of the demographics and health information of patients who were identified as high risk for OSA. Comparisons across the four OSA screening tools were also examined in terms of raw (positive, negative, and overall) agreements to convey their relative performance accuracy and Kappa statistics to expresses the extent of their concordance beyond what chance alone would predict. Conventional use of this statistic suggests a kappa exceeding 0.75 indicates excellent agreement, between 0.40 and 0.75 indicates moderate to good agreement, and lower than 0.40 indicates poor agreement.35 Table 2 shows that the levels of raw agreement were reasonably high, but the level of positive agreement was always lower than negative agreement for any combination of any two screening tools. On the other hand, the overall agreement revealed that the Berlin and STOP had the highest concordance (82.8%) followed by STOP-BANG and Flemons’ index (80.7%). The lowest overall agreement was between STOP and the Flemons’ index (65.5%). Moreover, the kappa values ranged from a low of 0.225 for the agreement between the STOP and the Flemons’ index to a high of only 0.611 between STOP-BANG and Flemons, indicating moderate to good agreement between these two screening tools.
Table 2.
Measures of agreement among OSA screeners in a sample of surgical patients (N = 14,962)
| OSA Screeners | Positive Agreement (95% CI) |
Negative Agreement (95% CI) |
Overall Agreement (95% CI) |
Kappa (95% CI) |
|---|---|---|---|---|
| Berlin vs. STOP | 63.2% (61.76% – 64.59%) | 88.7% (88.28% – 89.21%) | 82.8% (82.11% – 83.41%) | .524 (.507 – .541) |
| Berlin vs. STOP-BANG | 56.4% (55.22% – 57.57%) | 73.6% (72.82% – 74.34%) | 67.1% (66.29% – 67.90%) | .329 (.314 – .344) |
| Berlin vs. Flemons | 54.1% (52.82% – 55.36%) | 75.9% (75.17% – 76.62%) | 68.4% (67.58% – 69.20%) | .313 (.297 – .330) |
| STOP vs. STOP-BANG | 53.6% (52.32% – 54.86%) | 76.6% (75.93% – 77.33%) | 68.9% (68.12% – 69.71%) | .361 (.348 – .374) |
| STOP vs. Flemons | 42.8% (41.36% – 44.15%) | 75.3% (74.61% – 76.04%) | 65.5% (64.69% – 66.35%) | .225 (.209 – .241) |
| STOP-BANG vs. Flemons | 78.4% (77.55% – 79.23%) | 82.5% (81.82% – 83.19%) | 80.7% (79.98% – 81.36%) | .611 (.597 – .625) |
Postoperative morbidity
We compared the likelihood of going directly to an ICU postoperatively as opposed the PACU. Overall, 4.7% (696 of 14747) of patients were transferred directly to the ICU postoperatively. Having a history of OSA (6.3%, p<0.001) or a high risk score on the STOP (5.57%, p=0.004) and STOP-BANG (5.2%, p < 0.001) assessments was significantly associated with ICU admission. Non-adjusted odds ratios of going to ICU as opposed to the PACU with 95% confidence intervals were 1.439 [1.175 – 1.761] for patients with a history of OSA, 1.182 [0.986 – 1.416] for Berlin, 1.339 [1.100 – 1.631] for STOP, 1.409 [1.190 – 1.667] for STOPBANG, and 1.150 [0.884 – 1.495] for the Flemons high-risk group.
Backward logistic regression analysis using the aforementioned factors found that ASA status > 2E (p<0.001), history of congestive heart failure (p<0.001), Caucasian race (p < 0.001), male (p<0.001), history of coronary artery disease (p < 0.001), BMI > 25 (p=0.006), and history of alcohol consumption (p = 0.010) were predictive of increased postoperative ICU admission, while history of OSA (p = 0.003), and high risk scores on the STOP-BANG (p= 0.001), and Flemons’ index (p=0.041) were associated with decreased ICU admission. The c-statistic is equivalent to the area under a receiver operator curve, where one signifies a perfect model and 0.5 is random chance. The c-statistic of this model was 0.771.
Information on hospital length of stay was only available for 4,846 patients; there were no significant univariate differences based on OSA history or risk score. Backward linear regression analysis using the same predetermined factors found that male (p<0.001), older than 65 years (p=0.037), with ASA >2E (p<0.001), or ASA 1E – 2 (p=0.007), and BMI < 18 kg/m2 (p=0.001), as well as having a history of CHF (p<0.001), COPD (p<0.001), or arrhythmias (p=0.003), and tobacco smoking (p=0.003) were predictive of longer hospital stay, while being Caucasian (p=0.028) with a history of OSA (p<0.001), or scoring high on Flemons (p = 0.008) or STOP-BANG screeners (p=0.006) were associated with shorter hospital stay.
Patients with a history of OSA stayed longer in the ICU postoperatively (3.1 ± 4.8 days versus 2.4 ± 4.3 days), but no statistical differences were found between risk groups of the various screens. There were significant differences in PACU length of stay, however. Patients with history of OSA had an average stay of 134.3 ± 78.9 minutes, versus 128.4 ± 73.1 minutes for those without a history of OSA; Berlin high risk had an average of 133.3 ± 73.2 minutes, versus 126.5 ± 72.9 minutes in the low risk group; STOP high risk had an average of 136.9 ± 75.0 minutes, versus 126.4 ± 72.5 minutes in the low risk group; STOP-BANG high risk had an average of 132.6 ± 73.4 minutes, versus 124.7 ± 72.6 minutes in the low risk group (p<0.001), and Flemons’ high risk patients stayed an average of 132.9 ± 70.7 minutes, versus 127.8 ± 73.7 minutes in the intermediate and low risk groups. While these were statistically significant (p≤0.024) differences, the small absolute differences may have minimal clinical importance in terms of their overall prognostic value for practitioners.
Mortality
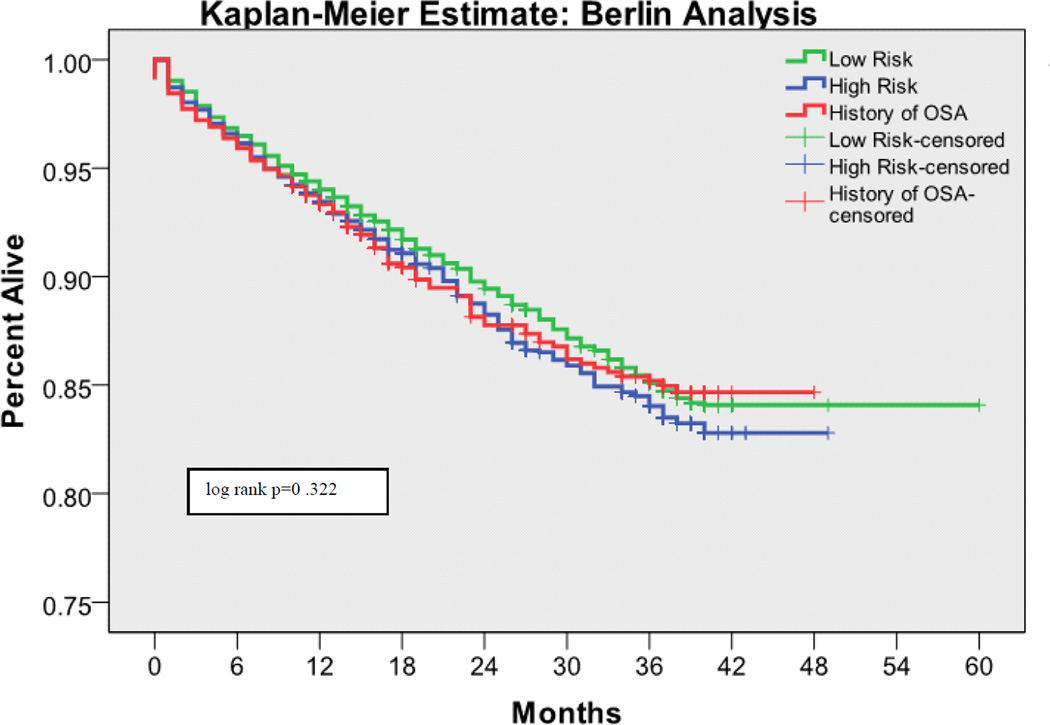
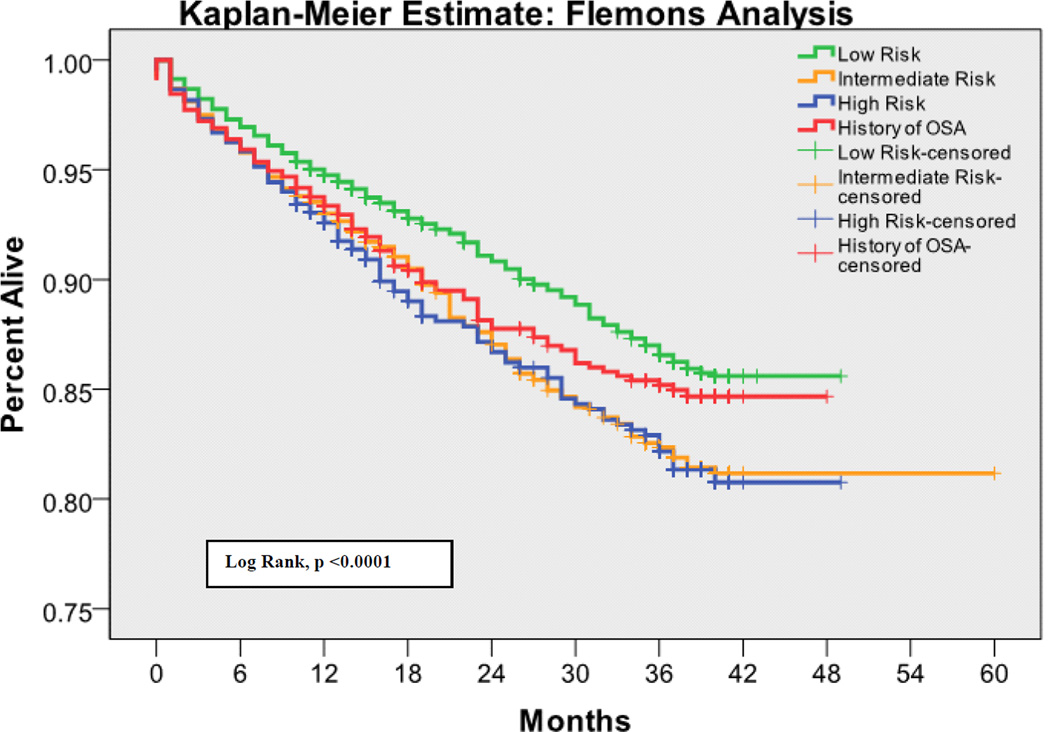
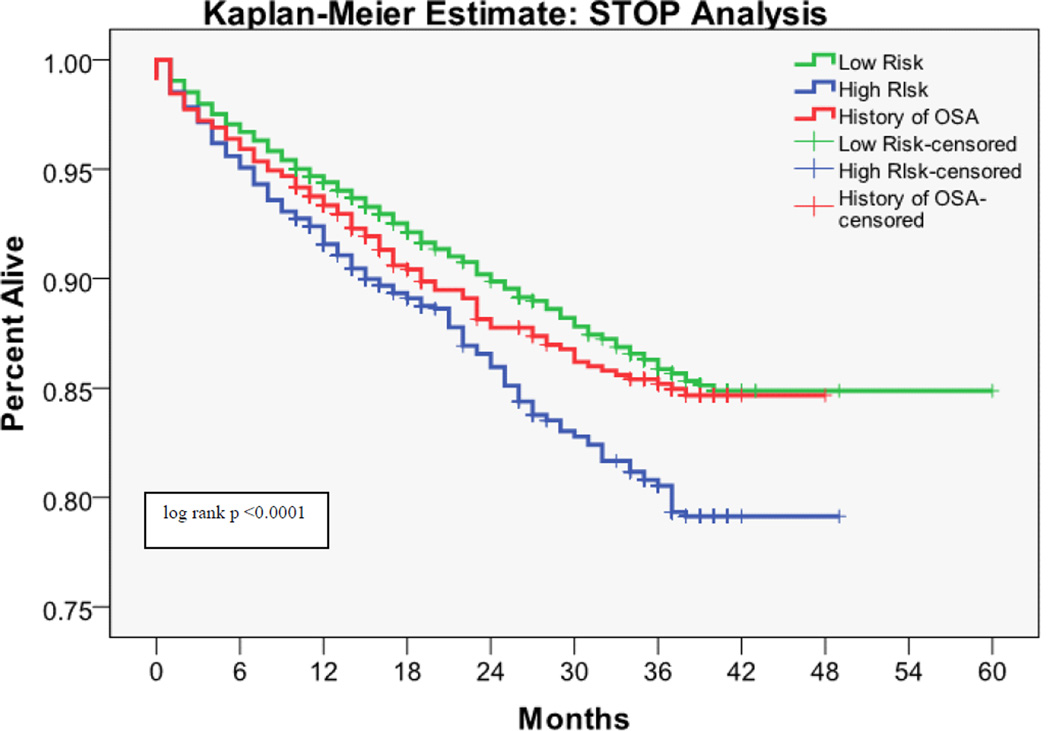
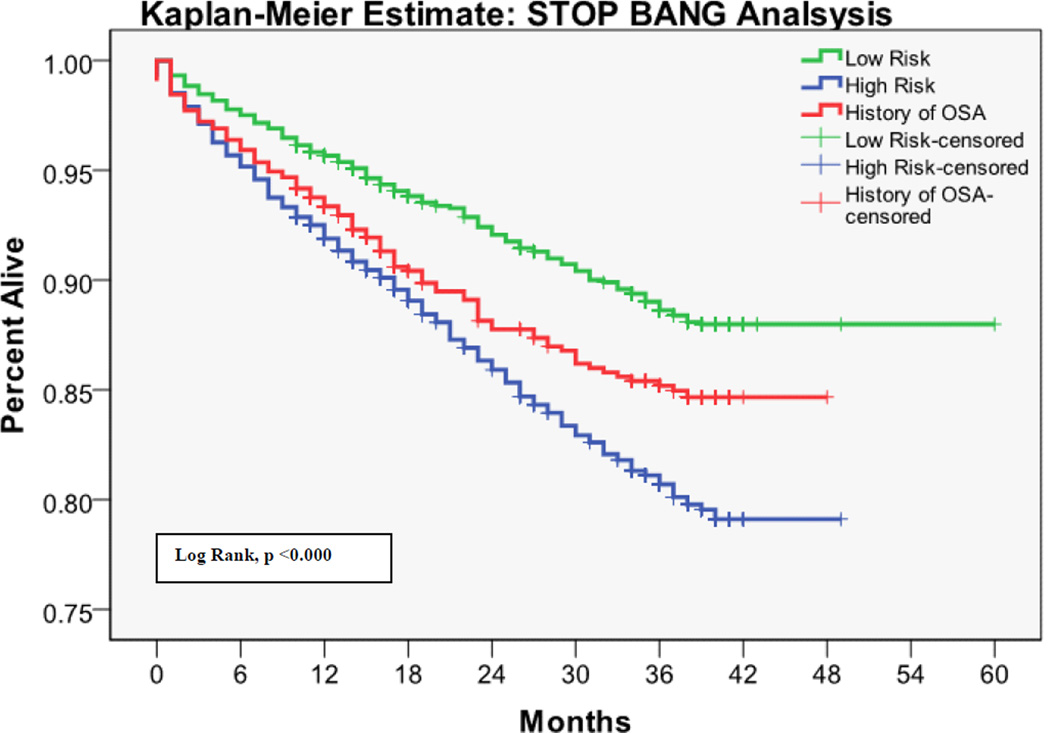
The 30 day mortality rate was 0.64%, the one year mortality rate was 5.78%, and the two year mortality rate was 7.77%. Kaplan-Meier survival analyses with log rank tests for each OSA screening tool are shown in Figures 1 – 4. Table 3 shows the predictive performance of the OSA screening tools for 30 day and one year mortality between patients as grouped by their screening scores. The results revealed no significant differences in 30 day mortality for each of the four screening tools. Significant differences in one year mortality were noted between the low and high-risk groups as identified by the Flemons’ (4.96% versus 6.91%; p <0.0001), STOP (5.28% versus 7.57%; p <0.0001) and STOP-BANG (4.13% versus 7.45%; p <0.0001) screens. There were no differences in mortality predictions between the Berlin screener groups. Additionally, comparisons of the incidence of mortality among patients who had versus those who did not have a history of OSA showed no significant difference in 30 day and one year mortality in this population.
Figure 1.
Kaplan-Meier estimates of mortality based on the risk of OSA as measured by the Berlin Screen
Figure 4.
Kaplan-Meier estimates of mortality based on the risk of OSA as measured by the Flemons’ Index
Table 3.
Predictive performances of OSA screeners among surgical patients (N = 14,962)
| OSA Screeners | 30-Day Mortality n(%) |
p-Value | One-Year Mortality n(%) |
p-Value |
|---|---|---|---|---|
| Berlin | ||||
| -Low Risk | 57(0.60%) | 525(5.57%) | ||
| -High Risk | 21(0.58%) | NS | 220(6.11%) | NS |
| STOP | ||||
| -Low Risk | 58(0.55%) | 556(5.28%) | ||
| -High Risk | 20(0.80%) | NS | 189(7.57%) | < 0.0001 |
| STOP-BANG | ||||
| Low Risk | 33(0.49%) | 281(4.13%) | ||
| -High Risk | 45(0.72%) | NS | 464(7.45%) | < 0.0001 |
| Flemons | ||||
| -Low Risk | 37(0.50%) | 368(4.96%) | ||
| -Intermediate Risk | 30(0.80%) | 241(6.42%) | ||
| -High Risk | 7(.49%) | NS | 98(6.91%) | < 0.0001 |
| Actual Incidence | ||||
| -History of OSA | 18(0.93%) | 120(6.19%) | ||
| -No History of OSA | 78(0.60%) | NS | 745(5.72%) | NS |
| -Overall | 96(0.64%) | 865(5.78%) |
The estimated hazards ratios from the multivariate Cox regression model were intended to evaluate the independent risk for postoperative mortality of each of the four OSA screening tool results, while controlling for previously established perioperative risk factors. None of the four investigated OSA screening tools was independently predictive of mortality rate up to one year postoperatively in this population. (Table 4)
Table 4.
Risk factors for One-year mortality across OSA screeners
| Hazard Ratio (95% Confidence Limits) | |||||
|---|---|---|---|---|---|
| Overall Sample (n = 14,962) |
Berlin (n = 3,601) |
STOP (n = 2,496) |
STOP-BANG (n = 6,226) |
Flemons (n =5,171) |
|
| Male | 1.48(1.29 – 1.71) | 1.74(1.31 – 2.31) | 281.76(1.29 – 2.39) | 1.72(1.36 – 2.18) | 1.65(1.25 – 2.17) |
| Age | |||||
| < 45 | - | - | - | - | - |
| 45 to 65 | 2.31(1.76 – 3.03) | 1.76(1.02 – 3.03) | * | 1.65(1.01 – 2.69) | 1.68(1.05 – 2.67) |
| > 65 | 3.52(2.67 – 4.65) | 3.03(1.73 – 5.29) | 3.01(1.49 – 6.03) | 2.55(1.56 – 4.17) | 2.54(1.59 – 4.08) |
| BMI | |||||
| < 18.5 | 3.49(2.50 – 4.88) | * | * | 3.22(1.83 – 5.68) | * |
| 18.5 to 24.9 | 1.69(1.42 – 2.01) | 1.82(1.27 – 2.60) | 1.98(1.35 – 2.89) | 1.84(1.44 – 2.36) | 1.51(1.07 – 2.13) |
| 25.0 to 29.9 | 1.20(1.02 – 1.42) | * | 1.42(1.01 – 2.00) | 1.25(1.00 – 1.56) | * |
| > 30.0 | - | - | - | - | - |
| ASA | |||||
| = 1 | - | - | - | - | - |
| 1E to 2 | 2.76(1.29 – 5.90) | * | * | * | * |
| ≥ 2E | 6.21(2.91 – 13.28) | * | * | 9.76(1.36 – 70.09) | * |
| Comorbid Conditions | |||||
| Having 2+Conditions | 1.18(1.02 – 1.38) | * | * | 1.26(1.03 – 1.55) | * |
| Risk Behaviors | |||||
| Past/Current Smoking | 1.28(1.11 – 1.48) | * | * | 1.24(1.02 – 1.51) | * |
| Alcohol Drinking | 1.10(0.95 – 1.26) | * | * | * | * |
Notes: '-' marks reference category with assumed hazard ratio = 1.00, and '*' marks not sigificant effect (P >.05).
3. Discussion
This study of unselected adult surgical patients support previous reported findings that there is a high prevalence of undiagnosed OSA among patients presenting for surgery at Barnes Jewish Hospital [17], which is likely to be reflective of similar populations in the United States. Interestingly, 13% of patients presented with a prior diagnosis of OSA, suggesting that awareness in the medical community and the general public about OSA may be increasing. Depending on which screening tool is used in patients without a known diagnosis of OSA, the percent identified as being at high risk for OSA varied considerably; 10% screened high risk with the Flemons’ index whereas, 42% screened high risk with the STOP-BANG assessment. All four screening tools demonstrated a moderately high overall percentage agreement, though they had higher concordance with each other identifying negative OSA cases than reporting positive ones. When adjusting for possible agreement by chance, only the STOP-BANG screener and the Flemons’ Index remained in fair agreement with each other. Despite their differences in prediction, the four screening tools agreed with one another on specific patient characteristics suggestive of sleep-disordered breathing associated with OSA. The most consistent characteristics common to patients who screened high risk for OSA with the four screens were male sex, older age, overweight, and comorbid conditions.
There is emerging evidence that OSA is a risk factor for perioperative morbidity [26]. The current study hypothesized that this morbidity would translate into higher 30 day postoperative mortality. A possible explanation for the lack of evidence supporting this hypothesis, is that the structured quality improvement interventions, which were implemented for patients with known OSA or who screened positive on the hospital OSA screen, might have prevented some postoperative complications and deaths. Notably, patients who screened at high risk for having OSA on the Flemons’, STOP, and STOP-BANG screens had increased cumulative one year mortality, as did those with a history of OSA. This was not true for the Berlin questionnaire. Whether the association between surgery and one year postoperative mortality is causal or coincidental (i.e., people with OSA have increased intermediate term mortality regardless of whether or not they undergo surgery) cannot be determined from this study.
Patients with either a history of OSA or a high risk score on the STOP and STOP-BANG assessments were more likely to be admitted to the ICU postoperatively. We do not have information about scheduled versus unanticipated ICU admissions, and it is possible that patients with OSA were selected preoperatively for ICU admission based either on their OSA risk or diagnosis, or on the basis of comorbidities. On its own this finding cannot be taken as an indication that postoperative complications were increased in patients with OSA. However, this finding could be consistent with studies that have suggested that patients with OSA have higher rates of postoperative pulmonary [12, 27], cardiac [13, 28], and neurologic [29] complications.
Patients with known OSA had shorter hospital stays and did not have the significant differences in one year mortality that was seen with high versus low risk by screening criteria. We do not have information about percentage of patients treated with or compliance with CPAP at home which would be an important consideration. There are many reported benefits of therapy with CPAP including positive effects on arterial stiffness [30] reduction of inflammatory mediators associated with OSA [31], reductions in systolic blood pressure and improved left ventricular systolic function [32, 33, 34], and reduced the risk of death and hospitalization among patients with HF and OSA [35]. While these are interesting findings, we cannot yet suggest that treatment of OSA is responsible for these observed shorter hospital stays and similarities one year mortality between those with and without history of OSA. The role of CPAP therapy in reducing perioperative morbidity and mortality in patients with OSA remains unclear.
The ASA issued guidelines in relation to the perioperative management of patients with known or suspected OSA [23]. These guidelines were disseminated with the goal of reducing adverse outcomes associated with OSA and improving perioperative care. In addition to the ASA, several authors have recommended routine preoperative screening for OSA. Adesanya et al. reviewed evidence that perioperative adverse events are associated with OSA, provided an algorithm, and suggested strategies for preoperative, intraoperative and postoperative care [11]. The Berlin questionnaire [36], ASA checklist [23], STOP screen and STOP-Bang screen [18] have all been studied in the perioperative setting. Flemons’ Index [37] has been shown to be useful for identifying patients at risk for oxygen desaturation after PACU discharge [38]. In general, there is paucity of evidence about which OSA screen should be used for adult patients, and even the evidence for routine screening is not compelling. This controversy is reflected in the diversity of approaches that have previously been adopted, and is reinforced by the current study that shows strikingly different results with various screens and no convincing association between OSA and postoperative complications. Interestingly, although the STOP and STOP-BANG screening tools had very similar predictive performances of one-year mortality, the STOP-BANG was the only screening tool in this study that showed OSA risk was directly associated with certain perioperative complications as well as intermediate postoperative morbidity and mortality. This study finding supports the concept that the additional risk factors (BMI > 35, age >50, neck circumference >40cm, and being a male) of OSA increase the specificity of the STOP-BANG screening tool [36].
There are several important limitations to this study. Information on cancer status was not included, and could be a potential confounder of mortality outcomes. Furthermore, patients were not stratified according to surgical risk and those with a history of OSA included those who self-reported a prior diagnosis. While many of these patients were diagnosed via polysomnography, these results were not confirmed. Another potential confounding factor relates to our targeted perioperative care of at-risk patients. All patients with either a history of OSA or with a positive hospital screen for OSA received a structured quality improvement intervention. Without a control group of high-risk patients that did not receive the intervention, it is difficult to determine the extent that this intervention might have prevented postoperative morbidity and mortality.
The B-J APNEAS trial was a large prospective cohort study that examined relationships among OSA screens, patient characteristics, and postoperative morbidity and mortality. The results of this study highlight important uncertainties and research priorities for the medical community. First, it is necessary to clarify whether OSA is indeed independently associated with increased perioperative morbidity and mortality. Second, it is essential to investigate which method of screening for OSA preoperatively is both practical and effective. Third, it is important to discover whether targeted or universal utilization of quality improvement initiatives should be implemented to mitigate the hypothesized perioperative risks associated with OSA.
Supplementary Material
Figure 2.
Kaplan-Meier estimates of mortality based on the risk of OSA as measured by the STOP Screen
Figure 3.
Kaplan-Meier estimates of mortality based on the risk of OSA as measured by the STOP-BANG Screen
Acknowledgements
The Institute of Quality Improvement, Research and Informatics (INQUIRI) at Washington University School of Medicine.
Funding: Grant from the Barnes Jewish Hospital Foundation # 01153-0308-01 to M. Avidan; TLRR024995, TL1 TR000449, UL1 TR000448 and UL1RR024992 to M. Willingham
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ellen M. Lockhart, Anesthesiology, Washington University School of Medicine, 660 S. Euclid, Box 8054, St. Louis, MO 63110, Tel: (314) 362-2628, Fax: (314) 362-1185, lockhare@wustl.edu.
Mark D. Willingham, Internal Medicine - Infectious Disease, Washington University School of Medicine, 660 S. Euclid, Box 8051, St. Louis, MO 63110.
Arbi Ben Abdallah, Anesthesiology, Washington University School of Medicine, 660 S. Euclid, Box 8054, St. Louis, MO 63110.
Daniel L. Helsten, Anesthesiology, Washington University School of Medicine, 660 S. Euclid, Box 8054, St. Louis, MO 63110.
Bahaa A. Bedair, Washington University School of Medicine, 660 S. Euclid, Box 8107, St. Louis, MO 63110.
James Thomas, Periop Performance Improvement Administration and Information Systems, Barnes-Jewish Hospital, 1 Barnes-Jewish Hospital Drive, St. Louis, MO 63110, Mail Stop: 90-72-408.
Stephen Duntley, Neurology, Washington University School of Medicine, 660 S. Euclid, Box 8111, St. Louis, MO 63110.
Michael S. Avidan, Anesthesiology, Washington University School of Medicine, 660 S. Euclid, Box 8054, St. Louis, MO 63110.
References
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14(1):179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 7.Chou KT, Huang CC, Chen YM, et al. Sleep apnea and risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. Am J Med. 125(4):374–380. doi: 10.1016/j.amjmed.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax. 2008;63(6):536–541. doi: 10.1136/thx.2007.085464. [DOI] [PubMed] [Google Scholar]
- 9.Chou YT, Lee PH, Yang CT, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant. 26(7):2244–2250. doi: 10.1093/ndt/gfq821. [DOI] [PubMed] [Google Scholar]
- 10.Rasche K, Keller T, Tautz B, et al. Obstructive sleep apnea and type 2 diabetes. Eur J Med Res. 15(Suppl 2):152–156. doi: 10.1186/2047-783X-15-S2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adesanya AO, Lee W, Greilich NB, Joshi GP. Perioperative management of obstructive sleep apnea. Chest. 2010;138(6):1489–1498. doi: 10.1378/chest.10-1108. [DOI] [PubMed] [Google Scholar]
- 12.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56(11):819–828. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 13.den Herder C, Schmeck J, Appelboom DJ, de Vries N. Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ. 2004;329(7472):955–959. doi: 10.1136/bmj.329.7472.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaw R, Golish J. Obstructive sleep apnea: what to do in the surgical patient? IMPACT consults. Cleve Clin J Med; Proceedings of the 2nd Annual Cleveland Clinic Perioperative Medicine Summit; 2006. pp. S15–S17. [DOI] [PubMed] [Google Scholar]
- 15.Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107(5):1543–1563. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 16.Ronald J, Delaive K, Roos L, Manfreda J, Bahammam A, Kryger MH. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225–229. doi: 10.1093/sleep/22.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10(7):753–758. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 19.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 108(5):768–775. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadi N, Chung SA, Gibbs A, Shapiro CM. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12(1):39–45. doi: 10.1007/s11325-007-0125-y. [DOI] [PubMed] [Google Scholar]
- 22.Mungan U, Ozeke O, Mavioglu L, et al. The Role of the Preoperative Screening of Sleep Apnoea by Berlin Questionnaire and Epworth Sleepiness Scale for Postoperative Atrial Fibrillation. Heart Lung Circ. doi: 10.1016/j.hlc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104(5):1081–1093. doi: 10.1097/00000542-200605000-00026. quiz 117–8. [DOI] [PubMed] [Google Scholar]
- 24.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2(1):2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sesso HD, Paffenbarger RS, Lee IM. Comparison of National Death Index and World Wide Web death searches. Am J Epidemiol. 2000;152(2):107–111. doi: 10.1093/aje/152.2.107. [DOI] [PubMed] [Google Scholar]
- 26.Kaw R, Michota F, Jaffer A, Ghamande S, Auckley D, Golish J. Unrecognized sleep apnea in the surgical patient: implications for the perioperative setting. Chest. 2006;129(1):198–205. doi: 10.1378/chest.129.1.198. [DOI] [PubMed] [Google Scholar]
- 27.Hwang D, Shakir N, Limann B, et al. Association of sleep-disordered breathing with postoperative complications. Chest. 2008;133(5):1128–1134. doi: 10.1378/chest.07-1488. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76(9):897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 29.Kaw R, Golish J, Ghamande S, Burgess R, Foldvary N, Walker E. Incremental risk of obstructive sleep apnea on cardiac surgical outcomes. J Cardiovasc Surg (Torino) 2006;47(6):683–689. [PubMed] [Google Scholar]
- 30.Kasai T, Inoue K, Kumagai T, et al. Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens. 24(4):401–407. doi: 10.1038/ajh.2010.248. [DOI] [PubMed] [Google Scholar]
- 31.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33(5):1195–1205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 32.Butt M, Dwivedi G, Shantsila A, Khair OA, Lip GY. Left ventricular systolic and diastolic function in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Circ Heart Fail. 5(2):226–233. doi: 10.1161/CIRCHEARTFAILURE.111.964106. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 34.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 35.Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133(3):690–696. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 36.Chung F, Ward B, Ho J, Yuan H, Kayumov L, Shapiro C. Preoperative identification of sleep apnea risk in elective surgical patients, using the Berlin questionnaire. J Clin Anesth. 2007;19(2):130–134. doi: 10.1016/j.jclinane.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Gali B, Whalen FX, Jr, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3(6):582–588. [PMC free article] [PubMed] [Google Scholar]
- 38.Adesanya AO, Lee W, Greilich NB, Joshi GP. Perioperative management of obstructive sleep apnea. Chest. 138(6):1489–1498. doi: 10.1378/chest.10-1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.