Abstract
Stabilized α-helices and nonpeptidic helix mimetics have emerged as powerful molecular scaffolds for the discovery of protein–protein interaction inhibitors. Protein-protein interactions often involve large contact areas, which are often difficult for small molecules to target with high specificity. The hypothesis behind the design of stabilized helices and helix mimetics is that these medium-sized molecules may pursue their targets with higher specificity because of a larger number of contacts. This protocol describes an optimized synthetic strategy for the preparation of stabilized α-helices that feature a carbon-carbon linkage in place of the characteristic N-terminal main-chain hydrogen bond of canonical helices. Formation of the carbon-carbon bond is enabled by a microwave-assisted ring-closing metathesis reaction between two terminal olefins on the peptide chain. The outlined strategy allows the synthesis and purification of a hydrogen bond surrogate (HBS) α-helix in ~1 week.
INTRODUCTION
The α-helix, first described by Pauling and Corey in 1951, is the most prevalent protein secondary structure1. α-Helices, when situated at protein surfaces, have a significant role in biomolecular recognition. In fact, protein-protein interactions are often mediated by α-helices2,3. Helix lengths tend to be relatively short (8–12 residues) at interfaces, thus providing the possibility to selectively modulate cellular processes with synthetic mimetics4,5.
The structure of an α-helix is characterized by a hydrogen bond between the C = O of the ith amino acid residue and the NH of the i + 4th amino acid residue of a peptide chain, which results in a right-handed helix with an average 3.6 residues per turn (Fig. 1). Unfortunately, the organization of peptides into this three-dimensional structure is energetically demanding6–8, and the simple excision of short peptide sequences from a parent protein leads not only to the loss of organized secondary structure but also to an increased susceptibility to proteolysis9,10.
Figure 1.
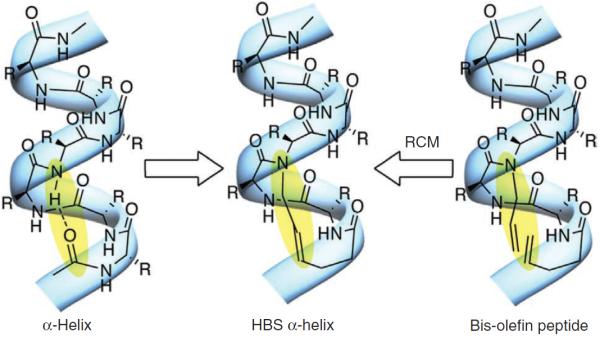
In an HBS helix, an N-terminal hydrogen bond is replaced with a carbon-carbon bond obtained from a ring-closing metathesis (RCM) reaction.
To address these issues, several strategies have been developed for the nucleation and stabilization of short peptide sequences into helices; they include helix capping11–13, non-natural amino acid substitutions14,15, side chain constraints9,16–23 and hydrogen bond surrogates (HBS)24. The HBS approach involves simple substitution of an intramolecular i→i + 4 hydrogen bond with a covalent linkage, and does not modify the solvent-exposed surfaces that may be required for molecular recognition. The HBS approach is particularly attractive for stabilizing short peptide sequences consisting of 7–18 residues.
Satterthwait and co-workers25 introduced the hydrogen bond mimic approach when they reported that a hydrazone linkage between the i and i + 4 residues resulted in the formation of stabilized α-helical peptides. Inspired by their finding, we devised an approach that involves the introduction of an i→i + 4 carbon-carbon bond through a ring-closing metathesis (RCM) reaction (Fig. 1). The metathesis-based method affords a stable and irreversible bond in comparison with the hydrazone strategy, and is applicable to a broader range of peptide sequences. The HBS helices have been shown to reproduce the conformation of protein α-helices, and have also been successfully used to target protein receptors in both cell-free and cell-culture assays10,26–29.
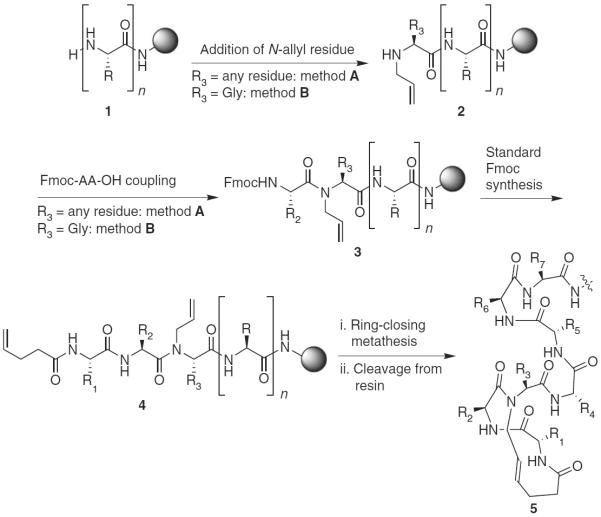
This protocol describes detailed synthetic procedures for the preparation of HBS α-helices. The RCM reaction is the key step in our synthesis of HBS peptides, and we have reported optimized procedures for this step in solid phase under oil-bath heating and microwave irradiation conditions30,31. Recently, we also reported high-yielding procedures for a difficult amide bond–formation step required in the synthesis32. Together, the optimized solid-phase methodology is compatible with all standard side chain–protecting groups and provides efficient synthesis of HBS helices (Fig. 2).
Figure 2.
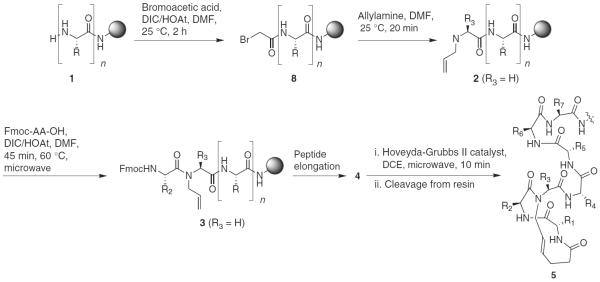
Synthesis of HBS α-helices on solid phase (Knorr or Rink amide resins). Depending on residue R3, two different procedures are employed for the synthesis of 3. R, R1–R7, amino acid side chain functionality.
Experimental design
The optimized procedures allow solid-phase synthesis of HBS helices using commercially available resins, protected amino acids and reagents. Formation of the key carbon-carbon bond is enabled by the incorporation of two terminal olefins on the peptide chain, which are used in a microwave-assisted RCM reaction to afford the desired alkene isostere. Coupling of 4-pentenoic acid at the N terminus allows the facile introduction of one olefin. An N-allyl group on the third amino acid residue from the N terminus provides the partner olefin. The synthesis of HBS helices begins with standard solid-phase synthesis of a peptide of n residues, which is modified with an N-allylamino acid to obtain 2 (Fig. 2). Sequential insertion of two amino acid residues followed by coupling of 4-pentenoic acid gives bis-olefin 4, which is treated with the metathesis catalyst to obtain the resin-bound stabilized helix. Cleavage of the peptide from resin followed by HPLC purification affords the desired HBS helix 5.
We use two different methods for the synthesis of N-allyl-bearing peptides 2 from 1, depending on the identity of the N-allylamino acid (Fig. 2)32. The most general approach, `method A', involves N-allylation of the third amino acid residue from the N terminus with the Tsuji-Trost reaction33,34. The subsequent peptide coupling, although difficult, is affected with triphosgene to provide 3. For sequences with glycine as the third residue, `method B' is used because it is procedurally more straightforward than method A and provides higher yields. In method B, the N-allyl group is incorporated by bromoacetylation of 1, followed by nucleophilic displacement of the bromide with allylamine. Subsequent amino acid coupling to N-allylglycine with DIC/HOAt (N,N′-diisopropylcarbodiimide/1-hydroxy-7-azabenzotriazole) affords the N-allylpeptide in high yields.
The reaction scale is limited by the size of the microwave vessels to 25–500 mg. Within this range, the procedure described here works efficiently for a diverse range of sequences, except those that feature coupling of amino acids to N-allylthreonine, N-allylvaline and N-allylisoleucine (β-branched) residues. The two critical steps for the synthesis of HBS helices involve triphosgene-mediated coupling of N-allylamino acids and the RCM. Both these reactions require anhydrous conditions. The yield of the coupling step is substantially improved by treating the N-allylpeptide with fresh aliquots of activated amino acid; the number of repetitions is dependent on the sequence and requires optimization.
MATERIALS
REAGENTS
! CAUTION Chemical safety considerations: Most of the reagents used in the protocol require protective goggles, gloves and lab coats.
Knorr Amide MBHA resin (solid support, capacity 0.4 mmol g−1; Novabiochem, cat. no. 855118)
N,N-dimethylformamide (DMF; Sigma-Aldrich, cat. no. 319937) ! CAUTION It is toxic.
Methylene chloride (DCM; Sigma-Aldrich, cat. no. 443484) ! CAUTION It is harmful.
1-Methyl-2-pyrrolidinone (NMP; Sigma-Aldrich, cat. no. M6762) ! CAUTION It is toxic.
9-Fluorenylmethyloxycarbonyl (Fmoc) amino acids (Novabiochem)
Piperidine (Sigma-Aldrich, cat. no. 104094) ! CAUTION It is highly flammable/toxic.
1-(Bis(dimethylamino)methylene)-1H-benzotriazolium hexafluorophosphate 3-oxide (HBTU; Novabiochem, cat. no. 851006) ! CAUTION It is an irritant/harmful.
N,N′-diisopropylethylamine (DIEA; Sigma-Aldrich, cat. no. 550043) ! CAUTION It is corrosive/highly flammable.
Ninhydrin (Sigma-Aldrich, cat. no. 151173) ! CAUTION It is harmful.
Ethanol (Sigma-Aldrich, cat. no. 459844) ! CAUTION It is highly flammable.
Phenol (Sigma-Aldrich, cat. no. P3653) ! CAUTION It is toxic/corrosive.
Potassium cyanide (KCN; Sigma, cat. no. 60178) ! CAUTION It is toxic/dangerous for the environment.
Pyridine (Sigma, cat. no. P3776) ! CAUTION It is highly flammable/harmful.
Chloranil (Fluka, cat. no. 23290) ! CAUTION It is an irritant/dangerous for the environment.
Acetaldehyde (Sigma-Aldrich, cat. no. 402788) ! CAUTION It is extremely flammable/harmful.
2-Nitrobenzenesulfonyl chloride (o-NsCl; Aldrich, cat. no. N11507) ! CAUTION It is corrosive
2,4,6-Collidine (Sigma-Aldrich, cat. no. 27690) ! CAUTION It is harmful. Use of a functional fume hood is required.
Triphenylphosphine (Fluka, cat. no. 93090) ! CAUTION It is harmful.
Tetrahydrofuran (THF; Sigma-Aldrich, cat. no. 494461) ! CAUTION It is highly flammable/irritant.
Tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3; Aldrich, cat. no. 328774)
Allyl methyl carbonate (Aldrich, cat. no. 381381) ! CAUTION It is an irritant.
Sodium diethyldithiocarbamate trihydrate (Sigma-Aldrich, cat. no. 228680) ! CAUTION It is harmful.
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU; Aldrich, cat. no. 139009) ! CAUTION It is corrosive.
2-Mercaptoethanol (Aldrich, cat. no. M6250) ! CAUTION It is toxic/dangerous for the environment. Use of a functional fume hood is required.
Bis(trichloromethyl) carbonate (Triphosgene; Aldrich, cat. no. 330752) ! CAUTION It is very toxic. Use of a functional fume hood is required.
Acetic anhydride (Sigma-Aldrich, cat. no. 320102) ! CAUTION It is corrosive.
Bromoacetic acid (Aldrich, cat. no. 259357) ! CAUTION It is toxic/corrosive/dangerous for the environment.
N,N′-diisopropylcarbodiimide (DIC; Aldrich, cat. no. D125407) ! CAUTION It is extremely flammable/toxic.
1-Hydroxy-7-azabenzotriazole (HOAt; Genscript, cat. no. C01568) ! CAUTION It is an irritant.
Allylamine (Aldrich, cat. no. 241075) ! CAUTION It is highly flammable/toxic/dangerous for the environment.
Methanol (Sigma-Aldrich, cat. no. 179337) ! CAUTION It is highly flammable/toxic.
4-Pentenoic acid (Aldrich, cat. no. 245925) ! CAUTION It is corrosive.
Hoveyda-Grubbs II (Aldrich, cat. no. 569747)
Anhydrous 1,2-dichloroethane (DCE, Sigma-Aldrich, cat. no. 284505) ! CAUTION It is highly flammable/toxic.
Trifluoroaetic acid (TFA; Sigma-Aldrich, cat. no. T62200) ! CAUTION It is extremely corrosive. Extra precautions are required.
Distilled water
Triisopropylsilane (TIPS; Sigma-Aldrich, cat. no. 233781) ! CAUTION It is an irritant.
Diethyl ether (Sigma-Aldrich, cat. no. 472492) ! CAUTION It is extremely flammable/harmful.
Acetonitrile (ACN; Fluka, cat. no. 00687) ! CAUTION It is highly flammable/harmful.
EQUIPMENT
Plastic syringe (10 ml) equipped with a frit column plate (or 10 ml solid-phase extraction tube equipped with a frit column plate)
Plastic syringe (2 ml) equipped with a frit column plate (or 2 ml solid-phase extraction tube equipped with a frit column plate)
Vacuum membrane pump
Microwave reaction vessel with caps (10 ml; CEM, cat. no. 908310)
Magnetic stir bars (gentle stirring allows homogenous mixing without damaging the resin)
Centrifuge tubes
HPLC vials
CEM Discover series microwave reactor with fiber-optic temperature probe and magnetic stirrer (CEM)
Rotary evaporator
Centrifuge
Vacuum desiccator
Analytical HPLC system (see EQUIPMENT SETUP)
Preparative HPLC system (see EQUIPMENT SETUP)
Liquid chromatograph–mass spectrometer (LCMS; Agilent 1100 Series Capillary LCMSD Trap XCT Spectrometer, Agilent)
Lyophilizer
CEM Liberty Series Microwave Peptide Synthesizer (optional; CEM)
Nitrogen gas
EQUIPMENT SETUP
CEM Discover Series microwave reactor
Use a CEM Discover series microwave reactor equipped with a fiber-optic temperature probe and magnetic stirrer. Table 1 shows the optimized parameters for specific synthetic steps.
TABLE 1.
Microwave synthesizer settings for Steps 4A, 4B and the metathesis reaction.
| Step 4A(iv) | Step 4A(xv) Step | 4A(xxi) | Step 4B(viii) | Step 11 | |
|---|---|---|---|---|---|
| Power (W) | 200 | 100 | 150 | 200 | 150 |
| Ramp time (min) | 2 | 1 | 2 | 2 | 2 |
| Hold time (min) | 15 | 5 | 30 | 10 | 10 |
| Temperature (°C) | 100 | 50 | 45 | 120 | 120 |
| Pressure (p.s.i.) | 250 | 150 | 250 | 250 | 250 |
Reactions were performed on a CEM Discover microwave system.
Analytical HPLC
Use an HPLC gradient system equipped with a detector (220 nm) and a reversed-phase C18 column (4.6 mm × 100 mm; 5 μm). Run a linear gradient of 5–95% (vol/vol) solvent B in solvent A for 12 min (flow rate 1 ml min−1; solvent A: 0.1% (vol/vol) TFA in H2O, solvent B: 0.1% (vol/vol) TFA in acetonitrile) as shown in Table 2. ! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
TABLE 2.
Analytical and semipreparative HPLC conditions for HBS α-helicesa.
| Analytical scale (flow rate 1 ml min−1) |
Semipreparative scale (flow rate 5 ml min−1) |
||
|---|---|---|---|
| Time (min) | Solvent B (% (vol/vol) in solvent A) | Time (min) | Solvent B (% (vol/vol) in solvent A) |
| 0 | 5 | 0 | 5 |
| 12 | 95 | 45 | 95 |
| 15 | 100 | 50 | 100 |
| 17 | 5 | 55 | 5 |
Solvent A: 0.1% (vol/vol) TFA in H20; solvent B: 0.1% (vol/vol) TFA in acetonitrile.
Semipreparative HPLC
Use an HPLC gradient system equipped with a detector (220 nm) and a reversed-phase C18 column (19 mm × 100 mm; 5 μm). Run a linear gradient of 5–95% (vol/vol) solvent B in solvent A for 45 min (flow 5 ml min−1; solvent A: 0.1% (vol/vol) TFA in H2O, solvent B: 0.1% (vol/vol) TFA in acetonitrile) as shown in Table 2. ! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
Liquid chromatograph–mass spectrometer
We used an Agilent 1100 Series Capillary LCMSD Trap XCT Spectrometer. Resin-bound peptide (15 mg) is cleaved as described in Steps 13–17. The solution is injected into the LCMS with 75% (vol/vol) acetonitrile in 0.1% aqueous formic acid as the eluent.
PROCEDURE
Resin preparation ● TIMING 30 min
-
1|
For 0.1 mmol-scale synthesis of an HBS helix, weigh 250 mg of Knorr Amide MBHA resin (0.4 mmol g−1) into a solid-phase extraction tube with a frit column plate and swell the resin in 3.0 ml of DCM for 20 min.
-
2|
Remove the solvent by vacuum filtration and wash the resin with 2.0 ml of DMF.
Peptide synthesis ● TIMING variable
-
3|
Resin-bound peptides of desired sequence are prepared using standard Fmoc solid-phase peptide synthesis protocols (Fig. 2)35,36. Briefly, the Fmoc group is removed by treatment with 20% (vol/vol) piperidine/DMF. Each Fmoc amino acid (0.5 mmol) is activated with HBTU (0.45 mmol) and 5% (vol/vol) DIEA/DMF and coupled for 2 h at 25 °C. Peptide synthesis can be performed using a synthesizer such as a CEM Liberty Series microwave peptide synthesizer.
▲ CRITICAL STEP The progress of peptide synthesis can be monitored using Kaiser or chloranil tests (Box 1)37,38, which provide qualitative assessments for the presence or absence of free primary and secondary amines; if necessary, 15 mg of peptide can be cleaved from the resin and analyzed by LCMS35,36.
-
4|
Synthesis of N-allylpeptide 3 can be performed using method A or method B depending on the sequence. Method A is a general approach suitable for any sequence; method B offers a streamlined, high-yield approach applicable when the R3 residue in the HBS sequence is glycine (Figs. 2 and 3).
Figure 3.
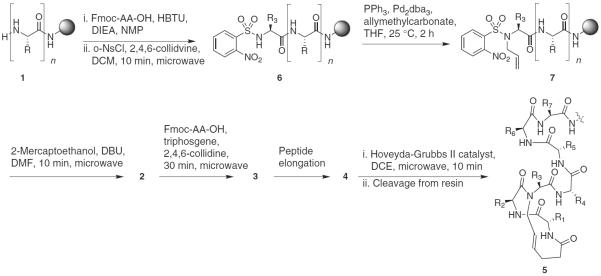
Synthesis of HBS helix 5 with method A. R, R1–R7, any amino acid side chain.
-
(A)
A general approach for the synthesis of N-allylpeptides
-
(i)
Preparation of nosyl-protected peptide 6 (Step 4A(i–vi)): Transfer the free amino, resin-bound peptide obtained from Step 3 to a microwave vessel equipped with a cap and magnetic stir bar. Add 3.0 ml of DCM to the resin and swell it for 10 min.
-
(ii)
Add 2-nitrobenzenesulfonylchloride (66 mg, 0.3 mmol) to the resin and stir the reaction mixture.
-
(iii)
Add 2,4,6-collidine (66 μl, 0.5 mmol) to the mixture from Step 4A(ii) and stir for an additional 5 min.
-
(iv)
Irradiate the mixture using the microwave parameters outlined in Table 1.
-
(v)
Filter the resin with a fritted solid-phase extraction tube. Wash the resin sequentially with DCM (5 ml × 3), DMF (5 ml × 3), methanol (5 ml × 3), DMF (5 ml × 3) and DCM (5 ml × 3).
▲ CRITICAL STEP Reaction progress can be monitored by the Kaiser test (Box 1)37, or if necessary, by cleaving 15 mg of peptide from resin and analyzing it with LCMS.
-
(vi)
Dry nosyl-protected peptide 6 in a vacuum desiccator overnight.
■ PAUSE POINT Dried resin containing 6 may be stored in a vacuum desiccator at 25 °C for about a month.
-
(vii)
Introduction of N-allyl group to 6 to obtain N-allylpeptide 7 (Step 4A(vii–xi)): Transfer the dried, nosyl-protected peptide from Step 4A(vi) to a 10-ml reaction vessel equipped with a septum cap, and add triphenylphosphine (21 mg, 0.08 mmol). Purge the reaction vessel with a continuous flow of argon gas for 30 min.
-
(viii)
Add 3 ml of anhydrous THF through a syringe to the argon-flushed reaction vessel and gently agitate or shake the reaction vessel to dissolve triphenylphosphine.
-
(ix)
Add Pd2(dba)3 (11 mg, 0.01 mmol) followed by allylmethylcarbonate (170 μl, 1.5 mmol), and gently agitate the mixture for 2 h.
-
(x)
Filter resin and wash it sequentially with DCM (5 ml × 3), DMF (5 ml × 3) and 0.2 M sodium diethyldithiocarbamate trihydrate (5 ml × 3), DMF (5 ml × 3) and DCM (5 ml × 3).
▲ CRITICAL STEP Reaction progress can be monitored by cleaving 15 mg of peptide from resin and analyzing it with LCMS.
-
(xi)
Dry resin in a vacuum desiccator overnight.
■ PAUSE POINT Dried resin containing 7 may be stored in a vacuum desiccator at 25 °C for about a month.
-
(xii)
Removal of nosyl group from 7 to obtain N-allylpeptide 2 (Step 4A(xii–xvii)): Transfer the dried resin obtained from Step 4A(xi) to a microwave vessel equipped with a cap and magnetic stir bar and purge the reaction vessel with a continuous flow of nitrogen gas for 30 min.
-
(xiii)
Add 2 ml anhydrous DMF to resin and stir for 10 min.
-
(xiv)
Add DBU (74 μl; 0.5 mmol) to the reaction mixture and stir for 2 min.
-
(xv)
Add 2-mercaptoethanol (70 μl; 1.0 mmol) to the reaction mixture and stir for 2 min at room temperature (RT, 25 °C); follow by microwave irradiation under conditions outlined in Table 1.
▲ CRITICAL STEP Deprotection of 2-nitrobenzenesulfonyl group releases a yellow chromophore, which provides visual confirmation of the reaction progress34.
? TROUBLESHOOTING
-
(xvi)
Filter resin with a fritted solid-phase extraction tube. Wash the resin sequentially with DCM (5 ml × 3), DMF (5 ml × 3), methanol (5 ml × 3), DMF (5 ml × 3) and DCM (5 ml × 3).
-
(xvii)
Dry the resin in a vacuum desiccator overnight.
▲ CRITICAL STEP The progress of this step can be monitored using the chloranil test (Box 1)38, which indicates presence of secondary amines; if necessary, 15 mg of peptide may be cleaved from the resin and analyzed by LCMS.
■ PAUSE POINT Dried resin containing 2 may be stored in a vacuum desiccator at 25 °C for about a month.
-
(xviii)
Triphosgene-mediated coupling of Fmoc amino acids to 2 to obtain 3 (Fig. 4) (Step 4A(xviii–xxiii)): Place the desired Fmoc amino acid (1.0 mmol) and triphosgene (0.098 mg; 0.33 mmol) in a microwave vessel equipped with a cap and magnetic stir bar. Purge the reaction vessel with nitrogen gas for 30 min.
-
(xix)
Add 2.1 ml of anhydrous THF to attain a triphosgene concentration of 0.15 M and stir the reaction mixture.
▲ CRITICAL STEP Use freshly distilled THF.
-
(xx)
Add 2,4,6-collidine (372 μl; 2.8 mmol) to the reaction mixture.
▲ CRITICAL STEP Formation of thick yellow-white precipitate of pyridinium salt must be observed after a few minutes of stirring (Fig. 4, middle panel).
-
(xxi)
Add activated Fmoc amino acid to the dried resin obtained from Step 4A(xvii), and subject to microwave irradiation using the parameters outlined in Table 1.
▲ CRITICAL STEP Washings between coupling cycles should be performed with DCM (5 ml × 3). Washing with other solvents should be avoided.
-
(xxii)
Repeat Step 4A(xviii–xxi) twice.
▲ CRITICAL STEP The progress of this step can be monitored using the chloranil test (Box 1)38, which indicates presence of secondary amines; if necessary, 15 mg of peptide may be cleaved from the resin and analyzed by analytical HPLC (Fig. 5) and/or LCMS.
-
(xxiii)
Filter the resin with a fritted solid-phase extraction tube. Wash the resin with DCM (5 ml × 3), DMF (5 ml × 3), methanol (5 ml × 3), 5% (vol/vol) DIEA in DMF (5 ml × 3) and DMF (5 ml × 3).
■ PAUSE POINT Dried resin containing 3 may be stored in a vacuum desiccator at 25 °C for about a month.
? TROUBLESHOOTING
-
(i)
-
(B)
Alternative approach for the preparation of N-allylglycinylpeptides (Fig. 6)
-
(i)
Synthesis of N-allylglycine peptide 2 (Step 4B(i–vi)): Prepare a solution containing bromoacetic acid (280 mg; 2 mmol), DIC (310 μl; 2 mmol) and HOAt (136 mg; 1 mmol) in 2.0 ml DMF.
▲ CRITICAL STEP The observation of a white precipitate indicates diisopropylurea formation.
-
(ii)
Add the solution prepared in the previous step to the free amino, resin-bound peptide obtained from Step 3, and shake at RT for 2 h.
-
(iii)
Filter the resin with a fritted solid-phase extraction tube.
-
(iv)
Wash the resin sequentially with DMF (5 ml × 3), DCM (5 ml × 3) and DMF (5 ml × 3).
-
(v)
Add 1 M allylamine (150 μl; 2 mmol) in DMF to the bromoacetylated, resin-bound peptide 8 and shake at RT for 20 min. Remove the solvent by vacuum filtration.
-
(vi)
Wash the resin sequentially with DMF (5 ml × 3), methanol (5 ml × 3) and DCM (5 ml × 3).
▲ CRITICAL STEP The progress of this synthesis can be monitored using the chloranil test (Box 1)38, which indicates presence of secondary amines; if necessary, 15 mg of peptide may be cleaved from the resin and analyzed by analytical HPLC and/or LCMS.
■ PAUSE POINT Dried resin containing 2 may be stored in a vacuum desiccator at 25 °C for about a month.
-
(vii)
Coupling of Fmoc amino acids to N-allylglycine peptide 2 to obtain 3 (Step 4B(vii–ix)): Dissolve the desired Fmoc amino acid (2.0 mmol), DIC (310 μl; 2 mmol) and HOAt (136 mg; 1 mmol) in DMF (3–5 ml) and allow the solution to stir for 15 min at RT.
▲ CRITICAL STEP The observation of a white precipitate indicates diisopropylurea formation.
-
(viii)
Transfer the resin bearing the N-allylglycine monomer 2 to a microwave vessel equipped with a cap and magnetic stir bar. Treat the resin with the solution prepared in Step 4B(vii) and irradiate microwave using the parameters outlined in Table 1.
-
(ix)
Filter the resin with a fritted solid-phase extraction tube. Wash the resin sequentially with DMF (5 ml × 3), DCM (5 ml × 3) and DMF (5 ml × 3).
▲ CRITICAL STEP The progress of this synthesis can be monitored using chloranil test (Box 1)38, which indicates presence of secondary amines; if necessary, 15 mg of peptide can be cleaved from the resin and analyzed by analytical HPLC and/or LCMS. For an incomplete coupling, repeat Steps 4B(vii–ix) until a negative chloranil test is observed.
■ PAUSE POINT Dried resin containing 3 may be stored in a vacuum desiccator at 25 °C for about a month.
-
(i)
Figure 4.
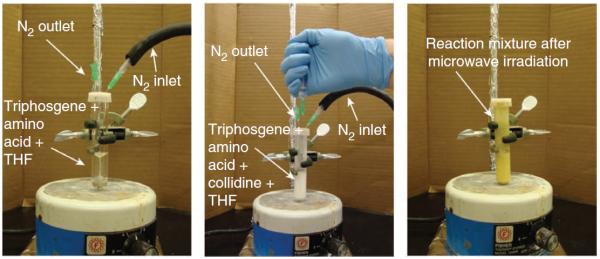
Reaction setup for the triphosgene-mediated coupling step. Left, reaction vessel with amino acid and triphosgene dissolved in THF; middle, appearance of the reaction mixture after addition of 2,4,6-collidine; and right, appearance of the reaction mixture after microwave irradiation.
Figure 5.
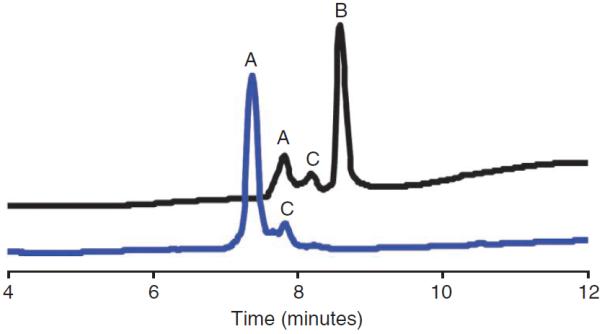
Representative analytical HPLC profile of the triphosgene-mediated coupling reaction (Step 4A(xix–xxiv)). A, N-allyl-AIYRLELL KAEEAN-NH2; B, FmocE-N-allyl-AIYRLELLKAEEAN-NH2; C, impurity resulting from resin cleavage.
Figure 6.
Synthesis of HBS helix 5 with method B. R, R1, R2, R4–R7, any amino acid side chain; R3, H.
Synthesis of bis-olefin peptide 4 from 3 ● TIMING 4–5 h
-
5|
Couple the next Fmoc amino acid (0.5 mmol) and 4-pentenoic acid (52 μl; 0.5 mmol) using standard Fmoc solid-phase peptide synthesis protocols35,36.
▲ CRITICAL STEP Reaction progress can be monitored by the Kaiser test (Box 1)37, or, if necessary, by cleaving a small amount of peptide from resin and analyzing it with LCMS.
-
6|
Remove solvent by vacuum filtration, and wash resin sequentially with DMF (5 ml × 3), DCM (5 ml × 3) and methanol (5 ml × 3).
-
7|
Dry bis-olefin peptide 4 in a vacuum desiccator overnight.
■ PAUSE POINT Dried resin containing 4 may be stored in a vacuum desiccator at 25 °C for about a month.
Preparation of HBS α-helix 5 using olefin metathesis reaction ● TIMING 2–5 h
-
8|
Transfer the dried resin-bound bis-olefin peptide 4 to a microwave vessel equipped with a cap and magnetic stir bar and purge the vessel with nitrogen gas for 1 h. The reaction setup for the metathesis step is shown in Figure 7.
▲ CRITICAL STEP Resin needs to be extensively dried for this reaction.
-
9|
Weigh 12.5 mg of the Hoveyda-Grubbs II catalyst (0.20 moles of catalyst for each mole of bis-olefin) and add to the microwave vessel containing the bis-olefin peptide 4. Allow the nitrogen gas to flow for an additional 30 min.
-
10|
Under nitrogen, add 2 ml anhydrous 1,2-dichloroethane per 0.10 mol of resin and stir for 15 min.
▲ CRITICAL STEP Use freshly distilled 1,2-dichloroethane.
-
11|
Irradiate the microwave vessel containing reaction mixture from Step 10 using the microwave parameters outlined in Table 1.
▲ CRITICAL STEP The progress of this step may be monitored by cleaving 15 mg of peptide from the resin and subsequent analysis by analytical HPLC (Fig. 8) and/or LCMS.
-
12|
Filter the resin with a fritted solid-phase extraction tube. Wash resin sequentially with DMF (5 ml × 3), DCM (5 ml × 3), methanol (5 ml × 3) and DCM (5 ml × 3), and allow the HBS peptide to air dry.
■ PAUSE POINT Dried resin containing 5 may be stored in a vacuum desiccator at 25 °C for about a month.
Figure 7.
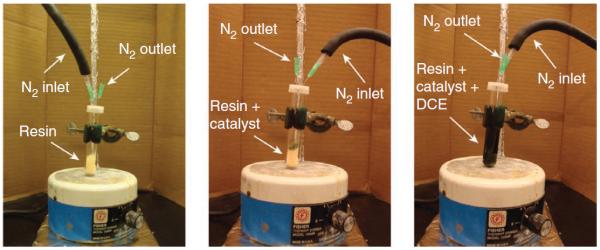
Reaction setup for the metathesis step. Left, reaction vessel with dried resin; middle, reaction vessel with resin and catalyst; right, appearance of the reaction mixture before microwave irradiation.
Figure 8.
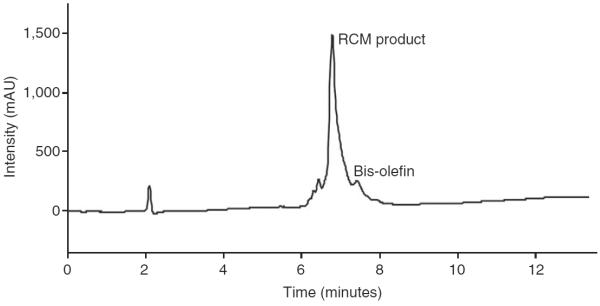
A representative HPLC chromatogram of the olefin metathesis reaction (Step 6). Bis-olefin: XFEG*IYRLELLKAEEAN-NH2, where X denotes 4-pentenoic acid residue and G* refers to N-allyl glycine; RCM product: reaction product after treatment of bis-olefin with the metathesis catalyst.
Cleavage of HBS helix 5 from resin ● TIMING 3 h
-
13|
Add 3.8 ml of the cleavage cocktail (TFA/H2O/TIPS, 95%/2.5%/2.5% (vol/vol/vol) to the dried, resin-bound HBS helix 5 and stir gently for 2 h.
! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
-
14|
Filter the cleavage mixture, wash resin with TFA (2 × 1 ml) and concentrate the combined filtrate using a rotary evaporator.
! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
-
15|
Slowly add 5 ml of cold diethyl ether to the cleavage mixture from Step 14 to precipitate the HBS peptide.
-
16|
Isolate the precipitate by centrifugation (5,000g for 5 min); carefully decant ether from the tube. Repeat ether wash two more times.
-
17|
Dissolve remaining solid from Step 16 in a mixture of 0.1% (vol/vol) TFA in water and acetonitrile, and lyophilize it.
! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
Purification and characterization of HBS α-helix 5 ● TIMING 1–2 d
-
18|
Dissolve 100 mg crude product in 0.4 ml of acetonitrile and 1.6 ml of 0.1% aqueous TFA. Inject the solution into a semipreparative HPLC system. Collect fractions (each 5 ml) corresponding to the main peak, and lyophilize aqueous solution to obtain the purified product.
! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
-
19|
Dissolve 1 mg of the purified HBS peptide in a 1 ml solution of 0.1% aqueous TFA (and acetonitrile, if needed), and inject 20 μl of the sample solution to an analytical HPLC and 6 μl of the sample solution to an LCMS to determine the purity and mass of the peptide.
! CAUTION TFA is extremely corrosive; wear eye protection, a lab coat and gloves.
? TROUBLESHOOTING
Step 4A(xv) If yellow color is not observed after the addition of 2-mercaptoethanol, wash the resin sequentially with DMF and DCM. Repeat Steps 4A(xii–xv) of nosyl deprotection.
Step 4A(xxiii) To avoid by-products from unreacted starting material, a capping step with acetic anhydride is suggested before further elongation of the peptide chain using standard procedures35,36.
● TIMING
Steps 1 and 2: 30 min
Step 3: Variable
Step 4A(i–vi): 45 min plus overnight drying time
Step 4A(vii–xi): 3 h plus overnight drying time
Step 4A(xii–xvii): 1 h plus overnight drying time
Step 4A(xviii–xxiii): 3–5 h
Step 4B(i–vi): 3 h
Step 4B(vii–ix): 1h
Steps 5–7: 4–5 h
Steps 8–12: 2–5 h
Steps 13–17: 3 h
Step 18: 1–2 d
Step 19: 30 min–1 h
Box 1: Kaiser test (7 min); Chloranil test (7 min)
ANTICIPATED RESULTS
This protocol offers an efficient approach for the synthesis of HBS-derived stabilized α-helices. The yield of purified HBS peptide depends on the specific sequence and the equipments used. Figure 8 shows representative HPLC profile for the corresponding crude product. Yields of several sequences that have been prepared with this method are shown in Table 3. The procedure works well for most sequences except for those that contain a β-branched residue at the N-allyl position, such as sequence 12 in Table 3. Coupling of such residues to the next amino acid is very low yielding. The identity of the peptides was confirmed by electrospray ionization mass spectrometry.
TABLE 3.
Representative yields of various HBS helices prepared by the outlined methods.
| HBS helix | Sequencea | % Yieldb |
|---|---|---|
| 9 | XFEA*IYRLELLKAEEAN-NH2 | 20 |
| 10 | XFEG*IYRLELLKAEEAN-NH2 | 25 |
| 11 | XEFL*LRLWLKAibEEAibN-NH2 | 17 |
| 12 | XRKA*YKRLAMK-NH2 | 10 |
| 12 | XQET*FSDLWKLLS-NH2 | Trace |
X denotes 4-pentenoic acid residue, and A*, G*, L* and T* refer to N-aLLyL A, G, L and T residues, respectively.
Amount of the desired helix after HPLC purification.
BOX 1 | COLORIMETRIC DETECTION OF RESIN-BOUND PRIMARY OR SECONDARY AMINES.
Kaiser test
The Kaiser test is a qualitative test used to detect the presence of primary amines based on the reaction of amines with ninhydrin. A positive test is indicated by a dark blue color, whereas a negative test is indicated by a colorless to pale-yellow color.
REAGENTS
Solution A: 5 g ninhydrin in 100 ml ethanol
Solution B: 80 g phenol in 20 ml ethanol
Solution C: 2 ml of 0.001 M KCN(aq) in 98 ml pyridine
PROCEDURE
Transfer a few resin beads into a small test tube.
Add 1 drop each of solutions A, B and C.
-
Mix well and heat to 100 °C for 5 min.
▲ CRITICAL STEP The test is not applicable to N-terminal secondary amines.
Chloranil test
The chloranil test is a qualitative test used to detect the presence of secondary amines. Dark blue to green beads indicate a positive result. Colorless to pale-yellow beads indicate a negative result.
REAGENTS
Solution A: 20 mg chloranil in 100 ml DMF
Solution B: 2 ml of acetaldehyde in 98 ml DMF
PROCEDURE
Transfer a few resin beads to a small test tube.
-
Add 1 drop each of solutions A and B and allow to stand at 25 °C for 5 min.
▲ CRITICAL STEP The beads will be dark blue to green only in the case of a positive result. The chloranil test will also detect primary amines.
ACKNOWLEDGMENTS
We are grateful for financial support from the NIH (GM073943). We also thank the National Science Foundation for equipment Grant CHE-0958457 and the NIH National Center for Research Resources (NIRR) for Research Facilities Improvement Grant C06 RR-16572.
Footnotes
AUTHOR CONTRIBUTIONS A.P. carried out the experiments as reported in the main paper; M.Z.M. tested the protocol; and A.P., M.Z.M., A.B.M. and P.S.A. wrote the article.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Pauling L, Corey RB, Branson HR. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl Acad. Sci. USA. 1951;37:205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones S, Thornton JM. Protein-protein interactions—a review of protein dimer structures. Prog. Biophys. Mol. Biol. 1995;63:31–65. doi: 10.1016/0079-6107(94)00008-w. [DOI] [PubMed] [Google Scholar]
- 3.Jochim AL, Arora PS. Assessment of helical interfaces in protein-protein interactions. Mol. Biosyst. 2009;5:924–926. doi: 10.1039/b903202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garner J, Harding MM. Design and synthesis of alpha-helical peptides and mimetics. Org. Biomol. Chem. 2007;5:3577–3585. doi: 10.1039/b710425a. [DOI] [PubMed] [Google Scholar]
- 5.Henchey LK, Jochim AL, Arora PS. Contemporary strategies for the stabilization of peptides in the alpha-helical conformation. Curr. Opin. Chem. Biol. 2008;12:692–697. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimm BH, Bragg JK. Theory of the phase transition between helix and random coil in polypeptide chains. J. Chem. Phys. 1959;31:526–535. [Google Scholar]
- 7.Lifson S, Roig A. On the theory of helix-coil transitions in polypeptides. J. Chem. Phys. 1961;34:1963–1974. [Google Scholar]
- 8.Qian H, Schellman JA. Helix-coil theories: a comparative study for finite length preferences. J. Phys. Chem. 1992;96:3987–3994. [Google Scholar]
- 9.Schafmeister CE, Po J, Verdine GL. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J. Am. Chem. Soc. 2000;122:5891–5892. [Google Scholar]
- 10.Wang D, Liao W, Arora PS. Enhanced metabolic stability and protein-binding properties of artificial alpha-helices derived from a hydrogen-bond surrogate: application to Bcl-xL. Angew. Chem. Int. Ed. Engl. 2005;44:6525–6529. doi: 10.1002/anie.200501603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin RE, et al. A template for stabilization of a peptide α-helix: synthesis and evaluation of conformational effects by circular dichroism and nmr. J. Am. Chem. Soc. 1997;119:6461–6472. [Google Scholar]
- 12.Chakrabartty A, Doig AJ, Baldwin RL. Helix capping propensities in peptides parallel those in proteins. Proc. Natl Acad. Sci. USA. 1993;90:11332–11336. doi: 10.1073/pnas.90.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp DS, Curran TP, Davis WM, Boyd JG, Muendel C. Studies of N-terminal templates for a-helix formation. Synthesis and conformational analysis of (2S,5S,8S,11S)-1-acetyl-1,4-diaza-3-keto-5-carboxy-10-thiatricyclo[2.8.1.04,8]-tridecane (Ac-Hel1-OH) J. Org. Chem. 1991;56:6672–6682. [Google Scholar]
- 14.Kaul R, Balaram P. Stereochemical control of peptide folding. Bioorg. Med. Chem. 1999;7:105–117. doi: 10.1016/s0968-0896(98)00221-1. [DOI] [PubMed] [Google Scholar]
- 15.Lyu PC, Sherman JC, Chen A, Kallenbach NR. α-Helix stabilization by natural and unnatural amino acids with alkyl side chains. Proc. Natl Acad. Sci. USA. 1991;88:5317–5320. doi: 10.1073/pnas.88.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackwell HE, Grubbs RH. Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew. Chem. Int. Ed. Engl. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Ghadiri MR, Choi C. Secondary structure nucleation in peptides—transition-metal ion stabilized alpha-helices. J. Am. Chem. Soc. 1990;112:1630–1632. [Google Scholar]
- 18.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. General-approach to the synthesis of short alpha-helical peptides. J. Am. Chem. Soc. 1991;113:9391–9392. [Google Scholar]
- 19.Osapay G, Taylor JW. Multicyclic polypeptide model compounds. 2. synthesis and conformational properties of a highly alpha-helical uncosapeptide constrained by 3 side-chain to side-chain lactam bridges. J. Am. Chem. Soc. 1992;114:6966–6973. [Google Scholar]
- 20.Phelan JC, Skelton NJ, Braisted AC, McDowell RS. A general method for constraining short peptides to an alpha-helical conformation. J. Am. Chem. Soc. 1997;119:455–460. [Google Scholar]
- 21.Harrison RS, et al. Downsizing human, bacterial, and viral proteins to short water-stable alpha helices that maintain biological potency. Proc. Natl Acad. Sci. USA. 2010;107:11686–11691. doi: 10.1073/pnas.1002498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd NE, Hoang HN, Abbenante G, Fairlie DP. Left- and right-handed alpha-helical turns in homo- and hetero-chiral helical scaffolds. J. Am. Chem. Soc. 2009;131:15877–15886. doi: 10.1021/ja9065283. [DOI] [PubMed] [Google Scholar]
- 23.Ma MT, Hoang HN, Scully CC, Appleton TG, Fairlie DP. Metal clips that induce unstructured pentapeptides to be alpha-helical in water. J. Am. Chem. Soc. 2009;131:4505–4512. doi: 10.1021/ja900047w. [DOI] [PubMed] [Google Scholar]
- 24.Patgiri A, Jochim AL, Arora PS. A hydrogen bond surrogate approach for stabilization of short peptide sequences in alpha-helical conformation. Acc. Chem. Res. 2008;41:1289–1300. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabezas E, Satterthwait AC. The hydrogen bond mimic approach: solid-phase synthesis of a peptide stabilized as an alpha-helix with a hydrazone link. J. Am. Chem. Soc. 1999;121:3862–3875. [Google Scholar]
- 26.Chapman RN, Dimartino G, Arora PS. A highly stable short alpha-helix constrained by a main-chain hydrogen-bond surrogate. J. Am. Chem. Soc. 2004;126:12252–12253. doi: 10.1021/ja0466659. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Chen K, Kulp JL, III, Arora PS. Evaluation of biologically relevant short alpha-helices stabilized by a main-chain hydrogen-bond surrogate. J. Am. Chem. Soc. 2006;128:9248–9256. doi: 10.1021/ja062710w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Wang D, Zheng Q, Lu M, Arora PS. Atomic structure of a short alpha-helix stabilized by a main chain hydrogen-bond surrogate. J. Am. Chem. Soc. 2008;130:4334–4337. doi: 10.1021/ja077704u. [DOI] [PubMed] [Google Scholar]
- 29.Henchey LK, et al. Inhibition of hypoxia inducible factor 1–transcription coactivator interaction by a hydrogen bond surrogate alpha-helix. J. Am. Chem. Soc. 2010;132:941–943. doi: 10.1021/ja9082864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman RN, Arora PS. Optimized synthesis of hydrogen-bond surrogate helices: surprising effects of microwave heating on the activity of grubbs catalysts. Org. Lett. 2006;8:5825–5828. doi: 10.1021/ol062443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimartino G, Wang D, Chapman RN, Arora PS. Solid-phase synthesis of hydrogen-bond surrogate-derived alpha-helices. Org. Lett. 2005;7:2389–2392. doi: 10.1021/ol0506516. [DOI] [PubMed] [Google Scholar]
- 32.Patgiri A, Witten MR, Arora PS. Solid phase synthesis of hydrogen bond surrogate derived alpha-helices: resolving the case of a difficult amide coupling. Org. Biomol. Chem. 2010;8:1773–1776. doi: 10.1039/c000905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trost BM, Van Vranken DL. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- 34.Miller SC, Scanlan TS. oNBS-SPPS: a new method for solid-phase peptide synthesis. J. Am. Chem. Soc. 1998;120:2690–2691. [Google Scholar]
- 35.Coin I, Beyermann M, Bienert M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007;2:3247–3256. doi: 10.1038/nprot.2007.454. [DOI] [PubMed] [Google Scholar]
- 36.Chan WC, White PD. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford University Press; 2000. [Google Scholar]
- 37.Kaiser E, Colescot RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in solid-phase synthesis of peptides. Anal. Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 38.Vojkovsky T. Detection of secondary amines on solid-phase. Peptide Res. 1995;8:236–237. [PubMed] [Google Scholar]