Abstract
Introduction
Obesity is a well-recognized risk factor for atrial fibrillation (AF) yet adiposity measures other than body mass index (BMI) have had limited assessment in relation to AF risk. We examined the associations of adiposity measures with AF in a biracial cohort of older adults. Given established racial differences in obesity and AF, we assessed for differences by black and white race in relating adiposity and AF.
Methods
We analyzed data from 2,717 participants of the Health, Aging, and Body Composition Study. Adiposity measures were BMI, abdominal circumference, subcutaneous and visceral fat area, and total and percent fat mass. We determined the associations between the adiposity measures and 10-year incidence of AF using Cox proportional-hazards models, and assessed for their racial differences in these estimates.
Results
In multivariable-adjusted models, 1-standard deviation increases in BMI, abdominal circumference and total fat mass were associated with a 13–16% increased AF risk (hazard ratio [HR]: 1.14, 95% confidence interval [CI]: 1.02–1.28, HR: 1.16, 95%CI: 1.04–1.28, and HR: 1.13, 95% CI: 1.002–1.27). Subcutaneous and visceral fat areas were not significantly associated with incident AF. We did not identify racial differences in the associations between the adiposity measures and AF.
Conclusion
BMI, abdominal circumference, and total fat mass are associated with risk of AF over 10 years among white and black older adults. Obesity is one of a limited number of modifiable risk factors for AF; future studies are essential to evaluate how obesity reduction can modify the incidence of AF.
Keywords: Atrial fibrillation, Obesity, Body Fat Distribution, Anthropometrics, Black, Older Adults
Introduction
Atrial fibrillation (AF) has increasing incidence in older adults.1 AF risk doubles with each progressive decade of age, reaching 11–18% in those age ≥85 years.2, 3 With improved survival and the overall aging of the population, it is estimated that the incidence of AF will increase from 1.2 million cases in 2010 to 2.6 million cases in 2030, with a corresponding rise in AF prevalence from 5.2 million in 2010 to 12.1 million in 2030.4 AF is associated with increased morbidity, mortality, and healthcare-associated expenses.5–7 Identifying and studying modifiable risk factors is a critical step for primary and secondary prevention of AF.8
Obesity is a well-established risk factor for AF.9 Body mass index (BMI - or its components) is part of two prominent prediction models for new-onset AF, and is one of a limited number of modifiable risk factors for AF.10–13 In a meta-analysis of 16 studies, obesity was associated with a 49% increased risk of AF (mean age of participants 50–68 years); the elevated risk was proportionate to BMI.14 Waist circumference and waist-to-hip circumference ratio also have been associated with higher risk of AF.15–18 Higher body fat mass has been associated with a 29–40% increase in AF risk over 13.5 years.15 Visceral adiposity is associated with incident cardiovascular disease independently of generalized adiposity.19 Yet, there are limited data assessing whether risk of AF is related to body fat distribution (subcutaneous versus visceral adiposity) or total fat mass. Body fat distribution and total fat mass are not fully captured by BMI or waist and hip circumference, and may have additional associations with prospective risk of AF.
The associations between obesity and AF risk have been studied predominantly in middle-aged cohorts that are primarily of European ancestry, with limited data existing in older adults and non-whites. Although the prevalence of obesity in blacks is higher compared to whites,20 the incidence of AF in blacks is lower.21, 22 Our study had 2 objectives: first, we sought to determine the associations between adiposity measures and 10-year risk of incident AF, and second, to examine if the associations between adiposity measures and AF varied by race. To pursue these objectives, we examined BMI, abdominal circumference, abdominal subcutaneous and visceral fat area, total fat mass and total percent fat in the Health, Aging, and Body Composition Study (Health ABC), a prospective biracial cohort of community-dwelling older adults. We hypothesized that adiposity measures other than BMI are associated with an increased risk of AF. Second, given the decreased incidence of AF in blacks despite the higher prevalence of obesity,20–22 we hypothesized that the associations between adiposity measures, and incident AF would be attenuated in Health ABC participants of black race.
Material and Methods
The Health Aging and Body Composition Study cohort
Health ABC is a longitudinal, community-based, biracial cohort study of 3,075 older adults, designed to examine the association of body composition with health outcomes. The details of the study have been reported previously.23, 24 Participants were recruited from a random sample of white Medicare beneficiaries residing in the Pittsburgh, Pennsylvania, and Memphis, Tennessee metropolitan areas and all age-eligible black residents in the same zip codes. Enrollment criteria were: age 70–79 years, white or black race, ability to perform mobility-related activities of daily living, absence of functional disability or life-threatening illness, and planning to remain in the geographic area for at least 3 years.25
Participants were evaluated during a baseline clinic visit and then prospectively with annual clinic visits and semi-annual telephone interviews. As part of the baseline clinic visit (1997–98), cohort participants had a set of standardized measures, including anthropometric measurements, electrocardiogram, imaging including computed tomography, and dual X-ray absorptiometry, as well as laboratory, neurocognitive, and mobility assessments. Blood samples were obtained after an 8-hour fast. Medications taken within the two weeks preceding baseline and follow-up clinical visits were brought to the visits and classified according to the Iowa Drug Information System.26
In the present analysis, participants with prevalent AF (n=134), BMI < 18.5 kg/m2 (n=37), missing covariates of interest (n=151), or lacking Center for Medicare and Medicaid data (n=36) were excluded from analysis. Health ABC’s study protocols were approved by the Institutional Review Boards at the University of Pittsburgh, University of Tennessee; the current study was approved by Boston University Medical Center. All participants provided written informed consent.
Measures of adiposity
In the present analysis measures of adiposity were collected during the baseline clinical visit. Health ABC adiposity measures are described in detail elsewhere.27, 28 In brief, body weight and height were measured without shoes in a hospital gown on a calibrated balance-beam scale and stadiometer. BMI was calculated by dividing weight by height squared. Abdominal circumference was measured between the lower margin of the last palpable rib and the top of the iliac crest, at the level of maximum circumference using a flexible inelastic fiberglass tape, with the tape parallel to the floor. Subcutaneous and visceral abdominal fat areas were quantified by computed tomography (University of Tennessee Health Science Center: Somatom Plus 4, Siemens, Erlangen, Germany, and PQ 2000S, Marconi Medical Systems, Cleveland, OH, USA; University of Pittsburgh: 9800 Advantage, General Electric, Milwaukee, WI, US). Total fat mass was measured using whole-body dual X-ray absorptiometry (Hologic QDR 4500 software, versions 8.2–12.5 Bedford, MA, USA) and percent fat mass was calculated by dividing total fat mass by total body mass.
Incident AF in Health ABC
Incident AF was determined by analysis of records linked to Health ABC through the Center for Medicare and Medicaid Services. Health ABC participants with International Classification of Diseases, Ninth Revision (ICD-9) principal or concomitant diagnosis codes of 427.31 (AF) or 427.32 (atrial flutter) acquired from the outpatient or inpatient setting from study baseline through 2008 were identified. Incident AF was identified when the ICD-9 codes 427.31 or 427.32 were identified during a hospitalization or twice during ambulatory visits within 12 months. This approach has been reliably used to identify AF by CMS coding.22, 29 The earliest date of onset was used as the date of incident AF. Health ABC study investigators did not have routine access to participants’ electrocardiograms or other rhythm assessments outside of the study and AF was not an adjudicated outcome in the study.
Covariates
Age, sex, race, and smoking status (current/former vs. non-smoker) were determined by self-report. Blood pressure was the average of two consecutive measurements in the seated position. Use of anti-hypertensive medications was determined by medication review. Total and high density lipoprotein cholesterol levels were measured in EDTA treated plasma using standardized assays (Ortho-Clinical Diagnostics, Rochester, NY). Prevalent congestive heart failure at the baseline exam was determined by self-reported history or use of diuretics, vasodilators, or digitalis. Coronary heart disease was determined by self-reported history of angina, myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, or presence of diagnostic Q-waves on electrocardiogram. Diabetes status was determined by self-reported history, use of medications to treat diabetes (including insulin), or fasting glucose ≥126 mg/dL. A baseline 12-lead electrocardiogram was performed to assess for heart rate, left ventricular hypertrophy, and PR interval using a standard approach (Marquette Electronics MAC PC, GE Healthcare, Wauwatosa, WI). Electrocardiogram tracings were analyzed by the St. Louis University Core Electrocardiography Laboratory (St. Louis, Missouri) as previously described.30 Electrocardiographic left ventricular hypertrophy was defined on the basis of R wave amplitude >2.6 mV in leads V5 or V6, >2.0 mV in leads I, II, III or aVF, or >1.2 mV in lead aVL. The PR interval was measured in lead II using digital magnification and was averaged between 2–3 consecutive beats.31
Statistical analysis
We determined descriptive statistics for baseline continuous variables as means ± standard deviation (SD) and categorical variables as absolute number (percentage). We calculated the10-year AF incidence rate and its 95% confidence intervals (CI). We stratified AF incidence using standard World Health Organization BMI categories (18.5–25, 25.1–30, 30.1–40 and >40 kg/m2) and quartiles of abdominal circumference, abdominal subcutaneous and visceral fat, and total and percent fat mass. We calculated the correlations between the varying adiposity measures using Spearman’s rho.
We utilized 3 pre-specified, multivariable-adjusted Cox proportional hazards models to relate the adiposity measures to the 10-year risk of incident AF. Model 1 included age, sex, race, and site. Model 2 included model 1 variables plus smoking, systolic and diastolic blood pressure, antihypertensive medication use, total to high density lipoprotein cholesterol ratio, heart rate, electrocardiographic left ventricular hypertrophy, PR interval, prevalent congestive heart failure, coronary heart disease and diabetes. Model 3 included model 2 variables plus BMI. Given the strong association of BMI with AF, we utilized model 3 to determine whether the distinct associations of adiposity measures with incident AF would persist after adjusting for BMI. Adiposity measures that were strongly correlated with BMI (rho>0.5) were excluded from model 3 to avoid multicollinearity. The proportionality of hazards assumption was verified.
To determine whether associations between adiposity measures and AF risk differ by race, we introduced two-way interaction terms for race with each adiposity measure in our Cox models. We also examined for interactions between sex and adiposity measures in regards to AF risk. Last, we examined for interactions between race and adiposity measures in sex-stratified models.
We examined the relations between adiposity measures and AF with restricted cubic splines using the cohort median of each adiposity measure as reference and incorporating knots at 25th, 50th, and 75th percentiles. The splines were adjusted for the same covariates included in model 2. A 2-sided P<0.05 was considered statistically significant. All statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
Research support
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103 and N01-AG-6-2106 and the Intramural Research Program of the NIH/NIA; NIH grants R01-AG028050, 5R03-AG045075, R01-NR012459, 2R01HL092577 and NINR grant R01-NR012459. Dr. Magnani is further supported by a Boston University School of Medicine Department of Medicine Career Investment Award. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. The content does not necessarily represent the official views of the National Institutes of Health or National Institutes of Ageing.
Results
Following exclusions, the analysis included 2,717 Health ABC participants (41.4% blacks, 51.7% women). The cohort’s mean age at baseline was 74±3 years. Mean BMI was 27.6±4.7 kg/m2 and mean abdominal circumference was 100.1±12.9 cm. Baseline characteristics of the cohort are presented in Table 1. Over 10-year follow-up, 371 participants developed AF (158 women, 111 blacks). The overall 10-year AF incidence was 16.6 (95% CI: 14.9–18.3) per 1000 person-years. The 10-year AF incidence was lower in blacks (12.1 per 1000 person-years 95% CI: 9.9–14.4), compared to whites (19.7 per 1000 person-years, 95% CI: 17.3–22.1). Table 2 presents the 10-year AF incidence rate per 1000 person-years across different BMI categories and quartiles of other adiposity measures. AF incidence was lower in blacks compared to whites across all BMI categories and other adiposity measures quartiles (Supplemental table 1).
Table 1.
Health ABC, baseline participant characteristics, overall and by race.
| Overall (N = 2717) | Whites (N = 1591) | Blacks (N = 1126) | |
|---|---|---|---|
|
|
|||
| Demographic Characteristics | |||
| Age, years | 74±3 | 74±3 | 73±3 |
| Men, N (%) | 1406 (51.7%) | 762 (47.9%) | 644 (57.2%) |
| Site: Memphis N (%) | 1385 (51%) | 837 (52.6%) | 548 (48.7%) |
| Smoking (current or former), N (%) | 1519 (55.9%) | 899 (56.5%) | 620 (55.1%) |
| Systolic blood pressure, mmHg | 136±21 | 134±20 | 139±22 |
| Diastolic blood pressure, mmHg | 71±12 | 70±11 | 74±12 |
| Antihypertensive medication, N (%) | 1444 (53.1%) | 749 (47.1%) | 695 (61.7%) |
| Total/HDL cholesterol Ratio | 4.1±1.3 | 4.2±1.3 | 3.9±1.3 |
| Heart failure, N (%) | 59 (2.2%) | 25 (1.6%) | 34 (3%) |
| Coronary heart disease, N (%) | 540 (19.9%) | 311 (19.5%) | 229 (20.3%) |
| Stroke, N (%) | 208 (7.7%) | 110 (6.9%) | 98 (8.7%) |
| Diabetes, N (%) | 396 (14.6%) | 161 (10.1%) | 235 (20.9%) |
| Heart rate, beats per minute | 65±11 | 64±10 | 66±11 |
| ECG LVH, N (%) | 230 (8.5%) | 74 (4.7%) | 156 (13.9%) |
| PR interval, ms | 172±29 | 171±30 | 172±29 |
| Adiposity Measures | |||
| Body mass index, Kg/m2 | 27.6±4.7 | 26.7±4.1 | 28.9±5.3 |
| Abdominal circumference (cm) | 100±13 | 99±12 | 101±13 |
| Abdomen subcutaneous fat area (cm2) | 290±120 | 269±102 | 319±137 |
| Abdomen visceral fat area (cm2) | 145±67 | 154±69 | 132±61 |
| Total fat (kg) | 27±9 | 26±7 | 29±10 |
| Total percent fat (%) | 35±8 | 35±7 | 36±9 |
Values are means ± SD for continuous variables and absolute number (percentage) for categorical variables. HDL indicates high density lipoprotein; LVH, left ventricular hypertrophy.
Table 2.
Ten-year atrial fibrillation incidence rate in Health ABC participants included in the analysis, per 1000 person-years follow-up (95% CI), across BMI categories and other adiposity measures quartiles.
| BMI Category | 18.5 to 25 kg/m2 | 25.1 to 30 kg/m2 | 30.1 to 40 kg/m2 | >40.1 kg/m2 |
|
|
||||
| 16.6 (13.6, 19.7) | 15.7 (13.2, 18.2) | 18.3 (14.2, 22.3) | 17.3 (11.0, 23.6) | |
| Adiposity Measure | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile |
|
|
||||
| Abdominal circumference | 14.0 (10.8, 17.1) | 17.8 (14.3, 21.3) | 14.8 (11.6, 18.0) | 19.9 (16.1, 23.6) |
| Subcutaneous abdominal fat | 19.7 (15.8, 23.6) | 14.8 (11.6, 18.1) | 15.7 (12.3, 19.0) | 14.6 (11.3, 17.8) |
| Visceral abdominal fat | 12.0 (9.1, 14.9) | 17.2 (13.8, 20.7) | 16.3 (12.9, 19.7) | 18.7 (15.0, 22.3) |
| Total Fat mass | 17.9 (14.3, 21.6) | 15.9 (12.6, 19.2) | 15.0 (11.8, 18.2) | 17.2 (13.8, 20.6) |
| Total percent fat | 18.5 (14.8, 22.2) | 20.1 (16.3, 23.9) | 14.0 (10.9, 17.1) | 13.2 (10.2, 16.2) |
Health ABC indicates Health, Aging and Body Composition Study; BMI, body mass index; 95% CI, 95% confidence interval.
In multivariable-adjusted Cox proportional hazards models, a 1-SD (4.7 kg/m2) increase in BMI was associated with a 14% increase in 10-year AF risk (HR: 1.14, 95% CI: 1.02 to 1.28). A 1-SD (12.9 cm) increase in abdominal circumference was associated with a 16% increase in 10-year AF risk (HR: 1.16, 95% CI: 1.04 to 1.28). Figure 1 presents restricted cubic splines of multivariable-adjusted AF hazard ratios across BMI and abdominal circumference percentiles. AF risk increases linearly as BMI and abdominal circumference increase without a significant interaction by race (Figure 1).
Figure 1.
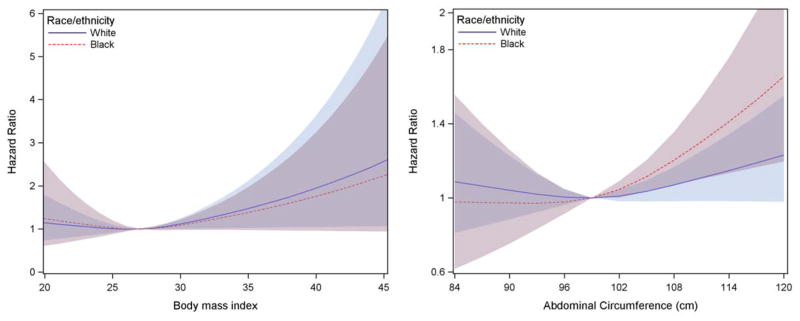
Multivariable-adjusted restricted cubic splines relating (a) BMI (b) abdominal circumference and risk of AF in whites and blacks participants of the Health ABC cohort. A BMI of 27 mg/kg2 and an abdominal circumference of 99 cm were used as a reference since they represent the median of each variable’s distribution. The solid line demonstrates a progressively increased AF risk in whites and the dashed line demonstrates a progressively increased AF risk in blacks. AF risk increases linearly as BMI and abdominal circumference increase, while there is no significant interaction by race. The shaded areas indicate the 95% confidence intervals.
A 1-SD (9 kg) increase in total fat mass was associated with a 13% increase in 10-year AF risk (HR: 1.13, 95% CI: 1.002 to 1.27). Abdominal circumference and total fat mass were strongly correlated with BMI (rho=0.79 and 0.87, p<0.01) and thus adjustment of the effect of total fat mass for BMI was not performed to avoid multi-collinearity. Visceral fat area was positively associated with 10-year AF risk in age-, race-, sex- and site-adjusted analysis (HR 1.11, 95% CI 1.003 to 1.23), but not in multivariable-adjusted models. Subcutaneous abdominal fat and percent fat mass were not associated with incident AF (Table 3).
Table 3.
Multivariable Cox proportional hazard models for AF per 1-SD increase in adiposity.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
|
|
|
|||
| Anthropometry | ||||
| BMI | 1.16 (1.04, 1.29) | 0.01 | 1.14 (1.02, 1.28) | 0.03 |
| Abdominal circumference | 1.16 (1.05, 1.27) | <0.01 | 1.16 (1.04, 1.28) | 0.01 |
| Computerized Tomography | ||||
| Subcutaneous abdominal fat | 1.12 (0.98, 1.27) | 0.10 | 1.11 (0.97, 1.27) | 0.13 |
| Visceral abdominal fat | 1.11 (1.003, 1.23) | 0.04 | 1.07 (0.96, 1.19) | 0.25 |
| Sum of subcutaneous and visceral abdominal fat | 1.13 (1.003, 1.26) | 0.04 | 1.10 (0.98, 1.25) | 0.11 |
| DXA scan | ||||
| Total fat mass | 1.15 (1.03, 1.29) | 0.01 | 1.13 (1.002, 1.27) | 0.04 |
| Total percent fat | 1.06 (0.90, 1.24) | 0.52 | 1.03 (0.87, 1.22) | 0.77 |
Model 1: adiposity measure, age, sex, race, and site. Model 2: model 1 + smoking, systolic and diastolic BP, treatment for hypertension, total to HDL cholesterol ratio, heart rate, ECG LVH, PR interval, prevalent heart failure, coronary artery disease, and diabetes. DXA, dual-energy X-ray absorptiometry; HR, hazard ratio; 95% CI, 95% confidence interval.
The sum of subcutaneous and visceral fat areas was positively associated with AF risk in age-, race-, sex- and site-adjusted analysis (HR 1.13, 95% CI 1.003–1.26), but not in multivariable-adjusted models. The sum of subcutaneous and visceral fat areas was moderately associated with abdominal circumference (rho=0.76, p<0.01) and strongly associated with BMI and total fat mass (rho=0.84 and 0.93, both p<0.01). There were no statistically significant interactions between adiposity measures and race with the outcome of AF when examining the whole cohort (all p-values >0.14, Supplemental table 2), and in sex-stratified analysis (all p-values >0.14, Supplemental table 3). There were no statistically significant interactions between adiposity measures and sex with the outcome of AF (all p-values >0.64).
Discussion
The present study examined the associations of obesity, anthropometric, body composition, and fat distribution parameters with the10-year AF risk in a biracial cohort of older adults. We found that BMI, abdominal circumference, and total fat mass are associated with a modest (13–16%) increase in risk of AF risk over 10-year follow-up.
Our results expand on findings from prior studies of obesity and AF to older individuals of both white and black race. Most of the available data to date have been collected from younger cohorts than Health ABC that have been comprised primarily of individuals of European ancestry. Furthermore, there has been limited assessment of associations between different types of obesity (visceral vs. subcutaneous) and AF. In a range of community-based cohorts, obesity and increased BMI have been associated with increased AF risk (HR: 1.65–2.35) in follow-up spanning 6 to 14 years.21, 32–35 In the Women’s Health Study (mean age 56±7 years) each 1 mg/kg2 increase in BMI was associated with a 4.7% increased risk of AF over 13-year follow-up.32 In the Women’s Health Initiative study (mean age 63±7 years) overweight status was identified as a risk factor for AF, contributing a 10-year population attributable risk of 12.1%.21 Blacks had a lower AF incidence rate by 41% compared to whites in the Women’s Health Initiative study.21 BMI >35 is also associated with increased AF risk by a HR of 3.50 in young (30.6±4.7 years), otherwise healthy women, in a nation-wide cohort study from Denmark.36
Waist and hip circumference, as well as waist-to-hip circumference ratio have been associated with AF risk.15, 17, 18 In community-dwelling cohorts, increased waist circumference and waist-to-hip circumference ratio have been associated with higher risk of AF, by 11–13% over a 13 to 15-year follow-up period.15, 17, 34 Elevated body fat mass has been recently associated with increased AF risk by 29% during a 13.5-year follow-up.15 In our analysis, conducted in Health ABC participants, we determined a lower hazard for incident AF than the analyses conducted in younger cohorts that are summarized here. First, we examined BMI as a continuous variable, rather than stratifying by BMI categories. Second, obesity and adiposity measures may compete with other risk factors for AF in older adults. It is possible that obesity is less prominent risk factor in older adults due to cumulative insults from aging and other prominent risk factors for AF (e.g. hypertension). Last, Health ABC enrolled only patients older than 70 years without known AF, so a survival bias may exist.
Several mechanisms may underlie the associations of obesity and adiposity measures with AF risk. Increased BMI is associated with increased left atrial size.37, 38 Increased left atrial size is associated with higher AF risk in whites and blacks.39 Second, inflammation is strongly associated with AF and has been proposed to have a pivotal role in the initiation and perpetuation of AF.40 Obesity is recognized as a chronic, low-grade systemic inflammatory state,41, 42 which may partially contribute to the association of obesity with AF. Third, increased epicardial fat thickness has been described in obese individuals43 and has been associated with altered atrial electrophysiology44 and sympathovagal imbalance of the atria.45 In small case-control studies, increased epicardial fat has been associated with AF.46, 47
Visceral obesity is associated with a more unfavorable metabolic phenotype, enhanced systemic inflammation, and higher cardiovascular risk compared to subcutaneous obesity.48 In our study, although visceral obesity was associated with AF risk in age-, sex-, race-, and site-adjusted analysis, it was not associated with AF in multivariable models. Our results suggest that increased BMI, rather than adipose distribution, has a strong association with AF risk. Last, obesity is associated with inter-atrial conduction delay and increased P-wave duration, terminal force,49 and PR interval31 that constitute intermediate phenotypes for AF.50
Studies have identified multiple exposures as associated with increased risk of AF. However, obesity is one of the few that are modifiable. Understanding the associations of obesity and different adiposity measures with AF has implications for public health. Treatment of obesity with weight loss has been associated with reduced AF episodes frequency, duration and severity.51 BMI is a universally available, easy to obtain measurement. It provides a measure that can be modified and followed prospectively, and does not require sophisticated testing. Whether changes in BMI are associated with changes in AF risk needs to be evaluated in future studies. Whether changes in the other adiposity measures examined here likewise modifies AF risk also merits investigation.
Our study has several strengths. Health ABC employs standardized protocols that facilitated accurate collection of adiposity measures and covariates. Health ABC’s biracial design enabled assessing for statistical interaction by race and conducting race-stratified analyses. Our study has several limitations. The most important limitation of our study is that Health ABC was not designed to identify incident AF. AF outcomes were based on Center for Medicare and Medicaid data and outcome adjudication was not performed. The reliance on Center for Medicare and Medicaid data for our outcome ascertainment could potentially introduce misclassification for AF.22 Inclusion of individuals with a good functional status may limit generalizability of our results to sicker or functionally impaired older adults who may have an increased risk for AF. However there is an increasing number of well-functioning older adults in the community thereby increasing the generalizability of our findings. We further note that abdominal circumference was associated with AF risk while the sum of subcutaneous and visceral adipose tissue was not. It may be that intramuscular fat or non-adipose tissue contributed towards the association between abdominal circumference and AF. We were not able to quantify intramuscular fat content or non-adipose tissue, and hence are unable to explore this finding. Last, although we adjusted for available covariates, we cannot exclude residual confounding.
In conclusion, we established that anthropometric, body composition and fat distribution parameters were associated with incident AF in a community-dwelling cohorts of older adults. As obesity prevalence continues to rise, it is essential to develop a deeper understanding of the associations and mechanisms that link obesity with AF and its associated morbidity. Obesity is one of the very few risk factors for AF that can be modified. Future studies are required to enhance our understanding of how weight loss and reducing obesity can affect the incidence, progression, and course of AF.
Supplementary Material
Acknowledgments
Sources of Funding: This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103 and N01-AG-6-2106 and the Intramural Research Program of the NIH/NIA; NIH grants R01-AG028050, 5R03-AG045075, R01-NR012459, and 2R01HL092577. NINR grant R01-NR012459. Dr. Magnani is further supported by a Boston University School of Medicine Department of Medicine Career Investment Award.
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 4.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124(18):1982–93. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 7.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–20. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 8.Van Wagoner DR, Piccini JP, Albert CM, Anderson ME, Benjamin EJ, Brundel B, et al. Progress toward the prevention and treatment of atrial fibrillation: A summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm. 2015;12(1):e5–e29. doi: 10.1016/j.hrthm.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnani JW, Hylek EM, Apovian CM. Obesity begets atrial fibrillation: a contemporary summary. Circulation. 2013;128(4):401–5. doi: 10.1161/CIRCULATIONAHA.113.001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107(1):85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy-Dicey A, Harris TB, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170(21):1909–17. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373(9665):739–45. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155(2):310–5. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Frost L, Benjamin EJ, Fenger-Gron M, Pedersen A, Tjonneland A, Overvad K. Body fat, body fat distribution, lean body mass, and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girerd N, Pibarot P, Fournier D, Daleau P, Voisine P, O’Hara G, et al. Middle-aged men with increased waist circumference and elevated C-reactive protein level are at higher risk for postoperative atrial fibrillation following coronary artery bypass grafting surgery. Eur Heart J. 2009;30(10):1270–8. doi: 10.1093/eurheartj/ehp091. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159(5):850–6. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. J Hypertens. 2012;30(5):1004–14. doi: 10.1097/HJH.0b013e3283522a51. [DOI] [PubMed] [Google Scholar]
- 19.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–5. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease C and Prevention. Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58(27):740–4. [PubMed] [Google Scholar]
- 21.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women’s Health Initiative Observational Study. Heart. 2013;99(16):1173–8. doi: 10.1136/heartjnl-2013-303798. [DOI] [PubMed] [Google Scholar]
- 22.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–7. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, et al. Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC Study. Nutr Metab Cardiovasc Dis. 2011;21(6):430–7. doi: 10.1016/j.numecd.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129–37. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 26.Rumschlag DHB. IDIS: Iowa Drug Information Service. CD-ROM World. 1993;6:80–83. [Google Scholar]
- 27.van den Borst B, Gosker HR, Koster A, Yu B, Kritchevsky SB, Liu Y, et al. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. Am J Clin Nutr. 2012;96(3):516–26. doi: 10.3945/ajcn.112.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66(8):888–95. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaitman BR, Zhou SH, Tamesis B, Rosen A, Terry AB, Zumbehl KM, et al. Methodology of serial ECG classification using an adaptation of the NOVACODE for Q wave myocardial infarction in the Bypass Angioplasty Revascularization Investigation (BARI) J Electrocardiol. 1996;29(4):265–77. doi: 10.1016/s0022-0736(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 31.Magnani JW, Wang N, Nelson KP, Connelly S, Deo R, Rodondi N, et al. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition study. Circ Arrhythm Electrophysiol. 2013;6(1):84–90. doi: 10.1161/CIRCEP.112.975342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study) J Am Coll Cardiol. 2010;55(21):2319–27. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118(5):489–95. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, et al. Physical Activity, Obesity, Weight Change and Risk of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Circ Arrhythm Electrophysiol. 2014 doi: 10.1161/CIRCEP.113.001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang TJ, Parise H, Levy D, D’Agostino RB, Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 36.Karasoy D, Bo Jensen T, Hansen ML, Schmiegelow M, Lamberts M, Gislason GH, et al. Obesity is a risk factor for atrial fibrillation among fertile young women: a nationwide cohort study. Europace. 2013;15(6):781–6. doi: 10.1093/europace/eus422. [DOI] [PubMed] [Google Scholar]
- 37.Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1997;29(3):651–8. doi: 10.1016/s0735-1097(96)00554-2. [DOI] [PubMed] [Google Scholar]
- 38.Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Doring A, et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009;54(21):1982–9. doi: 10.1016/j.jacc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123(4):375, e1–7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–70. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CK, Yang CY, Lin JW, Hsieh HJ, Chiu FC, Chen JJ, et al. The relationship among central obesity, systemic inflammation, and left ventricular diastolic dysfunction as determined by structural equation modeling. Obesity (Silver Spring) 2012;20(4):730–7. doi: 10.1038/oby.2011.30. [DOI] [PubMed] [Google Scholar]
- 43.Graner M, Seppala-Lindroos A, Rissanen A, Hakkarainen A, Lundbom N, Kaprio J, et al. Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am J Cardiol. 2012;109(9):1295–302. doi: 10.1016/j.amjcard.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Lin YK, Chen YC, Chang SL, Lin YJ, Chen JH, Yeh YH, et al. Heart failure epicardial fat increases atrial arrhythmogenesis. Int J Cardiol. 2013;167(5):1979–83. doi: 10.1016/j.ijcard.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Balcioglu AS, Cicek D, Akinci S, Eldem HO, Bal UA, Okyay K, et al. Arrhythmogenic evidence for epicardial adipose tissue: heart rate variability and turbulence are influenced by epicardial fat thickness. Pacing Clin Electrophysiol. 2015;38(1):99–106. doi: 10.1111/pace.12512. [DOI] [PubMed] [Google Scholar]
- 46.Stojanovska J, Kazerooni EA, Sinno M, Gross BH, Watcharotone K, Patel S, et al. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur Radiol. 2015 doi: 10.1007/s00330-015-3643-1. [DOI] [PubMed] [Google Scholar]
- 47.Acet H, Ertas F, Akil MA, Oylumlu M, Polat N, Yildiz A, et al. New inflammatory predictors for non-valvular atrial fibrillation: echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio. Int J Cardiovasc Imaging. 2014;30(1):81–9. doi: 10.1007/s10554-013-0317-4. [DOI] [PubMed] [Google Scholar]
- 48.Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53(4):577–84. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 49.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P wave indices, obesity, and the metabolic syndrome: the atherosclerosis risk in communities study. Obesity (Silver Spring) 2012;20(3):666–72. doi: 10.1038/oby.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009;2(1):72–9. doi: 10.1161/CIRCEP.108.806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–60. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


