Abstract
Background
The adenoma detection rate (ADR) is a validated and widely used measure of colonoscopy quality. There is uncertainty in the published literature on which colonoscopy examinations should be excluded when measuring a physician’s ADR.
Objective
To examine the impact of varying the colonoscopy exclusion criteria on physician ADR.
Design
We applied different exclusion criteria used in 30 prior studies to a dataset of endoscopy and pathology reports. Under each exclusion criterion, we calculated physician ADR.
Setting
A private practice colonoscopy center affiliated with the University of Illinois College of Medicine.
Patients
Data on 20,040 colonoscopy examinations and associated pathology notes performed by 11 gastroenterologists from July 2009 to May 2013.
Main Outcome Measurements
ADR across all colonoscopy exainations, each physician’s ADR, and ADR ranking.
Results
There were 28 different exclusion criteria used when measuring ADR. Each study used a different combination of these exclusion criteria. The fraction of all colonoscopy examinations in the dataset excluded under these combinations of exclusion criteria ranged from 0 to 93.1%. The mean ADR across all colonoscopy examination was 35.9%. The change in mean ADR after applying the 28 exclusion criteria ranged from −4.6 to +3.1 percentage points. However, the exclusion criteria impacted each physician’s ADR relatively equally, and therefore physicians’ rankings via ADR were stable.
Limitations
ADR assessment was limited to a single private endoscopy center.
Conclusions
There is wide variation in the exclusion criteria used when measuring ADR. Although these exclusion criteria can impact overall ADR, the relative rankings of physicians by ADR were stable. A consensus definition on which exclusion criteria are applied when measuring ADR is needed.
In 2014, almost 140,000 Americans will be diagnosed with colorectal cancer (CRC), the second-leading cause of cancer mortality in the United States.1 Effective screening can prevent a large fraction of CRC cases. Colonoscopy is the most widely used screening modality in the US,2 but the effectiveness of colonoscopy is limited by variation in physician quality. Prior research has observed a two- to three-fold discrepancy in the adenoma detection rate (ADR) across physicians and an inverse relationship between ADR and the incidence of subsequent, interval CRC.3,4
ADR has become the primary measure of colonoscopy quality. Clinical experts5 and the American Society of Gastrointestinal Endoscopy (ASGE)6 have recommended that physicians regularly measure ADR. The Centers for Medicare and Medicaid Services7 incorporated ADR, as measured by Gastrointestinal Quality Improvement Consortium (GIQuIC),8 as a quality measure for the 2014 Physician Quality Reporting System. Based on expert opinion, the ASGE/American College of Gastroenterology (ACG) Taskforce on Quality in Endoscopy proposed that the minimum acceptable ADR is ≥ 25% in men and 15% in women healthy asymptomatic patients undergoing screening colonoscopy examinations.6
When measuring a physician’s ADR, there is notable variation in which colonoscopy examinations are included and excluded. Because the proposed minimum standards for ADR focus on first-time screening ADR, some studies exclude non-screening colonoscopy examinations such as surveillance studies, diagnostic colonoscopy examinations (for example, those done for gastrointestinal bleeding), or colonoscopy examinations performed on patients whose age is outside the typical range for CRC screening.6,9,10 Other studies omit incomplete cases (for example, where the preparation is inadequate).10–12 Exclusion criteria have also been employed to address differences in patient populations across physicians. For example, some physicians specialize in care for inflammatory bowel disease and some studies exclude colonoscopy examinations of patients with inflammatory bowel disease to allow for a more homogenous comparison across physicians.13–15
The aim of this study is to explore the effect of various exclusion criteria on physician ADR. We first surveyed the literature to identify previously used exclusion criteria. We then applied each of these exclusion criteria to a dataset of approximately twenty thousand colonoscopy reports from 11 gastroenterologists in a private endoscopy center and assessed (1) what fraction of colonoscopy examinations were excluded, (2) the change in overall ADR across all physicians, and (3) the relative physician ranking by ADR.
METHODS
Identifying Different Definitions of Denominator for ADR
Prior work has used various combinations or sets of exclusion criteria to evaluate physician ADR. To identify commonly used exclusion criteria, we identified a convenience sample of prior studies; we did not feel it was critical to identify every study that uses ADR because our goal was to illustrate the impact of common exclusion criteria on ADR. Given their importance for quality measurement, we did include the ADR definitions used by GIQuIC16 and the American Gastroenterological Association.17 In total, we examined the exclusion criteria used in 30 prior studies.
We categorized the exclusion criteria by age, prior colonoscopy, incomplete colonoscopy, and indication. Indications were categorized into routine screening, high-risk screening (defined as family history, history of polyposis), surveillance procedures, and diagnostic procedures (defined as cases where patient had any symptoms reported, including cases where screening or surveillance was another indication).
Setting
We applied the various exclusion criteria employed in the literature to data from the Central Illinois Endoscopy Center (CIE). CIE is a private endoscopy center with 11 gastroenterologists in Peoria, Illinois and is affiliated with the University of Illinois College of Medicine at Peoria. All 11 gastroenterologists are generalists who do not subspecialize. We obtained all 20,040 colonoscopy and associated pathology reports from colonoscopy examinations performed between July 2009 and May 2013 at the endoscopy center. July 2009 was the earliest date available because this was when a new electronic health record was introduced. The reports were a combination of structured data and free text. Inpatient colonoscopy examinations were not included because they were not captured in the electronic health record.
Abstracting Relevant Information from Colonoscopy and Pathology Reports
Relevant data from the reports were abstracted using a previously developed Natural Language Processing (NLP) software application.18 Details of the development and testing of this tool are reported elsewhere.18,19 In brief, NLP is a field of computer science in which a computer “reads” unstructured text to extract relevant data. The accuracy of the NLP program was confirmed by comparing ADR in 453 colonoscopy and associated pathology reports which were analyzed both by the NLP program and manually abstracted by physicians.18
The NLP program extracted the following variables from each colonoscopy report: patient age, family history of colon cancer, documentation of whether the cecum was reached, documentation of whether there was a prior colonoscopy and the timing of any prior colonoscopy, and indication for procedure (up to three). From the pathology reports, the NLP program identified whether an adenoma was reported.
Analyses
For each exclusion criterion, we calculated the fraction of cases among the CIE reports that would be excluded and the average ADR among the excluded cases and the remaining cases. We assessed what fraction of colonoscopy examinations were excluded because it is important from a statistical perspective to have a sufficient quantity of colonoscopy examinations performed in order to accurately assess a physician’s ADR. We also calculated each physician’s ADR under each exclusion criterion. Finally, we ranked the 11 participating gastroenterologists according to their ADRs before and after applying each criterion.
RESULTS
Across the 30 studies, 28 different exclusion criteria were applied with some of the most common being surveillance procedures (63% of studies used this exclusion criteria), high-risk screening (57%), age less than 50 (50%), prior colonoscopy (47%), inflammatory bowel disease (40%), and incomplete colonoscopy (40%), (Table 1). The fraction of colonoscopy examinations excluded under each of the 28 criteria varied widely – surveillance (22% of colonoscopy examinations excluded), any prior colonoscopy (18%), colonoscopy examinations in patients younger than 50 years (13%), and colonoscopy exainations in patients older than 75 years (10%), (Table 2).
Table 1.
Exclusion criteria employed in Previous Studies
| Excluded subjects based on this criteria |
Number of studies that use this exclusion criteria n (%)” |
GIQuIC | AGA Registry | Raju | DenisA | Kahi (2013a) | Lee (2011) | SchoenfeldD | Boroff | Imperiale (2000) | Barclay | Jiang | Leffler | Gohel | Betes | Coe | Imperiale (2009) | Williams | Robertson | Lieberman | Nelson | Munson | Anderson | Chen | Sanchez | Millan | Kahi (2013b) | Corley | LeeE | Shaukat | KaminskiF | Hernandez |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 19 (63.33) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| <18H | 1 (3.33) | x | ||||||||||||||||||||||||||||||
| <40 | 3 (10.00) | x | x | x | ||||||||||||||||||||||||||||
| <50 | 15 (50.00) | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||
| >75B | 7 (23.33) | x | x | x | x | x | x | x | ||||||||||||||||||||||||
| Prior colonoscopy | 14 (46.67) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||
| Any prior colonoscopy | 9 (30.00) | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||
| If prior colonoscopyK | ||||||||||||||||||||||||||||||||
| <5yrs | 2 (6.67) | x | x | |||||||||||||||||||||||||||||
| <10yrsC | 4 (13.33) | x | x | x | x | |||||||||||||||||||||||||||
| Incomplete colonoscopy | 12 (40.00) | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Did not reach cecum | 10 (33.33) | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||
| Anatomy was not intactI | 1 (3.33) | x | ||||||||||||||||||||||||||||||
| Prep was inadequate | 7 (23.33) | x | x | x | x | x | x | x | ||||||||||||||||||||||||
| Indication | ||||||||||||||||||||||||||||||||
| High-risk screening | 17 (56.67) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Family hist of CRC/polyps | 5 (16.67) | x | x | x | x | x | ||||||||||||||||||||||||||
| Familial syndrome/ polyposisI | 10 (33.33) | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||
| Surveillance | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Personal historyJ | 19 (63.33) | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| Diagnostic | 18 (60.00) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Inflammatory bowel disease | 12 (40.00) | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||
| BleedingG | 10 (33.33) | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||
| Pain | 6 (20.00) | x | x | x | x | x | x | |||||||||||||||||||||||||
| Anemia | 4 (13.33) | x | x | x | x | |||||||||||||||||||||||||||
| Change in bowel habits | 5 (16.67) | x | x | x | x | x | ||||||||||||||||||||||||||
| Symptoms of colon disorders (ex. colitis) | 4 (13.33) | x | x | x | x | |||||||||||||||||||||||||||
| Weight loss | 3 (10.00) | x | x | x | ||||||||||||||||||||||||||||
| Abdominal radiationI | 1 (3.33) | x |
Denis et al did not include any exclusion criteria in this study as it was the goal of the study to identify better indicators for colonoscopy quality than their institution was currently using.
Schoenfeld et al established the upper age limit at 79 years
Schoenfeld et al excluded patients that had a positive colonoscopy study within the prior 10 from the starting point of their study
The prior colonoscopy exclusion criteria applies to screening colonoscopies, not to surveillance colonoscopies included in this study
The age limit in the study was between 60–74 years of age.
Upper limit for this study is 66 years of age
Exclusion criteria of bleeding for diagnostic colonoscopies includes rectal bleeding, hematochezia, and a positive FOBT
We did not use this criterion in our analyses because there was only one colonoscopy in our dataset performed on a patient <18
We could not identify and exclude these cases with sufficient accuracy using the data we abstract from each colonoscopy report. Therefore we did not include them in the rest of the analyses
Personal history of polyposis/polyps/prior polypectomy/previous colon resection/CRC
Some studies excluded patients with prior colonoscopy, but did not designate a cutoff on how long ago that colonoscopy was performed
Table 2.
Effect of applying exclusion criteria on the ADR in the Central Illinois Data set
| Excluded subjects based on this criteria | Colonoscopy examinations in dataset excluded N (%) | ADR among colonoscopy examinations excluded (95% confidence interval) | ADR among remaining colonoscopy exainations (difference in ADR compared to ADR when no exclusion criteria applied, 95% CI of the difference in ADR) |
|---|---|---|---|
| No Exclusion Criteria | 0 (0) | N/A | 35.9 (0, −0.9 to +0.9) |
| Age (excluded patients who were) | |||
| <40 | 1158 (5.7) | 10.9 (9.1–12.8) | 37.4 (+1.5, +0.5 to +2.5) |
| <50 | 2668 (13.3) | 15.4 (14.0–16.8) | 39.0 (+3.1, +2.1 to +4.1) |
| >75 | 2004 (10) | 70.8 (68.7–72.8) | 32.0 (−3.9, −3.1 to −5.0) |
| Prior colonoscopyA | |||
| Any prior colonoscopy | 3518 (17.6) | 50.7 (49.0–52.4) | 32.7 (−3.2, −2.2 to −4.2) |
| Incomplete colonoscopy | |||
| Did not reach cecum | 532 (2.7) | 21.2 (17.8–25.0) | 36.3 (+0.4, −0.6 to +1.4) |
| Prep was inadequate | 852 (4.3) | 28.2 (25.2–31.3) | 36.2 (+0.3, +0.2 to 2.2) |
| Indication | |||
| high-risk screening | |||
| family hist of CRC/polyps | 1725 (8.6) | 37.8 (35.5–40.1) | 35.7 (−0.2, −1.2 to +0.8) |
| surveillanceB | 4352 (21.7) | 52.6 (51.1–54.1) | 31.3 (−4.6, −3.6 to −5.6) |
| diagnostic | |||
| inflammatory bowel disease | 184 (0.9) | 13.0 (8.5–18.8) | 36.1 (+0.2, −0.7 to +1.1) |
| bleedingC | 1798 (9.0) | 27.0 (24.9–29.1) | 36.8 (+0.9, −0.6 to +1.9) |
| pain | 1026 (5.1) | 20.5 (18.3–23.1) | 36.7 (+0.8, −0.2 to +1.8) |
| anemia | 637 (3.2) | 30.9 (27.4–34.7) | 36.1 (+0.2, −0.8 to +1.2) |
| change in bowel habits | 735 (3.7) | 23.8 (20.8–27.1) | 36.4 (+0.5, −0.5 to 1.5) |
| weight loss | 176 (0.9) | 29.0 (22.4–37.3) | 36.0 (+0.1, −0.8 to +1.0) |
Prior colonoscopy includes any prior colonoscopy, colonoscopy greater than 5 years in past, colonoscopy greater than 10 years in past
Surveillance includes personal history of polyposis, prior polypectomy, previous colon resection
Exclusion criteria of bleeding includes rectal bleeding, hematochezia, and a positive FOBT
Across the 30 studies, there were 30 unique combinations of the 28 different exclusion criteria (Table 1). In one study, no exclusion criteria were applied.20 If we applied the most stringent combination of exclusion criteria used in a prior study, 93.1% (95% confidence interval (CI), 92.7%–93.4%) of colonoscopy examinations in our data were excluded.21 In this study colonoscopies were excluded for persons below 50 and above 75 years of age, any prior colonoscopy; incomplete colonoscopies (i.e., did not reach cecum or had inadequate preparation), any family history of CRC/polyps; familial syndrome/polyposis, patients with a personal history of polyps or CRC, or patients with symptoms (e.g., GI pain, abdominal bleeding) or inflammatory bowel disease.
The overall ADR when no exclusion criteria were applied was 35.9% (95% CI, 35.2%–36.7%), and the ADR among cases excluded under each criterion varied (Table 2). The ADR for the 1,158 colonoscopy examinations excluded due to an age <40 was 10.9% (95% CI, 9.1%–12.8%). The ADR for colonoscopy examinations excluded for an age <50 and age >75 was 15.4% (95% CI, 14.0%–16.8%), and 70.8% (95% CI, 68.7%–72.8%), respectively. The ADR among surveillance colonoscopy examinations was 52.6% (95% CI, 51.1%–54.1%).
We compared the overall ADR when there were no exclusion criteria vs. the ADR of the colonoscopy examinations remaining when we applied various individual exclusion criteria (Table 2). The change in ADR varied: if we excluded procedures in which the physician did not reach the cecum, the overall ADR was +0.4 percentage points (95% CI, −0.6 to +1.4 percentage points) higher among remaining colonoscopies than when no exclusion criteria were applied; age < 50 [+3.1 (+2.1 to +4.1)], > 75 [−3.9 (−3.1 to −5.0)], prior colonoscopy [−3.2 (−2.2 to −4.2)], family history [−0.2 (−1.2 to +0.8)], surveillance colonoscopy examinations [−4.6 (−3.6 to −5.6)] and inflammatory bowel disease [+0.2 (−0.7 to +1.1)]. Variation across providers, as measured by coefficient of variation, did not change appreciably across the different exclusion criteria.
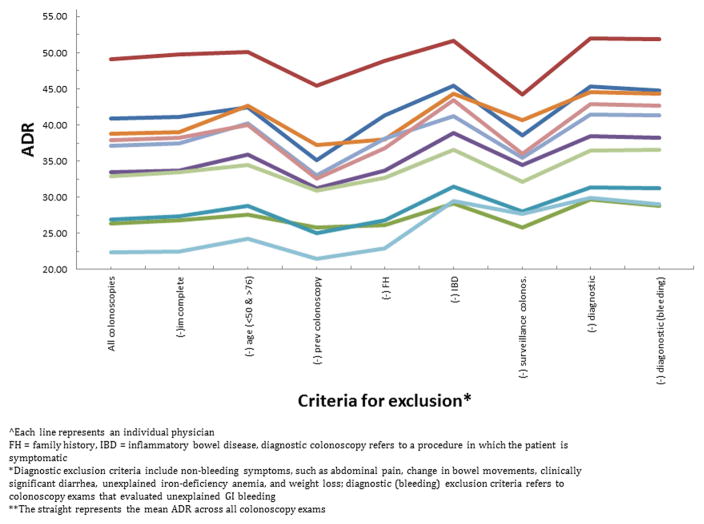
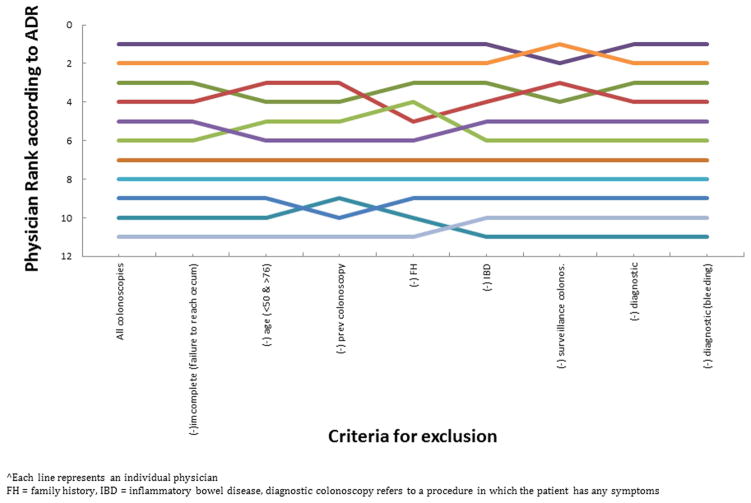
We calculated each physician’s ADR and ranking under each of the exclusion criteria. Although the exclusion criteria did impact a physician’s ADR, the physician ADRs moved in concert (Figure 1) and therefore the physician ranking by ADR was not substantially affected (Figure 2). The rankings across the 11 physicians were generally constant regardless of ADR denominator definition. Only two exclusion criteria, family history and inflammatory bowel disease, caused a single physician’s ADR ranking to change by more than one ranking, from fourth to sixth place, and from third to fifth place, respectively.
Figure 1.
Figure 2.
Discussion
Using data from over 20,000 colonoscopy examinations performed by 11 gastroenterologists, we evaluated the effect of applying various combinations of exclusion criteria on the overall ADR, the physician’s individual ADR, and a physician’s relative ADR. We found the individual exclusion criteria excluded up to approximately 20% of colonoscopy examinations. If we applied the combination of exclusion criteria used in the most stringent study, more than 90% of colonoscopy examinations in our data were excluded. Applying exclusion criteria did result in notable shifts in mean ADR. However, our analysis shows the exclusion criteria affected physicians relatively equally, and thus, the relative ranking in ADR was largely unchanged.
Given the growing use of ADR in the gastroenterology community for quality monitoring, credentialing, and reimbursement, and the need for generalizability across research studies, our results highlight the necessity of a consensus definition of ADR with a consistent set of exclusion criteria. This would facilitate comparison of the performance of gastroenterologists to others. To increase acceptance in the gastroenterology community, this consensus ADR definition should be developed with endorsement from specialty organizations and quality measurement organizations such as the National Quality Forum. Our results can help inform what combination of exclusion criteria might be used in this consensus standard.
Because physicians care for different patient populations, an ADR definition should attempt to address these differences to ensure an “apples to apples” comparison. Exclusion criteria have been used to create a more homogenous patient population to facilitate comparison. Although this goal is important, we recommend using relatively few exclusion criteria and instead using other methods to address differences in patient population. First, our data suggest that varying the exclusion criteria does not appreciably change physician ADR ranking. Second, any differences in patient population can be addressed via statistical risk adjustment instead of exclusion criteria. Risk adjustment is the norm in quality measurement in other areas of medicine.22–27 Third, using extensive exclusion criteria substantially reduces the number of colonoscopy reports used to generate a physician’s ADR. This results in wider confidence intervals around each physician’s ADR estimate, thus sacrificing precision. Fourth, limiting exclusion criteria reduces the potential for gaming, a problem that has existed in prior physician quality efforts.28 For example, when the New York State Coronary Artery Bypass Graft Surgery mortality public reporting initiative was implemented, physicians changed their coding of comorbidities.29,30 Regarding colonoscopy, a number of assessment criteria are subjective, such as the assessment of preparation quality28 and even procedure indication, where symptoms can be emphasized as a reason for the procedure or not. The use of fewer exclusion criteria reduces the chance for subjective manipulation of which colonoscopy examinations are included or excluded.
Although we advocate for relatively few exclusion criteria, there may be a role for a small number of exclusion criteria. The goal of the ADR is to capture the effectiveness of physicians in identifying and removing cancer precursors. Thus, on theoretical grounds, it may be appropriate to exclude colonoscopy examinations that are clearly not related to cancer prevention or are conducted for distinctly different reasons, such those performed in young patients who are being evaluated for evidence of inflammatory bowel disease.
There are several limitations of this study. Our analysis is limited to 11 general physicians from a private endoscopy group. At an academic medical center with gastroenterologists who subspecialize (for example, focus on inflammatory bowel disease) the relative ranking of physicians by ADR could change more under some exclusion criteria. Additional exclusion criteria which we did not identify may need to be evaluated. Despite prior research validating its performance, the use of NLP software to identify the characteristics of a colonoscopy may have created some bias in our findings, though it is reassuring that other groups have developed and tested NLP programs for the purposes of colonoscopy quality.31,32 We did not use a standardized reporting form for the colonoscopies and instead depended on the data provided in the colonoscopy report. Therefore key elements (e.g., adequacy of colon preparation) may be missing from some reports.
In summary, our data show that in a general GI practice, exclusion criteria can substantially reduce the number of colonoscopy reports included in the analysis of ADR. However, applying these criteria does not impact relative physician ranking. As ADR becomes a commonly used quality metric, our findings emphasize the need for a common set of exclusion criteria.
Acronyms
- ADR
Adenoma Detection Rate
- CRC
Colorectal Cancer
- GIQuIC
Gastrointestinal Quality Improvement Consortium
- CIE
Central Illinois Endoscopy Center
- NLP
Natural Language Processing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colorectal Cancer Prevention. [Accessed Jun 9 Mon, 2014]; at http://www.cancer.gov/cancertopics/pdq/prevention/colorectal/HealthProfessional.
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski MF, JREK Quality indicators for colonoscopy and the risk of interval cancer. New England Journal of Medicine. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. New England Journal of Medicine. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman DA. A call to action – measuring the quality of colonoscopy. The New England Jornal of Medicine. 2003;355:2588–9. doi: 10.1056/NEJMe068254. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 7.Medicaid CfM. 2014 Physician Quality Reporting System Qualified Clinical Data Registries. 2014 www.cms.gov.
- 8. [Accessed 2014 May 2014];GIQuIC Qualified Clinical Data Registry Measures. 2014 at http://giquic.gi.org/docs/GIQuIC_Measure_Submission.pdf.
- 9.Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointestinal Endoscopy. 2002;55:307–14. doi: 10.1067/mge.2002.121883. [DOI] [PubMed] [Google Scholar]
- 10.Imperiale TF, Wagner D, Lin CY, Larkin GN, Rogge JD, Ransohoff DE. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. New England Journal of Medicine. 2000;343:169–74. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 11.Raju GS, Vadyala V, Slack R, et al. Adenoma detection in patients undergoing a comprehensive colonoscopy screening. Cancer medicine. 2013;2:391–402. doi: 10.1002/cam4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 13.Raju GS, Vadyala V, Slack R, et al. Adenoma detection in patients undergoing a comprehensive colonoscopy screening. Cancer Med. 2013;2:391–402. doi: 10.1002/cam4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahi CJ, Ballard D, Shah AS, Mears R, Johnson CS. Impact of a quarterly report card on colonoscopy quality measures. Gastrointest Endosc. 2013;77:925–31. doi: 10.1016/j.gie.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–7. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 16.GIQuIC Qualified Clinical Data Measures. (Accessed at http://giquic.gi.org/docs/GIQuIC_Measure_Submission.pdf.)
- 17.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Harkema H, Chapman WW, Saul M, Dellon ES, Schoen RE, Mehrotra A. Developing a natural language processing application for measuring the quality of colonoscopy procedures. Journal of American Medical Informatics Association. 2011;18:i150–6. doi: 10.1136/amiajnl-2011-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrotra A, Dellon ES, Schoen RE, et al. Applying a natural language processing tool to electronic health records to assess performance on colonoscopy quality measures. Gastrointestinal Endoscopy. 2012;75:1233–9. doi: 10.1016/j.gie.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denis B, Sauleau EA, Gendre I, Piette C, Bretagne JF, Perrin P. Measurement of adenoma detection and discrimination during colonoscopy in routine practice: an exploratory study. Gastrointest Endosc. 2011;74:1325–36. doi: 10.1016/j.gie.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Gohel TD, Burke CA, Lankaala P, et al. Polypectomy rate: a surrogate for adenoma detection rate varies by colon segment, gender, and endoscopist. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1137–42.1. doi: 10.1016/j.cgh.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Woodhead C, Ashworth M, Schofield P, Henderson M. Patterns of physical co- /multi-morbidity among patients with serious mental illness: a London borough-based cross-sectional study. BMC family practice. 2014;15:117. doi: 10.1186/1471-2296-15-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health care financing review. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis RP, Pope GC, Iezzoni L, et al. Diagnosis-based risk adjustment for Medicare capitation payments. Health care financing review. 1996;17:101–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Goldfield N, Averill R, Eisenhandler J. Payment and provider profiling of episodes of illness of clinical illnesses involving rehabilitation. Journal of outcome measurement. 2000;4:706–20. [PubMed] [Google Scholar]
- 26.Kronick R, Gilmer T, Dreyfus T, Lee L. Improving health-based payment for Medicaid beneficiaries: CDPS. Health care financing review. 2000;21:29–64. [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen AK, Reid R, Broemeling AM, Rakovski CC. Applying a risk-adjustment framework to primary care: can we improve on existing measures? Annals of family medicine. 2003;1:44–51. doi: 10.1370/afm.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerard DP, Foster DB, Raiser MW, Holden JL, Karrison TG. Validation of a new bowel preparation scale for measuring colon cleansing for colonoscopy: the chicago bowel preparation scale. Clinical and translational gastroenterology. 2013;4:e43. doi: 10.1038/ctg.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahian DM, Edwards FH, Jacobs JP, et al. Public reporting of cardiac surgery performance: Part 1--history, rationale, consequences. The Annals of thoracic surgery. 2011;92:S2–11. doi: 10.1016/j.athoracsur.2011.06.100. [DOI] [PubMed] [Google Scholar]
- 30.Chassin MR, Hannan EL, DeBuono BA. Benefits and hazards of reporting medical outcomes publicly. The New England journal of medicine. 1996;334:394–8. doi: 10.1056/NEJM199602083340611. [DOI] [PubMed] [Google Scholar]
- 31.Imler TD, Morea J, Kahi C, Imperiale TF. Natural Language Processing Accurately Categorizes Findings From Colonoscopy and Pathology Reports. Clinical Gastroenterology and Hepatology. 2013;11:689–94. doi: 10.1016/j.cgh.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denny JC, Choma NN, Peterson JF, et al. Natural language processing improves identification of colorectal cancer testing in the electronic medical record. Medical decision making : an international journal of the Society for Medical Decision Making. 2012;32:188–97. doi: 10.1177/0272989X11400418. [DOI] [PMC free article] [PubMed] [Google Scholar]