Abstract
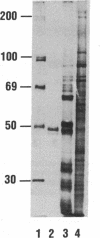
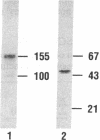
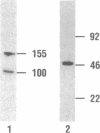
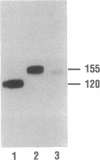
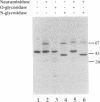
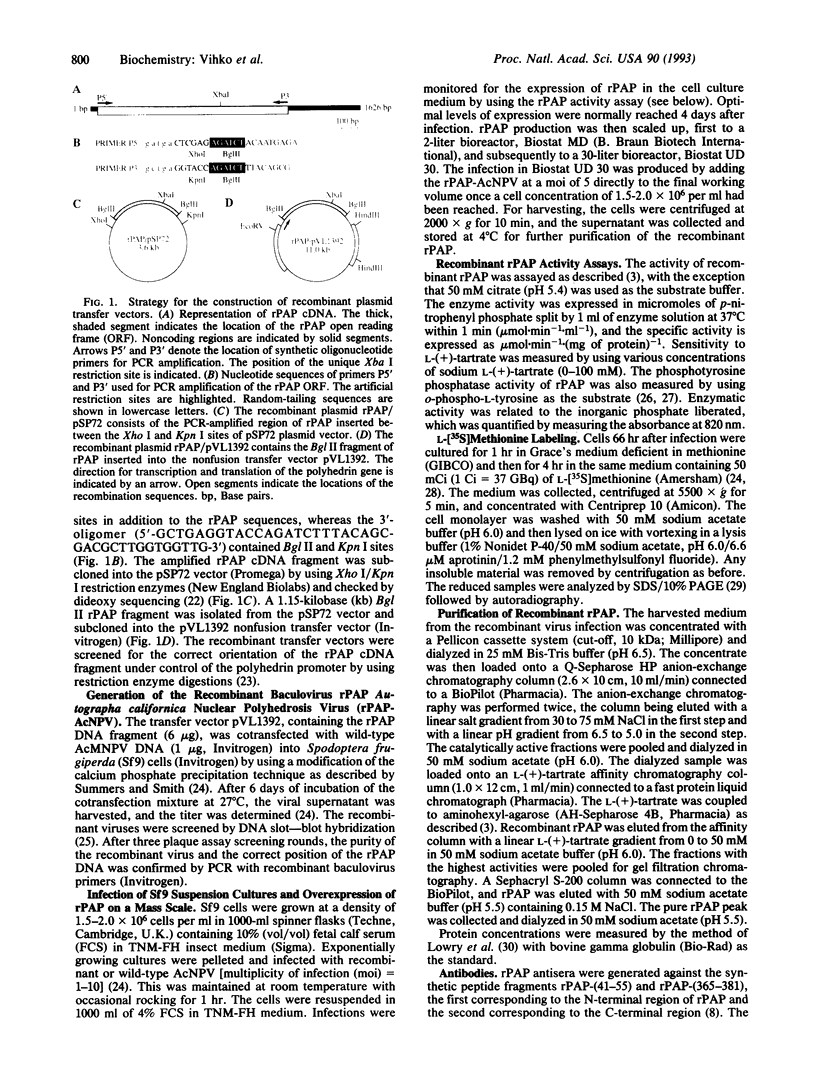
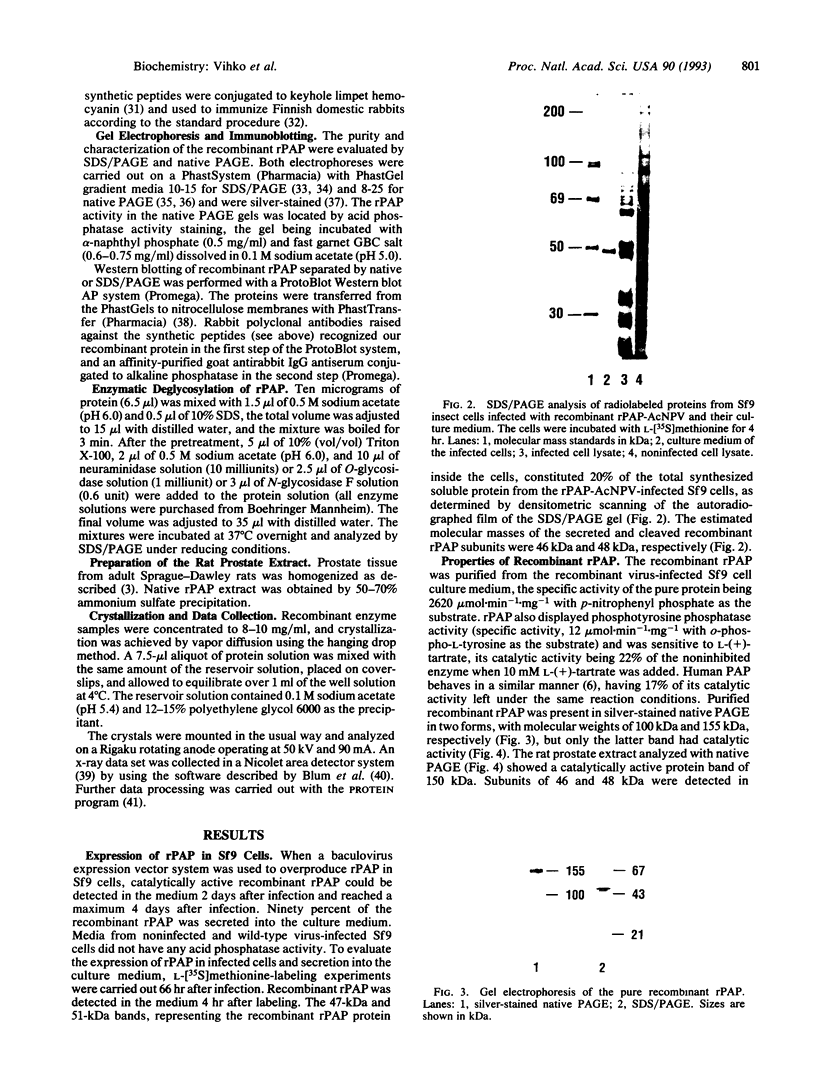
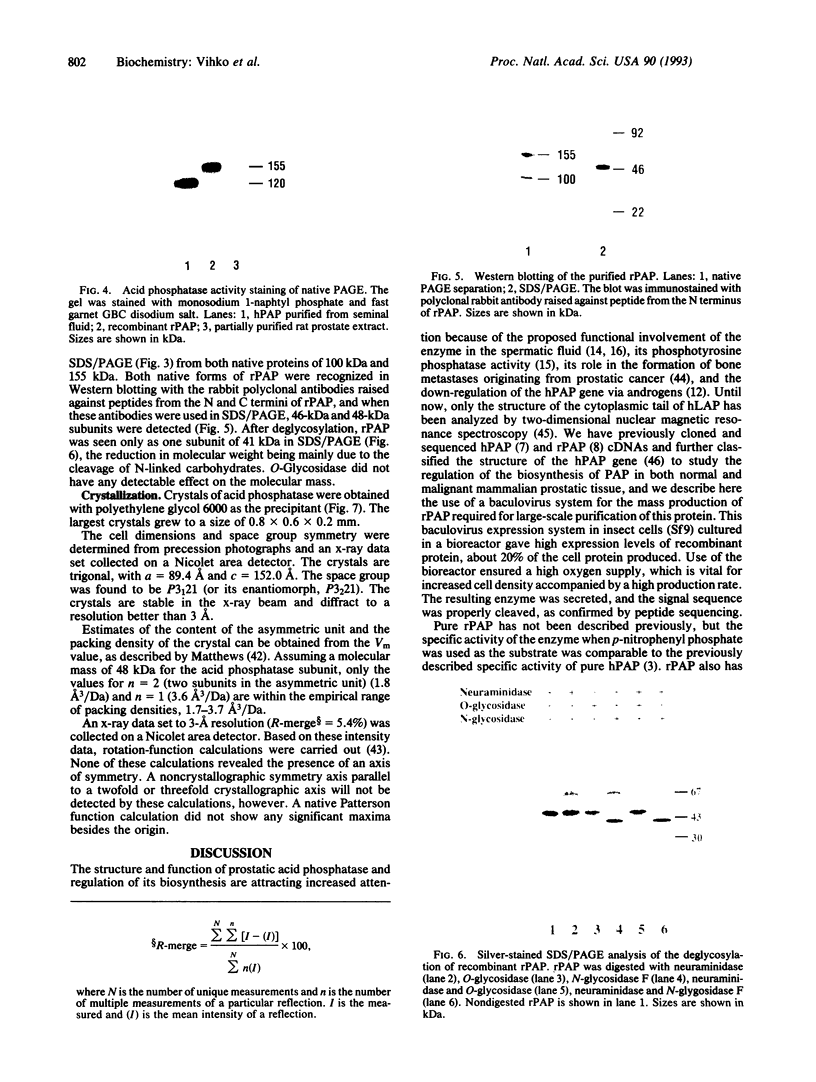
Rat prostatic acid phosphatase (rPAP; orthophosphoric-monoester phosphohydrolase (acid optimum), EC 3.1.3.2) was expressed in the baculovirus expression vector system. Recombinant protein was secreted into the medium at a high yield by infected insect cells, which were cultured at high density in a 30-liter bioreactor allowing high oxygen content for rapidly growing cells. About 20% of the cell protein produced was rPAP. Partial sequence determination of the N terminus of the purified recombinant secreted protein revealed identity to the native secreted protein, showing that the signal peptide is recognized and properly cleaved in insect cells. The enzyme was purified by using L-(+)-tartrate affinity chromatography. The purified protein had a high specific activity of 2620 mumol.min-1.mg-1 with p-nitrophenyl phosphate at the substrate, and it also showed phosphotyrosine phosphatase activity. The molecular mass of the recombinant rPAP was 155 kDa. Two subunits of 46 kDa and 48 kDa could be detected in SDS/PAGE, but only one subunit of 41 kDa was present after digestion with N-glycosidase. The active enzyme is a trimer of subunits differing only in glycosylation. When recombinant rPAP was crystallized with polyethylene glycol 6000 as the precipitant, the crystals were trigonal (space group P3(1)21) with cell dimensions a = 89.4 A and c = 152.0 A. The observed diffraction pattern extends to a resolution of at least 3 A.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaway G. P., Vivino A. A., Kohn L. D., Notkins A. L., Prabhakar B. S. Characterization of the 70KDA component of the human Ku autoantigen expressed in insect cell nuclei using a recombinant baculovirus vector. Biochem Biophys Res Commun. 1990 Apr 30;168(2):747–755. doi: 10.1016/0006-291x(90)92385-d. [DOI] [PubMed] [Google Scholar]
- Bona C., Hooghe R., Cazenave P. A., Leguérn C., Paul W. E. Cellular basis of regulation of expression of idiotype. II. Immunity to anti-MOPC-460 idiotype antibodies increases the level of anti-trinitrophenyl antibodies bearing 460 idiotypes. J Exp Med. 1979 Apr 1;149(4):815–823. doi: 10.1084/jem.149.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff J., Schievella A. R., Jost C. A., Erikson R. L., Neel B. G. Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2735–2739. doi: 10.1073/pnas.87.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Durbin R. M., Burns R., Moulai J., Metcalf P., Freymann D., Blum M., Anderson J. E., Harrison S. C., Wiley D. C. Protein, DNA, and virus crystallography with a focused imaging proportional counter. Science. 1986 May 30;232(4754):1127–1132. doi: 10.1126/science.3704639. [DOI] [PubMed] [Google Scholar]
- Eberle W., Sander C., Klaus W., Schmidt B., von Figura K., Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell. 1991 Dec 20;67(6):1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- Ek-Rylander B., Bill P., Norgård M., Nilsson S., Andersson G. Cloning, sequence, and developmental expression of a type 5, tartrate-resistant, acid phosphatase of rat bone. J Biol Chem. 1991 Dec 25;266(36):24684–24689. [PubMed] [Google Scholar]
- Geier C., von Figura K., Pohlmann R. Structure of the human lysosomal acid phosphatase gene. Eur J Biochem. 1989 Aug 15;183(3):611–616. doi: 10.1111/j.1432-1033.1989.tb21090.x. [DOI] [PubMed] [Google Scholar]
- Gieselmann V., Hasilik A., von Figura K. Tartrate-inhibitable acid phosphatase. Purification from placenta, characterization and subcellular distribution in fibroblasts. Hoppe Seylers Z Physiol Chem. 1984 Jun;365(6):651–660. doi: 10.1515/bchm2.1984.365.1.651. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A. B., Gutman E. B. AN " ACID " PHOSPHATASE OCCURRING IN THE SERUM OF PATIENTS WITH METASTASIZING CARCINOMA OF THE PROSTATE GLAND. J Clin Invest. 1938 Jul;17(4):473–478. doi: 10.1172/JCI100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henttu P., Liao S. S., Vihko P. Androgens up-regulate the human prostate-specific antigen messenger ribonucleic acid (mRNA), but down-regulate the prostatic acid phosphatase mRNA in the LNCaP cell line. Endocrinology. 1992 Feb;130(2):766–772. doi: 10.1210/endo.130.2.1370795. [DOI] [PubMed] [Google Scholar]
- Himeno M., Fujita H., Noguchi Y., Kono A., Kato K. Isolation and sequencing of a cDNA clone encoding acid phosphatase in rat liver lysosomes. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1044–1053. doi: 10.1016/0006-291x(89)90779-1. [DOI] [PubMed] [Google Scholar]
- Ishibe M., Rosier R. N., Puzas J. E. Human prostatic acid phosphatase directly stimulates collagen synthesis and alkaline phosphatase content of isolated bone cells. J Clin Endocrinol Metab. 1991 Oct;73(4):785–792. doi: 10.1210/jcem-73-4-785. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. 3. Further analysis and new forms of discontinuous buffer systems. Biochemistry. 1973 Feb 27;12(5):890–898. doi: 10.1021/bi00729a016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Fillers W. S., Iyengar M. R. Phosphocreatine, an intracellular high-energy compound, is found in the extracellular fluid of the seminal vesicles in mice and rats. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7265–7269. doi: 10.1073/pnas.85.19.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. F., Clinton G. M. Human prostatic acid phosphatase has phosphotyrosyl protein phosphatase activity. Biochem J. 1986 Apr 15;235(2):351–357. doi: 10.1042/bj2350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Naz R. K., Ahmad K., Kumar R. Role of membrane phosphotyrosine proteins in human spermatozoal function. J Cell Sci. 1991 May;99(Pt 1):157–165. doi: 10.1242/jcs.99.1.157. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Chapdelaine A., Chevalier S. Prostatic acid phosphatase in serum of patients with prostatic cancer is a specific phosphotyrosine acid phosphatase. Clin Chem. 1990 Aug;36(8 Pt 1):1450–1455. [PubMed] [Google Scholar]
- Pen J., Welling G. W., Welling-Wester S. An efficient procedure for the isolation of recombinant baculovirus. Nucleic Acids Res. 1989 Jan 11;17(1):451–451. doi: 10.1093/nar/17.1.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Krentler C., Schmidt B., Schröder W., Lorkowski G., Culley J., Mersmann G., Geier C., Waheed A., Gottschalk S. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J. 1988 Aug;7(8):2343–2350. doi: 10.1002/j.1460-2075.1988.tb03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur B., Russo-Marie F. An automated Western blot analysis using the phastsystem. Anal Biochem. 1988 Aug 1;172(2):338–343. doi: 10.1016/0003-2697(88)90453-8. [DOI] [PubMed] [Google Scholar]
- Roiko K., Jänne O. A., Vihko P. Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatases. Gene. 1990 May 14;89(2):223–229. doi: 10.1016/0378-1119(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Rönnberg L., Vihko P., Sajanti E., Vihko R. Clomiphene citrate administration to normogonadotropic subfertile men: blood hormone changes and activation of acid phosphatase in seminal fluid. Int J Androl. 1981 Jun;4(3):372–378. doi: 10.1111/j.1365-2605.1981.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Saini M. S., Van Etten R. L. A homogeneous isoenzyme of human liver acid phosphatase. Arch Biochem Biophys. 1978 Dec;191(2):613–624. doi: 10.1016/0003-9861(78)90399-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solin T., Kontturi M., Pohlmann R., Vihko P. Gene expression and prostate specificity of human prostatic acid phosphatase (PAP): evaluation by RNA blot analyses. Biochim Biophys Acta. 1990 Jan 30;1048(1):72–77. doi: 10.1016/0167-4781(90)90024-v. [DOI] [PubMed] [Google Scholar]
- Susi F. R., Goldhaber P., Jennings J. M. Histochemical and biochemical study of acid phosphatase in resorbing bone in culture. Am J Physiol. 1966 Oct;211(4):959–962. doi: 10.1152/ajplegacy.1966.211.4.959. [DOI] [PubMed] [Google Scholar]
- Van Etten R. L., Davidson R., Stevis P. E., MacArthur H., Moore D. L. Covalent structure, disulfide bonding, and identification of reactive surface and active site residues of human prostatic acid phosphatase. J Biol Chem. 1991 Feb 5;266(4):2313–2319. [PubMed] [Google Scholar]
- Vanha-Perttula T., Niemi R., Helminen H. J. Separate lysosomal and secretory acid phosphatases in the rat ventral prostate. Invest Urol. 1972 Jan;9(4):345–352. [PubMed] [Google Scholar]
- Vihko P., Kontturi M., Korhonen L. K. Purification of human prostatic acid phosphatase by affinity chromatography and isoelectric focusing. Part I. Clin Chem. 1978 Mar;24(3):466–470. [PubMed] [Google Scholar]
- Vihko P., Virkkunen P., Henttu P., Roiko K., Solin T., Huhtala M. L. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett. 1988 Aug 29;236(2):275–281. doi: 10.1016/0014-5793(88)80037-1. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]