Summary
CD8+ T cells contribute to the control of HIV, but it is not clear whether initial immune responses modulate the viral set point. We screened high-risk uninfected women twice a week for plasma HIV RNA and identified twelve hyperacute infections. Onset of viremia elicited a massive HIV-specific CD8+ T cell response, with limited bystander activation of non-HIV memory CD8+ T cells. HIV-specific CD8+ T cells secreted little interferon-γ, underwent rapid apoptosis and failed to upregulate the interleukin 7 receptor, known to be important for T cell survival. The rapidity to peak CD8+ T cell activation and the absolute magnitude of activation induced by the exponential rise in viremia were inversely correlated with set point viremia. These data indicate that rapid, high magnitude HIV-induced CD8+ T cell responses are crucial for subsequent immune control of acute infection, which has important implications for HIV vaccine design.
Introduction
Human and animal studies of acquired immune deficiency syndrome (AIDS) virus infections provide unequivocal evidence that CD8+ T cells contribute to immune containment (reviewed in (Walker and McMichael, 2012)). The HIV viral set point is the stable viral load that is established after acute infection. In acute HIV infection in humans, HIV-specific CD8+ T cell responses, measured by interferon-γ (IFNγ) secretion, appear as the viral load is declining to the set point, suggesting that these cells contribute to initial viral control (Borrow et al., 1994; Koup et al., 1994). Moreover, depletion of CD8+ T cells in acute AIDS virus infection in macaques leads to persistent high-magnitude viremia, which declines as these cells reappear (Jin et al., 1999; Schmitz et al., 1999). Viral evolution in response to HIV-specific CD8+ T cell responses as the viral set point is reached provides further evidence of early immune pressure (Goonetilleke et al., 2009; Liu et al., 2013). The viral set point following acute HIV infection is predictive of subsequent disease progression (Lyles et al., 2000), suggesting that early responses play a crucial role in the subsequent control of viremia, but whether initial immune responses modulate the viral set point has not been determined.
Studies of acute HIV infection have largely been conducted as viral load is declining from the peak (Appay et al., 2002; Goonetilleke et al., 2009; Liu et al., 2013; Trautmann et al., 2012; Turnbull et al., 2009), and therefore little is known about the initial phase of the CD8+ T cell response. Such studies have been challenging since hyperacute infection, defined here as the period between onset of detectable plasma viremia and peak viral load, remains poorly characterized due to the difficulty of identifying infections prior to peak viremia. Pre-peak viral dynamics have been measured in plasma blood donors, but the unavailability of cells from that cohort has left questions regarding corresponding T cell dynamics unanswered (Freel et al., 2010; Ribeiro et al., 2010). T cell studies performed in the early stages of acute HIV infection have shown that antiviral CD8+ T cell responses measured by IFN-γ secretion are narrowly directed and of low magnitude (Dalod et al., 1999; Radebe et al., 2011; Streeck et al., 2009; Turnbull et al., 2009). This contrasts with the high magnitude of CD8+ T cell activation that have been noted during the period from peak viremia to viral set point in HIV infection (Appay et al., 2002; Pantaleo et al., 1994), in excess of measurements of virus-specific immunity by IFN-γ Enzyme-Linked ImmunoSpot ELISPOT). Early T cell activation has been attributed to bystander activation induced by HIV (Bangs et al., 2006; Doisne et al., 2004), but studies of TCR repertoire showing oligoclonal expansions imply that they could be antigen-specific (Pantaleo et al., 1994; Wilson et al., 1998). Indeed, following yellow fever or vaccinia virus immunization, a massive activation of virus-specific CD8+ T cells is induced, without appreciable bystander activation (Miller et al., 2008). The relatively weak antigen-specific CD8+ T cell responses reported in early HIV infection seem inconsistent with the observed rapid decline in plasma viral load (pVL), typically in excess of 10,000 fold. Likewise, although the magnitude of initial CD8+ T cell responses to a given epitope is associated with a more rapid time to immune escape, relatively weak IFN-γ ELISPOT responses are observed even for immunodominant epitopes at the time of rapid viral load decline (Borrow et al., 1997; Brumme et al., 2008; Goonetilleke et al., 2009; Liu et al., 2013; Radebe et al., 2014).
In this study, we sought to define the onset, magnitude and evolution of CD8+ T cell responses and their relation to viral load dynamics during the period from onset of HIV viremia to pVL set point. We established a cohort of young HIV-negative women at very high risk of HIV-1 clade C virus infection in KwaZulu-Natal, South Africa, where the reported rate of HIV-prevalence in those from 15 to 49 years of age is 27% (Delva and Abdool Karim, 2014). This study, termed FRESH, for Females Rising through Education, Support and Health, had two interlinked objectives. One was to establish a pathway out of the socioeconomic constraints that put women at risk of HIV infection (Kalichman et al., 2006), by provision of an intensive empowerment, life-skills and job readiness curriculum, coupled with HIV prevention education, through classes taken twice a week over the course of a year. The second objective was to cryopreserve baseline blood samples, screen each participant twice a week by finger-prick plasma HIV RNA testing for evidence of acute HIV infection, and examine the relationship between onset of viremia and adaptive CD8+ T cell responses. Here we report results on the first 12 subjects identified. Contrary to the notion that early T cell responses are low in magnitude, our results indicate that acute HIV infection elicits a massive HIV-specific CD8+ T cell response, with limited bystander activation of memory CD8+ T cell responses to other pathogens. These expanded cells then undergo rapid apoptosis and lack phenotypic markers associated with long-term memory development. Importantly, the data show that the magnitude and rapidity of induction of activated CD8+ T cells are crucial factors in modulating subsequent immune control.
Results
Analysis of 12 subjects with hyperacute HIV infection demonstrates exponential rise in viremia coincident with CD4+ T cell decline
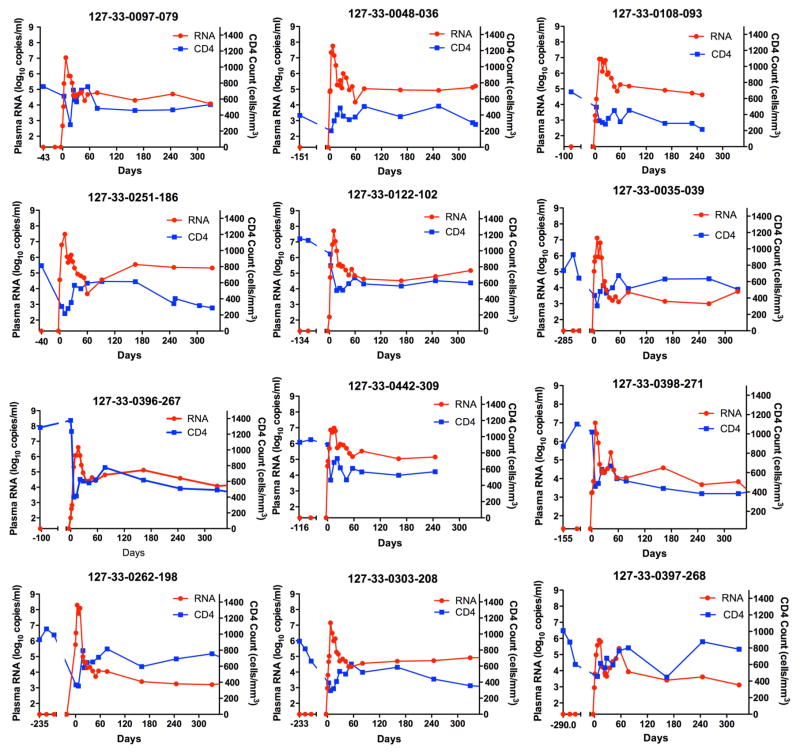
Finger prick blood samples were obtained from high-risk uninfected women twice a week for quantitative HIV RNA testing. Twelve subjects were identified with hyperacute infection, defined by detection of HIV RNA prior to peak viremia, representing an incidence of 7.3 (95% CI=3.8–12.8) per 100 person-years. Of the 11 subjects for whom we could test the earliest sample, all were in Fiebig Stage I (Fiebig et al., 2003). The time from enrollment in the study to the first detection of viremia ranged from 39–284 days. pVL measurements from each of the 12 infected subjects were obtained at 3–6 time points during the rise to peak viremia (Fig. 1). The median pVL at first detection was 3.2 log10 (IQR 2.66–4.57) HIV RNA copies/ml. The median peak pVL was 7.01 log10 (IQR 6.68–7.66) RNA copies/ml, more than 10-fold higher than the median peak pVL of 5.80 log10 observed in plasma donors infected with clade B virus (Ribeiro et al., 2010). Despite differences in peak pVL compared to plasma donors, the calculated median viral doubling time was 16.4 h corresponding to 0.68 days (IQR 0.65–0.78 days) (Table S1), which is comparable to a median doubling time of 0.65 days in plasma donors from a clade B endemic region (Ribeiro et al., 2010). R0, the basic reproductive number that defines the number of infected cells a single cell gives rise to when target cells are not limiting, ranged from 5.5 to 14.9 (Table S1). Frequent measurement of peripheral blood CD4 cell counts revealed a rapid decline in cell numbers coincident with peak viremia, reaching a nadir that was on average 37% of the documented pre-infection average value. CD4 cell counts rebounded but always to numbers below pre-infection numbers as the viral load set point was achieved (Fig. 1). These data demonstrate the feasibility of identifying hyperacute HIV infection, and document the exponential rise in acute viremia coincident with the rapid decline in CD4+ T cell counts.
Figure 1. Dynamics of absolute CD4 count and plasma viral loads during hyperacute HIV infection.
Plasma HIV-1 RNA magnitude (red) and absolute CD4 counts (blue) before HIV infection and following onset of detectable plasma viremia in 12 subjects with hyperacute HIV infection that were not receiving antiretroviral treatment. See also table S1
The rapidity and magnitude of CD8+ T cell activation correlate with set point viremia
To elucidate the nature of the CD8+ T cell response induced following onset of plasma viremia, we analyzed the expression patterns of CD38 and HLA-DR, markers that have been used to define T cell activation during the acute phase of viral infections and following vaccination (Appay et al., 2002; Doisne et al., 2004; Miller et al., 2008). Both markers had low pre-infection cell surface expression, allowing for the unequivocal identification of an emerging activated CD8+ T cell population induced by acute HIV infection. Within 1 to 3 days following onset of plasma viremia, (DFOPV), and prior to peak viremia, CD8+ T cell activation was already detectable, ultimately increasing from less than 1% pre-infection to a peak as high as 77% (IQR 41.8%–67.4%) of total CD8+ T cells (Fig. 2a, b). Peak activation was delayed compared to peak viremia by a median of 2 weeks, reaching maximal values approximately 3 weeks after first detection of viremia (Fig. 2c), and then contracting in all 11 participants evaluated, reaching a quasi-steady state at a median of 80 days (IQR 73–107) (Fig. 2b).
Figure 2. CD8+ T cell activation following onset of plasma viremia.
Longitudinal analysis of CD38 and HLA-DR expression on CD8+ cells after HIV infection. (a) Representative FACS plots gated on CD8+ cells expressing CD38 and HLA-DR. Days following onset of plasma viremia (DFOPV) are indicated (b) Longitudinal dynamics of activated (CD38+HLA-DR+) CD8+ cells for 11 donors in whom samples were available for analysis. (c) Representative relationship between HIV-induced CD8+ cell activation kinetics and contemporaneous viral load. (d) Correlation between time to peak activation and viral load setpoint. (e) Correlation between frequency of activated CD8+ cells at peak activation and viral load set point. Spearman’s rank correlation was used in (d) and (e).
The observed differences in the kinetics of peak activation of CD8+ T cells prompted us to determine whether the rapidity and magnitude of CD8+ T cell activation correlated with set point viremia, a known predictor of subsequent disease progression (Lyles et al., 2000). As shown in Fig. 2d, greater time to peak activation positively correlated with higher pVL set point (Spearman r=0.7, p=<0.03). In contrast, the frequency of activated (CD38+HLA DR+) CD8+ T cells at peak activation inversely correlated with HIV viral set point (Spearman r=−0.8, p=0.009) (Fig. 2e). Although CD4 count is a strong predictor of disease progression in chronic HIV infection (Phillips and Lundgren, 2006), nadir CD4 counts did not correlate with set point pVL (data not shown, Spearman r=0.15, p=0.65). Because pVL set point predicts subsequent HIV disease progression (Lyles et al., 2000), these data show that the kinetics and magnitude of HIV-induced CD8+ T cell activation in hyperacute infection impact subsequent immune control of HIV.
The ability of HIV-specific CD8+ T cells to proliferate is strongly correlated with viral control in chronic infection (reviewed in (Migueles and Connors, 2015)). To further define the expansion and subsequent contraction of CD8+ T cells in hyperacute infection, we measured intracellular markers associated with cellular proliferation and cell survival. Ki67 is upregulated in cells undergoing division due to recent antigen stimulation (Gerdes et al., 1984; Hazenberg et al., 2000), whereas the anti-apoptotic protein Bcl-2 is down regulated in effector cells (Grayson et al., 2000). Pre-infection CD8+ T cells from the 11 donors evaluated had a resting phenotype that was Ki67negBcl-2high, with only a median of 0.4% (IQR 0.1%–0.8%) displaying a cycling phenotype (Ki67high Bcl-2low) (Fig. 3a). Upon HIV infection a population of Ki67highBcl-2lowCD8+ T cells rapidly emerged, reaching a median of 25.4% (IQR 13.5–33.5%) (Fig. 3b). Peak expression of these markers was delayed by approximately one week compared to peak viremia (representative example, Fig. 3c). Simultaneous analysis of the expression pattern for the four markers evaluated (CD38, HLA-DR, Ki67 and Bcl-2) on CD8+ T cells confirmed that Ki67high and Bcl-2low population was a subset of the CD38, HLA-DR double positive population (Fig. 3d). We observed a negative relationship between the frequency of Ki67high Bcl2low CD8+ T cells and pVL set point (Spearman r= −0.7, p=0.03) (Fig. 3e). These data indicate that the activated cells are also undergoing vigorous proliferation, and indicate that CD8+ T cell proliferation induced by acute HIV infection is associated with subsequent viral control.
Figure 3. CD8+ T cell proliferation and apoptosis following onset of viremia.
(a) Representative FACS plots gated on CD8+ cells expressing Ki-67 and Bcl-2. (b) Summary data for the longitudinal analysis of proliferating and pro-apoptotic CD8+ cells for 11 donors evaluated. (c) Representative relationship between Ki67negBcl-2lowCD8+ T cells and contemporaneous viral load. (d) CD8+T cell co-expression of CD38, HLA-DR, Ki67 and Bcl-2. (e) Correlation between peak Ki67negBcl-2lowCD8+ T cells and viral load set point. Spearman’s rank correlation was used in (e).
Hyperacute activated CD8+ T cells are HIV-specific
The observed correlation between the kinetics of activated CD8+ T cells and pVL set point suggested that these cells might be HIV-specific. HIV-specific CD8+ T cell responses were detectable by intracellular cytokine staining (ICS) for IFN-γ production as early as 3 days after detection of viremia in some subjects (Fig. S1a), but were weak at peak activation, and appeared to increase as activation was decreasing (Fig. S1b). Using IFN-γ ELISPOT to determine responses to the entire proteome, we found that five of 9 donors tested had a detectable HIV-specific CD8+ T cell response by day 7–10 following the onset of plasma viremia, when CD8+ T cell activation was peaking (Fig. S1c) with many of the early responses targeting Gag, Pol and Nef (Fig. S1d). Longitudinal analysis revealed the transient nature of many of the early responses given that a median of 31% (IQR 11% to 52%) of the early responses measurable in the first 30 DFOPV became undetectable at later time points, when new responses were arising (Fig. S2).
The above studies measured the ability of CD8+ T cells to produce cytokines in response to synthetic HIV peptides, but did not measure the recognition of endogenously processed epitopes. To address this, autologous expanded CD4+ T cells were infected with a patient-derived clade C HIV virus and used to stimulate peripheral blood mononuclear cells (PBMCs). Representative data from one subject (Fig. 4a) show that nearly 20% of total CD8+ T cells at the time of peak activation mobilized CD107a upon exposure to HIV infected cells, compared to a background of 2.9% cells in a pre-infection sample. Incubation of CD8+ T cells in interleukin-2 (IL-2) supplemented media for 5 days resulted in an average 30-fold increase in antigen-specific IFN-γ production (Fig. 4b) despite negligible ex vivo proliferation during the incubation period (Fig. S3). Summary data for 6 donors using CD8+ T cells rested in IL-2-supplemented media showed significantly greater CD107a expression compared to IFN-γ, TNF-α, and IL-2 responses (Kruskal–Wallis p<0.0001) (Fig. 4c). Importantly, CD107a expression at peak activation was not significantly different from the median frequency of in vivo proliferating cells defined by Ki67high, BCL-2low (p=0.6) (Fig. 4d). These results suggest that the majority of proliferating CD8+ T cells were HIV-specific effector cells with degranulation potential.
Figure 4. Hyperacute HIV infection induces strong CD8+ T cell responses with degranulation potential.
HIV-specific CD8+ T cell responses were measured by ICS after overnight incubation with autologous HIV-infected CD4+ cells. Uninfected autologous CD4+ cells co-cultured with CD8+ cells were used as a negative control. (a) Representative flow plots demonstrating CD107a expression on CD8+ cells. (b) Upper panel shows CD107a expression on ex vivo CD8+ cells from 14 DFOPV incubated for 12 hours with HIV infected autologous CD4+ T cells. The lower panel shows CD107a expression on CD8+ cells from 14 DFOPV incubated in IL-2 containing media for 5 days prior to co-culture with infected autologous CD4+ T cells. (c) Proportion of CD8+ cells secreting cytokines following stimulation with HIV infected autologous CD4+ cells. Background responses in the uninfected co-culture condition are subtracted. Statistical significance was determined using Mann-Whitney and Kruskal Wallis tests. (d) Comparison between proliferating CD8+ T cells and CD107a positive cells at peak activation time points (also see supplementary figures). Statistical significance was determined using Mann-Whitney test. See also Figure S1, S2 and S3
To further define the specificity of activated CD8+ T cells, we used HLA class I tetramers to directly stain antigen-specific cells. Despite the limited availability of tetramers, individual responses of 10% or greater were detected in three of four subjects evaluated. In subject 208, for whom the most tetramers (five) were available, 36% of all CD8+ T cells were HIV-specific, at a time when 51% of all CD8+ T cells were activated (Fig. 5a). Similarly, 3 tetramers identified 34% of total CD8+ T cells as HIV-specific in a second subject at a time when 50% of CD8+ T cells were activated (Fig. 5b). In a third donor, only 2 HLA-matched tetramers were available, but these identified 12% of total CD8+ cells as HIV-specific for a sample with 66% activated CD8+ T cells (Fig. 5c). The forth donor did not have detectable responses to 4 HIV tetramers tested, but HIV-specific CD8+ T cell responses were clearly present, as evidenced by weak IFN-γ ELISPOT responses (donor 309 in fig. S1 and S2), suggesting that the tetramers used did not match in vivo epitopes.
Figure 5. Analysis of HIV-specific CD8+ T cell responses during hyperacute HIV infection using MHC class I tetramers.
PBMCs were stained with MHC class I tetramers specific for HIV and antibodies against CD38 and HLA-DR. All plots are gated on CD8+ T cells. Upper panels show frequencies of HIV-specific CD8+ T cells for each HIV tetramer tested. Blue dots depict the frequency of tetramer+ cells co-expressing CD38 and HLA-DR overlaid on total CD8+ T cells (red dots). Data for subjects 208, 079 and 093 are shown in panels (a), (b) and (c), respectively. (d) Flow plots show combined tetramer staining and cytokine secretion measured by ICS. Representative data for one of the four donors tested are shown. (e) Proportion of HIV-specific and CMV-specific tetramer+ cells secreting IFN-γ following optimal peptide stimulation of a peak activation sample. Statistical significance was determined using Mann-Whitney test.
The tetramer data indicated much higher frequencies of HIV-specific CD8+ T cells than did the IFN-γ ELISPOT assay. Dual staining by tetramer and IFN-γ at peak activation revealed that on average only 20% of tetramer+ cells produced measurable amounts of IFN-γ, whereas comparative analysis showed that CMV tetramer+ cells secreted significantly more IFN-γ (p=0.01) (Fig. 5d and 5e). Given that only a fraction of known epitopes were tested, and that over half of the epitopes targeted in acute HIV infection have not been previously identified (Liu et al., 2013), these data strongly support the hypothesis that the majority of expanded CD8+ T cells observed during the hyperacute phase of the infection are HIV-specific.
Limited CD8+ T cell bystander activation during acute HIV infection
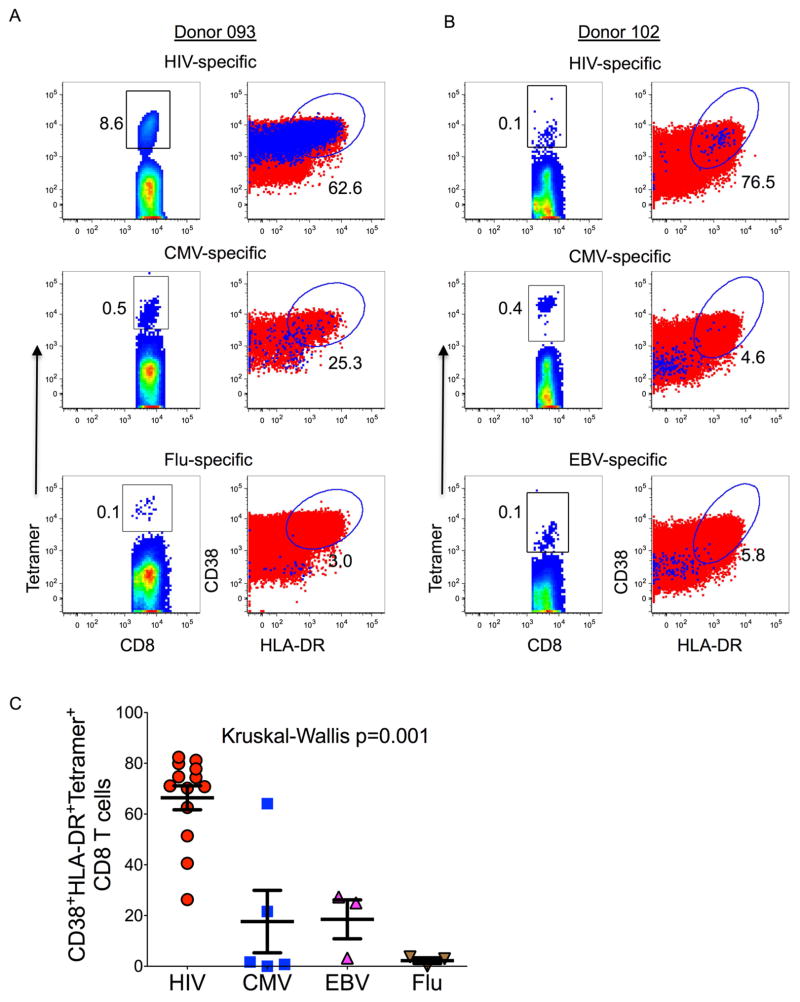
Having confirmed that a major fraction of activated proliferating cells were HIV-specific by tetramer staining, we next investigated whether CD8+ T cell bystander activation might account for some of the additional CD8+ T cell activation. Comparative analyses focused on cytomegalovirus (CMV), influenza and Epstein-Barr virus (EBV) specific CD8+ T cells in four of the acutely infected subjects. For donor 093, 62.6% of HIV-specific tetramer+ cells were activated at peak activation, whereas only 25.7% of CMV-specific and 3.1% of influenza specific tetramer+ cells were activated (Fig. 6a). Similarly, in donor 102, 76.5% of HIV specific cells displayed an activated phenotype, with minimal activation of CMV- and EBV-specific CD8+ T cell responses (Fig. 6b). Higher magnitude activation was observed for a total of 13 different HIV-specific tetramers tested in 4 study participants compared to 5 CMV-, 3 influenza- and 3 EBV-specific responses (Kruskal-Wallis p=0.001) (Fig. 6c). Together, these data suggest that there was minimal contribution of bystander activation to the observed HIV-induced CD8+ T cell expansion.
Figure 6. Assessment of bystander CD8+ T cell activation during hyperacute HIV infection.
Intra-donor activation (CD8+ CD38+ HLA-DR+ cells) profiles of HIV, CMV, EBV and influenza virus specific (tetramer+) CD8+ T cells. Data for two donors with detectable tetramer+ cells specific for three different pathogens are shown in panels (a) and (b). The first columns show flow plots gated on tetramer+ cells, the second column shows flow plots gated on tetramer+ cells that are double positive for CD38 and HLA-DR (blue dots) overlaid on total CD8+ T cells (red dots). (c) Activation data for 13 HIV, 5 CMV, 3 EBV and 3 influenza specific tetramer+ cells are graphed. Statistical significance was determined using Kruskal Wallis test.
Marked apoptosis of HIV-specific CD8+ T cells in hyperacute infection
Our data indicate that the majority of the activated CD8+ T cells in hyperacute infection are HIV-specific, and that some of the earliest antigen-specific responses do not persist. We next investigated if the early massive activation accelerated cell death given that apoptosis is intimately linked with short-lived effector cells in the acute phase of a viral infection (Badovinac et al., 2002). Consistent with the observed decrease in Bcl-2 expression in activated and proliferating CD8+ T cells (Fig. 7a, b), activated cells also expressed high magnitude of CD95 expression, a potent inducer of apoptosis and one of the major effector mechanisms of activated CD8+ T cells involved in cell killing (Fig. 7a) (Kagi et al., 1994). Additionally, the activated CD8+ T cells failed to up regulate CD127 (Fig. 7c), which is important for cell survival during the contraction phase of an acute immune response (Kaech et al., 2003; MacPherson et al., 2001). Dual annexin V and propidium iodide (PI) staining revealed that CD8+ T cells from peak activation failed to survive overnight incubation compared to pre-infection (p=0.02) and post CD8+ T cell activation (p=0.03) samples (Fig. 7d). No survival defect was observed for CD4+ T cells (Fig. 7e). Tetramer immunophenotyping confirmed that HIV-specific cells were selectively more susceptible to apoptosis compared to bystander CD8+ T cell responses (Fig. 7f). Combined, these data show that newly differentiated effector CD8+ T cells underwent significant contraction despite ongoing viremia, consistent with activation induced cell death and failure to upregulate the CD127 molecule involved in long term CD8+ T cell memory formation.
Figure 7. Activation profiles of CD8+ T cells in hyperacute HIV infection.
(a) Phenotypic analysis of HIV specific tetramer+ CD8+ cells. The first flow plot is gated on tetramer+ cells, the second, third and fourth plots depict tetramer+ cells (blue dots) overlaid on total CD8+ cells. Data showing frequencies of tetramer+ cells that are (b) Ki67high, BCL2low and (c) CD127high are graphed. Statistical significance was determined using Mann-Whitney and Kruskal Wallis tests. (d) Frequencies of dead CD8+ T cells (annexin-V+, PI+ CD8+ cells). Statistical significance was determined using Mann-Whitney test. (e) Frequencies of dead CD4+ T cells (annexin-V+, PI+ CD4+ cells) after overnight incubation in R10. Statistical significance was determined using Mann-Whitney test. (f) Flow cytometric data for two donors showing ex vivo expression of annexin V by HIV tetramer+ and CMV tetramer+ cells at peak activation.
Discussion
The relationship between the onset of HIV viremia, adaptive immune responses and subsequent viral control has not been well defined, due to difficulty obtaining samples prior to and at peak viremia. Here we identified HIV infected subjects at the onset of plasma viremia by screening high-risk uninfected women in an area of extremely high incidence of infection twice a week. Our results indicated that hyperacute HIV infection defined as the period between first detectable and peak plasma viremia, elicited vigorous activation and proliferation of CD8+ T cells, with up to 77% of peripheral CD8+ T cells expressing HLA-DR and CD38. We determined that the majority of these early-activated cells were HIV-specific, as inferred by their ability to degranulate when stimulated with autologous HIV-infected CD4+ T cells, by HLA class I tetramer staining, and by lack of appreciable bystander activation. Moreover, we showed that this initial robust CD8+ T cell response impacted viral control, consistent with what has been observed in SIV rhesus macaque immunization models (Hansen et al., 2011; Hel et al., 2002). Overall these data indicated that the onset of plasma HIV viremia is markedly immunogenic for HIV-specific CD8+ T cell responses that control acute viremia, and that traditional measures of IFN-γ production are an inadequate measure of these responses in the acute phase of infection.
A unique aspect of this study was the availability of samples from the time of onset of viremia, allowing accurate definition of kinetics and magnitude of CD8+ T cell activation. Both the promptness of induction and the peak magnitude of activated CD8+ T cells impacted the rate of early virus replication, indicating that these responses have direct antiviral function. The ability of these cells to proliferate also impacted pVL set point, indicating that the ability to proliferate is critical in acute infection, as has been shown in chronic infection (Migueles et al., 2002; Ndhlovu et al., 2013). However, increased apoptosis and lack of CD127 expression was observed on the activated CD8+ T cells, indicating that impaired long-term memory may contribute to the inability to fully suppress the virus despite robust induction of HIV-specific CD8+ T cells elicited by onset of viremia, an important focus for future studies. In addition, persistent antigenic exposure may affect resolution of CD8+ T cell activation resulting in impaired CD8+ T cell function and faster HIV disease progression (Cossarizza et al., 2012; Trautmann et al., 2012).
Previous studies of CD8+ T cell responses measured by IFN-γ secretion to HIV peptide antigens in acute infection have largely focused on the period following peak viremia (Appay et al., 2002; Goonetilleke et al., 2009; Liu et al., 2013; Radebe et al., 2014; Trautmann et al., 2012; Turnbull et al., 2009). Those studies suggested that the initial CD8+ T cell response is weak, as measured by ICS or ELISPOT. Here, availability of pre-infection samples and twice weekly sampling allowed us to more precisely define the onset of viremia and the evolution of CD8+ T cell responses. By IFN-γ staining we found that responses were weak during peak activation, and increased once activation returned to baseline. In contrast, the early HIV-specific CD8+ T response was robust by tetramer staining. In one subject for whom 5 different tetramers were available, 36% of all CD8+ T cells were HIV-specific. As responses to many additional potential HIV epitopes could not be tested due to lack of additional epitopes for the class I tetramers used, and lack of any tetramers for four of the subject’s class I alleles, these data likely underestimated the magnitude of HIV-specific responses. The use of tetramers also enabled us to determine that acute infection was associated with impaired IFN-γ production, but this increased following the return of CD8+ T cell activation to baseline, consistent with earlier studies (Radebe et al., 2011; Streeck et al., 2014; Trautmann et al., 2012). It is conceivable that the aforementioned limitations of IFN-γ as readout contributed to the lack of association between breadth or magnitude and viral load set point reported in recent acute infection studies (Radebe et al., 2011; Streeck et al., 2014). Our data show that the use of autologous infected cells as targets and the use of tetramers allowed for a more sensitive estimation of virus-specific CD8+ T cell responses during the early phase of the infection. Previous studies in chronic infection show that the majority of in vivo primed, circulating HIV-specific CD8+ T cells are discordant for cytolysis and cytokine secretion, notably IFN-γ (Kostense et al., 2002; Varadarajan et al., 2011). Defining the mechanism accounting for the impaired IFN-γ production during acute infection will be an important objective of future studies.
In contrast to the robust activation of HIV-specific CD8+ T cells, CD8+ T cells specific for pathogens such as CMV, EBV and influenza virus were minimally activated even when a very high proportion of total CD8+ T cells displayed an activated phenotype. These data suggested that very limited bystander activation was occurring during the acute phase of HIV infection, consistent with previous reports in other acute viral infections and following immunization (Butz and Bevan, 1998; Miller et al., 2008; Murali-Krishna et al., 1998). The modest activation of CMV- and EBV-specific responses, which tend to reactivate during acute viral infections, further supports the notion that most of the observed activation is antigen-driven rather than cytokine-driven bystander activation.
These studies also provide insight into the fate of the earliest CD8+ T cell responses in acute infection. Activated HIV-specific CD8+ T cells expressed CD95, a death receptor that leads to programmed cell death, and down-regulated the pro-apoptotic protein Bcl-2, underscoring their susceptibility to activation induced cell death. Additionally, even in this very early stage of infection HIV-specific CD8+ T cell responses did not upregulate the IL-7 receptor, which promotes long-term survival (Kaech et al., 2003). These data confirm previous reports in humans and SIV models showing a survival defect and the skewed phenotype with a preponderance of pro-apoptotic effector CD8+ T cells in the first few weeks of infection (Mueller et al., 2007; Trautmann et al., 2012). Spontaneous cell death coupled with the pro-apoptotic phenotype exhibited by early responses suggest that premature cell death may be one reason for lack of persistence of early responses and consequently incomplete virus suppression. Although immune escape has also been shown to lead to decreases in HIV-specific CD8+ T cell responses (Liu et al., 2013), this is likely to occur over a more protracted time course and be different for different epitopes, suggesting that apoptosis is the major driver of the uniform decline in activated CD8+ T cells observed here.
In conclusion, these results show that longitudinal sampling of high risk uninfected persons can identify cases of hyperacute infection, allowing for the assessment of the earliest interactions between virus and host. Our data indicate that onset of HIV viremia induces vigorous CD8+ T cell activation and proliferation, the majority of which is HIV specific but would be missed by traditional IFN-γ ELISPOT assays. The more rapidly peak activation is achieved, and the greater the peak activation attained, the lower the subsequent viral load set point. These data thus indicate that rapid, high magnitude HIV-induced CD8+ T cell responses are critical for subsequent immune control of acute infection, and thus have important implications for HIV vaccines.
MATERIALS AND METHODS
Study population and blood samples
Subjects were 18–23 year old HIV-negative women at very high risk of HIV-1 clade C infection in Durban, South Africa. Blood and female reproductive tract (FRT) samples were obtained at pre-infection, and participants attended twice-weekly sessions in which a comprehensive empowerment, life skills and HIV prevention curriculum was provided by trained counselors. At each twice-weekly visit, finger prick monitoring for plasma HIV RNA was performed, with results provided within 24 hours. Upon detection of viremia by NucliSens EasyQ v2.0 assay, participants were contacted, health counseling provided, and additional blood samples collected. For each donor, a pre-infection sample and serial post-infection samples were obtained. Studies of the FRT are reported elsewhere (Anahtar et al., 2015). All the participants provided written informed consent for and the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal and the institutional review board of Massachusetts General Hospital approved the study.
Viruses and synthetic viral peptides
Primary HIV clade C virus was prepared according to a previously described protocol (Ndung’u et al., 2006). Four hundred and ten overlapping 18-mer consensus clade C peptide sequences spanning the entire expressed HIV proteome as well as optimal class I restricted epitopes (http://hiv-web.lanl.gov) were synthesized at the MGH Peptide Core Facility on an automated peptide synthesizer using F-moc technology.
Flow cytometry analysis of T cell activation and proliferation
Fresh or previously frozen PBMCs were first stained with viability dye for 10 minutes, washed and surface stained at room temperature for 20 min. Cells were permeabilized and fixed with intracellular staining reagents according to the manufacturer’s instructions (BD Bioscience) before intracellular staining with antibodies to Bcl-2 and Ki-67. Samples were then washed before acquisition and analysis on a BD LSRFortessa flow cytometer (BD Biosciences). Data analysis was performed using FlowJo software (Treestar).
ELISPOT and ICS staining
ELSPOT assays were performed as described in a matrix system allowing for identification of targeted peptides (Kiepiela et al., 2007). Responses were regarded as positive if they had at least 3 times the mean number of SFC in the 3 negative control wells; positive responses also had be at least 50 SFC/106 PBMCs. For ICS, cells were first stimulated overnight with HIV peptides and stained according to the BD Bioscience ICS protocol. ‘Fluorescence minus one’ (FMO) staining was used to define the cutoff for positivity.
Tetramer/ICS studies
Stimulated PBMCs were stained with HLA class I-peptide tetrameric complexes for 45 min at room temperature, washed in PBS, 0.5 mM EDTA, 1% BSA and surface stained with appropriate surface antibodies for 15 min at room temperature in the dark. Cells were then permeabilized, fixed and intracellularly stained with IFN-γ, IL-2 and TNF-α and analyzed on a BD LSRFortessa flow cytometer.
Generation of CD4+ T cell targets and CD8+ effector T cells
CD4+ T cells were enriched by depleting CD8+ T cells using CD8+ microbeads (Miltenyi Biotech) and rested for 4 hours. CD4+ T cells were stimulated with 5μg/ml of CD3+/CD8+ bi-specific antibody (Chen et al., 2012) in R10/50 for 72 hours before infection with patient isolated clade C viruses for 48 hours. Infection was monitored by flow cytometry after surface staining with CD4 antibody and intracellular staining for HIV-Gag (Kc57-FITC, Beckman Coulter). CD8+ T cells were kept in R10/50 for 5 days prior to the co-culture experiment.
HIV infected cell recognition assay
Freshly isolated or cultured CD8+ T cells were co-cultured with infected or uninfected (mock) autologous CD4 T target cells at a 1:1 ratio. Anti CD107a antibody (BD Bioscience) and the secretion inhibitor monensin used according to the manufacturer’s instructions were added at the beginning of the 12-h stimulation. After overnight incubation, cells were surface stained fixed, permeabilized and intracellularly stained with IFN-γ, TNF- α and IL-2 monoclonal antibodies. Cells were analyzed by flow cytometry and data analysis was performed using FlowJo software.
Statistical analyses
Descriptive statistics, Kruskal Wallis tests, Spearman rank-correlation and Mann-Whitney tests were performed using GraphPad Prism version 5.0b. All tests were two-tailed and p-values of p<0.05 were considered significant. Estimates of rates of viral expansion, viral decline following peak-viremia and initial rate of CD4 decline were obtained by fitting linear mixed-effects models on acute phase data as described before (Ribeiro et al., 2010). The fitting was performed using the Statistical Analysis Toolbox in Matlab (Version R2014a). Viral load set point was defined as average viral loads measured between 2 to 8 months following onset of plasma viremia because this is the interval when viral loads for all are study participants reached steady state (Huang et al., 2012).
Supplementary Material
Acknowledgments
We would like to thank the FRESH participants, as well as the FRESH and HIV Pathogenesis Programme (HPP) laboratory staff. This work was supported by the Bill and Melinda Gates Foundation and the Collaboration for AIDS Vaccine Discovery, the Witten Family Foundation, Dan and Marjorie Sullivan, the Mark and Lisa Schwartz Foundation, Ursula Brunner, Gary and Loren Cohen, the International AIDS Vaccine Initiative (IAVI) (UKZNRSA1001), the NIAID (R37AI067073), and an NIH funded Center for AIDS Research (P30 AI060354), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, and OAR, and the Howard Hughes Medical Institute. We would like to thank Drs. Alan S Perelson and Ruy M Rubeiro for their expert advice on viral growth kinetics calculations, and Dr. Daniel E. Kaufmann, MD for critical reading of the manuscript.
Footnotes
Author contribution
Z.M.N. was responsible for the overall conduct of the study under the supervision of B.D.W.; Z.M.N., T.N. and B.D.W. contributed to the experimental design. Z.M.N. and P.K. did the experiments and Z.M.N analyzed the data. K.D, T.N and B.D.W provided clinical samples. T.N performed virus isolation and titration. S.B., A.M, P.J.R.G., H.K. and A.L. provided tetramers and technical expertise. Z.M.N and H.K did the tetramer staining experiments. Z.M.N and B.D.W wrote the paper and all co-authors contributed to the revisions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Papagno L, Spina CA, Hansasuta P, King A, Jones L, Ogg GS, Little S, McMichael AJ, Richman DD, Rowland-Jones SL. Dynamics of T cell responses in HIV infection. J Immunol. 2002;168:3660–3666. doi: 10.4049/jimmunol.168.7.3660. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nature immunology. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Bangs SC, McMichael AJ, Xu XN. Bystander T cell activation--implications for HIV infection and other diseases. Trends Immunol. 2006;27:518–524. doi: 10.1016/j.it.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. Journal of virology. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nature medicine. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Brumme ZL, Brumme CJ, Carlson J, Streeck H, John M, Eichbaum Q, Block BL, Baker B, Kadie C, Markowitz M, et al. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. Journal of virology. 2008;82:9216–9227. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, et al. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nature immunology. 2012;13:691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Bertoncelli L, Nemes E, Lugli E, Pinti M, Nasi M, De Biasi S, Gibellini L, Montagna JP, Vecchia M, et al. T cell activation but not polyfunctionality after primary HIV infection predicts control of viral load and length of the time without therapy. PLoS One. 2012;7:e50728. doi: 10.1371/journal.pone.0050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Dupuis M, Deschemin JC, Goujard C, Deveau C, Meyer L, Ngo N, Rouzioux C, Guillet JG, Delfraissy JF, et al. Weak anti-HIV CD8(+) T-cell effector activity in HIV primary infection. The Journal of clinical investigation. 1999;104:1431–1439. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva W, Abdool Karim Q. The HIV epidemic in Southern Africa - Is an AIDS-free generation possible? Current HIV/AIDS reports. 2014;11:99–108. doi: 10.1007/s11904-014-0205-0. [DOI] [PubMed] [Google Scholar]
- Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M, Venet A. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. Journal of immunology. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, Ochsenbauer C, Edmonds TG, Kappes JC, Cunningham CK, et al. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. Journal of virology. 2010;84:4998–5006. doi: 10.1128/JVI.00138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. Journal of immunology. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. The Journal of experimental medicine. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. Journal of immunology. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. Journal of immunology. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. The Journal of experimental medicine. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Kagee A, Toefy Y, Jooste S, Cain D, Cherry C. Associations of poverty, substance use, and HIV transmission risk behaviors in three South African communities. Social science & medicine. 2006;62:1641–1649. doi: 10.1016/j.socscimed.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature medicine. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Kostense S, Vandenberghe K, Joling J, Van Baarle D, Nanlohy N, Manting E, Miedema F. Persistent numbers of tetramer+ CD8(+) T cells, but loss of interferon-gamma+ HIV-specific T cells during progression to AIDS. Blood. 2002;99:2505–2511. doi: 10.1182/blood.v99.7.2505. [DOI] [PubMed] [Google Scholar]
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of virology. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MK, Hawkins N, Ritchie AJ, Ganusov VV, Whale V, Brackenridge S, Li H, Pavlicek JW, Cai F, Rose-Abrahams M, et al. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. The Journal of clinical investigation. 2013;123:380–393. doi: 10.1172/JCI65330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, Margolick JB, Phair JP, Mellors JW. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. The Journal of infectious diseases. 2000;181:872–880. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2001;28:454–457. doi: 10.1097/00042560-200112150-00008. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Connors M. Success and failure of the cellular immune response against HIV-1. Nature immunology. 2015;16:563–570. doi: 10.1038/ni.3161. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature immunology. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science (New York, N Y. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Ndhlovu ZM, Chibnik LB, Proudfoot J, Vine S, McMullen A, Cesa K, Porichis F, Jones RB, Alvino DM, Hart MG, et al. High-dimensional immunomonitoring models of HIV-1-specific CD8 T-cell responses accurately identify subjects achieving spontaneous viral control. Blood. 2013;121:801–811. doi: 10.1182/blood-2012-06-436295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndung’u T, Sepako E, McLane MF, Chand F, Bedi K, Gaseitsiwe S, Doualla-Bell F, Peter T, Thior I, Moyo SM, et al. HIV-1 subtype C in vitro growth and coreceptor utilization. Virology. 2006;347:247–260. doi: 10.1016/j.virol.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM, Sekaly RP, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Current opinion in HIV and AIDS. 2006;1:43–49. doi: 10.1097/01.COH.0000194106.12816.b1. [DOI] [PubMed] [Google Scholar]
- Radebe M, Gounder K, Mokgoro M, Ndhlovu ZM, Mncube Z, Mkhize L, van der Stok M, Jaggernath M, Walker BD, Ndung’u T. Broad and persistent Gag-specific CD8+ T-cell responses are associated with viral control but rarely drive viral escape during primary HIV-1 infection. Aids. 2014 doi: 10.1097/QAD.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radebe M, Nair K, Chonco F, Bishop K, Wright JK, van der Stok M, Bassett IV, Mncube Z, Altfeld M, Walker BD, Ndung’u T. Limited immunogenicity of HIV CD8+ T-cell epitopes in acute Clade C virus infection. The Journal of infectious diseases. 2011;204:768–776. doi: 10.1093/infdis/jir394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, Perelson AS. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. Journal of virology. 2010;84:6096–6102. doi: 10.1128/JVI.00127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science (New York, N Y. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Streeck H, Jolin JS, Qi Y, Yassine-Diab B, Johnson RC, Kwon DS, Addo MM, Brumme C, Routy JP, Little S, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. Journal of virology. 2009;83:7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H, Lu R, Beckwith N, Milazzo M, Liu M, Routy JP, Little S, Jessen H, Kelleher AD, Hecht F, et al. Emergence of individual HIV-specific CD8 T cell responses during primary HIV-1 infection can determine long-term disease outcome. Journal of virology. 2014 doi: 10.1128/JVI.02016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Mbitikon-Kobo FM, Goulet JP, Peretz Y, Shi Y, Van Grevenynghe J, Procopio FA, Boulassel MR, Routy JP, Chomont N, et al. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120:3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull EL, Wong M, Wang S, Wei X, Jones NA, Conrod KE, Aldam D, Turner J, Pellegrino P, Keele BF, et al. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. Journal of immunology. 2009;182:7131–7145. doi: 10.4049/jimmunol.0803658. [DOI] [PubMed] [Google Scholar]
- Varadarajan N, Julg B, Yamanaka YJ, Chen H, Ogunniyi AO, McAndrew E, Porter LC, Piechocka-Trocha A, Hill BJ, Douek DC, et al. A high-throughput single-cell analysis of human CD8(+) T cell functions reveals discordance for cytokine secretion and cytolysis. The Journal of clinical investigation. 2011;121:4322–4331. doi: 10.1172/JCI58653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B, McMichael A. The T-Cell Response to HIV. Cold Spring Harbor perspectives in medicine. 2012 doi: 10.1101/cshperspect.a007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, Ogg GS, Allen RL, Goulder PJ, Kelleher A, Sewell AK, O’Callaghan CA, Rowland-Jones SL, Callan MF, McMichael AJ. Oligoclonal expansions of CD8(+) T cells in chronic HIV infection are antigen specific. The Journal of experimental medicine. 1998;188:785–790. doi: 10.1084/jem.188.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.