Abstract
Background:
Multidrug resistant strains of Acinetobacter baumannii (MDR-AB) have emerged as alarming nosocomial pathogens among patients with burning.
Objectives:
The current study aimed to determine the susceptibility of A. baumannii species, carbapenems resistance patterns, and their association with ISAba1 and ISAba4 elements upstream of the blaOXA-like genes, and the distribution of international clone (IC) of A. baumannii isolates among patients with burning in Tehran, Iran.
Materials and Methods:
In the current study, 62 A. baumannii species isolates from patients with burning in Tehran, Iran, in 2012 were evaluated for the antimicrobial susceptibility, genetic relationships, ICs, carbapenemase encoding genes, and insertion elements ISAba upstream of blaOXA-like genes.
Results:
The highest rates of susceptibility were observed with colistin (88.7%) and tigecycline (82.2%). The extensively drug-resistance and pan drug-resistance were observed in 37.1% and 8.1% of the isolates, respectively. About 98.3% of 17 genotypes categorized into three distinct clusters. Thirty-six of the 62 isolates (58%) belonged to the IC II lineage. The most prevalent acquired OXA-type carbapenemase was blaOXA-23-like (62.9%). ISAba1 and ISAba4 were detected upstream of blaOXA-23-like genes in 45.1% and 12.9% of isolates, respectively. In 32.2% of all isolates, ISAba1 laid upstream of blaOXA-51-like genes. The PCR results were negative for carbapenemase genes of Ambler class A and B, except blaVIM-2. (1.6%).
Conclusions:
It was the first study that attempted to detect the insertion elements ISAba and IC lineages in MDR-AB species isolated from patients with burning in Iran.
Keywords: Drug Resistance, beta-Lactamases, Molecular Epidemiology, Carbapenemase, IS elements, Acinetobacter baumannii
1. Background
Acinetobacter baumannii is an aerobic Gram-negative organism widely distributed in the environment and is also an important nosocomial pathogen. Acinetobacter baumannii outbreak, involving multidrug-resistant (MDR) strains, occurs worldwide. In hospitalized patients, A. baumannii frequently colonize the skin and upper respiratory tract and is isolated from human sputum, urine, feces, and blood. This bacterium also persist for a long time on inanimate hospital environment such as surfaces and medical equipment and is isolated from various locations, including tap water faucets, air, bedside urinals, gloves, ventilators, and catheters (1). Multidrug Drug-Resistant (MDR) and Extensively Drug-Resistant (XDR) strains of A. baumannii emerge as formidable nosocomial pathogens among patients with burning (1, 2). In the developing countries such as Iran, clinicians face serious challenges to manage patients with burning and MDR-A. baumannii (MDR-AB) infections, which present significant healthcare challenges by prolonging hospitalization, treatment failure, and increased mortality (3).
Carbapenems are currently the drug of choice; however, clonal outbreaks of carbapenems resistant A. baumannii (CR-AB) have led to an inadequacy of therapeutic choices in treating MDR-AB infections among patients in the developing countries (4). While expression of blaOXA-like genes play a major role in carbapenem resistance among CR-AB (5); blaOXA–mediated carbapenem resistance requires enhancement of gene expression either by insertion of element ISAba1 (upstream of blaOXA-51-like genes), or ISAba1 and/or ISAba4 (upstream of blaOXA-23-like genes) (6-8). ISAba1 and/or ISAba4 (upstream of blaOXA-like genes) are increasingly reported worldwide in the strains of the epidemic international clones (IC), the so-called European Clones (EC) I, II and III (9, 10). Despite a few reports on the distribution and/or frequency of resistant genes among CR-AB isolated from patients with burning in Iran (11, 12); the scarcity of molecular epidemiologic data renders the national efforts to control the spread of CR-AB infections unsuccessful. To date, no study has addressed spreading ISAba and distribution of the dominant ICs in MDR-AB strains that cause infection in patients with burning in Iran.
2. Objectives
The current study investigated (I) the susceptibility of A. baumannii isolates, (II) the carbapenems resistance patterns, and their association with ISAba1 and ISAba4 elements upstream of the blaOXA-like genes, and (III) the distribution of IC of A. baumannii species isolated among patients with burning in Tehran, Iran.
3. Materials and Methods
3.1. Bacterial Isolates
In 2012, a total of 62 non-repetitive clinical species of A. baumannii were collected from the patients with burning of a tertiary burn center in Tehran, Iran (1).
3.2. Identification of Acinetobacter Species
Initially, isolates were identified as A. baumannii using API-20NE system (bioMérieux, Marcy-l'Etoile, France), and were later confirmed by detection of blaOXA-51 like carbapenemase gene and gyrB-multiplex Polymerase Chain Reaction (PCR) (13, 14). All the clinical Isolates were stored at -20°C in CRYOBANK™ (Copan Diagnostics Inc., Canada) until further use.
3.3. Susceptibility Testing
The Clinical and Laboratory Standards Institute (CLSI, 2013) disk agar diffusion (DAD) method (15) and CLSI breakpoint interpretation were used to assess susceptibility of A. baumannii isolates to the current antimicrobial agents used in the treatment of MDR-AB infections. Minimum Inhibitory Concentration (MIC) values of all isolates for colistin and tigecycline were determined by the microbroth dilution method as described by the CLSI guidelines. MICs of colistin were interpreted according to the CLSI breakpoints. However, no breakpoints for tigecycline were available in the CLSI guidelines; therefore, the criteria for interpretation of the MIC values of tigecycline were determined according to the European committee on antimicrobial susceptibility testing (EUCAST) for members of the Enterobacteriaceae spp. All antimicrobial agents were purchased from Mast Co. Diagnostics Ltd., except tigecycline (purchased from Sigma-Aldrich). During each test, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control microrganisms to ensure accuracy of the antimicrobial susceptibility assays. Isolates of A. baumannii were defined as multidrug-resistant (MDR) when the organism was resistant to at least one agent in three or more antimicrobial categories. Species were considered extensively drug-resistant (XDR) when they were non-susceptible to one or more of the agents in all but two or less categories. Pandrug-Resistant (PDR) was defined as non-susceptibility to all antimicrobial agents (16).
3.4. Detection of Carbapenemase Encoding Genes
A multiplex-PCR, referred to as hexaplex-PCR (h-PCR), was optimized for simultaneous detection of frequent carbapenemase genes of Ambler class A (blaKPC, blaGES) and B metallo-β-lactamases (blaIMP-1, blaVIM-2, blaNDM-1, and blaSPM-1) in positive controls (Figure 1). PCR primers (Table 1) were designed using Primer 3 software (version 4.0; http://primer3.wi.mit.edu/; accessed 05.06.11). Reference gene sequences were extracted from GenBank (http://www.ncbi.nlm.nih.gov/GenBank [blaKPC: GQ140348, blaGES: GU207844, blaIMP-1: EF375699, blaVIM-2: GQ288396, blaNDM-1: JN794561, and blaSPM-1: HM370523; accessed 04.06.11]).
Figure 1. Detection of Genes Encoding Carbapenemase in the Molecular Classes A and B.
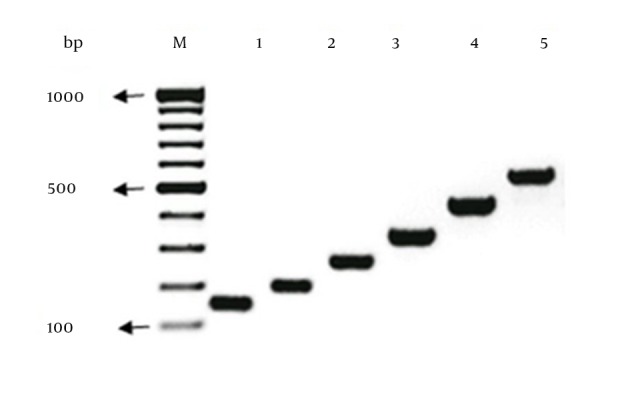
Ambler; blaKPC, blaGES, blaIMP-1, blaVIM-2, blaNDM-1, and blaSPM-1 by multiplex PCR. Clinical isolates harboring blaIMP-1 (Pseudomonas aeruginosa; lane 2), blaVIM-2 (Pseudomonas aeruginosa; lane 3), NDM-1 (Klebsiella pneumonia; lane 4), KPC (Klebsiella pneumonia; lane 5), blaGES (Pseudomonas aeruginosa; lane 6) and blaSPM-1 (Pseudomonas aeruginosa; lane 7) were used as PCR controls. The molecular size marker (lane 1) was a 100 bp ladder.
Table 1. Applied Primers Sequences a.
| Name/Primer | Sequence (5’ - 3’) | Annealing Temperature, °C | Size of Ampelicon, bp | Use (s) |
|---|---|---|---|---|
| hexaaplex PCR (h- PCR) | 52 | Detection of carbapenemase in the molecular classes A and B | ||
| IMP-1F | CTATCCCCACGTATGCATCTGA | 174 | ||
| IMP-1R | TTTCAGGCAACCAAACCACTA | |||
| VIM-2F | TGGTCTATTTGACCGCGTCTAT | 210 | ||
| VIM-2R | CATCACCATCACGGACAATGAG | |||
| NDM-1F | ACTGGATCAAGCAGGAGATCAA | 279 | ||
| NDM-1R | CGGTGATATTGTCACTGGTGTG | |||
| KPCF | ATATCTGACAACAGGCATGACG | 315 | ||
| KPCR | GTCGTGTTTCCCTTTAGCCAAT | |||
| bla GESF | TCGAAAACTTTCATATGGGCCG | 411 | ||
| bla GESR | TCTTTAGGAAAACCCGCTCGTA | |||
| SPM-1F | ATTGATTCGGACGTTTTCGTCG | 540 | ||
| SPM-1R | CAGATAACCAAGTTCCTTCGGC | |||
| ISAba1F/OXA-51 R | 51 | 227 | ISAba1detection upstream of bla OXA-51 -like genes | |
| ISAba1F | AAGCATGATGAGCGCAAAG | |||
| OXA-51 R | GGTGAGCAGGCTGAAATAAAA | |||
| ISAba1F/OXA-23 R | 44 | 321 | ISAba1 detection upstream of bla OXA-23 -like genes | |
| ISAba1F | TGAGATGTGTCATAGTATTC | |||
| OXA-23 R | AGAGCATTACCATATAGATT | |||
| ISAba4F/OXA-23 R | 41 | 327 | ISAba4detection upstream of bla OXA-51 -like genes | |
| ISAba4F | CACAATTTCTGATAAAGATA | |||
| OXA-23 R | TTTATTAAATTATGCTGAAC |
a All References are belong to this study.
The h-PCR was performed using Master Mix reagents (Ampliqon, Denmark) and primers, shown in Table 1, (SinaClon, Tehran, Iran) under the following conditions: preheat at 95°C for five minutes; 35 cycles of 94°C for one minute, 52°C for 40 seconds, and 72°C for 45 seconds; followed by 72°C for five minutes. Figure 1 shows representative agarose gel analysis of specific blaIMP-1, blaSPM-1, blaGES, blaKPC, blaNDM-1 and blaVIM-2 target sequence amplicons. The Ambler Class D type carbapenemase genes (blaOXA-23, 40, 51, 58, 143-like genes) were detected (17).
3.5. Detection of ISAba1and ISAba4 Sequences
To determine the frequency of ISAba1 and ISAba4 elements upstream of the blaOXA-23-like genes, as well as ISAba1 upstream of the blaOXA-51-like genes novel sets of primers (ISAba1F/OXA-23R and ISAba4F/OXA-23R) and ISAba1F/OXA-51R were used, respectively (Table 1). PCR primers were designed using Primer 3 software (version 4.0; http://primer3.wi.mit.edu/; accessed 05.06.11). Reference gene sequences were extracted from GenBank (http://www.ncbi.nlm.nih.gov/GenBank [ISAba1F/OXA-23R: AB849270.1, ISAba4F/OXA-23R: EF059914.1, and ISAba1F/OXA-51R: HQ637467.1; accessed 04.06.11]). PCR assays were performed under standard conditions (18) with modifications in annealing temperatures, as per Table 1.
3.6. AFLP Genomic Fingerprint Analysis
Epidemiological types of all A. baumannii isolates were determined by Amplified Fragment Length Polymorphism (AFLP) (19). Briefly, genomic DNA from isolates were double digested with Mbo I and Mse I (Fermentas, Lithuania) and ligated to their corresponding adaptors. Ligation products served as templates for preliminary-PCR amplification using PreAmp-Mbo and PreAmp-Mse primers. Diluted preliminary-PCR products were then used for selective PCR amplification to generate AFLPs profiles. To determine the AFLP genotypes, the selective PCR products were separated by electrophoresis on 2% (w/v) agarose gels. Gel images were analyzed by BioNumerics version 5.10 (Applied Maths) using A. baumannii NCTC 12156 as a normalization reference. The similarity between the band patterns was calculated using the Dice coefficient (with an optimization of 0.5% and a position tolerance of 1%). The AFLP clusters and type identification were defined by groups formed at 60% and 90% Dice similarity cutoffs, respectively, on a dendrogram constructed by the unweighted-pair group method using average linkages (UPGMA) (19).
3.7. Determination of Clonal Type
International clone (IC) lineages were determined using three-locus dual assay multiplex PCR (M-PCR) (20). The M-PCR assays selectively amplified the outer-membrane protein A (ompA), chaperone–subunit usher E (csuE) and intrinsic carbapenemase (blaOXA-51-like) genes of A. baumannii isolates. Identification of an isolate as a member of IC I or IC II needed the amplification of all three fragments in the corresponding M-PCR, and an absence of any amplification by the other M-PCR. IC III strains were defined as the amplification of only the ompA fragment in the IC II M-PCR, and the amplification of only the csuE and blaOXA-51-like fragments in the IC I M-PCR. Standard A. baumannii strains of IC type I, II, and III served as controls. Strains double negation either IC type I, II, or type III were reported as variant (V) clonal type.
3.8. Statistical Analysis
To determine associations between blaOXA-like genes and carbapenems resistant phenotype, statistical analysis including the chi-squire and Fisher’s exact tests were performed by the SPSS software (version 18, Chicago, IL, USA). In all of the experiments, P-values < 0.05 were considered significant.
4. Results
4.1. Isolates
In the current study, all 62 non-repetitive isolates identified as A. baumannii in the API 20NE system were also positive for intrinsic blaOXA-51-like genes.
4.2. Susceptibility Testing
In the antimicrobial susceptibility test, A. baumannii isolates showed resistance to phenotype (including intermediate susceptibility) 5 - 12 (median of 6) of all 12 test antimicrobials; all isolates were resistant to ceftriaxone and cefepime (Table 1). All isolates were MDR. In the current study, 23 (37.1%), and 5 (8.1%) of A. baumannii isolates were XDR and PDR, respectively. The highest rates of susceptibility were observed to colistin (88.7%; n = 55), tigecycline (82.2%; n = 51), and minocycline (72.5%; n = 45). All colistin resistant isolates were susceptible to tigecycline and/or tobramycin. Fortunately, despite the alarming rates of imipenem resistance, only 3.2% of imipenem-resistant A. baumannii isolates were resistant to colistin. Two imipenem- and colistin-resistant A. baumannii isolates were sensitive to tigecycline. The drug resistance pattern showed that the MICs of colistin and tigacycline ranged between 0.25 - 16 and 0.5 – 8 µg/mL, with MIC 90 of 8 and 4 µg/mL, respectively. According to the results of MIC for imipenem, 4 isolate was intermediate and 38 isolates were resistant to imipenem. The MICs for imipenem varied between 0.5 and 128 µg/mL.
4.3. Detection of Carbapenemase Encoding Genes
All 62 CRAB isolates included in the study were positive for blaOXA-51-like genes. The most prevalent acquired OXA-type carbapenemase were blaOXA-23-like genes isolated from 39 (62.9%) burning wounds (Table 2). Distribution of OXA-23 was not significantly different between AFLP clusters (P > 0.05) and blaOXA-23-like harboring isolates belonging to all AFLP-types. Another acquired OXA carbapenemase, blaOXA-58-like, was less common and isolated from 9 (14.5%) burning wounds. Despite OXA-23 encoding genes, blaOXA-58-like genes were more commonly isolated from burning wounds in clusters A, and D (P < 0.05). The blaOXA-51-like and blaOXA-23-like genes were detected among 20 (32.2%) and 6 (9.6%) imipenem non-susceptible isolates, respectively. Co-existence of blaOXA-23-like/blaOXA-23-40-like, blaOXA-23-23-like/blaOXA-23-58-like, blaOXA-23-40-like/blaOXA-23-58-like, blaOXA-23-23-like/blaOXA-23-40-like/blaOXA-23-58-like and blaOXA-23-23-like/blaOXA-23-40-like/blaOXA-23-58-like/blaOXA-23-40-like/blaOXA-23-58-like/blaOXA-23-143-like genes were detected in 19.3%, 4.8%, 1.6%, 3.2% and 1.6% of isolates, respectively which correlated with imipenem MICs > 32 µg/mL (P < 0.05). The PCR results were negative for blaKPC, blaGES, blaIMP-1, blaNDM-1, and blaSPM-1 type carbapenemase genes for all of the evaluated isolates, and blaVIM-2 encoding gene was observed in 1.6% of the isolates.
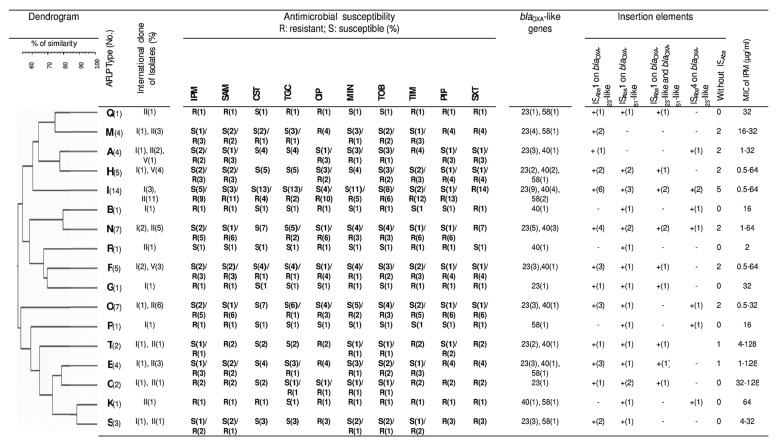
Table 2. AFLP-based Dendogramand Resistance Profiles of Isolated Acinetobacter baumannii .
| AFLP Type (No.) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q (1) | M (4) | A (4) | H (5) | I (14) | B (1) | N (7) | R (1) | F (5) | G (1) | O (7) | P (1) | T (2) | E (4) | C (2) | K (1) | S (2) | |
| IC and Variants of Isolates (No.) | II (1) | I (1), II (3) | I (1), II (2), V2 (1) | I (1), V1 (1), V4 (3) | I (3), II (11) | I (1) | I (2), II (5) | II(1) | I (2), V3 (1), V4 (2) | I (1) | I (1), II (6) | I (1) | I (1), II (1) | I (1), II (3) | I (1), II (1) | II (1) | I (1), II (1) |
| No. of Isolates with bla OXA -like Genes | |||||||||||||||||
| Only OXA-51 | 3 | 1 | 1 | 1 | |||||||||||||
| OXA-51, OXA-23 | 1 | 4 | 3 | 2 | 6 | 5 | 3 | 1 | 5 | 2 | 3 | 1 | 2 | ||||
| OXA-51, OXA-40 | 1 | 2 | 4 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| OXA-51, OXA-58 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | |||||||||
| OXA-51, OXA-143 | 1 | ||||||||||||||||
| OXA-51, OXA-23, OXA-40 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||
| OXA-51, OXA23, OXA-58 | 1 | 1 | 1 | ||||||||||||||
| OXA-51, OXA-40, OXA-58 | 1 | ||||||||||||||||
| OXA-51, OXA-23, OXA-40, OXA-58 | 1 | 1 | |||||||||||||||
| No. of Isolates With Insertion Elements | |||||||||||||||||
| OXA-51, OXA-23 ,OXA-40, OXA-58, OXA-143 | 1 | ||||||||||||||||
| ISAba1 on blaOXA-23-like | 1 | 2 | 1 | 2 | 6 | 4 | 3 | 1 | 3 | 1 | 3 | 1 | 2 | ||||
| ISAba1 on blaOXA-51-like | 1 | 2 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | ||
| ISAba1 on blaOXA-23-like and blaOXA-51-like | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||
| ISAba4 on blaOXA-23-like | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Without ISAba | 2 | 2 | 2 | 5 | 2 | 2 | 2 | 1 | 1 | ||||||||
| MIC of IPM, µg/mL | |||||||||||||||||
| 0.5 - 4 | 3 | 2 | 8 | 3 | 1 | 2 | 4 | 1 | 1 | ||||||||
| 8-Apr | 1 | 1 | 1 | 1 | |||||||||||||
| ≥ 8 | 1 | 4 | 1 | 3 | 5 | 1 | 4 | 3 | 1 | 3 | 1 | 1 | 2 | 1 | 1 | 2 | |
4.4. Detection of ISAba1 and ISAba4
Since it is also reported that ISAba4 raises MIC of isolates against carbapenems, in order to represent a more complete analysis of the blaOXA-like genes harboring A. baumannii isolates from Iran, detection of ISAba4 as well as ISAba1 by PCR were included. ISAba1 and ISAba4 were detected as upstream of blaOXA-23-like genes in 45.1% and 12.9%, respectively (Figure 2). In 32.2% of all isolates ISAba1 laid upstream of blaOXA-51-like genes. In all AFLP genotypes of the current study, ISAba1 and ISAba4 imparting imipenem resistance in A. baumannii isolates except two AFLP genotypes, E and S, which they were imipenem susceptible genotypes. One (1.6%) of A. baumannii isolates harboring blaOXA-23-like genes with high MIC for imipenem (16 μg/mL) was negative for ISAba1 and ISAba4 genes in the upstream of blaOXA-23-like genes; 11 (17.7%) of the isolates with high MIC for imipenem (64 - 128 μg/mL) harbored ISAba1 in both upstream of blaOXA-51-like genes and blaOXA-23-like genes. Eight (13%) A. baumannii isolates harboring blaOXA-23-like genes with high MIC for imipenem (32 - 128 μg/mL) also harbored ISAba4 genes in the upstream of blaOXA-23-like genes.
Figure 2. Dendrogram of AFLP analysis of genomic DNA from 62 non-repetitive A. baumannii isolates. Susceptibility to select antimicrobials, frequency of blaOXA-like genes, international clones, ISAba presence and MIC against imipenem is also indicated.
4.5. AFLP Genomic Fingerprint Analysis
Dendrogram constructed the following AFLP pattern preparation and the results of AFLP analysis of isolates were shown in Figure 2 using a similarity index of ≥ 60% since the threshold categorized 98.3% of the isolates into three distinct clusters (A, B and D) (Figure 2). Cluster D was more heterogeneous with six AFLP types (C, E, K, P, S, and T). AFLP represented 17 distinct AFLP types (A-Q) with predominance of genotype I, in 14 patients (Figure 2). AFLP types were between 1 and 14 isolates. Each of the AFLP types B, G, K, P, Q, and R was observed in one patient solely. Strain O also displayed a very low level of similarity (55%) to the others and was clearly far less related to any of the other 61 isolates.
4.6. Determination of International Clone Types
International Clone (IC) I, II and four other different variants were identified among the 62 A. baumannii isolates (Figure 2). thirty-six isolates (58%) belonged to the IC II and 18 (29%) isolates belonged to IC I. Eight (13%) isolates pertained to variants of clonal types, defined according to the new combination of amplified products obtained from the two separate multiplex PCRs that did not correspond to the previously defined IC I, II, and III (European Clone I, II, and III, respectively). Acinetobacter baumannii isolates with the variant of ICs were assigned to various AFLP types (A, F and H) (Figure 2). In the current study, no IC III was found in the isolates. According to the best of the authors’ knowledge, it is the first time that the prevalence of ICs is described in the bacteria isolated from burning wounds of Iranian patients.
4.7. Correlation of Acinetobacter baumannii Genotypes with Antibiotype and IC Lineages
The AFLP type analysis of all colistin-resistant A. baumannii (CoR-AB) strains revealed that five out of seven (71.4%) isolates belonged to the cluster A (AFLP types F, I, K and M). Among seven CoR-AB strains, three (42.8%) included in the study had distinct AFLP type (I) and antimicrobial resistance profiles (IPM, SAM, CST, TGC, CIP, MIN, TOB, TZP, PIP and SXT). Two CoR-AB strains had the same AFLP type (M) with distinctive antibiotypes (ampicillin-sulbactam, ciprofloxacine, colistin, imipenem, minocycline, piperacillin, piperacillin-tazobactam, tigecycline, tobramicin, and trimethoprim-sulfamethoxazole and ampicillin-sulbactam, ciprofloxacine, colistin, piperacillin, piperacillin-tazobactam, tobramicin, and trimethoprim-sulfamethoxazole). The obtained data revealed that PDR strains belonged to the cluster A, and B; three of five (60%) PDR isolates were assigned to AFLP type I. AFLP types B, G, K, P, Q, and R corresponded to a unique IC comprising IC1 and GC2 strains. The other AFLP types were present in more than one GC (Figure 2). The AFLP types of the CoR-AB strains identified in the three clusters A, B, and D were further investigated, and the results were shown in Figure 2. Four of 7 (57.1%) CoR-AB isolates which belonged to cluster A, were categorized to the same genotype (I)
5. Discussion
Nosocomial infections caused by multidrug resistant strains of A. baumannii (MDR-AB) are currently among the most difficult to treat, and they continue to present serious challenges to clinicians’ empirical and therapeutic decisions in patient with burning (1). Outbreaks of extensively, and pan drug-resistant A. baumannii (XDR, and PDR, respectively) are currently reported worldwide. In the current study, the high prevalence of XDR and PDR A. baumannii isolates (37.1% and 8.1%, respectively) from patient with burning, is consistent with the previous reports (1, 11). The increasing prevalence of XDR and PDR A. baumannii strains and limited treatment options prompt the use of antibiotics combinations like tigecycline and colistin as therapeutic regimens (21-23).
The present study revealed that 17.8% vs. 41.8% and 11.3% vs. 0% of A. baumannii isolates from patient with burning are resistant to tigecycline and colistin, respectively, compared to the other studies in Iran (11, 12). In a timely manner, antimicrobial resistance surveillance and strict infection control strategies are still absent in burn wards in Iran, despite the alarming emergence of MDR-AB strains, particularly among the isolates that are not susceptible to colistin. The emergence of colistin-resistant A. baumannii in the current study might be due to differences in A. baumannii strains available in the study or excessive use of colistin. However, it is still unknown whether this obvious resistance to colistin in A. baumannii isolates of patient with burning in Iran was due to increase of A. baumannii virulence or other intervening factors. Interestingly, all colistin resistant isolates were susceptible to Tigecycline and/or tobramycin. It is very important to treat serious infections caused by Colistin resistance isolates. However, this combination still needs to be validated in animal model and clinical trials. The results of the current study were consistent with a recent report in which a number of combinations exhibited potent activity against Multidrug resistant strains of A. baumannii (MDR-AB) (24).
It is well known that the blaOXA-23-like genes are among the most prevalent acquired carbapenemase-encoding genes worldwide, which can be on the chromosome or plasmids in different genetic structures (25). Analysis of carbapenemase encoding genes prevalence demonstrated that 30 MDR-AB isolates (62.9%) were positive for blaOXA-23-like genes. It was consistent with the results of the study by Pajand et al. (11), which showed that blaOXA-23-like genes were the most common genes encoding carbapenemase in patients with burning, suggesting that to prevent the spread of blaOXA-23-like genes in A. baumannii will be a major concern for both clinicians and local communities. In contrast to the study by Shahcheraghi et al. (26), in the current study blaSPM-1 and blaGES-1 were not found among the clinical strains of A. baumannii. This dissimilarity might be due to differences in locations of sample collection. The NDM-1 metallo-β-lactamase detected recently in A. baumannii, especially in patients from India and Pakistan (25), the neighboring countries of Iran, was not detected in any of the current study isolates. It is clearly demonstrated that ISAba1 in the upstream of blaOXA-like genes provides a promoter sequence with enhancing properties on OXA–enzymes expression (27). In contrast to the study by Pajand et al. (11) in northwest of Iran, ISAba1 was found upstream of the blaOXA-51-like genes in 32.2% of the isolates in the present study. This dissimilarity might be due to differences in the locations of sample collection and A. baumannii strains available for the study.
Most IC1 and IC II A. baumannii isolates are MDR, and are important opportunistic pathogens associated with life threatening nosocomial infections and hospital outbreaks worldwide (28, 29). In the current study, 36 of 62 (58%) MDR- AB strains belonged to the IC II lineage, indicating the clonal relationship of MDR-AB isolates in patients with burning in Tehran, Iran. In the current study, V4 had the same pattern of a variable group described in the previous studies (19), which yielded ompA fragment in the Group 1 PCR and csuE fragment in the Group 2 PCR. Although the presence of ISAba in the IC lineages seem to play a substantial role in antimicrobial resistance in MDR-AB isolates, according to the authors’ best knowledge there is no data on the presence of ISAba in MDR-AB strains isolated from patients with burning in Iran. However, further investigation is required to assess the prevalence of ISAba in MDR-AB strains isolated from Iran.
AFLP analysis grouped the obtained isolates from patients with burning into 17 distinct AFLP types. Cluster analysis of the AFLP patterns suggested that the outbreak MDR-AB isolates were quite diverse, and that outbreak strains of A. baumannii were low clonal, with a high diversity that may be caused by movement of insertion elements, a feature already recognized as being important in MDR-AB isolates (30). These data support the view that the Iranian MDR-AB isolates are more diverse than those of the other parts of the world (9, 22). Finally, a number of important limitations need to be considered. First, the number of patients was relatively small. Second, the isolates were collected from teaching hospitals, which may not represent the epidemiology of MDR, XDR and PDR A. baumannii at private hospitals. Based on the current study findings, studies should be planned to further explain the number and types of ISAba in carbapenems resistant strains of A. baumannii.
In conclusion, the obtained data support the idea that tigecycline alone or plus tobramycin exhibited a potent activity against colistin-resistant A. baumannii species isolated from patients with burning. Acinetobacter baumannii strains isolated from Iranian patients with burning are heterogeneous and this is the first report on spreading the ISAba -type genomic island and ISAba in the upstream of blaOXA-like genes in MDR-AB isolates belonging to different IC lineages among patients with burning in Iran. It was also shown that MDR-AB IC I and II were present among patients with burning in Iran at almost the same time that they were described worldwide.
Acknowledgments
We are greatly thankful to Dr. J. Kharazi for statistical analysis.
Footnotes
Authors’ Contributions:This work was carried out in collaboration between all authors. Abbas Bahador, Reza Raoofian and Davood Esmaeili designed the study, wrote the protocol, the first draft of the manuscript and revised the final draft of the manuscript. Azad Khaledi contributed to sampling. Sara Rahimi and Masoumeh Mokhtaran performed the microbiological tests and molecular procedures. Zahra Farshadzadeh and Leyli Beitollahi performed the statistical analysis.
Funding/Support:This study was supported by the grant from Tehran University of Medical Sciences (TUMS) (grant No: 89-01-30/10430).
References
- 1.Yali G, Jing C, Chunjiang L, Cheng Z, Xiaoqiang L, Yizhi P. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns. 2014;40(3):402–7. doi: 10.1016/j.burns.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Rezaei E, Safari H, Naderinasab M, Aliakbarian H. Common pathogens in burn wound and changes in their drug sensitivity. Burns. 2011;37(5):805–7. doi: 10.1016/j.burns.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(2):233–8. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez A, Gattarello S, Rello J. New treatment options for infections caused by multiresistant strains of Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli. Semin Respir Crit Care Med. 2011;32(2):151–8. doi: 10.1055/s-0031-1275527. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz M, Marti S, Fernandez-Cuenca F, Pascual A, Vila J. High prevalence of carbapenem-hydrolysing oxacillinases in epidemiologically related and unrelated Acinetobacter baumannii clinical isolates in Spain. Clin Microbiol Infect. 2007;13(12):1192–8. doi: 10.1111/j.1469-0691.2007.01825.x. [DOI] [PubMed] [Google Scholar]
- 6.Bogaerts P, Cuzon G, Naas T, Bauraing C, Deplano A, Lissoir B, et al. Carbapenem-resistant Acinetobacter baumannii isolates expressing the blaOXA-23 gene associated with ISAba4 in Belgium. Antimicrob Agents Chemother. 2008;52(11):4205–6. doi: 10.1128/AAC.01121-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HY, Chang RC, Su LH, Liu SY, Wu SR, Chuang CH, et al. Wide spread of Tn2006 in an AbaR4-type resistance island among carbapenem-resistant Acinetobacter baumannii clinical isolates in Taiwan. Int J Antimicrob Agents. 2012;40(2):163–7. doi: 10.1016/j.ijantimicag.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, et al. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006;258(1):72–7. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.He C, Xie Y, Fan H, Kang M, Tao C, Zhang R, et al. Spread of imipenem-resistant Acinetobacter baumannii of European clone II in Western China. I J Antimicrobial Agents. 2011. doi: 10.1016/j.ijantimicag.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Nigro SJ, Post V, Hall RM. The multiresistant Acinetobacter baumannii European clone I type strain RUH875 (A297) carries a genomic antibiotic resistance island AbaR21, plasmid pRAY and a cluster containing ISAba1-sul2-CR2-strB-strA. J Antimicrob Chemother. 2011;66(8):1928–30. doi: 10.1093/jac/dkr213. [DOI] [PubMed] [Google Scholar]
- 11.Pajand O, Rezaee MA, Nahaei MR, Mahdian R, Aghazadeh M, Soroush MH, et al. Study of the carbapenem resistance mechanisms in clinical isolates of Acinetobacter baumannii: comparison of burn and non-burn strains. Burns. 2013;39(7):1414–9. doi: 10.1016/j.burns.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Asadollahi P, Akbari M, Soroush S, Taherikalani M, Asadollahi K, Sayehmiri K, et al. Antimicrobial resistance patterns and their encoding genes among Acinetobacter baumannii strains isolated from burned patients. Burns. 2012;38(8):1198–203. doi: 10.1016/j.burns.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Higgins PG, Lehmann M, Wisplinghoff H, Seifert H. gyrB multiplex PCR to differentiate between Acinetobacter calcoaceticus and Acinetobacter genomic species 3. J Clin Microbiol. 2010;48(12):4592–4. doi: 10.1128/JCM.01765-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Chen T, Yu R, Lu X, Zong Z. Acinetobacter pittii and Acinetobacter nosocomialis among clinical isolates of the Acinetobacter calcoaceticus-baumannii complex in Sichuan, China. Diagn Microbiol Infect Dis. 2013;76(3):392–5. doi: 10.1016/j.diagmicrobio.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Cockerill FR, Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement;[... provides updated tables for... M02-A11, M07-A9, and M11-A8]. N C Clin Lab Standards; 2013. [Google Scholar]
- 16.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Higgins PG, Lehmann M, Seifert H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2010;35(3):305. doi: 10.1016/j.ijantimicag.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Mullis KB, editor. Target amplification for DNA analysis by the polymerase chain reaction.; Annales de biologie clinique.; 1989; pp. 579–82. [PubMed]
- 19.Bahador A, Taheri M, Pourakbari B, Hashemizadeh Z, Rostami H, Mansoori N, et al. Emergence of rifampicin, tigecycline, and colistin-resistant Acinetobacter baumannii in Iran; spreading of MDR strains of novel International Clone variants. Microb Drug Resist. 2013;19(5):397–406. doi: 10.1089/mdr.2012.0233. [DOI] [PubMed] [Google Scholar]
- 20.Turton JF, Gabriel SN, Valderrey C, Kaufmann ME, Pitt TL. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin Microbiol Infect. 2007;13(8):807–15. doi: 10.1111/j.1469-0691.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 21.Safari M, Saidijam M, Bahador A, Jafari R, Alikhani MY. High prevalence of multidrug resistance and metallo-beta-lactamase (MbetaL) producing Acinetobacter baumannii isolated from patients in ICU wards, Hamadan, Iran. J Res Health Sci. 2013;13(2):162–7. [PubMed] [Google Scholar]
- 22.Chang KC, Lin MF, Lin NT, Wu WJ, Kuo HY, Lin TY, et al. Clonal spread of multidrug-resistant Acinetobacter baumannii in eastern Taiwan. J Microbiol Immunol Infect. 2012;45(1):37–42. doi: 10.1016/j.jmii.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed NM, Youssef AA. In vitro activity of tigecycline and comparators against gram-negative bacteria isolated from a tertiary hospital in Alexandria, Egypt. Microb Drug Resist. 2011;17(4):489–95. doi: 10.1089/mdr.2010.0195. [DOI] [PubMed] [Google Scholar]
- 24.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54(12):4971–7. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusradze I, Diene SM, Goderdzishvili M, Rolain JM. Molecular detection of OXA carbapenemase genes in multidrug-resistant Acinetobacter baumannii isolates from Iraq and Georgia. Int J Antimicrob Agents. 2011;38(2):164–8. doi: 10.1016/j.ijantimicag.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Shahcheraghi F, Abbasalipour M, Feizabadi M, Ebrahimipour G, Akbari N. Isolation and genetic characterization of metallo-beta-lactamase and carbapenamase producing strains of Acinetobacter baumannii from patients at Tehran hospitals. Iran J Microbiol. 2011;3(2):68–74. [PMC free article] [PubMed] [Google Scholar]
- 27.Sung JY, Kwon KC, Cho HH, Koo SH. Antimicrobial resistance determinants in imipenem-nonsusceptible Acinetobacter calcoaceticus-baumannii complex isolated in Daejeon, Korea. Korean J Lab Med. 2011;31(4):265–70. doi: 10.3343/kjlm.2011.31.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Choi JY, Kim HW, Kim SH, Chung DR, Peck KR, et al. Spread of carbapenem-resistant Acinetobacter baumannii global clone 2 in Asia and AbaR-type resistance islands. Antimicrob Agents Chemother. 2013;57(11):5239–46. doi: 10.1128/AAC.00633-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins N, Dalla-Costa L, Uehara AA, Riley LW, Moreira BM. Emergence of Acinetobacter baumannii international clone II in Brazil: reflection of a global expansion. Infect Genet Evol. 2013;20:378–80. doi: 10.1016/j.meegid.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemec A, Krizova L, Maixnerova M, Diancourt L, van der Reijden TJ, Brisse S, et al. Emergence of carbapenem resistance in Acinetobacter baumannii in the Czech Republic is associated with the spread of multidrug-resistant strains of European clone II. J Antimicrob Chemother. 2008;62(3):484–9. doi: 10.1093/jac/dkn205. [DOI] [PubMed] [Google Scholar]