Abstract
Background:
Recent studies have shown that benzene extract of Ocimum sanctum (O. sanctum) leaves induces the ultrastructural changes in the epithelial cells of the cauda epididymis, its subsequent recovery in the seminiferous epithelium and fertility of male albino rats.
Objective:
Our aim was to investigate the effect of benzene extract of O.sanctum leaves on the cauda epididymal sperm parameters, morphology and their organelles at the ultrastructural level in albino rats.
Materials and Methods:
Wistar male rats (n=20) were allocated into two groups of control (n=10) and test group (n=10). The test group received benzene extract of O.sanctum leaves (250mg/kg/day) for 48 consequence days. Five animals from each group were used for fertility test. Twenty-four hours after the last dose, the rest of the control (n=5) and treated (n=5) animals were sacrificed by cervical dislocation and then the cauda epididymal plasma was used for sperm analysis, scanning electron microscopy (SEM) and transmission electron microscopic (TEM) studies.
Results:
Sperm analysis of test group exhibited significant (p≤0.001) decrease in the sperm count, motility, speed and increase in sperm anomalies when compare to control group. SEM and TEM observation in treated animals indicated the morphological changes in plasma membrane as well as in the acrosomal membrane of spermatozoa, formation of a balloon-like cytoplasmic droplet in the mid-region of abnormal tail and disorganization or degeneration of mitochondria of sperm mitochondrial sheaths.
Conclusion:
The effects observed in this study may have resulted from a general alteration in the cauda epididymal milieu, probably due to androgen deficiency consequent to the anti-androgenic property of O.sanctum leaves.
Key Words: Ocimum sanctum, Epididymis, Spermatozoa, Fertility, Electron microscope, Rats
Introduction
Fertility control is an issue of global and national public health concern. Through increased public awareness, statements supporting research on male methods and greater involvements of men reproductive health have been forthcoming from several quarters, including international women's organizations. The clinical and scientific basis for the research has been reviewed in recent years. Apart from research for finding harmless chemical drugs as effective oral contraceptives in the western countries, the crude vegetal drugs used by tribal people are being closely looked into for their possible efficiency to find out safe and effective oral drugs for controlling human fertility. Many of the plants which are common in India are reported to possess antifertility activity as spermicidal, abortifacient or antiandrogenic in nature (1-7). Therefore, it has become necessary to use biologically active botanical substances or fertility-regulating agents of plant origin which are ecofriendly in approach and interfere with the natural patterns of reproduction (8).
The plants of genus Ocimum belonging to family Labiatae are very important for their unique properties. Ocimum sanctum L. (Tulsi), Ocimum gratissium (Ram Tulsi), Ocimum canum (Dulal Tulsi), Ocimum basilicum (Ban Tulsi), Ocimum kilimandscharicum, Ocimum ammericanum, Ocimum camphora and Ocimum micranthum are examples of known important species of genus Ocimum which grow in different parts of the world and are known to have medicinal properties (9-11). Ocimum sanctum is, a small herb seen throughout India, commonly cultivated in gardens. In traditional systems of medicine, different parts (leaves, stem, flower, root, seeds and even whole plant) of Ocimum sanctum, have been recommended for the treatment of bronchitis, bronchial asthma, malaria, diarrhea, dysentery, skin diseases, arthritis, painful eye diseases, chronic fever, insect bite etc. The Ocimum sanctum L. has also been suggested to possess antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic and diaphoretic actions.
In addition, the leaves of O. sanctum significantly altered the sperm count, motility, velocity and fructose contained in the cauda epididymis (12), reduce the mating behaviour of both male and female albino rats (13-15). Recent studies shown that benzene extract of Ocimum sanctum leaves induces the ultrastructural changes in the epithelial cells of the cauda epididymis, its subsequent recovery, after withdrawal of treatment, in the process of spermatogenesis and fertility of male albino rats (16,17) and morphological changes in the rat cauda epididymal sperms upon graded dose treatment (18).
As there is little information concerning the influence of O. sanctum leaves on the cauda epididymal sperm at the ultrastructural level, the present investigation is designed to study whether benzene extract of O.sanctum leaves could cause some of the sperm parameters, morphological alterations in cauda epididymal spermatozoa and its organelles by electron microscopic studies and fertility of male of albino rats as this medicinal plant has anti-spermatogenic and anti-androgenic like properties (12-15).
Materials and methods
Preparation of test material
Fresh O.sanctum leaves were collected and dried in shade. A voucher specimen (Zoo/herb/File No.47-Acc.No.22) was deposited at Zoology Department, Karnatak University, Dharwad, India. The dried leaves were coarsely powdered and subjected to soxheltation process to get the benzene extract. Extract thus obtained was allowed to dry and stored in a dessicator at 4ºC. The benzene extract is then mixed with propylene glycol as required and administered orally (gavage) to the experimental animals (19).
Experimental Animals
Colony bred healthy adult male albino rats (Wistar strain) weighing 190-200g were utilized for experiments. All animals were proven fertility and obtained from the rat colony maintained in the department. They were housed at a temperature of 262oC and exposed to 13-14 h of daylight and maintained on a standard rat pellet diet (Gold Mohar, Hindustan Level Ltd., Hyderabad) and water was given ad libitum. The animals were acclimatized to the laboratory conditions before conducting experiments and the care of the laboratory animals was taken as per the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) regulations.
Study protocol
The control group (n=10) were administered 1 ml propylene glycol/rat/day orally for 48 days and test group (n=10), that received benzene extract of Ocimum sanctum leaves (250mg/kg/day) orally for 48 consequence days. The effective dose of 250mg/kg body weight has been arrived at after preliminary studies on dose and duration of 48 days is concerned to the spermatogenic cycle of rat in response studies in our laboratory and reported (12).
Five animals from each group were used for fertility test. Twenty-four hours after the last dose, rest of the control (n=5) and treated (n=5) animals were sacrificed by cervical dislocation and then the cauda epididymal plasma was used for sperm analysis, scanning and transmission electron microscopic studies.
Sperm analysis
The cauda epididymis was chopped into phosphate buffered glucose saline (PBGS) [composition: NaCl 50 mM/l; Na2HPO4 200 mM/l; glucose 200 mM/l and KH2PO4 26 mM/l]. The debris was removed and a clear suspension, the epididymal plasma was used for the analysis of total sperm count, sperm motility, forward velocity and relative percentage of abnormal sperms in male albino rats. The total sperm count and motility were calculated according to the method of Besley et al (20) using Neubauer’s haemocytometer. Briefly, to increase the accuracy of sperm count, the epididymal plasma was diluted with a spermicidal solution, prepared by dissolving 5 g of sodium bicarbonate (NaHCO3) and 1 ml of 40% formaldehyde in 100 ml of normal saline.
A twenty times dilution was made using W.B.C pipette, which was thoroughly mixed and one drop was added to both sides of Neubauer haemocytometer. The spermatozoa were allowed to settle down in the haemocytometer by keeping them in a humid chamber for one hour. The sperm count was done in R.B.C counting 5 major squares. The total number of sperms were counted in all the major squares and calculated as follows.
Similarly the total number of motile sperms was calculated, using phosphate buffer saline instead of spermicidal solution. The forward velocity of the sperm was calculated according to the method of Ratnasoorya (21). Briefly, the epididymal plasma was suspended in phosphate buffer saline, cleared the tissue debris and a clear solution was used for the assessment of average forward velocity of sperms. The assessment was made under light microscope, fitted with a movable mechanical stage and a calibrated ocular micrometer, at 400 X magnification. A drop of sperm suspension was transferred to a clean glass slide and the initial place and time of each sperm was recorded. The time taken for forward movement of sperm from the initial place within microscopic field was recorded using a stop watch. The procedure was repeated for 10 spermatozoa in each sample and the average forward velocity of sperm was calculated and expressed as m/sec. The relative proportion of abnormal sperms was analyzed according to the method of Bauer et al (22). Briefly, equal volume of cauda epididymal plasma and 5% NaHCO3 were taken in a centrifuge tube, mixed well and centrifuged for 5 minutes at 4000g. The supernatant was discarded and 5 ml of normal saline was added to the precipitate, mixed well and centrifuged again. The procedure was repeated 2 to 3 times and a clear precipitate was obtained. To the final precipitate few drops of normal saline were added, mixed thoroughly and a smear was prepared on a clean slide. The smear was dried at room temperature, fixed by heating it over the flame for two to three seconds. Then the smear was flushed with 95% alcohol, drained and dried. It was stained in Ziehl Neelson's Carbol Fuchsin diluted with equal volume of 95% alcohol for 3 minutes and counter stained with 1:3 (v/v) aqueous solution of Loeffer's methylene blue for 2 minutes. After staining, the smear was rinsed in water and dried in air. The abnormal sperms included categories like double tailed, detached head, detached tail, mid piece bending and irregular head. The relative proportion of the normal and abnormal sperms was from the smear and expressed in terms of percentage.
Preparation of spermatozoa for SEM study
Preparation of rat spermatozoa for SEM studies was performed as described elsewhere (23). Briefly, a drop of cauda epididymal plasma was fixed in 2% glutaraldehyde, centrifuged and washed with 0.1 M Sodium cacodylate buffer (pH=7.2), centrifuged again in distilled water till the buffer solution was washed out and a thin film was applied on a cover slip, dried, sputter coated with gold and finally observed under scanning electron microscope (Model. LEO 435 VP Detector SL.1. LEO Electron Microscopy ltd Cambridge, England).
Preparation of cauda epididymal spermatozoa for TEM study
Preparation of rat spermatozoa for TEM studies was performed as described elsewhere (3). Briefly, the epididymis was removed, rapidly fixed in 3% glutaraldehyde in phosphate buffer (pH=7.4; 0.1 M) for 4 hr at 4°C, washed in phosphate buffer and post-fixed in 1% osmium tetraoxide in phosphate buffer (pH=7.4; 0.1M) for 6 hr. The fixed epididymis was washed several times in distilled water, stained en bloc in 2% aqueous uranyl acetate for 6 hr, dehydrated in acetone series, infiltered in epon-araldite mixture for 10 hr and embedded in the same media in a beam capsule. The blocks were cut in LKB Bromma ultramicrotome. Semithin sections of 1 µm thickness were stained with toludine blue for identification of stages. Ultrathin sections were cut at 100-300 A, mounted on copper grids and stained with 1% aqueous uranyl acetate and lead citrate (24). The stained sections were scanned in Jeol-TEM 100 C X II electron microscope for ultrastructural observations.
Fertility test
To assess the fertility rate with reference to the number of implantations, the female rats of proven fertility exhibiting regular estrous or early proestrus stage were separately housed with the control and treated males overnight. The appearance of sperm in the vaginal smear next morning confirmed the mating and is considered as day1 of the pregnancy. After 8 days, the females were laparotomized and the numbers of implantations and pups were recorded.
Statistical analysis
The data were statistically analysed and expressed as Mean± Standard error (25). The comparison of data for statistically significant differences was done using student's t-test and a probability level of p≤0.001 was considered as significant.
Results
Sperm analysis
Analysis of sperm parameters, such as total sperm count, total number of motile sperm, forward velocity of the sperm and percentage of abnormal sperm of the cauda of epididymal plasma were carried out in the control and all the treated animals. The control rats showed 56.40×104 total numbers of sperm/ml epididymal fluid, 52.40×104 numbers of motile sperm/ml epididymal fluid with a speed of 127.63 m/sec and 11.40% of abnormal sperm were recorded. Whereas in the 250 mg/kg body weight of benzene extract treated animals showed a highly significant decrease (p≤0.001) in total sperm count (56%), total number of motile sperm (45%), forward velocity of sperm (49%) and a highly significant increase (p≤0.001) in the percentage of abnormal sperm (544%) when compared to control animals (Table I).
Table I.
Effect of O.sanctum leaves (benzene extract) on various sperm parameters of cauda epididymal plasma in albino rats (values are expressed as SEM of 5 animals).
| Group | Treatment |
Sperm count
(Total no. x 10 4 /ml) |
Motile sperm
(Total no. x 10 4 /ml) |
Forward velocity
(µm/sec) |
Abnormal sperms (%) |
|---|---|---|---|---|---|
| I | 1 ml propylene glycol | 56.40 ± 1.39 (100%) |
52.40 ± 2.15 (100%) |
127.63 ± 2.75 (100%) |
11.40 ± 0.26 (100%) |
| II | 250 mg/kg body weight of benzene extract O.sanctum leaves | 32.00 ±1.22*** (56%) |
24.00 ±1.70*** (45%) |
63.16 ±1.71*** (49%) |
62.03 ± 1.95*** (544%) |
p ≤ 0.001
Scanning electron microscopic (SEM) observations of rat sperms from cauda epididymal plasma
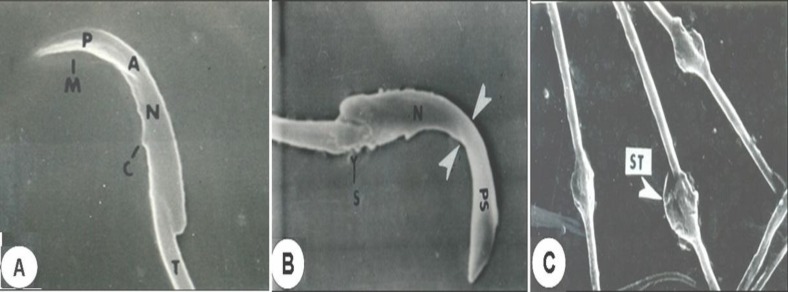
SEM observations of the cauda epididymal sperms of control rats showed normal parts (Figure 1A). Perforatorium and acrosome are covered with the plasma membrane. A distinguish acrosome is covered with acrosomal membrane. The whole spermatozoon is intact with all the membranes and organelles. However, in 250 mg/kg body weight of benzene extract, treated animals showed disturbance in the plasma membrane as well as in the acrosomal membrane in the most of sperm heads. It is rather difficult to differentiate the acrosomal membrane as well as the plasma membrane. Serrations in the head region of the spermatozoa are observed. The shape and size of the sperm head has also changed considerably.
Figure 1.
Scanning electron micrographs (SEM) of cauda epididymal spermatozoa of control (Fig.A) and treated with 250mg/kg body weight of benzene extract rat (Figs. B&C)
A. Spermatozoa of control rat exhibiting normal parts of acrosome (A); post nuclear cap (C); plasma membrane (M); nucleus (N) perforatium (P) and tail region (T). 4.56kx.
B. The perforatium (sub-acrosomal material), swells (PS) and the middle region of the sperm head is constricted dorsoventrally (arrows). There is serration (S) at the connective piece of the spermatozoa. A5.56kx.
C. There is increase of swelling at tail region in the mid portion of the tail region rat spermatozoa (arrows, ST). 5.93 kx.
There was acute dorsoventrally constriction in the mid-head region of most sperms. The perforatorium (Sub acrosomal material) is bulged/swelled (Figure 1B). Most of the spermatozoa showed a splitting of the tail and distinct visibility of balloon-like cytoplasmic droplets in the mid-region of the tail (Figure 1C).
Transmission electron microscopic (TEM) observations of Cauda epididymal spermatozoa
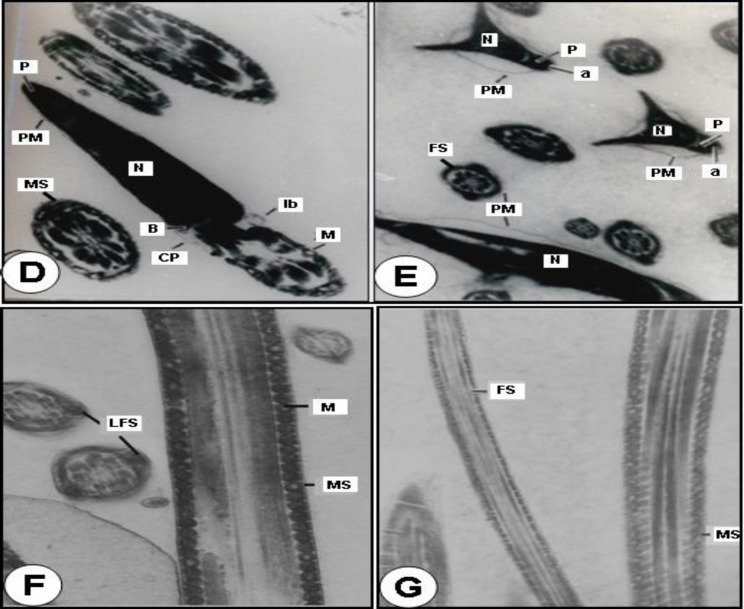
Observations with the TEM revealed that different parts of the cauda epididymal sperm of control rats exhibited normal features (Figure 2D-G).Where as in the animals treated with 250mg/kg body weight of benzene leaf extract, the sperm heads exhibited disrupted plasma membrane, acrosomal membrane and surface coating with fuzzy material. The tip of the sperm head showed disruption of the plasma membrane and acrosome.
Figure 2.
Transmission electron micrographs (TEM) of the control (D-G).
D: A median section through the base of the sperm head illustrating nucleus (N) covered with perforatorium (P), basal plate (B), plasma membrane (PM) and connecting piece of tail (CP). Caudal portion of the nucleus illustrating lamellar body (lb) and mitochondria (M) are seen, in oblique section, in contact with connecting piece. Cross sections(CS) of mid-piece of spermatozoa flagellum with spatial arrangement of the 9 outer dense fibres to one another, to the centrally located axoneme composed of the 9 plus 2 arrangements of microtubules and to the mitochondrial sheath (MS) X 29,000.
E. C.S. of anterior portion of sperm head illustrate normal features of nucleus (N); perforatorium (P); acrosome (a); plasma membrane (PM) and principal piece are with normal features of fibrous sheath (FS) and arrangement of outer fibres and axonemal component X 21,700.
F-G: Longitudinal section (LS) of mid piece and principal piece illustrating well-preserved mitochondrial sheath (MS) and fibrous sheath (FS) and are intact with plasma membrane. Mitochondria (M) appear normal. C.S. of principal piece is with normal features of longitudinal fibrous sheath (LFS) and arrangement of outer fibres and axonemal component X 15,000.
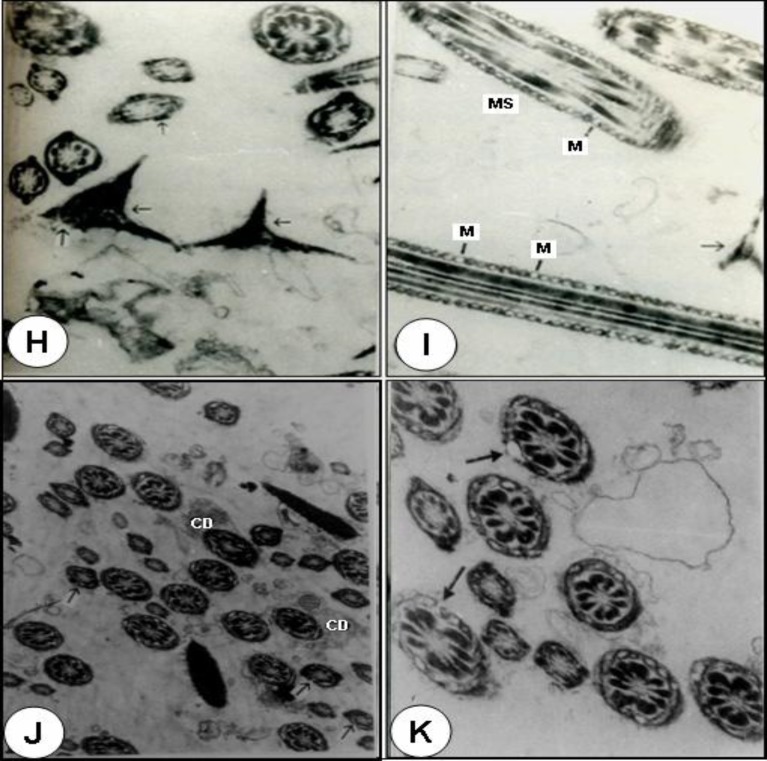
The perforatorium was condensed and most of its surface was covered by a thin portion of acrosomal sac. The anterior and caudal portion of the sperm heads revealed disruption or loss of plasma membrane, acrosome, perforatorium and small vesicles on the ventral surface of the perforatorium, which is probably part of the acrosomal system. Most of caudal portion of the sperm head revealed the disturbance of lamellar body and centrioles. The basal plate, posterior ring and post-nuclear cap appeared normal (Figure 3H and I).
Figure 3.
Treated with 250mg/kg body weight of benzene extract rat (Figs. H-K).
H: C.S. of the different parts of sperm head (←) showing disruption in normal features plasma membrane, acrosome and perforatorium. Fuzzy material is seen on their surface. X 15,000.
I: L.S and C.S. of the mid piece shows the loss of plasma membrane. Most of mitochondria (M) are hypertrophied or have started degeneration. The loss of plasma membrane is seen in the sperm head region (←) X 28,000.
J: C.S. of the mid piece showing abnormal pattern of mitochondrial sheath and loss of plasma membrane. Most of mid-piece show increased cytoplasmic droplets (CD) displaced on one side are clearly seen. Coating of fuzzy material is seen on the surface of head and tail sections X 10,300.
K: C.S of the mid piece shows abnormal pattern and degeneration of mitochondrial sheath (long arrows) along the length of the structure and displaced mitochondrial sheath on one side. The discontinuation of fibrous sheath is also noticed in principal piece X 17,500.
The middle part showed disruption and degeneration of the mitochondrial sheath and a loss of plasma membrane. The disorganization, or commencement of degeneration of the mitochondria, was observed in most of the abnormal mitochondrial sheaths. Some showed a displaced mitochondrial sheath on one or both sides and there was abnormal pattern of outer dense fibres. Different parts of the principle part of the tail exhibited loss and discontinuation of plasma membrane and fibrous sheath, respectively (Figure 3J-L). Most of the tail sections showed retention of cytoplasmic droplets around the middle part on one side (Figure 3K).
Fertility test
Results of fertility performance test showed that female rats mated with control male rats illustrated that the numbers of implantations were 10.20±1.07 on day 8 of pregnancy and number of pups obtained 9.60±1.08. However, no implantations were observed in the female rats mated with benzene extract O. sanctum leaves treated male rats.
Discussion
In the present study of benzene extract of O. sanctum leaves, treated animals exhibits significant increase in sperm anomalies with reducing sperm count, motility and sperm speed. The epididymis plays an active role in sperm development, and sperm maturation is dependent on the unique luminal environment of the epididymis, including specific proteins synthesized and secreted by the epididymal epithelium (26). Although several epididymis-specific secretory proteins have been identified, little is known about the sperm maturation events in the epididymis (27).
Various plants like methanol sub-fraction of the seeds of Carica papaya (4); methanol extract of Dendrophthoe falcate (5); ethanolic leaf extract of Aegle marmelos (6); hydroalcoholic extract from Lantana camara leaves (28); aqueous extracts of Ruta graveolens L. solution (29); aqueous extract of Peganum harmala (30); and marjoram volatile oil and grape seed extract of Vitis vinifera (31) have been reported to possess antifertility activity by reducing above said sperm parameters in mice and rats to support our results. It was suggested that the extract causes androgen depletion at the target level, particularly in the cauda epididymis thereby affecting physiological maturation of the sperm (32). Present observations of increased abnormal sperms, reduced sperm count, motility and sperm speed on treatment with benzene extract of O. sanctum leaves, it can be suggested that sperm anomalies in albino rats may have resulted from the alteration in the epididymal milieu due to androgen deficiency following antiandrogenic property of the leaf extract.
Ultra study of cauda epididymal spermatozoa (SEM and TEM)
Androgens are essential for survival and motility of spermatozoa in the rat epididymis. Sperm motility is an important attribute of sperm quality as there is a good correlation between sperm motility on one hand and plasma membrane integrity and conception rates on the other. There is an evidence of acrosomal loss or damage as well as other abnormalities observed in caudal region of rat sperm due to this treatment suggesting that these sperms were probably unable to fertilize the ovum (33). From the literature of medicinal plants, it has been shown that different parts of the plant source affecting aspects of male reproduction, brings about the effect through either of two mechanisms namely, estrogenic or antiandrogenic effect and extensively established that these plant sources cause impairing sperm parameters results exhibit androgen is essential for the maturation, motility and survival of sperms in the epididymis (1, 2, 32, 34).
The acrosome contains several enzymes which are secreted by the Golgi apparatus and endoplasmic reticulum. The production of enzymes destined for the acrosome is regulated to some degree by testosterone (35). From the histochemical evidence, the presence of carbohydrates or polysaccharides in the head of the spermatozoa, which are associated with various enzymatic activities, is indicated. Earlier studies have been reported that morphological changes in the head of spermatozoa in general and the acrosome in particular may have resulted from an alteration in the epididymal milieu of rats treated with crude leaf extract of Azadirachta indica (23) and alcohol seed extract of Momordica charantia (2) suggested that these changes are due to a general disturbance of carbohydrates or polysaccharides present in the acrosome of the sperm head (23). In the SEM, observation of present study revealed that most of the sperms shown deformity includes dorsoventrally constricted with the bulged sub acrosomal material in the mid region of sperm heads and disrupted plasma membrane and acrosomal membrane particularly at the anterior region on treatment with benzene extract of O. sanctum leaves are probably due to the general disturbance in the proteins.
Specialized structural features of the spermatozoa are a reflection of its unique functional activities. Various authors have suggested that in spite of morphological variations, the main structures present in the sperm head of mammals are the nucleus, the acrosome or acrosomal cap and the membranous envelops (36). The acrosome is unique organelle (37) that is required for fertilization in mammals. It has been predicted that the post-nuclear cap plays a protective role in maintaining the head shape and this structure is very resistant to any extractive agent. The mitochondrial sheath is believed to be the source of energy for sperm motility and outer dense fibres might be contractile because of their close association with the axoneme and play an active role in flagellar motion (37). These outer dense fibres provide added strength to protect sperm from damage by shear forces encountered during epididymal transit or ejaculation (38). Reports on different plants extract namely gossypol, Solanum xanthocarpum, Carica papaya cause the sperm abnormalities by exhibiting acrosomal damage and mid-piece anomalies which results in complete inhibition of fertility in rats and mice (32, 39). Crude extract of Echeveria gibbiflora on guinea pig sperm, results in the formation of a huge bubble by distension of the plasma membrane and dispersion of the acrosome content with disappearance of the external acrosomal membrane at the sperm head level (40). Triptolide isolated from Tripterygium wilfordii cause all cauda epididymal sperm to exhibit a complete absence of the plasma membrane over the entire middle and principal piece and prematured decondensation of the nuclei in rats (41). Similarly, crude extract of A.indica leaves (3); aqueous decoction of Chenopodium album seeds (7); chloroform extract of Carica papaya seeds (42); and a hydroalcoholic extract of Lantana camara leaves (28) were shown morphological abnormalities in the head of spermatozoa along with mid-piece anomalies in rats and rabbits.
The spermatozoa of the cauda epididymis in the present study of benzene extract of O. sanctum leaves treated animals revealed several abnormalities. Abnormal patterns of the outer dense fibres and components of axoneme are displaced on one side or both sides, complete absence of plasma membrane of the entire middle and principal pieces and disorganization of mitochondrial sheath in several spermatozoa of rats were observed. The missing segment of the mitochondrial sheath probably represents a weak point in the structural collar, which supports the axial fibre-bundle during the contractional wave, leading to splitting of the axial bundle and subsequent dislocation of its fibres (37, 38). Recent ultra study of aflatoxin B1, a food borne mycotoxin, has shown that the sections of the testis and cauda epididymidis revealed sperm flagella missing one or more outer dense fibres and the associated axonemal microtubule doublets. Severe mitochondrial pathologies in spermatozoa and elongated spermatids, suggesting a link between AFB1-induced sperm mitochondrial pathology due to possibility that AFB1 treatment would disrupt the cytoskeletal proteins of the flagellum in male albino rats (43). It has been suggested that the extract might cause an androgen deprived effect to target organs resulting in alterations in the internal milieu, especially of cauda epididymis (32).
From both SEM and TEM observations of treated animals showed that the tail portion has balloon like cytoplasmic droplets. Ejaculates containing a high proportion of spermatozoa with attached cytoplasmic droplet can be correlated with altered epididymal function and reduced fertility (44, 45). The presence of high proportion of spermatozoa with attached cytoplasmic droplets in benzene extract of O. sanctum leaves treated animals may be due to altered epididymal function. Similar observations were made in studies of combination of progestagen and androgen, Carica papaya, vincristine and aflatoxin B1 treated animals (1, 32, 33, 46). Agnes and Akbarsha (46) showed in their experimental study a higher percentage of cauda epididymal spermatozoa retained the cytoplasmic droplets in albino mouse. Cytoplasmic droplets contained electron-dense spherical inclusions, which were hypothesized as lipid inclusions produced from the lamellae through the spherical vesicles of the cytoplasmic droplets.
It is known that spermatozoa carrying cytoplasmic droplets would be inhibited in motility and may not fertilize the ova (47). Hence, in the present study, in the light of the pathological changes observed, it is suggested that benzene extract of O.sanctum leaves damages the spermatozoa in the epididymis, leading to reduced fertilizing ability of the sperm. The fertility studies reveal that the male rats treated with O.sanctum leaves are unable to fertilize the female rats probably because the male gametes are affected thereby, establishing the antifertility property of the plant studied.
Conclusion
It was demonstrated that the administration of benzene extract of O. sanctum leaves can induce the morphological changes in the head and tail region of albino rat sperms.This benzene extract of O. sanctum leaves was may be due to general disturbance of proteins and alteration in the epididymal milieu probably due to androgen deficiency consequent upon the antiandrogenic property of this plant. However, these conclusions are based on the preliminary study, where the rats are force fed with the benzene extract of O. sanctum leaves. More refined and sophisticated study is needed for identification of active principles and their effects on androgen dependent parts of the spermatozoa in albino rats which are under progress.
Acknowledgment
Authors are thankful to Department of Zoology, Karnatak University, India and Union Grant Commission under SAP and COSIST program, New Delhi, India for support to carry out this research and also grateful to the whole TEM unit, National Institute of Mental Health And Neurosciences (NIMHANS) Bangalore and SEM unit, Central Food Technological Research Institute (CFTRI), Mysore, respectively, for providing us the EM facility and their kind encouragement.
References
- 1.Akbarsha MA, Averal HI. Epididymis as a target organ for the toxic effect of vincristine: light microscopic changes in the epididymis epithelial cell types. Biomedical Letters. 1996;54:133–146. [Google Scholar]
- 2.Girini MM, Ahamed RN, Aladakatti RH. Effect of graded doses of Momordica charantia seed extract on rat sperm: scanning electron microscope study. J Basic Clin Physiol Pharmacol. 2005;16:53–66. doi: 10.1515/jbcpp.2005.16.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Aladakatti RH, Ahamed RN. Ultrastructural changes in Leydig cell and cauda epididymal spermatozoa induced by Azadirachta indica leaves in albino rats. Phytother Res. 2005;19:756–766. doi: 10.1002/ptr.1710. [DOI] [PubMed] [Google Scholar]
- 4.Lohiya NK, Manivannan B, Garg S. Toxicological investigations on the methanol sub-fraction of the seeds of Carica papaya as a male contraceptive in albino rats. Reprod Toxicol. 2006;22:461–468. doi: 10.1016/j.reprotox.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RS, Kachhawa JB. Evaluation of contraceptive activity of methanol extract of Dendrophthoe falcata stem in male albino rats. J Ethnopharmacol. 2007;112:215–218. doi: 10.1016/j.jep.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan A, Agarwal M. Reversible changes in the antifertility induced by Aegle marmelos in male albino rats. Syst Biol Reprod Med. 2008;54:240–246. doi: 10.1080/19396360802516856. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Biswas S, Mandal D, Roy HN, Chakraborty S, Kabir SN, et al. Chenopodium album seed extract: a potent sperm-immobilizing agent both in vitro and in vivo. Contraception. 2007;75:71–78. doi: 10.1016/j.contraception.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Dixit VP. Plant products/non-steroidal compounds affecting fertility in the Indian desert gerbil, Meriones hurrianae Jerdon. In: Prakash I, Ghosh PK, editors. Rodents in Indian Agriculture. 1st Ed. Jodhpur India: Scientific Publishers; 1992. pp. 595–604. [Google Scholar]
- 9.Chopra RN, Chopra IC, Handa KL, Kapoor LD. Indigenous drugs of India. Calcutta: UN Dhar, Pvt. Ltd; 1993. [Google Scholar]
- 10.Rajeshwari S, editor. Current Medical Scene. Bombay Central, Bombay: Cipla Ltd; March-April 1992. Ocimum sanctum. The Indian home remedy. [Google Scholar]
- 11.Gupta SK, Prakash J, Srivastava S. Validation of claim of Tulsi, Ocimum sanctum Linn as a medicinal plant. Indian J Exp Biol. 2002;40:765–773. [PubMed] [Google Scholar]
- 12.Ahmed M, Ahamed RN, Aladakatti RH, Ghosesawar MG. Reversible anti-fertility effect of benzene extract of Ocimum sanctum leaves on sperm parameters and fructose content in rats. J Basic Clin Physiol Pharmacol. 2002;13:51–59. doi: 10.1515/jbcpp.2002.13.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Khanna S, Gupta SR, Grover JK. Effect of long term feeding of Tulsi (Ocimum sanctum Linn) on reproductive performance of adult albino rats. Indian J Exp Biol. 1986;24:302–304. [PubMed] [Google Scholar]
- 14.Kantak NM, Gogate MG. Effect of short term administration of Tulsi (Ocimum sanctum Linn ) on reproductive behaviour of adult male rats. Indian J Physiol Pharmacol. 1992;36:109–111. [PubMed] [Google Scholar]
- 15.Sardessai SR, Borker AS, Abraham ME. Effects of short term administration of Tulsi leaves on sexual behaviour in female rats. Indian J Physiol Pharmacol. 1999;43:398–400. [PubMed] [Google Scholar]
- 16.Ahmed M, Aladakatti RH, Deepthi KR, Nazeer Ahamed R. Reversible histoarchitecture study of testis and cauda epididymis and changes in cauda epididymal epithelial cell types on treatment with benzene extract of Ocimum sanctum leaves in albino rats. Oriental Pharm Exp Med. 2008;8:111–124. [Google Scholar]
- 17.Ahmed M, Nazeer Ahamed R, Aladakatti RH. Effect of benzene extract of Ocimum sanctum leaves on cauda epididymis of albino rats. J Basic Clin Physiol Pharmacol. 2009;20:29–41. doi: 10.1515/jbcpp.2009.20.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Mukhtar Ahmed, Nazeer Ahamed R, Aladakatti RH Ghodesawar MG. Dose dependent effect of benzene extract of Ocimum sanctum leaves on cauda epididymal spermatozoa of albino rats. Oriental Pharm Exp Med. 2009;9:339–349. [Google Scholar]
- 19.WHO Protocol, LG-06 Extraction and fractionation for biological and phytochemical studies. A.P.J.F/I.P, A. 1983:1001–1083. [Google Scholar]
- 20.Besley MA, Eliarson R, Gallegos AJ, Moghissi KS, Paulsen CA, Prasad M RN. Laboratory manual for the examination of human semen and semen cervical mucus interaction. Singapore: WHO Press concern; 1980. [Google Scholar]
- 21.Ratnasooriya WD. Effect of Atropine on fertility of female rat and sperm motility. Indian J Exp Boil. 1984;22:463–466. [PubMed] [Google Scholar]
- 22.Bauer JD, Ackermen PG, Toro G. Clinical Laboratory Methods. St Louis: C.V. Mosty co.; 1974. [Google Scholar]
- 23.Aladakatti RH, Ahamed RN. Effect of Azadirachta indica leaves on rat spermatozoa. Indian J Exp Biol. 1999;37:1251–1254. [PubMed] [Google Scholar]
- 24.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostle B. Statistics in Research: Basic Concepts and Techniques for Research Workers. 4thEd. Ames: Iowa State University Press; 1988. p. 664. [Google Scholar]
- 26.Cooper TG. Epididymis. In: Neill JD, Knobil E, editors. Encyclopedia of reproduction . 2nd Ed. San Diego: Academic Press; 1998. pp. 1–17. [Google Scholar]
- 27.Kirchhoff C. Molecular aspects of epididymal function and sperm maturation. In: Waites GMH, Frick J, Baker GWH, editors. Current advances in andrology. Bologna Italy: Monduzzi; 1997. pp. 253–259. [Google Scholar]
- 28.de Mello FB, Jacobus D, de Carvalho KC, de Mello JR. Effects of Lantana camara (Verbenaceae) on rat fertility. Vet. Hum Toxicol. 2003;45:20–23. [PubMed] [Google Scholar]
- 29.Khouri NA, El-Akawi Z. Antiandrogenic activity of Ruta graveolens L in male Albino rats with emphasis on sexual and aggressive behavior. Neuro Endocrinol Lett. 2005;26:823–829. [PubMed] [Google Scholar]
- 30.El-Dwairi QA, Banihani SM. Histo-functional effects of Peganum harmala on male rat's spermatogenesis and fertility. Neuro Endocrinol Lett. 2007;28:305–310. [PubMed] [Google Scholar]
- 31.El-Ashmawy IM, Saleh A, Salama OM. Effects of marjoram volatile oil and grape seed extract on ethanol toxicity in male rats. Basic Clin Pharmacol Toxicol. 2007;101:320–327. doi: 10.1111/j.1742-7835.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 32.Chinoy NJ, D'Souza JM, Padman P. Contraceptive efficacy of Carica papya seed extract in male mice (Mus musculus) Phytothera Res. 1995;9:30–36. [Google Scholar]
- 33.Rao MV, Roy GK. Biochemical and morphological changes of spermatozoa in progestin and androgen injected rats. Indian J Exp Biol. 1993;31:12–15. [PubMed] [Google Scholar]
- 34.Aladakatti RH, Nazeer Ahamed R, Ahmed M, Ghodesawar MG. Sperm parameters changes induced by Azadirachta indica in albino rats. J Basic Clin Physiol Pharmacol. 2001;12:69–77. doi: 10.1515/jbcpp.2001.12.1.69. [DOI] [PubMed] [Google Scholar]
- 35.Morton DB. Acrosomal enzymes: Immunochemical localization of acrosin and hyaluronidase in ram spermatozoa. J Reprod Fertil. 1975;45:375–378. doi: 10.1530/jrf.0.0450375. [DOI] [PubMed] [Google Scholar]
- 36.Breed WG. Sperm head structure in the hydromyinae (Rodentia: Muridae): A further evolutionary development of the subacrosomal space in mammals. Gamete Res. 1984;10:31–34. [Google Scholar]
- 37.Fawcett DW. The mammalian spermatozoon. Dev Biol. 1975;44:394–436. doi: 10.1016/0012-1606(75)90411-x. [DOI] [PubMed] [Google Scholar]
- 38.Baltz JM, Williams PO, Cone RA. Dense fibres protect mammalian sperm against damage. Biol Reprod. 1990;43:485–491. doi: 10.1095/biolreprod43.3.485. [DOI] [PubMed] [Google Scholar]
- 39.Rao MV. Effect of alcoholic extract of Solanum xanthocarpum seeds in adult male rats. Indian J Exp Biol. 1988;26:95–98. [PubMed] [Google Scholar]
- 40.Delgado NM, Sánchez-Vázquez ML, Hernández O, Reyes R. Correlation between sperm membrane destabilization by heparin and aniline blue staining as membrane integrity index. Arch Androl. 1998;40:147–152. doi: 10.3109/01485019808987937. [DOI] [PubMed] [Google Scholar]
- 41.Hikim AP, Lue YH, Wang C, Reutrakul V, Sangsuwan R, Swerdloff RS. Post testicular antifertility action of triptolide in the male rat: evidence for severe impairment of cauda epididymal sperm ultrastructure. J Androl. 2000;21:431–437. [PubMed] [Google Scholar]
- 42.Lohiya NK, Manivannan B, Mishra PK, Pathak N, Sriram S, Bhande SS, et al. Chloroform extract of Carica papaya seeds induces long-term reversible azoospermia in langur monkey. Asian J Androl. 2002;4:17–26. [PubMed] [Google Scholar]
- 43.Faisal K, Periasamy VS, Sahabudeen S, Radha A, Anandhi R, Akbarsha MA. Spermatotoxic effect of aflatoxin B1 in rat: extrusion of outer dense fibres and associated axonemal microtubule doublets of sperm flagellum. Reproduction. 2008;135:303–310. doi: 10.1530/REP-07-0367. [DOI] [PubMed] [Google Scholar]
- 44.Cummins JM. The effect of artificial cryptorchidism in the rabbit on the transport and survival of spermatozoa in the female reproductive tract. J Reprod Fertil. 1973;33:469–479. doi: 10.1530/jrf.0.0330469. [DOI] [PubMed] [Google Scholar]
- 45.Bedford JM. Adaptations of male reproductive tract and the fate of spermatozoa following vasectomy in the rabbit, rhesus monkey, hamster and rat. Biol Reprod. 1976;14:118–142. doi: 10.1095/biolreprod14.2.118. [DOI] [PubMed] [Google Scholar]
- 46.Agnes VF, Akbarsha MA. Spermatotoxic effect of aflatoxin B (1) in the albino mouse. Food Chem Toxicol. 2003;41:119–130. doi: 10.1016/s0278-6915(02)00171-0. [DOI] [PubMed] [Google Scholar]
- 47.Hermo L, Dworkin J, Oko R. Role of epithelial clear cells of the rat epididymis in the disposal of the contents of cytoplasmic droplets detached from spermatozoa. Am J Anat. 1988;183:107–124. doi: 10.1002/aja.1001830202. [DOI] [PubMed] [Google Scholar]