Abstract
Rationale
There is a paucity of large cohort studies examining the association of obstructive sleep apnea(OSA) with clinical outcomes including all-cause mortality, coronary heart disease(CHD), strokes and chronic kidney disease(CKD).
Objectives
We hypothesized that a diagnosis of incident OSA is associated with higher risks of these adverse clinical outcomes.
Methods, Measurements
In a nationally representative cohort of over 3 million(n=3,079,514) US veterans(93% male) with baseline estimated glomerular filtration rate (eGFR)≥60 ml/min/1.73m2, we examined the association between the diagnosis of incident OSA, treated and untreated with continuous positive airway pressure(CPAP), and: 1) all-cause mortality, 2) incident CHD, 3) incident strokes, 4)incident CKD defined as eGFR<60 ml/min/1.73m2, and 5)slopes of eGFR.
Main Results
Compared to OSA negative patients, untreated and treated OSA was associated with 86% higher mortality risk,(adjusted hazard ratio and 95% confidence interval: 1.86(1.81-1.91)) and 35% (1.35(1.21-1.51)), respectively. Similarly, untreated and treated OSA was associated with 3.5 times(3.54(3.40-3.69)) and 3 times(3.06(2.62-3.56)) higher risk of incident CHD; 3.5 times higher risk of incident strokes(3.48(3.28-3.64) and 3.50(2.92-4.19)) for untreated and treated OSA, respectively. The risk of incident CKD was also significantly higher in untreated(2.27(2.19-2.36)) and treated(2.79(2.48-3.13)) OSA patients. The median (interquartile range) of the eGFR slope was −0.41(−2.01 - 0.99), −0.61(−2.69 - 0.93) and −0.87(−3.00 - 0.70)ml/min/1.73m2 in OSA negative, untreated and treated OSA positive patients, respectively.
Conclusions
In this large and contemporary cohort of more than 3 million US veterans, a diagnosis of incident OSA was associated with higher mortality, incident CHD, stroke and CKD and with faster kidney function decline.
Keywords: coronary heart disease, chronic kidney disease, continuous positive airway pressure, kidney function, mortality, obstructive sleep apnea, stroke
Introduction
Obstructive sleep apnea (OSA) is one of the most clinically important forms of sleep-related breathing disorders. The prevalence of moderate and severe obstructive OSA, defined as an apnea hypopnea index (AHI)>15 and the presence of daytime symptoms of OSA is 10% in the general population.1
Previous studies have shown that OSA is associated with a higher risk of mortality2-4, but some studies did not confirm this association in elderly patients with prevalent OSA5-7. In addition to increased mortality risk, OSA has also shown positive associations with cardiovascular and cerebrovascular events.3,8-10 However, most of these studies were limited by small sample size and low event rates.9,11
In patients with chronic kidney disease (CKD), OSA is associated with accelerated atherosclerosis, hypertension and vascular damage.12 The complex pathophysiology that links OSA to cardiovascular risk may also have a detrimental effect on kidney function. Patients with OSA experience glomerular hyperfiltration, which is alleviated by short-term continuous positive airway pressure (CPAP) treatment,13 but our previous studies in kidney transplant recipients found no association between OSA and deterioration of kidney function.14,15 Only one recently published study assessed the association of OSA with kidney function decline and progression to CKD in non-transplant patients.16
We examined the associations of incident treated and untreated OSA diagnosis with all-cause mortality, the incidences of coronary heart disease (CHD), ischemic stroke, and CKD, and the rate of kidney function decline in a large, nationally representative contemporary cohort of US veterans. Based on previous findings, we hypothesized that a diagnosis of incident OSA is associated with higher risks of these adverse clinical outcomes.
Methods
Study Setting and Cohort Definition
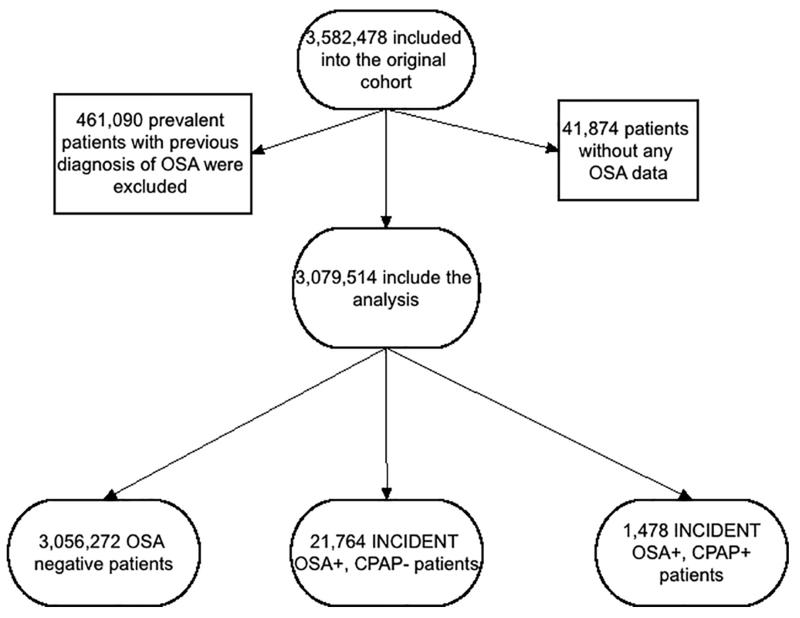
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Data were obtained from the Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study, which examines risk factors in patients with incident CKD in US veterans, and which was previously described in detail.17,18 Diagnoses of incident OSA, CPAP and polysomnography (overnight sleep study) were identified from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic and procedure codes and Current Procedural Terminology (CPT) codes (Table S1).19 The algorithm for cohort definition is shown in Figure 1. Patients were included in the study if they had a baseline estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73m2 and did not have a diagnosis of OSA at the first encounter in the inclusion period (October 1, 2004-September 30, 2006), while other comorbidities were listed in that encounter. The final cohort included 3,079,514 patients.
Figure 1.
Flow chart of patients’ selection
Exposure and Covariates
Incident OSA was defined as a new ICD9-CM code for OSA during the inclusion period, without such a diagnosis at the first encounter of the inclusion period. Among the 3,079,514 patients, 21,764 had incident diagnosis of OSA but without CPAP treatment (OSA+/CPAP−) and 1,478 had incident diagnosis of OSA treated with CPAP (OSA+/CPAP+) (Figure 1).
Socio-demographic characteristics, comorbid conditions and laboratory characteristics were obtained, as previously described.20-23 Information about age, gender and race were obtained through the VA Corporate Data Warehouse (CDW) and from Medicare through the VA-Medicare data merge project.24 Information about comorbidities was collected from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic and procedure codes and Current Procedural Terminology (CPT) codes (Table S2).19 Prevalent comorbidities were defined as those diagnosed during October 1, 2004-September 30, 2006.
Outcomes
We defined five different outcomes: 1) all-cause mortality, 2) incident CHD, 3) incident ischemic stroke, 4) incidence of CKD, and 5) slopes of eGFR.
Data on all-cause mortality was obtained from the VA Vital Status Files (VSF), which contain dates of death or last medical/administrative encounter from all sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, respectively, as compared to the National Death Index. Incident CHD was defined as the composite outcome of a first occurrence of an ICD-9-CM or CPT code for acute myocardial infarction, coronary artery bypass grafting, or percutaneous angioplasty after October 1, 2006 in patients without such diagnoses prior to this date. Incident stroke was defined as the first occurrence of ICD-9-CM codes for ischemic after October 1, 2006 in patients without such diagnoses prior to this date (Table S3-S4). Incident CKD was defined as two consecutive eGFR levels <60 ml/min/1.73m2 separated by ≥90 days, and a >25% decrease from baseline eGFR. Estimated GFR was calculated from serum creatinine measurements using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation. Slopes of eGFR were calculated in each patient from all available eGFR values derived from outpatient serum creatinine measurements and using least squares regression. The median (interquartile range (IQR)) number of serum creatinine measurements used to calculate eGFR slopes was 10 (5-17). Rapid deterioration was defined as slopes of <-5 ml/min/1.73m2/year (i.e. loss of eGFR of >5 ml/min/1.73m2/year).
Statistical Analysis
Data were summarized using proportions, means ± SD, or median (interquartile range (IQR)) as appropriate. Continuous variables were compared using the Student’s t-test and Mann-Whitney U test according to data type. Predictors of incident diagnosis of OSA were assessed using logistic regression analyses. The associations between diagnoses of incident OSA -with and without CPAP treatment-with outcomes were assessed using the Kaplan-Meier method, and Cox proportional hazard models (for time to event analyses) and logistic regressions (for slopes).
The start of the follow-up period was the date of the first eGFR ≥60 ml/min/1.73m2 during October 1, 2004-September 30, 2006. Patients diagnosed with OSA at a subsequent date were considered as part of the non-OSA group for the time period between cohort entry and diagnosis of OSA. Patients were followed until different end-points or were censored at the date of last healthcare or administrative visit, or on July 26, 2013.
In post-event (CHD or stroke) mortality analyses, the start of the follow-up period was the date of the event. Incident CHD and stroke events were identified after October 1, 2006 in patients without such diagnoses prior to this date; therefore, to avoid immortal time bias, the start of the follow-up period for these end points was October 1, 2006.
All associations were examined in both unadjusted and adjusted models. Models were adjusted for the following confounders based on a priori considerations: model 1: age, gender, race/ethnicity; model 2: model 1 variables and baseline eGFR; model 3: model 2 variables and comorbidities at baseline (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS and depression) and measures of quality of care (number of administered cholesterol measurements and influenza vaccinations); and model 4: model 3 variables and income, marital status and body mass index (BMI). The final fully adjusted model 4 analyses were repeated in different a priori selected subgroups.
Several sensitivity analyses were also performed. We repeated all analyses in a cohort of patients where the OSA diagnosis was based on the above-mentioned ICD-9 codes and confirmed by polysomnography. Because death and other outcomes are competing events, a competing risk regression model was used in a propensity-matched cohort of patients (n=42,340) with and without a diagnosis of incident OSA. Our events of interest were incident CHD, ischemic stroke and CKD and the competing event was death (Table S5). In our study we used the Fine and Gray method,25 which extends the Cox proportional hazards model to competing-risks data by considering the subdistribution hazard. The competing risk models’ goal was to estimate the cumulative incidence function, which is defined by the probability of events (Figure S1).
Statistical analyses were performed using Stata MP version 12 (Stata Corporation, College Station, TX).
Results
Baseline characteristics
The mean±SD age of the cohort at baseline was 60.5±14.4 years, 93% were male, 79% and 17% of patients were white and black, respectively, 22% of the patients were diabetic and the mean baseline eGFR was 83.6±15.4 ml/min/1.73m2. Baseline characteristics of patients categorized by OSA status are shown in Table 1. Patients with a diagnosis of incident OSA were slightly younger, more likely to be divorced and to have lower income, and had higher body mass index (BMI), higher prevalence of hypertension, diabetes mellitus, CVD, congestive heart failure (CHF), cerebrovascular disease, depression and higher eGFR.
Table 1.
Baseline characteristics of study population
| OSA negative (n=3,056,272) | OSA positive without treatment (n=21,764) | OSA positive with treatment (n=1,478) | |
|---|---|---|---|
| Age (years) | 61±14 | 59±11 | 57±10 |
| Gender (male) | 2,840,692 (93) | 20,932 (96) | 1,421 (96) |
| Outcomes: | |||
| Death | 671,645 (22) | 5,996 (28) | 323 (22) |
| Incident CHD event* | 68,268 (2) | 2,537 (12) | 167 (12) |
| Incident stroke event** | 50,333 (2) | 1,601 (8) | 122 (9) |
| New CKD | 290,037 (10) | 5,486 (25) | 434 (29) |
| Race: | |||
| White | 2,153,744 (79) | 17,020 (80) | 1,082 (74) |
| African-American | 465,810 (17) | 3,402 (16) | 333 (23) |
| Hispanic | 63,138 (2) | 413 (2) | 16 (1) |
| Other Race | 57,445 (2) | 410 (2) | 25 (2) |
| Marital status: | |||
| Married | 1,606,708 (55) | 10,341 (50) | 735 (52) |
| Single | 335,237 (11) | 2,112 (10) | 166 (12) |
| Divorced | 749,305 (26) | 6,858 (33) | 435 (31) |
| Widow | 229,756 (8) | 1,538 (7) | 77 (5) |
| Other sociodemographic: | |||
| Income (USD) | 22,849 (11,522-36,929) | 20,327 (11,563-31,104) | 19,881 (11,805-30,893) |
| Service connection | 1,154,717 (38) | 11,208 (51) | 778 (53) |
| Baseline eGFR (ml/min./1.73m2) | 84±15 | 85±15 | 87±16 |
| BMI (kg/m2) | 28.3±4.9 | 32.0±5.8 | 33.1±5.4 |
| Comorbidities: | |||
| Hypertension | 1,763,151 (58) | 15,881 (73) | 1,124 (76) |
| Diabetes mellitus | 674,318 (22) | 8,675 (40) | 644 (44) |
| Cardiovascular Disease*** | 333,638 (11) | 4,105 (19) | 308 (21) |
| Congestive Heart Failure | 121,312 (4) | 2,691 (12) | 202 (14) |
| Cerebrovascular Disease | 186,119 (6) | 1,910 (9) | 146 (10) |
| Peripheral Arterial Disease | 164,507 (5) | 1,959 (9) | 140 (9) |
| Chronic Lung Disease | 524,645 (17) | 6,592 (30) | 450 (30) |
| Dementia | 27,440 (0.9) | 190 (0.9) | 12 (0.8) |
| Rheumatologic Disease | 42,154 (1.4) | 370 (1.7) | 30 (2.0) |
| Peptic ulcer disease | 56,591 (1.8) | 502 (2.3) | 34 (2.3) |
| Liver Disease | 37,795 (1.2) | 411 (1.9) | 22 (1.5) |
| All malignancies | 327,064 (11) | 2,296 (11) | 138 (9) |
| AIDS/HIV | 19,958 (0.6) | 104 (0.5) | 5 (0.3) |
| Depression | 249,958 (8) | 3,325 (15) | 294 (20) |
Dichotomous/dummy variables are presented as number of patients and percentage; continous variables are presented as mean±SD or median (interquartile range, IQR)
in cardiovascular disease (see below) free patients at baseline
in stroke disease free patients at baseline
Cardiovascular Disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention
Abbreviations: AIDS: Acquired immundeficiency syndrome; BMI: Body mass index; CHD: coronary heart disease; CKD: Chronic Kidney Disease; eGFR: Estimated glomerular filtration rate; HIV: Human Immundeficiency Virus; IQR: Interquartile range; SD: Standard deviation
Predictors of the diagnosis of incident OSA
In our adjusted logistic regression model, younger age, female gender, African-American or Hispanic-American race, unmarried status, higher BMI and most of the comorbidities (such as diabetes, hypertension, CVD, CHF) were associated with the presence of a diagnosis of incident OSA (Table 2).
Table 2.
Predictors of a diagnosis of incident OSA using logistic regression analysis
| Odds Ratio (OR) | 95% confidence interval of OR | |
|---|---|---|
|
| ||
| Age (+10 year) | 0.90 | 0.89-0.91 |
|
| ||
| Gender: female vs male (ref.) | 0.49 | 0.45-0.53 |
|
| ||
| Race: | ||
| White (ref.) | 1.00 | 1.00-1.00 |
| African-American | 0.90 | 0.86-0.93 |
| Hispanic | 0.86 | 0.77-0.95 |
| Other Race | 0.95 | 0.86-1.05 |
|
| ||
| Income (+1 log) | 0.94 | 0.93-0.96 |
|
| ||
| Marital status: Unmarried vs married (ref.) | 1.12 | 1.09-1.16 |
|
| ||
| Baseline eGFR (+10 ml/min./1.73m2) | 1.06 | 1.05-1.07 |
|
| ||
| Presence of diabetes vs absence of diabetes (ref.) | 1.36 | 1.32-1.40 |
|
| ||
| Presence of hypertension vs absence of hypertension (ref.) | 1.33 | 1.28-1.37 |
|
| ||
| Presence of Cardiovascular Disease vs absence of Cardiovascular Disease (ref.) | 1.35 | 1.30-1.40 |
|
| ||
| Presence of Congestive Heart Failure vs absence of Congestive Heart Failure (ref.) | 1.97 | 1.88-2.06 |
|
| ||
| Presence of Cerebrovascular Disease* vs absence of Cerebrovascular Disease* (ref.) | 1.26 | 1.20-1.33 |
|
| ||
| Presence of Peripheral Arterial Disease vs absence of Peripheral Arterial Disease (ref.) | 1.29 | 1.23-1.36 |
|
| ||
| Presence of Chronic Lung Disease vs absence of Chronic Lung Disease (ref.) | 1.91 | 1.85-1.98 |
|
| ||
| Presence of dementia vs absence of dementia (ref.) | 1.06 | 0.92-1.23 |
|
| ||
| Presence of Rheumatologic Disease vs absence of Rheumatologic Disease (ref.) | 1.36 | 1.22-1.51 |
|
| ||
| Presence of malignancy vs absence of malignancy (ref.) | 1.05 | 1.00-1.10 |
|
| ||
| Presence of AIDS/HIV vs absence of AIDS/HIV (ref.) | 0.93 | 0.76-1.14 |
|
| ||
| Presence of depression vs absence of depression (ref.) | 1.71 | 1.64-1.78 |
|
| ||
| Body mass index (+1 kg/m2) | 1.14 | 1.13-1.14 |
Cardiovascular Disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention
Abbreviations: AIDS: Acquired immundeficiency syndrome; eGFR: Estimated glomerular filtration rate; HIV: Human Immundeficiency Virus
Mortality
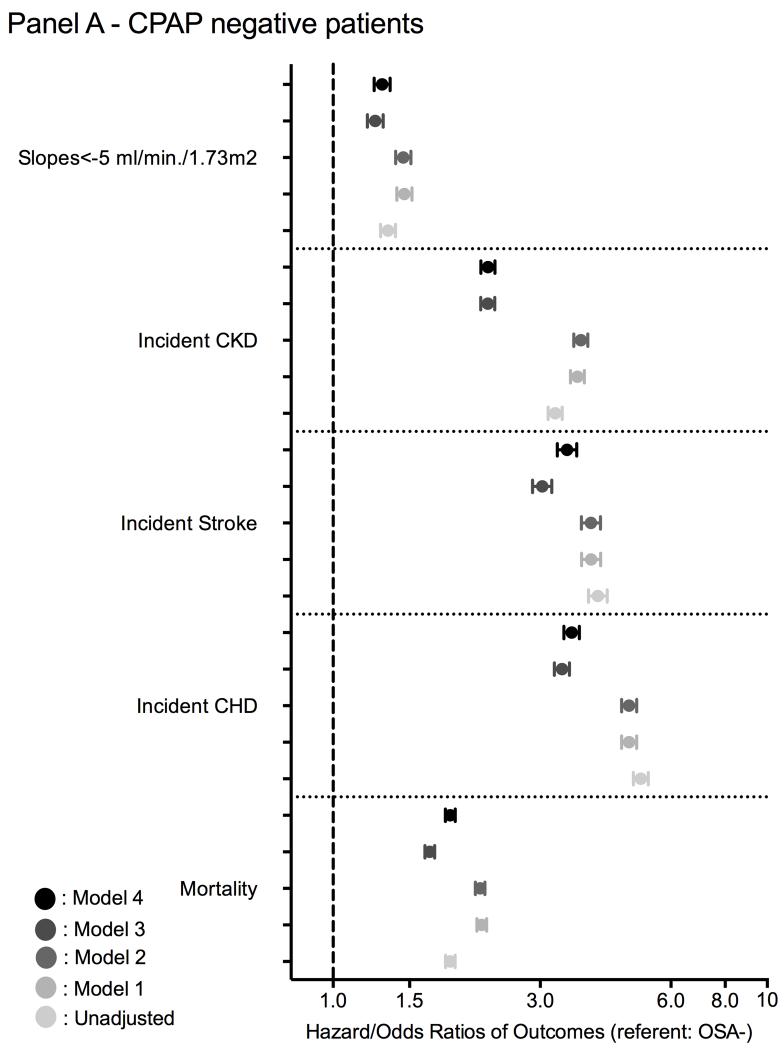
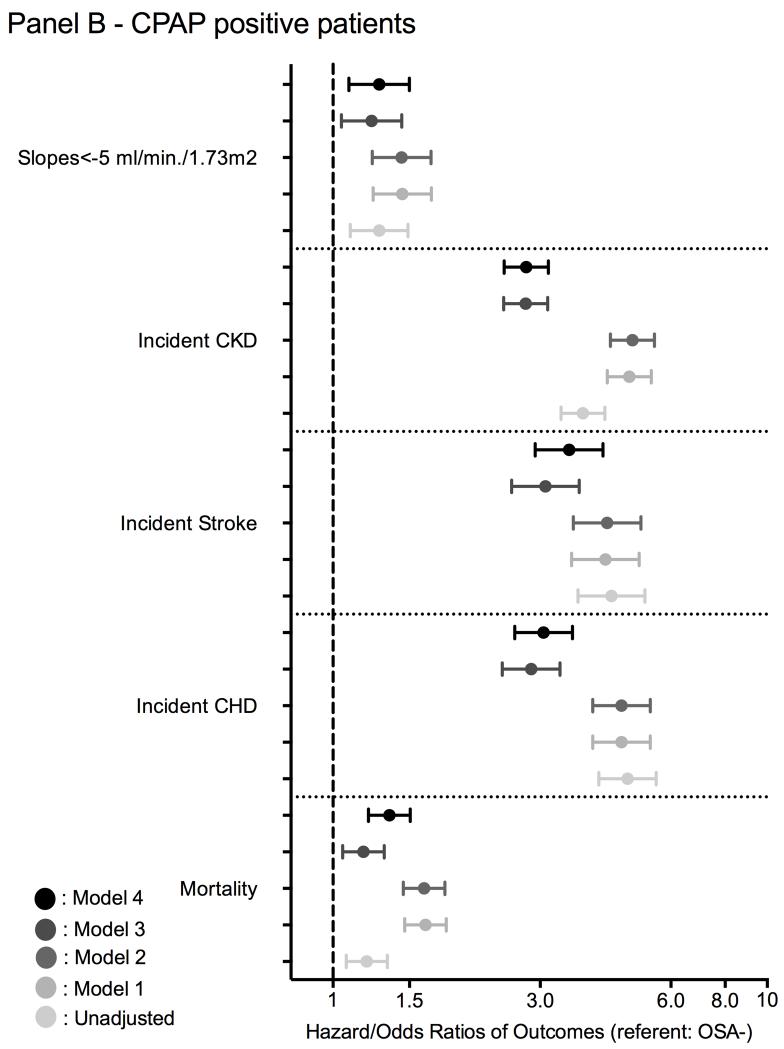
The median follow-up time was 7.74 years (IQR: 5.99-8.37 years). There were 671,645 deaths (22%, mortality rate 32.6 [32.5-32.7]/1000 patient-years) in the OSA negative group, 323 deaths (22%, 62.3 [60.7-63.9]/1000 patient-years) in the treated OSA group and 5,996 deaths (28%, 39.9 [35.8-44.5]/1000 patient-years) in the untreated OSA group. Figures 2 and 3 show the associations between the diagnosis of incident OSA and mortality in unadjusted and adjusted models. Untreated OSA was associated with higher mortality in the unadjusted (hazard ratio (HR): 1.86, 95% confidence interval (CI): 1.81-1.91) and fully adjusted model (HR: 1.86, 95% CI: 1.81-1.91) (Figure 3 panel A). Treated OSA was also associated with higher mortality in the unadjusted (HR: 1.20, 95%CI: 1.07-1.33) and fully adjusted model (HR: 1.35, 95% CI: 1.21-1.51) (Figure 3 panel B). Similar results were present in all subgroups in untreated OSA+ patients and most of the treated OSA+ subgroup (Figure S1).
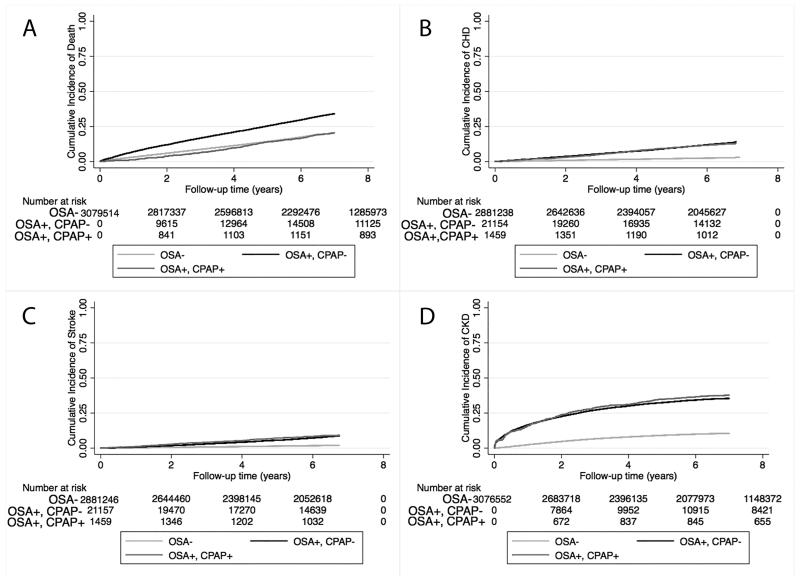
Figure 2.
Association between a diagnosis of incident OSA and outcomes (panel A: cumulative incidence of death; panel B: cumulative incidence of CHD; panel C: cumulative incidence of ischemic stroke event and panel D: cumulative incidence of CKD) using Kaplan-Meiers curves
Figure 3.
Association between a diagnosis of incident OSA (panel A: without CPAP treatment; panel B: with CPAP treatment) and outcomes using time-dependent Cox regression (mortality and incidend CKD), Cox proportional regression (incident CHD and ischemic stroke event) and logistic regression analysis (for slope) compared to OSA negative patients
model 1: adjusted for age, gender, race/ethnicity;
model 2: adjusted for model 1 variables and baseline eGFR;
model 3: adjusted for model 2 variables and comorbidities at baseline (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS and depression) and measures of quality of care (number of administered cholesterol measurements and influenza vaccinations);
model 4: adjusted for model 3 variables and income, marital status and body mass index (BMI).
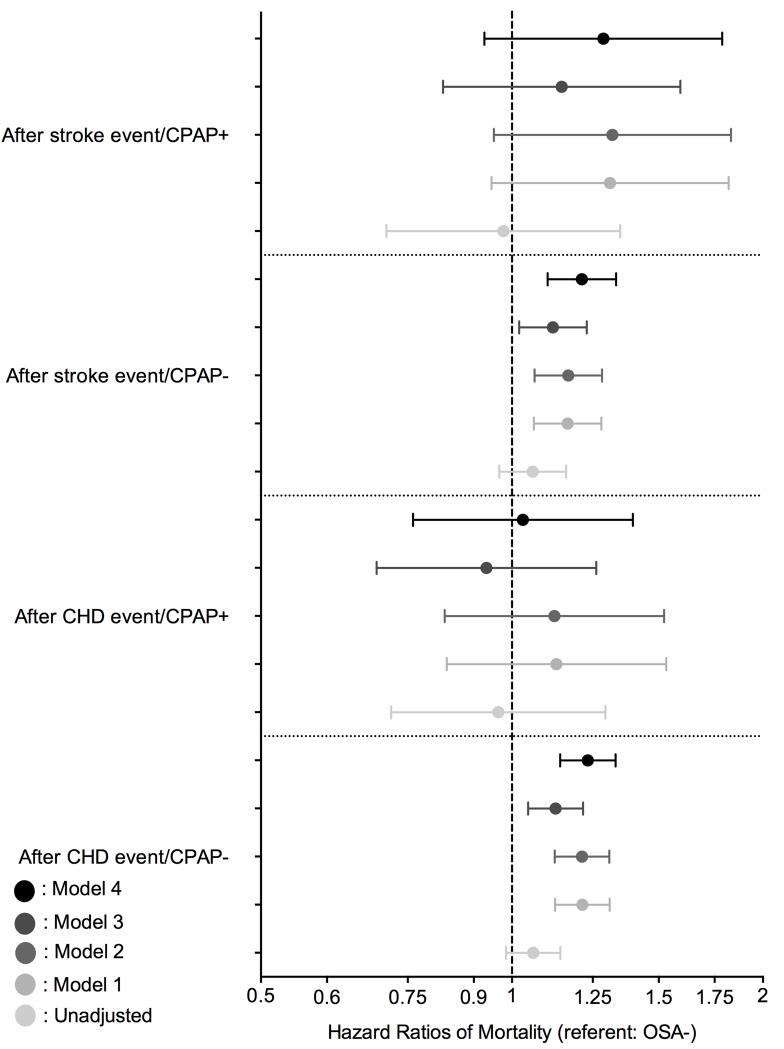
Figure 4 shows the association between a diagnosis of incident OSA and post-event mortality. Untreated OSA was associated with significantly higher post-CHD event mortality only in the fully adjusted model (HR: 1.23, 95% CI: 1.14-1.33). However, the post-CHD event mortality was similar between OSA negative and untreated OSA positive patients (Figure 4). Compared to OSA negative patients, patients with treated OSA exhibited a trend toward higher post-stroke mortality in the unadjusted model (HR: 1.06, 95% CI: 0.97-1.16), and a significantly higher post-stroke mortality in the fully adjusted model (HR: 1.21, 95% CI: 1.10-1.33) (Figure 4). However, the post-stroke mortality risk was similar between OSA negative and treated OSA positive patients (Figure 4).
Figure 4.
Association between a diagnosis of incident OSA and post-CHD/post-ischemic stroke event mortality using Cox proportional regression analysis compared to OSA negative patients
model 1: adjusted for age, gender, race/ethnicity;
model 2: adjusted for model 1 variables and baseline eGFR;
model 3: adjusted for model 2 variables and comorbidities at baseline (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS and depression) and measures of quality of care (number of administered cholesterol measurements and influenza vaccinations);
model 4: adjusted for model 3 variables and income, marital status and body mass index (BMI).
Incident CHD
There were 68,268 incident CHD events (2.3%, event rate 4.17 [4.14-4.20]/1000 patient-years) in the OSA negative group, 167 incident CHD events (12.1%, 20.1 [17.2-23.4]/1000 patient-years) in the treated OSA group, and 2,537 incident CHD events (12.4%, 21.5 [20.7-22.3]/1000 patient-years) in the untreated OSA group. Figures 2 and 3 show the associations between a diagnosis of incident OSA and incident CHD events in unadjusted and adjusted models. Untreated OSA was associated with 5-fold higher risk of incident CHD in the unadjusted (HR: 5.11, 95% CI: 4.91-5.32) and 3.5-fold higher risk in the fully adjusted model (HR: 3.54, 95% CI: 3.40-3.69) (Figure 3 panel A). Treated OSA was associated with a nearly 5-fold higher risk for incident CHD in the unadjusted (HR: 4.77, 95% CI: 4.10-5.55) and 3-fold higher risk in the fully adjusted model (HR: 3.06, 95% CI: 2.62-3.56) (Figure 3 panel B). Similar results were present in all subgroups (Figure S2) and in our competing risk regression model (Figure S1 and Table S5).
Incident stroke
There were 50,333 incident strokes (1.8%, event rate 3.07 [3.04-3.10]/1000 patient-years) in the OSA negative group, 122 incident strokes (9.2%, 14.6 [12.2-17.4]/1000 patient-years) in the treated OSA group, and 1,601 incident strokes (8.1%, 13.3 [12.7-14.0]/1000 patient-years) in the untreated OSA group. Figures 2 and 3 show the association between a diagnosis of incident OSA and incident stroke in unadjusted and adjusted models. Untreated OSA was associated with 4-fold higher risk of incident stroke in the unadjusted (HR: 4.07, 95% CI: 3.87-4.28) and 3.5-fold higher risk in the fully adjusted model (HR: 3.48, 95% CI: 3.28-3.64) (Figure 3 panel A). Treated OSA was associated with nearly 4-fold higher risk for incident stroke in the unadjusted (HR: 4.38, 95% CI: 3.67-5.23) and 3.5-fold higher risk in the fully adjusted model (aHR: 3.50, 95% CI: 2.92-4.19) (Figure 3 panel B). Similar results were found in all subgroups (Figure S3) and in our competing risk regression model (Figure S1 and Table S5).
Incidence of eGFR <60 ml/min/1.73m2
There were 290,037 incident CKD events (10%, event rate 15.05 [14.99-15.11]/1000 patient-years) in the OSA negative group, 434 incident CKD events (29%, 46.26 [41.19-51.96]/1000 patient-years) in the treated OSA group, and 5,486 incident CKD events (25%, 38.31 [36.93-39.74]/1000 patient-years) in the untreated OSA group. Figures 2 and 3 show the associations between a diagnosis of incident OSA and incident CKD in unadjusted and adjusted models. Untreated OSA was associated with 3-fold higher risk of incident CKD in the unadjusted (HR: 3.25, 95%CI: 3.13-3.37) and 2-fold higher risk in the fully adjusted model (HR: 2.27, 95%CI: 2.19-2.36) (Figure 3 panel A). Treated OSA was associated with nearly 3.8-fold higher risk of incident CKD in the unadjusted (HR: 3.76, 95% CI: 3.35-4.23) and 2.8-fold higher risk in the fully adjusted model (HR: 2.79, 95% CI: 2.48-3.13) (Figure 3 panel B). Similar results were found in all subgroups (Figure S4) and in our competing risk regression model (Figure S1 and Table S5).
Deterioration of kidney function
The median (IQR) of the eGFR slope was −0.41 (−2.01; 0.99); −0.61 (−2.69; 0.93) and −0.87 (−3.00; 0.70) in OSA negative, untreated and treated OSA positive patients, respectively. 292,825 veterans had slopes steeper than −5 ml/min/1.73m2/year. Figure 3 shows the association between a diagnosis of incident OSA status and the slope of deterioration of the kidney function. Untreated OSA positive patients had significantly higher risk of rapid deterioration of kidney function in the unadjusted (odds ratio (OR): 1.34, 95% CI: 1.29-1.39) and fully adjusted model (OR: 1.30, 95% CI: 1.24-1.35) (Figure 3 panel A). Treated OSA positive patients had also significantly higher risk of rapid deterioration of kidney function in the unadjusted (OR: 1.28, 95% CI: 1.10-1.49) and fully adjusted model (OR: 1.28, 95% CI: 1.09-1.50) (Figure 3 panel B). Similar results were found in most subgroups (Figure S5).
In sensitivity analyses using polysomnography as secondary criteria of the diagnosis instead of the ICD-9-CM diagnosis code, qualitatively and quantitatively similar results were found (results not shown).
Discussion
In a large cohort of US veterans with baseline eGFR ≥60 ml/min/1.73m2, we examined the association of a diagnosis of incident untreated/treated OSA with higher risk of all-cause and post-ischemic event mortality, incident CHD and stroke, incident eGFR<60 ml/min/1.73m2 and the rate of kidney function decline. Incident diagnosis of OSA was associated with higher risk of all clinical outcomes.
Higher mortality associated with OSA was reported in several previous studies, which used the gold standard of polysomnography for diagnosing OSA,2-4,11 but some studies performed in prevalent elderly patients did not confirm this association.5-7 This discrepancy could be explained by survival bias,5-7 or it could be related to the pathophysiology of OSA. There is increasing evidence showing that patients with OSA can have a survival advantage secondary to ischemic preconditioning.26-30 The defense mechanism for the repetitive milder ischemic events during OSA can result in structural and functional changes in brain cells and myocardial cells, preparing them for survival in the face of a major ischemic event through a mechanism of ischemic preconditioning,28-30 however, this seems not to protect the kidney.31 Our post-ischemic event mortality analyses support this hypothesis. When comparing the effect sizes of all-cause mortality vs. post-event mortality, those for untreated OSA patients were significantly smaller and became non-significant in treated OSA patients. This observation supports the hypothesis that “well-prepared” patients may have a better chance to survive a major ischemic event, while those without an ischemic preconditioning defense mechanism may have higher post-event mortality. This can result in a selection bias in studies examining the elderly, which would have an accumulation of these survivors in their study population. This hypothesis needs to be tested in the future.
The associations of incident diagnosis of OSA with higher risk of incident CHD and stroke can be explained by increased sympathetic activity, intermittent hypoxia, hypertension, accelerated atherosclerosis, or a combination of these in patients suffering from OSA. Our observations support those made in previous smaller studies.3,32,33
We report strong associations of incident diagnosis of OSA with incident low eGFR and progressive loss of kidney function. OSA can cause increased renal sympathetic activity, intermittent hypoxia, hypertension, accelerated atherosclerosis, production of pro-inflammatory cytokines, endothelial dysfunction and proteinuria, which could all contribute to the development and progression of CKD. Previous studies have reported a significant association of OSA with the progression of kidney disease.34,35 In a study comparing 27 OSA patients with 32 healthy controls, those with OSA had higher filtration fraction suggesting the presence of glomerular hyperfiltration.13 Short-term CPAP therapy significantly decreased the filtration fraction, suggesting that CPAP may prevent nephropathy by decreasing OSA-related glomerular hyperfiltration.13 Recent study from Taiwan reported 2-times higher CKD and ESRD risk in patients with OSA compared to OSA free counterparts.16
Our study is notable for its large sample size and event numbers, and for it being representative of veterans who received care in the VA system in the entire US. To our knowledge, this is the largest study to find substantial associations between a diagnosis of incident OSA and both kidney function decline and incident decrease in eGFR. This study also has several limitations that need to be acknowledged. This being an observational study, we can only report associations, and we cannot claim that OSA was indeed the cause of the worse clinical outcomes. Additionally, models could only be adjusted for identified confounders for which we had available data. Therefore, we cannot rule out residual confounding. Our study is limited by the use of diagnostic codes to define OSA, CPAP treatment and polysomnography. The diagnostic performance of these codes is not known. However, the significant predictors of incident diagnosis of OSA in our study were similar to those found in previous studies.36 Moreover, sensitivity analyses using polysomnography to define OSA led to similar results. We were unable to assess the associations of OSA severity with various outcomes as we did not have detailed polysomnography data. The proportion of patients who received CPAP treatment in our study was smaller than expected, which raises the possibility that patients received CPAP treatment outside the VA health system, refused CPAP treatment, or had mild/positional OSA, or received surgical treatment, or received CPAP treatment after the end of our follow-up period. Moreover, our study population consisted of mostly male US veterans, so the result should be applied with caution to female and to patients in other countries. Because we did not have information about causes of death, we could not analyze associations with cause-specific mortality. Additionally, we did not have data about albuminuria in our database; consequently we can only comment on associations between OSA and kidney function decline, but not on associations with properly defined incident CKD.
Conclusions
In our large and contemporary cohort of more than 3 million US veterans, a diagnosis of incident OSA was associated with higher risk of mortality, incident CHD, stroke and CKD, and faster kidney function decline. Improvement of the diagnostics and early detection as well the effect of proper therapy of OSA on preventing these clinical events need to be tested in clinical trials.
Supplementary Material
What is the key question?
We assessed the association between a diagnosis of incident OSA and adverse clinical outcomes such as incident chronic kidney disease (CKD) and mortality.
What is the bottom line?
In this large and contemporary cohort of more than 3 million US veterans, a diagnosis of incident OSA was associated with higher mortality, incident CHD, stroke and CKD and with faster kidney function decline.
Why read on?
This is the first study to show an association of a diagnosis of incident OSA with CKD and with faster kidney function decline.
Acknowledgments
None.
Funding Sources
This study is supported by grant 1R01DK096920 from the NIH to CPK and KKZ, and by resources from the US Department of Veterans Affairs. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). OAA was supported by Veni career grant 916.96.059 from the Netherlands Organization for Scientific Research.
Footnotes
Conflict of interest
None.
Disclosures
CPK, AXF, KKZ and KMH are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part.
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013;177(9):1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Peppard PE, et al. Sleep Disordered Breathing and Mortality: Eighteen-Year Follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31(8):1071–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. The New England journal of medicine. 2005;353(19):2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Marshall NS, Wong KK, Cullen SR, et al. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–62. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. Journal of sleep research. 2009;18(4):397–403. doi: 10.1111/j.1365-2869.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 6.Rich J, Raviv A, Raviv N, et al. All-cause mortality and obstructive sleep apnea severity revisited. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;147(3):583–7. doi: 10.1177/0194599812450256. [DOI] [PubMed] [Google Scholar]
- 7.Johansson P, Alehagen U, Svanborg E, et al. Clinical characteristics and mortality risk in relation to obstructive and central sleep apnoea in community-dwelling elderly individuals: a 7-year follow-up. Age and ageing. 2012;41(4):468–74. doi: 10.1093/ageing/afs019. [DOI] [PubMed] [Google Scholar]
- 8.Seicean S, Strohl KP, Seicean A, et al. Sleep disordered breathing as a risk of cardiac events in subjects with diabetes mellitus and normal exercise echocardiographic findings. The American journal of cardiology. 2013;111(8):1214–20. doi: 10.1016/j.amjcard.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS medicine. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. American journal of respiratory and critical care medicine. 2010;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge X, Han F, Huang Y, et al. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PloS one. 2013;8(7):e69432. doi: 10.1371/journal.pone.0069432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira Rodrigues CJ, Marson O, Tufic S, et al. Relationship among end-stage renal disease, hypertension, and sleep apnea in nondiabetic dialysis patients. American journal of hypertension. 2005;18(2 Pt 1):152–7. doi: 10.1016/j.amjhyper.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Kinebuchi S, Kazama JJ, Satoh M, et al. Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci (Lond) 2004;107(3):317–22. doi: 10.1042/CS20040074. [DOI] [PubMed] [Google Scholar]
- 14.Molnar MZ, Lazar AS, Lindner A, et al. Sleep apnea is associated with cardiovascular risk factors among kidney transplant patients. Clin J Am Soc Nephrol. 2010;5(1):125–32. doi: 10.2215/CJN.04030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornadi K, Ronai KZ, Turanyi CZ, et al. Sleep apnea is not associated with worse outcomes in kidney transplant recipients. Scientific reports. 2014;4:6987. doi: 10.1038/srep06987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YC, Hung SY, Wang HK, et al. Sleep Apnea and the Risk of Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Sleep. 2014 doi: 10.5665/sleep.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2014 doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosmanova EO, Lu JL, Streja E, et al. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64(5):951–7. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VIReC Research User Guide. VHA Medical SAS Inpatient Datasets FY 2006-2007. Hines IUSDoVAVIRC; 2007. [Google Scholar]
- 20.Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. ACE Inhibitor and Angiotensin Receptor Blocker Use and Mortality in Patients with Chronic Kidney Disease. Journal of the American College of Cardiology. 2014;63(7):650–58. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Lott EH, Lu JL, et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125(5):677–84. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Lott EH, Lu JL, et al. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. Journal of the American College of Cardiology. 2013;61(15):1626–33. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006-2007. US Department of Veterans Affairs VA Information Resource Center; Hines, IL: 2007. [Google Scholar]
- 24.US Department of Veterans Affairs VA Information Resource Center . Data Quality Update: Race. 2009. [Google Scholar]
- 25.Fine J, Gray R. A proportional hazards model for subdistribution of a competing risk. Journal of American Statistical Association. 1999;94:496–509. [Google Scholar]
- 26.Bartlett DJ, Rae C, Thompson CH, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep medicine. 2004;5(6):593–6. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia - Revisited - The bad ugly and good: Implications to the heart and brain. Sleep medicine reviews. 2014 doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig I, Kempton MJ, Crum WR, et al. Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning? PloS one. 2013;8(12):e83173. doi: 10.1371/journal.pone.0083173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Current opinion in pulmonary medicine. 2014;20(6):565–71. doi: 10.1097/MCP.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 30.Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep & breathing = Schlaf & Atmung. 2013;17(2):819–26. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nature medicine. 2011;17(11):1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joo BE, Seok HY, Yu SW, et al. Prevalence of sleep-disordered breathing in acute ischemic stroke as determined using a portable sleep apnea monitoring device in Korean subjects. Sleep & breathing = Schlaf & Atmung. 2011;15(1):77–82. doi: 10.1007/s11325-009-0325-8. [DOI] [PubMed] [Google Scholar]
- 33.Arzt M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. American journal of respiratory and critical care medicine. 2005;172(11):1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szentkiralyi A, Czira ME, Molnar MZ, et al. High risk of obstructive sleep apnea is a risk factor of death censored graft loss in kidney transplant recipients: an observational cohort study. Sleep medicine. 2011;12(3):267–73. doi: 10.1016/j.sleep.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Stehouwer CD, Gall MA, Twisk JW, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–65. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 36.Sharma SK, Malik V, Vasudev C, et al. Prediction of obstructive sleep apnea in patients presenting to a tertiary care center. Sleep & breathing = Schlaf & Atmung. 2006;10(3):147–54. doi: 10.1007/s11325-006-0062-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.