Abstract
Background
Neurogenesis in the brain of adult mammals occurs throughout life in two locations: the subventricular zone of the lateral ventricle and the subgranular zone of the dentate gyrus in the hippocampus. RNA interference mechanisms have emerged as critical regulators of neuronal differentiation. However, to date, little is known about its function in adult neurogenesis.
Results
Here we show that the RNA interference machinery regulates Doublecortin levels and is associated with chromatin in differentiating adult neural progenitors. Deletion of Dicer causes abnormal higher levels of Doublecortin. The microRNA pathway plays an important role in Doublecortin regulation. In particular miRNA-128 overexpression can reduce Doublecortin levels in differentiating adult neural progenitors.
Conclusions
We conclude that the RNA interference components play an important role, even through chromatin association, in regulating neuron-specific gene expression programs.
Electronic supplementary material
The online version of this article (doi:10.1186/s12868-015-0198-7) contains supplementary material, which is available to authorized users.
Keywords: RNAi, Dicer, Argonaute, Doublecortin, miRNA-128, Adult neurogenesis
Background
The precise regulation of proliferation, survival, migration and differentiation of neural stem cells (NSCs) and neural progenitors is crucial for proper formation of the mammalian brain during embryonic, postnatal and adult stages [1–5]. In the adult mouse brain, NSCs persist throughout life in the subgranular zone of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) of the lateral ventricle. In these regions, NSCs generate mature neurons through a complex sequence of developmental steps including the division of a precursor cell and a multi-step process (proliferation, differentiation, migration, targeting, and synaptic integration) that ends with the formation of a postmitotic functionally integrated new neuron [6]. These developmental stages can be distinguished on the basis of the expression of stage-specific marker proteins including the microtubule binding protein Doublecortin (Dcx). In the adult mouse brain, Dcx is almost exclusively expressed by immature newborn neurons in the DG and the SVZ/Olfactory Bulb-system and is commonly used to distinguish immature neurons from non-neuronally committed precursors and to estimate neurogenic activity [7–10]. Perturbation of adult neurogenesis is associated with learning memory deficits, dysregulation of mood, and has been implied in the pathogenesis of neuropsychiatric disorders [11, 12]. Hence, revealing the regulation of genetic programs crucial for NSCs expansion and lineage commitment in the adult brain can provide the molecular basis for developing effective methods for NSC-based and adult neurogenesis-targeted therapies.
RNA interference (RNAi) mechanisms are a well-conserved phenomenon in eukaryotes and act at multiple levels to regulate gene expression [13–16]. A large number of small RNA classes have been identified, including microRNAs (miRNAs), small interfering RNAs (siRNAs) and Piwi-interacting RNAs (piRNAs). These classes differ in their biogenesis, their modes of target regulation and in the biological pathways they regulate [17–19]. In mammals small RNA silencing pathways depend on two groups of genes encoding the key proteins of the Dicer and Argonaute families [18].
The RNase III enzyme Dicer is required for the processing of short (21–22 nucleotides) micro-RNAs (miRNAs) and small interfering RNAs (siRNAs) from double stranded RNA precursors. Dicer-generated RNAs trigger the degradation of complementary mRNAs or prevent their translation [13–15]. In addition accumulating evidence indicates that RNAi components and small RNAs participate in nuclear processes such as transposon regulation, heterochromatin formation, genome stability and transcription [20–23]. Defining the role of Dicer generated small RNAs in mammalian development is complicated by embryonic lethality of constitutive Dicer knockouts in mice [24, 25]. Mouse embryonic stem cells in the absence of Dicer fail to differentiate in vitro and do not contribute to mouse development in vivo [26]. This highlights the importance of siRNAs and miRNAs in the regulation of gene expression during cell differentiation.
Here we show that the RNAi machinery regulates Dcx levels and is associated with chromatin in differentiating adult neural progenitors. Deletion of Dicer causes abnormal higher levels of Dcx expression and miRNA-128 over-expression can down-regulate Dcx levels in differentiating adult neural progenitors. In addition a chromatin-binding assay reveals that some of Dicer and Argonaute-2 associate to chromatin in differentiating adult neural progenitors. We conclude that the Dicer/Argonaute-2 RNAi pathway plays an important role, potentially even through chromatin association, in regulating gene expression programs in the neural stem cell lineage.
Results
Adult neural stem cells lacking Dicer have higher level of Doublecortin
To study RNAi machinery function in mouse adult neural stem cells we isolated neural progenitor cells (NPCs) from the SVZ of 8 weeks old Dicer cKO (Dicerflox/flox) mice [27]. The deletion of the LoxP-flanked exons 20–21 in the Dicer locus was induced by transducing NPCs with the HTN-Cre protein, a recombinant fusion protein able to cross the cell and nuclear membrane [28]. Consistent with the almost complete deletion of the Dicer locus (Additional file 1: Fig. S1A) the HTN-Cre treated cells showed a marked decrease in the levels of mature miRNAs (Fig. 1a), of Dicer transcript (Fig. 1b), and of Dicer protein (Fig. 1c) indicating that activity of the RNAi machinery was severely impaired. Along with the Dicer transcript we analyzed the levels of additional transcripts (Fig. 1b), namely Actin as a housekeeping gene, Lin41 as miRNA-dependent gene [29], Dcx and Sox11 as possible miRNA targets based on the miRNA target prediction algorithm TargetScan [30]. The Actin transcript levels were almost unchanged; Lin41 and Sox11 transcripts were slightly up-regulated, but strikingly Dcx showed a 47.5 (±9.7 SEM) fold increase in Dicer null cells compared to control cells. Then we asked if the increase of the Dcx transcript in Dicer null cells resulted also in an increase of the Dcx protein level. In control NPCs, the Dcx protein was detectable only in differentiating cells (Fig. 1c) but, consistent with the qPCR data, the Dicer −/− NPCs had already detectable Dcx protein. Most of the Dicer −/− NPCs die upon induction of differentiation and therefore this condition was not included in the further analysis.
Fig. 1.
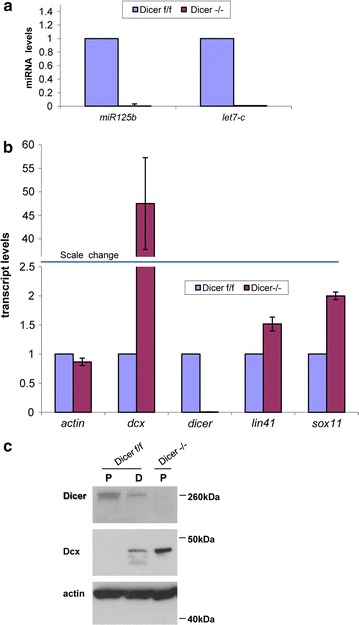
Dcx is up-regulated upon Dicer deletion in adult neural stem cells. a–c The samples analyzed were adult neural progenitor cells (NPCs) Dicer flox/flox (f/f) or Dicer −/−. Dicer deletion was obtained by transducing Dicer f/f cells with HTN-Cre protein. a Taqman quantitative RT-PCR of miR125b and let7-c mature miRNA transcripts. Transcript levels were normalized against the housekeeping gene snoRNA55 and calculated respect to Dicer f/f (control) that is set to 1. n = 3, bars represent the mean ± standard error of the mean. b Quantitative RT-PCR of the indicated transcripts. Transcript levels were normalized against the housekeeping gene Srp14 and calculated respect to Dicer f/f (control) that is set to 1. The blue horizontal bar indicates the scale change in the y-axis. n = 3, bars represent the mean ± standard error of the mean. c Western blot showing a representative picture of the Dicer, Dcx and Actin (loading control) protein levels in NPCs (P) or differentiating NPCs (D; 4 days after growth factor withdrawal) of the indicated genotype. Most of the Dicer −/− cells die upon induction of differentiation and therefore are not included in the analysis. Three independent biological samples have been analyzed. Shown are representative pictures
Overall our results show that in NPCs the ablation of Dicer and the consequent impairment of the RNAi machinery result in the up-regulation of the Dcx transcript and protein.
Doublecortin is upregulated upon Dicer deletion in hippocampal newborn neurons
In order to understand if the RNAi machinery impairment can deregulate Dcx expression also in the in vivo context we induced Dicer deletion in the adult hippocampal neurogenic lineage by injecting adult (8 weeks old) Dicer cKO (Dicerflox/flox) mice with a recombinant mouse moloney leukemia-retrovirus bicistronically encoding for GFP and Cre-recombinase into the septal hippocampal dentate gyrus. Heterozygous Dicer cKO (Dicerflox/+) mice injected with the same retroviral preparation served as controls. Animals were sacrificed at 28 days post injection (dpi). Brain sections were immunostained for the immature neuronal marker Dcx and for GFP, to identify virus-transduced cells (Fig. 2a, b). Consistent with previous studies most of virus-transduced cells in heterozygous Dicer cKO readily down regulated Dcx at 28 dpi [31, 32]. Interestingly, we found a significant increase (Fig. 2c) of Dcx/GFP double-positive cells in the Dicer cKO. We also checked another immature neuronal marker, Sox11, but did not observe significant differences of Sox11/GFP double positive cells in the Dicer cKO respect to the control (control: 49.6 % ± 11.3 SEM; Dicer cKO: 63.9 % ± 9.7 SEM; n = 4, two-tailed t test p = 0.4). The lack of significant change in Sox11 expression is in line with our in vitro analysis (Fig. 1b) where Dicer −/− NPCs show minor increase in Sox11 expression. Additionally we observed a consistent reduction (about 40 %) of virally transduced GFP positve cells in the Dicer cKO (not shown). Although in the present experimental set up we cannot fully exclude differences in the virus transduction efficiency between different experimental groups this would be suggestive of reduced viability of Dicer deficient neurons. Taken together these data indicate that in newborn neurons Dcx levels are sensitive to the levels of Dicer and thus to the functionality of the RNAi machinery.
Fig. 2.
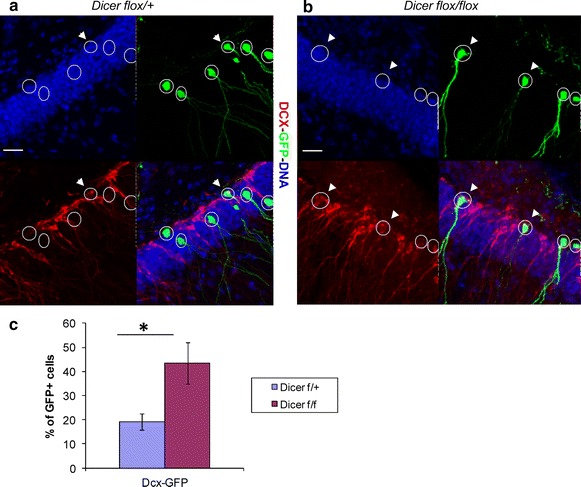
Dcx is up-regulated upon Dicer deletion in hippocampal new born neurons. a, b Representative pictures of the immunostaining for Dcx and GFP on hippocampal regions of Dicer cKO mice transduced with Cre-recombinase. a Dicer flox/+ mice (control) 28 dpi. b Dicer flox/flox mice (experiment) 28 dpi. Transduced cells are GFP positive (green) and identified by white circles. Arrowheads indicate Dcx-expressing (red) among the Cre-transduced (green) cells. Single channel and overlay pictures are shown. Scale bar 25 µm. c Quantification of Dcx/GFP double positive cells among the virus-transduced cells. Bars show the average ± the standard error of the mean (n = 4). At least 100 virus-transduced cells per group of four animals were analyzed. Two-tailed t test was applied for statistical analysis. Asterisks indicate statistically relevant differences; p < 0.05
Overexpression of miRNA-128 reduces Doublecortin levels in differentiating adult neural stem cells
Our in vitro and in vivo (Figs. 1, 2) experiments show that the impairment of the RNAi machinery through the deletion of Dicer in NPCs is paralleled by abnormally elevated levels of Dcx. The Dcx transcript has been shown to be regulated by the RNAi machinery through miRNA-dependent pathways that are severely reduced upon Dicer deletion (Fig. 1a). Specifically, miR-134 [33] was shown to regulate Dcx in mouse embryonic brain tissues, while miR-128 [34] was found to modulate Dcx levels in a human neuroblastoma cell line. Of these, we found only miR-128 to be expressed in adult mouse NPCs (Additional file 2: Fig. S2) in agreement with a previous report [35] showing the presence of miR-128 but not miR-134 in the neurogenic areas of the mouse adult brain. We next asked whether miR-128 could target and regulate Dcx levels in adult NPCs. To this purpose we created a miR128-RFP expression vector (see “Methods”). To check its functionality we transfected this vector into Neuro2A cells, which resulted in a marked increase in miR-128 levels (Fig. 3a) along with decreased levels of Dcx protein but not the transcript (Fig. 3b, c; Additional file 3: Fig. S3). This indicates that the vector is able to increase the miR-128 levels leading to Dcx down-regulation by translational repression as it has been described for many miRNAs [36]. We then transfected the miR-128-RFP or miR-Ctr-RFP (negative control containing a scrambled miRNA sequence) in adult NPCs (derived from the SVZ of 8-weeks old mice) and let them differentiate for 6 days. After fixation the cells were immunostained for Dcx, GFAP (a marker for astroglial cells) and RFP marking the transfected cells (Fig. 4a). Interestingly, we found a significant decrease in the fraction of Dcx positive cells among the transfected cells when miR-128 is overexpressed (Fig. 4b). Notably, the fraction of GFAP positive cells among the transfected ones was not significantly affected. Our results show that miR-128 overexpression reduces the levels of Dcx in differentiating NPCs indicating that miR-128 can target and potentially take part in the regulation of Dcx levels in adult neurogenesis.
Fig. 3.
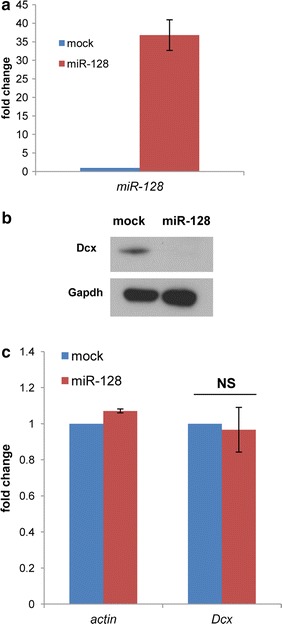
Overexpression of miRNA128 reduces Dcx protein levels in N2A cells. a–c The samples analyzed were mock-treated Neuro2A cells (control transfection without miRNA-128-RFP expressing plasmid) or Neuro2A cells treated with miRNA-128-RFP expressing plasmid. a Taqman quantitative RT-PCR of the mature miRNA-128 transcripts normalized against the housekeeping small non-coding RNA snoRNA55. Transcript levels are indicated as fold change respect to the control (mock) that is set to 1. n = 3, bars represent the mean ± standard error of the mean. b Western blot showing the Dcx and Gapdh (loading control) protein levels in mock and miRNA-128 over-expressing cells. c Quantitative RT-PCR of the indicated transcripts. Transcript levels are indicated as fold change respect to the control (mock) that is set to 1. Transcripts were normalized against the housekeeping gene Srp14. n = 4, bars represent the mean ± standard error of the mean. Two-tailed t test was applied for statistical analysis. NS not significant; p = 0.4
Fig. 4.
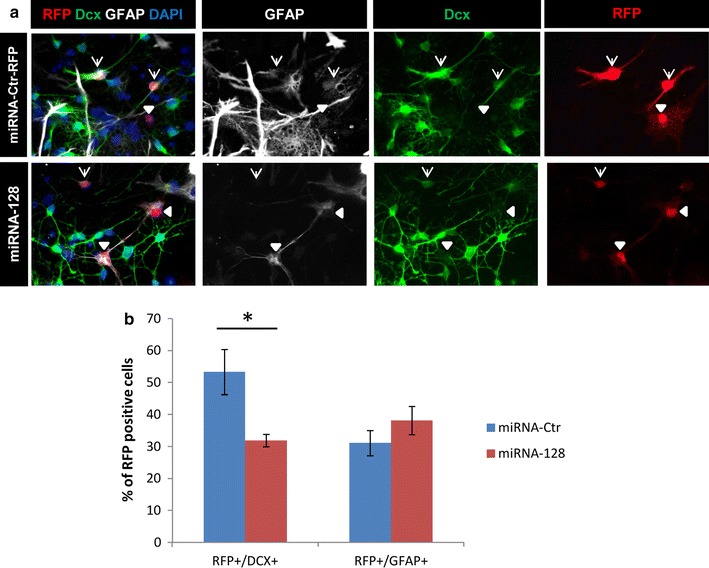
Overexpression of miRNA128 reduces Dcx protein levels in “in vitro” differentiating adult neural stem cells. a, b Adult neural progenitor cells were transfected with miRNA-Ctr-RFP (control) or mirRNA-128-RFP (experiment) expressing plasmids and induced to differentiate by growth factor withdrawal. Analysis was done 6 days later. a Representative pictures of the immunostaining of Dcx, GFAP and RFP on control and experiment cells. DAPI visualizes the DNA. Transfected cells are RFP positive. Arrows indicate Dcx/RFP positive cells; arrowheads indicate GFAP/RFP positive cells. b Quantification of Dcx and GFAP among the transfected cells. Bars show the average ± the standard error of the mean (n = 3). The percentage of marker-positive cells among the total RFP-positive population was calculated on three biological replicates. At least 100 cells per experimental group were analyzed. Two-tailed t test was applied for statistical analysis. Asterisks indicate statistically relevant differences; p < 0.05
RNAi components are associated with chromatin in differentiating adult neural stem cells
The RNAi machinery regulates gene expression mainly in the cytoplasm but has been reported to act also in the nucleus [20, 22, 23, 37, 38]. In order to assess if the main RNAi components could operate in the nucleus of NPCs we used a chromatin binding assay [23, 39] on undifferentiated and differentiating NPCs. This assay has been already used by us and others to predict the chromatin association of RNAi components [22, 23]. Chromatin associated proteins will be found in TritonX-100 resistant (P1) and, subsequently, in DNase and high salt (S2) extracted fractions (Fig. 5a). Indeed, a substantial portion of RNA Polymerase II (Pol II) is detected in these fractions (Fig. 5b). In contrast, most of Dicer and Argonaute-2 (Ago2) are found in the TritonX-100 soluble fraction (S1), along with β-catenin and glyceraldehyde 3-phosphate dehydrogenase (Gapdh), a marker for proteins not associated with chromatin (Fig. 5b). In differentiating NPCs (Fig. 5c) the markers Pol II and Gapdh are found in the same fraction as in undifferentiated cells. In contrast to undifferentiated NPCs, β-catenin was detected also in the chromatin fractions (P1, S2) consistent with the activation of the Wnt pathway during NPCs differentiation [40–42]. Interestingly, also Dicer and Ago2 display a differential fractionation profile depending on the differentiation status of the NPCs; thus Dicer and Ago2 were also found in the chromatin fraction P1 in differentiating NPCs. In contrast to Pol II and β-catenin, Dicer and Ago2 are found almost exclusively in the P1 but barely in the S2 chromatin fraction indicating that TritonX-100 is sufficient to fully release these proteins most likely due to a looser chromatin association of Dicer and Ago2 compared to Pol II and β-catenin.
Fig. 5.
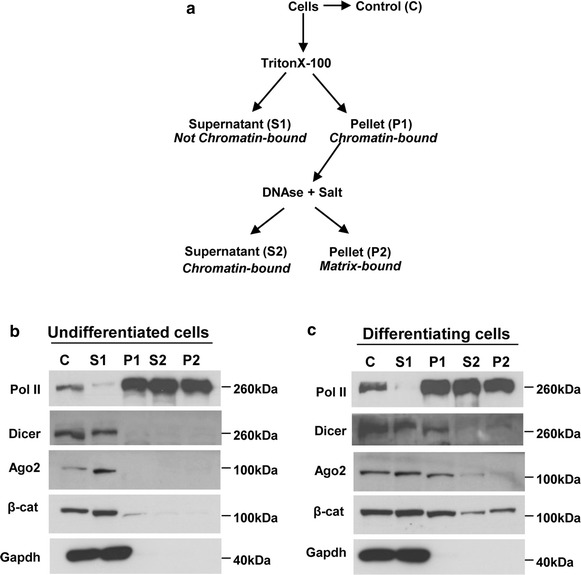
RNAi components are associated with chromatin in differentiating adult neural stem cells. a Scheme of the procedure followed to fractionate neural progenitor cells. Insoluble fractions (pellets) were dissolved in RIPA buffer (see “Methods”). Chromatin associated proteins should be found in fractions P1 and S2. b, c Equivalent amounts of the resulting protein samples from undifferentiated or differentiating (3 days) adult neural progenitor cells were analyzed by western blot for the presence of the indicated proteins. Gapdh (glyceraldehyde 3-phosphate dehydrogenase) serves as a chromatin unbound marker; Pol II (RNA Polymerase II) is a chromatin bound marker. β-cat β-catenin, Ago2 Argonaute-2. Three independent biological samples have been analyzed. Shown are representative pictures
Thus, the Dicer/Ago2 complex displays differential association with chromatin of adult NPCs depending on their differentiation status, raising the possibility that nuclear RNAi may contribute to the control of gene expression during specific developmental stages of adult neurogenesis.
Discussion
The RNAi machinery has emerged as an important regulator of neuronal differentiation but to date its contribution in adult neurogenesis is still not fully understood. We further probed the function of the RNAi machinery by loss-of-function, gain-of-function and biochemical approaches.
We show that in adult NPCs the ablation of Dicer results in a massive up-regulation of the Dcx transcript and protein (Figs. 1, 2). This suggests that Dcx levels are heavily influenced directly or indirectly by RNAi activity. In favor of a direct regulation, Dcx mRNA has been shown to be targeted by different miRNAs [33, 34], which—in absence of Dicer—would not be matured and consequently would not be able to target Dcx mRNA for degradation or translational inhibition. In line with this view we show that overexpression of the miR-128 in differentiating adult NPCs causes the reduction of the Dcx levels (Fig. 4). However miR-128 and Dcx are not mutually exclusive in our in vitro cultured NPCs. Indeed miR-128 maintains similar level of expression also in differentiating NPCs (Additional file 2: Fig. S2) when Dcx is physiologically expressed (Fig. 1c). Like many miRNAs, miR-128 has been show to have relatively modest effects on its targets [43]. Thus, in differentiating NPCs, miR-128 is likely responsible to fine-tune rather than switching off Dcx, by repressing but without eliminating it. Notably the miRNA algorithm TargetScan (http://www.targetscan.org) identifies, in addition to miR-128, let-7 and miR-29 as potential regulators of Dcx. Both these miRNA are, similarly to miR-128, expressed in the neurogenic areas of the adult mouse brain [35] making them possible additional regulators of Dcx expression.
Argonaute directly binds the mature miRNA processed by Dicer and seeks target mRNAs that have complementarity to the miRNA. If complementarity exists in the central region of the miRNA (nucleotides 9–11) then the mRNA target can be cleaved via the endonuclease activity of Ago2. Differently Argonaute is recruited to a complex containing GW182 within cytoplasmic P bodies where translational repression occurs [36]. Notably miR128 lacks this complementarity (nucleotides 9–11) in Dcx target sites predicted by the miRNA algorithms TargetScan and microRNA.org [44] (http://www.microrna.org). In agreement with this prediction we found only the Dcx protein but not the transcript being down regulated upon miR128 overexpression (Fig. 3). Therefore the massive up-regulation of the Dcx transcript observed in NPCs upon Dicer deletion (Fig. 1) is hardly explained only by a decrease of miRNAs targeting Dcx but it likely involves additional layers of RNAi-dependent regulation.
miRNAs have been implied in the modulation of differentiation and proliferation of adult NPCs [45–50]. We kept adult Dicer-null NPCs in culture for weeks without visible defects but we observed a massive cell death upon stimulation of differentiation by growth factor withdrawal (data not shown), arguing that in particular differentiating NPCs are highly dependent on Dicer. In line with a particular importance of Dicer for differentiating cells, previous studies [51, 52] showed that Dicer is strictly required for embryonic NPCs differentiation. The ability of Dicer-null NPCs to generate both neurons and glia was, however, restored by the reintroduction of Dicer, demonstrating that these cells were not irreversibly transformed despite the absence of Dicer and microRNAs [51]. Similarly, reintroduction of Dicer was able to rescue the differentiation impairment of mouse embryonic stem cells Dicer −/− [53]. The dependency of NPCs differentiation on Dicer may not only be related to Dicer’s crucial function in miRNA biogenesis. RNA interference components including Dicer have been implicated in nuclear related functions such as transposon regulation, heterochromatin formation, genome stability and transcription [20–23]. Notably, by using a chromatin binding assay we observed that some of the key RNAi components, Dicer and Argonaute-2, associate to chromatin in differentiating adult NPCs (Fig. 5). Although a ChIP-seq experiment (chromatin immunoprecipitation followed by next generation sequencing) will be required to reveal the Dicer and Ago-2 binding sites on chromatin, this already suggests that the critical role of RNAi in adult NPCs differentiation might be also linked to its chromatin-association.
Interestingly, transposable elements are expressed and active in the brain. LINE-1 elements, for example, are actively mobilized in normal mammalian brains during neurogenesis [54–56], leading to genetic heterogeneity, which might be of functional relevance. However, because transposable elements are capable of jumping into new positions in the genome they are potentially detrimental. Transposable element activation has been correlated with several neurodegenerative diseases [57–64]. Interestingly it has been recently shown that mutations in the RNAi key component Argonaute-2 resulted in increased transposons expression in the Drosophila brain leading to detrimental effects such as accelerate memory impairment and shortened life span [65]. It is tempting to speculate that RNAi components might have a similar function in the mammalian brain by acting as a barrier against excessive transposable element activity during neurogenesis.
Conclusions
Our data clearly show that RNAi machinery takes part in regulating neuron-specific developmental programs, in which the key RNAi components Dicer and Ago-2 display a dynamic chromatin association.
Methods
Animals
All experiments were performed in accordance with the European Communities Council Directive (86/609/EEC). Stereotactic injections of retroviruses into the brain of adult mice were approved by the Government of Upper Bavaria. According to German law, the approval of the animal experiments is given by a committee of the Government of Upper Bavaria, which checks (1) the conformity of the proposed study with German animal protection law, and (2) the relevance and ethical conformity. The relevant department in the Government of Upper Bavaria that issued the license for the experiments can be found at:
http://www.regierung.oberbayern.bayern.de/aufgaben/umwelt/verbraucher/ansprech/.
8–12-week-old Dicer cKO [27] or C57BL/6 mice were used. Mice were grouped housed in standard cages under a 12 h light/dark cycle and had ad libitum access to food and water.
Retrovirus preparation
pCAG–GFP–IRES–Cre was generated from the pCAG–GFP vector [66] by replacing the GFP coding sequence with cDNA for GFP and Cre recombinase as well as an internal ribosomal entry site (IRES). Retroviruses were generated as described previously [67]. Virus-containing supernatant was harvested four times every 48 h after transfection and concentrated by two rounds of ultracentrifugation. The obtained viral titers ranged between 105 and 108 colony forming units (cfu)/ml, dependent on the harvest number and the construct.
Stereotactic injections
All experiments were conducted under the condition that animals have ad libitum access to a running wheel until day 6 post injection. This measure was necessary to enhance proliferation and thus the number of transduced cells. Mice were deeply anesthetized with a mixture of fentanyl (0.05 mg/kg bodyweight), midazolam (5 mg/kg bodyweight) and medetomidine (0.5 mg/kg bodyweight). Anaesthesia was reverted with a mixture of buprenorphine (0.1 mg/kg bodyweight), atipamezole (2.5 mg/kg bodyweight) and flumazenil (0.5 mg/kg bodyweight). Mice were stereotactically injected with 1 µl of the CAG–GFP–IRES–Cre retroviruses (titer 1 × 108 cfu/ml) into the left and right dentate gyrus (coordinates from bregma were −1.9 mm anterior/posterior, ±1.6 mm medial/lateral, −1.9 mm dorsal/ventral from dura). Group size was n = 4 mice for each experimental group.
Tissue processing
Mice were sacrified using CO2. They were perfused with PBS, pH 7.4, for 5 min, followed by 4 % paraformaldehyde (PFA) for 5 min. Brains were removed and post fixed in 4 % PFA for 12 h at 4 °C and were subsequently transferred to a 30 % sucrose solution. Forty-micrometer-thick coronal brain sections were cut using a sliding microtome (Leica Microsystems). Brain sections were stored at −20 °C in cryoprotectant solution (0.05 M phosphate butter, 25 % ethylenglycol v/v, 25 % glycerol v/v).
Histology and counting procedures
Sections were blocked in Tris buffered saline (TBS) supplemented with 3 % normal donkey serum and 0.25 % Triton X-100 for 60 min. Brain sections were incubated in blocking solution containing the primary antibodies at the appropriate dilutions at 4 °C for 48 h. Primary antibodies against the following antigens were used: Dcx (rabbit, 1:250; Abcam ab18723), GFP (chicken, 1:500; Aves Labs GFP-1020), Sox11 (goat, 1:500; Santa Cruz sc-17347). After three washes in TBS, samples were incubated in blocking solution containing the secondary antibody coupled to Cy3, Cy5 or FITC, for 2 h at room temperature. Secondary antibodies were obtained from The Jackson Laboratory and were used at a dilution of 1:250 after resuspension in 200 µl of H2O and 200 µl of glycerol. Samples were washed three times with TBS, incubated in 10 mg/ml DAPI (4′,6-diamidino-2-phenylindole; 1:10,000; Sigma-Aldrich) for 10 min, and mounted in Aqua PolyMount (Polysciences). To characterize the phenotype of the retrovirally transduced cells, equidistant sections containing the dentate gyrus were selected and stained. Transduced cells were identified based on the expression of GFP and were analyzed by confocal microscopy for immunoreactivity of the respective markers (n > 100 cells per group of at least four animals). The counting procedure was carried out blindly. Confocal single-plane images and Z-stacks were taken on a Leica SP5 confocal microscope.
Cell culture
Neural stem/progenitor cells were isolated from adult (8–12 weeks) hippocampal tissue or subventricular area using the protocol described in [68]. Cells were cultured on poly-d-lysine (PDL; 10 µg/ml; Sigma) and laminin (5 µg/ml; Invitrogen) coated plates in DMEM/F-12 medium (Invitrogen) containing B27 supplement (Invitrogen), 8 mM HEPES buffer, 1× penicillin/streptomycin/fungizone (Invitrogen), 20 ng/ml epidermal growth factor (EGF; PeproTech), and 20 ng/ml basic fibroblast growth factor (FGF2; PeproTech). Cultures were supplemented with growth factors every other day. Differentiation was induced by growth factor withdrawal.
Dicer deletion by HTN-Cre
For deletion experiments, Dicer cKO neural stem/progenitor cells were seeded on PDL/laminin-coated 6-well plates and after 6 h transduced with 1 µM HTN-Cre protein [28]. At 18 h after transduction, growth medium was replaced and the cells expanded to reach the required amount. The Cre-mediated deletion of the Dicer locus was verified by DNA PCR using the following primer pairs: for Dicer-flox, forward 5′-CCATTTGCTGGAGTGACTCTG-3′ and reverse 5′-TAAATCTGGCAAGCGAGACG-3′ (product size about 400 bp); for Dicer-Δ: forward 5′-AGTAATGTGAGCAATAGTCCCAG-3′ and reverse as Dicer-flox (it gives a product only in case of recombination, size is about 350 bp). PCR amplifications were carried out in presence of 5 % (v/v) dimethyl sulfoxide. Differentiation was induced by growth factor withdrawal and harvested for analysis 4 days later. For western blot 20 µg of protein extract were analyzed. Proteins of interest were detected with the following antibodies: rabbit anti-Dicer [1:1000, kindly provided by Chrysi Kanellopoulou [26]; rabbit anti-Dcx (Abcam ab18723); rabbit anti-Actin (1:4000, Abcam ab8227)].
miRNA-128 overexpression
Neural stem/progenitor cells were isolated from the subventricular zone of 8-weeks old C57BL/6 mice as previously described [69] with the only difference that FGF2 and EGF (Invitrogen) were added both at 20 ng/ml to the B27-supplemented culture medium. Cells were seeded on Matrigel-coated 24-well plates (BD Bioscience) at the density of 1.5 × 10e5 cells per well and transfected 24 h later with a miRNA-128-RFP plasmid or a control miRNA-Ctr-RFP plasmid using Lipofectamine 2000 (Life Technologies; ratio DNA/lipofectamine 1:4). Differentiation was induced by growth factor withdrawal 24 h after transfection. Cells were harvested for analysis 7 days after transfection. Cultures were fixed with 4 % PFA for 15 min. Primary antibodies against the following antigens were used: DCX (guinea pig, 1:2000, Millipore AB5910), GFAP (mouse, 1:500; Sigma G3893). Primary antibodies were applied overnight at 4 °C in 10 % serum, 0.5 % Triton X-100 in PBS. Fluorescent secondary antibody was used according to the manufacturer’s protocol (Jackson ImmunoResearch). Single-plane images were taken on a Zeiss Axioplan fluorescence microscope. The percentage of marker-positive cells among the total RFP-positive population was determined for three wells within three different biological replicates. Approximately from ten to twelve randomly selected fields were evaluated for each condition in each experiment (n > 100 cells per experimental group). The counting procedure was carried out blindly.
Neuro2A cells were grown in Opti-MEM medium (Life Technologies). Cells were seeded on 6-well plates and after 24 h transfected with or without miRNA-128-RFP plasmid using Lipofectamine 2000 (Life Technologies; ratio DNA/lipofectamine 1:4). Cells were harvested for analysis 48 h after transfection. For western blot 20 µg of protein extract were analyzed. Proteins of interest were detected with the following antibodies: rabbit anti-Dcx (Abcam ab18723); mouse anti-Gapdh (1:000, clone 6C5, Santa Cruz 32233).
Dcx knock-down
Neuro2A cells were grown in Opti-MEM medium (Life Technologies). Cells were seeded on 10 cm plates and after 24 h transfected with a shRNA-Dcx plasmid or a control shRNA-Ctr plasmid using Lipofectamine 2000 (Life Technologies; ratio DNA/lipofectamine 1:5). Cells were harvested for analysis 72 h after transfection. shRNA-expressing vectors were previously described [70]. For western blot 20 µg of protein extract were analyzed. Proteins of interest were detected with the following antibodies: rabbit anti-Dcx (Abcam ab18723); rabbit anti-Actin (1:4000, Abcam ab8227).
RNA isolation and RT-qPCR
Total RNA was isolated using Trizol reagent (Invitrogen). cDNA was synthesized using QuantiTect Reverse Transcription Kit (Qiagen) including DNA elimination step. Real-time qPCRs were performed on a StepOne device (Applied Biosystems). Power SYBR Green PCR Master Mix (Applied Biosystems) was used for detection. Primers for qPCR were as follows: Sox11 forward, 5′-CCC TGT CGC TGG TGG ATA AG-3′ and reverse, 5′-GGT CGG AGA AGT TCG CCT C-3′; DCX forward, 5′-TGC TCA AGC CAG AGA GAA CA-3′ and reverse, 5′-CTG CTT TCC ATC AAG GGT GT-3′; Srp14 forward, 5′-CAG CGT GTT CAT CAC CCT CAA-3′ and reverse, 5′-GGC TCT CAA CAG ACA CTT GTT TT-3′; Actin forward, 5′-TTG CTG ACA GGA TGC AGAAG-3′ and reverse, 5′-ACA TCT GCT GGA AGG TGG AC-3′; Lin41 forward, 5′-CCC TTC TCC ATT CTC TCG GTG-3′ and reverse, 5′-AGA TGG GGA CAG AGC AGG TGT-3′; Dicer forward, 5′-AAT TGG CTT CCT CCT GGT TAT-3′ and reverse, 5′-GTC AGG TCC TCC TCC TCC TC-3′; Sox2 forward, 5′-GCG GAG TGG AAA CTT TTG TCC-3′ and reverse, 5′-CGG GAA GCG TGT ACT TAT CCT T-3′; Nestin forward, 5′-CCT TTC TTC TGT GGC TCA CC-3′ and reverse, 5′-TCA TCA TTG CTG CTC CTC TG-3′. Quantification was normalized to the housekeeping gene Srp14.
miRNA cDNA synthesis and amplification was performed with the TaqMan miRNA assay (Life Technologies) allowing the specific detection of the mature miRNA of interest. Real-time qPCRs were performed on the same StepOne device. Quantification in this case was normalized to the small non-coding genes snoRNA55 or snoRNA135. The IDs of the TaqMan Probe used were: miR-125 (000449); let-7c (000379); miR-134 (001186); miR-128 (002216); snoRNA55 (001228); snoRNA135 (001230). Relative expression levels were calculated using the following equation: A = 2[Ct(ref) − Ct(ref-control)] − [Ct(sample) − Ct(sample-control)].
miRNA overexpressing plasmids
The generation of CAG-RFP-miRNA-IRES-RFP vectors was done as previously described [70]. Additionally the following miRNA128/cloning primers were used:
miRNA128 Fwd: 5′-TGCTG tca cag tga acc ggt ctc ttt GTT TTG GCC ACT GAC TGA CAA AGA GAC CTT CAC TGTGA-3′.
miRNA128 Rew: 5′-CCT GTCA CAG TGA AGG TCT CTT TGT CAG TCA GTG GCC AAAAC aaa gag acc ggt tca ctg tgaC-3′ (in lower case is the mature miRNA128 sequence).
Chromatin binding assay
The procedure was used essentially as previously described [23, 39]. Neural stem/progenitor cells (7× confluent 10 cm plates of undifferentiated cells or 20× 10 cm plates of 3-days differentiating cells) were washed with cold PBS, detached from the plates with Accutase (Millipore), washed again with PBS and finally resuspended in 6 ml of cold PBS. One tenth of the cell suspension (control fraction, C), was centrifuged at 400g and resuspended in RIPA buffer [150 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.5 % DOC, 0.1 % (w/v) SDS, 1 % (v/v) NP-40, Roche protease inhibitor cocktail] and left 30 min on ice. The remaining fraction was lysed for 15 min on ice in cold CSKI buffer [10 mM Pipes, pH 6.8, 100 mM NaCl, 1 mM EDTA, 300 mM sucrose, 1 mM MgCl2, 1 mM DTT, 0.5 % (v/v) Triton X-100, Roche protease inhibitor cocktail, 1 mM PMSF]. The cell lysate was divided into two portions, which were centrifuged at 500g at 4 °C for 3 min. The supernatants (S1 fraction), which contain Triton-soluble proteins, were further analyzed. One of the pellets was washed twice in CSKI buffer and then resuspended in RIPA buffer (the P1 fraction). The second pellet, after 2× CSKI washes, was resuspended in CSK II buffer (10 mM Pipes pH 6.8, 50 mM NaCl, 300 mM sucrose, 6 mM MgCl2, 1 mM DTT, Roche protease inhibitor cocktail), treated with DNase for 30 min followed by extraction with 250 mM NH2SO4 for 10 min at 25 °C. The sample treated with DNase (Promega) and salt was then centrifuged at 1200g for 6 min at 4 °C and the supernatant (S2 fraction), and pellet (P2 fraction), were collected. P2 after 2× washes in CSKII buffer was also resuspended in RIPA buffer. 20 µg of all fractions were analyzed by immunoblotting. Proteins of interest were detected with the following antibodies: mouse anti-RNA Polymerase II (1:1000, clone 4H8, Abcam 5408); rabbit anti-Dicer [1:1000, kindly provided by Chrysi Kanellopoulou [26]; rat anti-Argonaute-2 (1:50; clone 6F4), kindly provided by Elisabeth Kremmer [71]; mouse anti-β-catenin (1:2000, BD Biosciences 610153); mouse anti-Gapdh (1:1000, clone 6C5, Santa Cruz 32233)].
Authors’ contributions
Conceived and designed the experiments: FMC, DCL. Performed the experiments: FMC, RDG. Analyzed the data: FMC, RDG, SC, DCL. Contributed reagents: FR. Wrote the paper: FMC, DCL. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Chrysi Kanellopoulou (NIH, Bethesda USA) and Elisabeth Kremmer (Helmholtz Zentrum München, Germany) for the Dicer and Argonaute-2 antibodies, respectively. We also thank Lifang Miu for help with stereotactic injections; Katharina Merz and Fabian Gruhn for help with tissue processing; Lucia Berti for help with isolation of neural progenitor cells; Kathrin Wassmer for retrovirus preparation. Funding was provided by Grants to D. C. L: the European Young Investigator Award of the European Science Foundation (DFG 858/6-3), the Bavarian Research Networks on Adult Neural Stem Cells “FORNEUROCELL” and on Human induced Pluripotent Stem Cells “FORIPS”, the Helmholtz Alliance for Mental Health in an Ageing Society, the BMBF Network “Cell Based Regenerative Medicine”, and the University Hospital Erlangen (IZKF Grants E12 and E16). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- SEM
standard error of the mean
- ChIP-seq
Chromatin immunoprecipitation followed by next generation sequencing
- NSCs
neural stem cells
- DG
dentate gyrus
- SVZ
subventricular zone
- Dcx
Doublecortin
- RNAi
RNA interference
- miRNAs
microRNAs
- siRNAs
small interfering RNAs
- piRNAs
Piwi-interacting RNAs
- dpi
days post injection
- DAPI
4′,6-diamidino-2-phenylindole
- PDL
poly-d-lysine
- FGF
fibroblast growth factor
Additional files
Additional file 1: Figure S1. Dicer deletion in adult neural stem cells. a–b The samples analyzed were adult neural stem cells Dicer flox/flox (f/f) or Dicer −/−. Dicer deletion was obtained by transducing the Dicer f/f cells with HTN-Cre protein. a) Representative picture of PCR genotyping of adult neural stem cells Dicer f/f and Dicer −/−. Efficient Dicer deletion was obtained by transducing the Dicer f/f cells with HTN-Cre protein. Primers-Δ only amplify in case of recombination. b) Quantitative RT-PCR. The cycle threshold numbers are plotted for the indicated genes. Srp14 is a housekeeping gene. The cycle threshold numbers are reciprocally correlated to the amount of template at the start of linear amplification. Nestin, Sox2 and Srp14 expression levels are very similar in both Dicer f/f or Dicer −/− cells. The mean values from 3 independent experiments are shown. Error bars indicate the standard error of the mean.
Additional file 2: Figure S2. miRNA-128 levels in adult neural progenitors cells. Shown is a Taqman quantitative RT-PCR on undifferentiated (undiff) or differentiating (diff; 4 days after growth factor withdrawal) adult neural progenitor cells. Levels of mature miR-128 were calculated as fold change relative to the housekeeping gene snoRNA55. n = 3, bars represent the mean ± standard error of the mean. Three independent biological samples have been analyzed.
Additional file 3: Figure S3. miR128 over-expression reduces Dcx protein in N2 cells. Western blot showing the Dcx (arrow) and Actin (loading control) protein levels in (from far right to left): mock (control transfection without miRNA-128-RFP expressing plasmid), miRNA-128 (cells treated with miRNA-128-RFP expressing plasmid), shRNA–Dcx (cells treated with shRNA targeting Dcx) and shRNA-Ctr (cells treated with control shRNA). The band corresponding to Dcx is reduced in both shRNA–Dcx and miR-128 samples. Dcx KD = Dcx knockdown; miR-128 OE = mirR-128 over-expression; MW = Molecular weight marker.
Contributor Information
Filippo M. Cernilogar, Email: filippo.cernilogar@med.uni-muenchen.de
Rossella Di Giaimo, Email: digiaimo@unina.it.
Frederick Rehfeld, Email: Frederick.Rehfeld@charite.de.
Silvia Cappello, Email: silvia_cappello@psych.mpg.de.
D. Chichung Lie, Email: chi.lie@fau.de.
References
- 1.Kokovay E, Shen Q, Temple S. The incredible elastic brain: how neural stem cells expand our minds. Neuron. 2008;60(3):420–429. doi: 10.1016/j.neuron.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18(6):704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Perrier AL, Studer L. Making and repairing the mammalian brain–in vitro production of dopaminergic neurons. Semin Cell Dev Biol. 2003;14(3):181–189. doi: 10.1016/S1084-9521(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- 7.Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 8.Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, et al. Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci. 2006;24(6):1535–1545. doi: 10.1111/j.1460-9568.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- 9.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 11.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33(6):1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 12.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15(12):1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Baulcombe D. RNA silencing in plants. Nature. 2004;431(7006):356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 15.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431(7006):343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 16.Bantignies F, Cavalli G. Polycomb group proteins: repression in 3D. Trends Genet TIG. 2011;27(11):454–464. doi: 10.1016/j.tig.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318(5851):761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- 18.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mello CC, Conte D., Jr Revealing the world of RNA interference. Nature. 2004;431(7006):338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 20.Castel SE, Martienssen RA. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet. 2013;14(2):100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li LC. Chromatin remodeling by the small RNA machinery in mammalian cells. Epigenet Off J DNA Methylation Soc. 2014;9(1):45–52. doi: 10.4161/epi.26830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang V, Zheng J, Qi Z, Wang J, Place RF, Yu J, Li H, Li LC. Ago1 interacts with RNA polymerase II and binds to the promoters of actively transcribed genes in human cancer cells. PLoS Genet. 2013;9(9):e1003821. doi: 10.1371/journal.pgen.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cernilogar FM, Onorati MC, Kothe GO, Burroughs AM, Parsi KM, Breiling A, Lo Sardo F, Saxena A, Miyoshi K, Siomi H, et al. Chromatin-associated RNA interference components contribute to transcriptional regulation in Drosophila. Nature. 2011;480(7377):391–395. doi: 10.1038/nature10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35(3):215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 25.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280(10):9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 26.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19(4):489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O’Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201(9):1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolden L, Edenhofer F, Haupt S, Koch P, Wunderlich FT, Siemen H, Brustle O. Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nat Methods. 2006;3(6):461–467. doi: 10.1038/nmeth884. [DOI] [PubMed] [Google Scholar]
- 29.Ecsedi M, Grosshans H. LIN-41/TRIM71: emancipation of a miRNA target. Genes Dev. 2013;27(6):581–589. doi: 10.1101/gad.207266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci Off J Soc Neurosci. 2009;29(25):7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, Schinder AF. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci Off J Soc Neurosci. 2011;31(21):7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaughwin P, Ciesla M, Yang H, Lim B, Brundin P. Stage-specific modulation of cortical neuronal development by Mmu-miR-134. Cereb Cortex. 2011;21(8):1857–1869. doi: 10.1093/cercor/bhq262. [DOI] [PubMed] [Google Scholar]
- 34.Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, Galardi S, Bue MC, Massalini S, McDowell HP, Messi E, et al. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J Off Publ Fed Am Soc Exp Biol. 2009;23(12):4276–4287. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- 35.Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14(3):432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. [DOI] [PMC free article] [PubMed]
- 37.Taliaferro JM, Aspden JL, Bradley T, Marwha D, Blanchette M, Rio DC. Two new and distinct roles for Drosophila Argonaute-2 in the nucleus: alternative pre-mRNA splicing and transcriptional repression. Genes Dev. 2013;27(4):378–389. doi: 10.1101/gad.210708.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gromak N, Dienstbier M, Macias S, Plass M, Eyras E, Caceres JF, Proudfoot NJ. Drosha regulates gene expression independently of RNA cleavage function. Cell Rep. 2013;5(6):1499–1510. doi: 10.1016/j.celrep.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llano M, Vanegas M, Hutchins N, Thompson D, Delgado S, Poeschla EM. Identification and characterization of the chromatin-binding domains of the HIV-1 integrase interactor LEDGF/p75. J Mol Biol. 2006;360(4):760–773. doi: 10.1016/j.jmb.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 40.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 41.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2011;108(14):5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruno IG, Karam R, Huang L, Bhardwaj A, Lou CH, Shum EY, Song HW, Corbett MA, Gifford WD, Gecz J, et al. Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol Cell. 2011;42(4):500–510. doi: 10.1016/j.molcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36(Database issue):D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6(5):433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C, Teng ZQ, McQuate AL, Jobe EM, Christ CC, von Hoyningen-Huene SJ, Reyes MD, Polich ED, Xing Y, Li Y, et al. An epigenetic feedback regulatory loop involving microRNA-195 and MBD1 governs neural stem cell differentiation. PLoS One. 2013;8(1):e51436. doi: 10.1371/journal.pone.0051436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16(4):365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun G, Ye P, Murai K, Lang MF, Li S, Zhang H, Li W, Fu C, Yin J, Wang A, et al. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12(4):399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson T, Rahman S, Sansom SN, Alsio JM, Kaneda M, Smith J, O’Carroll D, Tarakhovsky A, Livesey FJ. Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS One. 2010;5(10):e13453. doi: 10.1371/journal.pone.0013453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawase-Koga Y, Low R, Otaegi G, Pollock A, Deng H, Eisenhaber F, Maurer-Stroh S, Sun T. RNAase-III enzyme Dicer maintains signaling pathways for differentiation and survival in mouse cortical neural stem cells. J Cell Sci. 2010;123(Pt 4):586–594. doi: 10.1242/jcs.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciaudo C, Jay F, Okamoto I, Chen CJ, Sarazin A, Servant N, Barillot E, Heard E, Voinnet O. RNAi-dependent and independent control of LINE1 accumulation and mobility in mouse embryonic stem cells. PLoS Genet. 2013;9(11):e1003791. doi: 10.1371/journal.pgen.1003791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435(7044):903–910. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- 55.Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, Morell M, O’Shea KS, Moran JV, Gage FH. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460(7259):1127–1131. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baillie JK, Barnett MW, Upton KR, Gerhardt DJ, Richmond TA, De Sapio F, Brennan PM, Rizzu P, Smith S, Fell M, et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature. 2011;479(7374):534–537. doi: 10.1038/nature10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lathe R, Harris A. Differential display detects host nucleic acid motifs altered in scrapie-infected brain. J Mol Biol. 2009;392(3):813–822. doi: 10.1016/j.jmb.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 58.Muotri AR, Marchetto MC, Coufal NG, Oefner R, Yeo G, Nakashima K, Gage FH. L1 retrotransposition in neurons is modulated by MeCP2. Nature. 2010;468(7322):443–446. doi: 10.1038/nature09544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeong BH, Lee YJ, Carp RI, Kim YS. The prevalence of human endogenous retroviruses in cerebrospinal fluids from patients with sporadic Creutzfeldt-Jakob disease. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2010;47(2):136–142. doi: 10.1016/j.jcv.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Coufal NG, Garcia-Perez JL, Peng GE, Marchetto MC, Muotri AR, Mu Y, Carson CT, Macia A, Moran JV, Gage FH. Ataxia telangiectasia mutated (ATM) modulates long interspersed element-1 (L1) retrotransposition in human neural stem cells. Proc Natl Acad Sci USA. 2011;108(51):20382–20387. doi: 10.1073/pnas.1100273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol. 2011;69(1):141–151. doi: 10.1002/ana.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471(7338):325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan H, Qurashi A, Poidevin M, Nelson DL, Li H, Jin P. Retrotransposon activation contributes to fragile X premutation rCGG-mediated neurodegeneration. Hum Mol Genet. 2012;21(1):57–65. doi: 10.1093/hmg/ddr437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Jin Y, Prazak L, Hammell M, Dubnau J. Transposable elements in TDP-43-mediated neurodegenerative disorders. PLoS One. 2012;7(9):e44099. doi: 10.1371/journal.pone.0044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Prazak L, Chatterjee N, Gruninger S, Krug L, Theodorou D, Dubnau J. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci. 2013;16(5):529–531. doi: 10.1038/nn.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci Off J Soc Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 68.Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cel Neurosci. 2006;31(3):560–573. doi: 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Ortega F, Costa MR, Simon-Ebert T, Schroeder T, Gotz M, Berninger B. Using an adherent cell culture of the mouse subependymal zone to study the behavior of adult neural stem cells on a single-cell level. Nat Protoc. 2011;6(12):1847–1859. doi: 10.1038/nprot.2011.404. [DOI] [PubMed] [Google Scholar]
- 70.Merz K, Lie DC. Evidence that Doublecortin is dispensable for the development of adult born neurons in mice. PLoS One. 2013;8(5):e62693. doi: 10.1371/journal.pone.0062693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu JY, Strehle M, Frohn A, Kremmer E, Hofig KP, Meister G, Adler H. Identification and analysis of expression of novel microRNAs of murine gammaherpesvirus 68. J Virol. 2010;84(19):10266–10275. doi: 10.1128/JVI.01119-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


