Abstract
Past studies have focused on aggregate lupus disease activity during pregnancy and have produced conflicting results. Our study evaluated lupus activity based on involvement of five specific organ systems during the six months prior to conception and during pregnancy. We assessed 147 pregnancies among 113 women followed at Brigham and Women’s Lupus Center, 1990–2013. Organ-specific activity included hematologic disorder, nephritis, skin disease, arthritis, and serositis. We hypothesized that the presence of organ-specific activity six months prior to conception would increase the risk for that same type of activity during pregnancy. Our study population was 68% White; 100% had a positive ANA and 30% had a history of nephritis. Among women with organ-specific lupus activity during the six months before conception, the crude odds for the same type of activity during pregnancy was 7.7 to 32.5-fold higher compared to women without that type of activity immediately before conception. An adjusted logistic regression model also indicated significantly higher odds of organ-specific activity during pregnancy if that type of activity were present six months before conception. Approaching lupus based on specific organ systems may be a useful way for women and their physicians to consider the potential risk for disease activity during pregnancy.
Keywords: Systemic lupus erythematosus, pregnancy, nephritis, haematologic changes
Introduction
Past studies of aggregate systemic lupus erythematosus (lupus) disease activity during pregnancy have produced conflicting results. Some reports have demonstrated the presence of increased disease activity in pregnancy,1, 2 while others have not.3–5 In attempt to more accurately evaluate potential lupus flares in the context of physiologic changes during pregnancy, composite scales for lupus activity have been modified. These include the SLE-Pregnancy Disease Activity Index (SLEPDAI),6 Lupus Activity Index-Pregnancy (LAI-P),7 BILAG-P,8 and Modification of the Systemic Lupus Activity Measure (m-SLAM).9 While there is not agreement on whether or not pregnancy causes lupus to flare, there is consensus that active lupus nephritis in the six months prior to conception is associated with both an increased risk of maternal lupus flare during pregnancy and adverse pregnancy outcomes, including pre-eclampsia and preterm delivery.10–12 With the exception of lupus nephritis, however, studies have focused on total lupus activity–rather than specific organ-system activity–before conception and during pregnancy.
In the present study, we aimed to address the question of whether a woman with lupus who has active disease involving a specific organ system in the six months prior to conception will continue to have the same type of lupus organ system activity during her pregnancy. We hypothesized that the presence of organ-specific lupus disease activity during the six months prior to conception would increase the risk of having the same type of organ-specific disease activity during pregnancy, and that this might be a useful way of approaching the heterogeneity of lupus itself when informing women of their risks during pregnancy.
Methods
Study design
We performed a retrospective cohort study of women with a diagnosis of lupus based on four or more 1997 American College of Rheumatology (ACR) classification criteria and followed at the Brigham and Women's (BWH) Lupus Center; lupus diagnosis was confirmed by the review of a second rheumatologist.13 Of the 1127 women with more than two visits to the BWH Lupus Center between 1990 and 2013, those with one or more pregnancies during the study period were included in our analysis. The Department of Obstetrics and Gynecology at BWH is one of the oldest and largest, and performs 8000 deliveries per year; the majority of women included in our study delivered at BWH.
The primary outcome was the presence of any of five types of organ-specific lupus activity during pregnancy: hematologic, nephritis, skin disease, arthritis, and serositis. Given that we were interested in lupus activity during the entire duration of pregnancy, we restricted our analyses to include pregnancies that progressed past the first trimester (12 weeks gestation).
Data collection
For each pregnancy, we recorded the date of conception as documented in the electronic medical record; if not clearly documented in the medical record, this date was calculated based on the gestational age at completion of pregnancy. Using the date of conception as the index date, we determined the six-month period prior to the date of conception. We reviewed the electronic medical record from the time of the woman’s initial visit to the Brigham and Women’s Lupus Center, through the duration of pregnancy, and recorded information on demographics (age at lupus diagnosis, age at conception, race/ethnicity), medication use (whether a range of individual lupus-related medications had been prescribed ever, within six months of conception, and/or during pregnancy), and laboratory data (lupus serologies and antiphospholipid antibodies [APLAb] collected prior to conception; complete blood counts, complement levels, and urinalysis with sediment at each assessment during the six months prior to conception and during pregnancy).
Lupus clinical history was reviewed at each clinic visit for the six months prior to conception and during pregnancy, and was categorized into five organ-specific types of lupus activity: hematologic disorder (WBC <4000/mm3 not attributed to medication; hemolytic anemia; or platelet count <100 × 109/L),13 nephritis (new-onset or worsening proteinuria >0.5g/24h; >5 WBC/hpf or >5 RBC/hpf in the absence of infection or stone; or granular or cellular casts),14 skin disease (malar rash or discoid lesions),13 arthritis (synovitis in >1 joint),13 and serositis (pleurisy or pleural effusion; or pericarditis or pericardial effusion).13 The threshold for leukopenia was chosen after considering that WBC <4000/mm3 is the cutoff in the 1997 ACR classification criteria,13 and that the lower limit of WBC is 5600/mm3 during normal pregnancy.15 Thrombocytopenia was attributed to lupus activity after excluding medication-related cytopenia and pre-eclampsia based on a detailed review of the medical record. If new-onset or worsened proteinuria (>0.5g/24h) occurred during the first trimester, this was attributed to lupus nephritis. If new or worsened proteinuria occurred in the second or third trimesters, we assessed whether this was attributable to lupus nephritis or pre-eclampsia by incorporating information about C3 and C4 levels (low values supporting lupus nephritis, and normal or high values supporting pre-eclampsia), blood pressure (>140/90 on two or more occasions suggesting pre-eclampsia), the presence of urinary casts (supporting lupus nephritis, with the absence of casts suggesting pre-eclampsia), and elevated serum uric acid (supporting pre-eclampsia). Lupus nephritis was characterized as mild in the presence of non-nephrotic-range proteinuria, few RBC or WBC, and no cellular casts; and as moderate-to-severe in the presence of new nephrotic-range proteinuria, many RBC or WBC on urinary sediment, or cellular casts.
We recorded each type of organ-specific activity as present or absent at any point before conception, during the six-month period before conception, and at any point during pregnancy. If organ-specific activity was present during pregnancy, we classified it as new, recurrent, stable, or worsened.
Statistical Methods
Baseline characteristics of women with and without organ-specific activity during pregnancy were compared with Fisher’s exact test for proportions and Wilcoxon rank-sum test for continuous variables. The crude risk of each type of organ-specific disease activity during pregnancy was calculated as a percentage among those pregnancies preceded by the same type of organ-specific activity within six months of conception, and among those pregnancies without organ-specific activity within six months of conception. This latter group was subsequently used as the reference group for the odds ratio for organ-specific activity during pregnancy. Crude odds ratios (95% confidence interval) were calculated using logistic regression, and Wald chi-square testing was performed with a threshold of p<0.05 for statistical significance.
The crude risks and odds ratios were first performed using data from all pregnancies during the study period. In order to eliminate inter-individual clustering that could potentially occur if one woman with multiple pregnancies had the same lupus disease activity in each pregnancy, these calculations were then repeated including only the first pregnancy carried by each woman during the study period.
Multivariable logistic regression analyses were performed to determine if the presence of each type of organ-specific lupus activity in the six months before conception would predict the presence of the same type of activity during pregnancy after adjusting for age, race, history of anti-dsDNA elevation, and medication prescriptions in the six months before conception. Models were run separately for each of the five types of organ-specific lupus activity. In a sensitivity analysis adjusting for differences in the standard of care for lupus pregnancy over time, based on time period of conception, multivariable models included a term for conception prior to or in/after the calendar year 2006.
Corticosteroid and/or azathioprine (AZA) prescription within six months of conception, and hydroxychloroquine prescription within six months of conception, were a priori specified to be included in the multivariable model. The reason for a priori inclusion of these variables is as follows: Corticosteroid and/or AZA prescription in the months prior to conception was considered a marker for active lupus in one or more organ system, and was therefore expected to be associated with the presence of the same type of lupus activity during pregnancy. At the same time, corticosteroid and/or AZA use was also expected to decrease lupus activity via immunosuppression. Therefore, we considered corticosteroid and/or AZA prescription within the six months prior to conception to be a bidirectional confounder of organ-specific lupus activity during pregnancy. We also performed a sensitivity analysis adjusting for use of corticosteroids, methotrexate, rituximab, mycophenolate, and/or cyclophosphamide in the six months before conception, as these medications were thought to be a marker for the most active disease prior to conception.
Women using hydroxychloroquine (HCQ) during pregnancy have been shown to have lower overall lupus activity compared to women who discontinue HCQ just before or while pregnant;16, 17 therefore, we anticipated that a prescription for HCQ in the months before conception would be a negative confounder of lupus disease activity during pregnancy. Given that cessation of hydroxychloroquine has been associated with lupus flare, we performed a sensitivity analysis adjusting the multivariable model for HCQ cessation during pregnancy.18 Other lupus-related medications were prescribed infrequently and therefore were not included in the model. Results are presented as median [interquartile range] for continuous data, and n (%) for binary and categorical data. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
We identified 113 women who collectively carried 147 pregnancies past the first trimester during the study period. Baseline characteristics are presented in Table 1. Median age was 23.7 years at lupus diagnosis and 31.3 years at conception. Four women were diagnosed with lupus while pregnant. Each of these four recently diagnosed women had clinical manifestations of lupus during the six months prior to conception, so they were included in the analyses. The majority of women were White. Thirty-six percent (54/147) of women had activity in one or more organ system during the six months prior to conception, and 37% of pregnancies were documented to have been planned pregnancies. A history of anti-dsDNA elevation was significantly more common among women with organ-specific activity during pregnancy; otherwise, pre-pregnancy characteristics did not significantly differ between women with and without organ-specific activity during pregnancy.
Table 1.
Characteristics of women with ≥1 pregnancy
| 113 women | Total, n=113 |
Any organ-specific activity during pregnancy, n=43 |
No organ-specific activity during pregnancy, n=70 |
p value |
| Median age at lupus diagnosis [IQR], years | 23.7 [19.5, 27.8] | 24.6 [19.8, 27.5] | 22.9 [18.1, 28.5] | 0.66 |
| Median no. ACR criteria at diagnosis [IQR] | 5 [4,6] | 5 [4,6] | 5 [4,6] | 0.44 |
| Antiphospholipid antibody syndrome, n (%) | 5 (4.4) | 1 (2.3) | 4 (5.7) | 0.64 |
| History of lupus nephritis, n (%) | 34 (30.0) | 14 (32.6) | 20 (28.6) | 0.68 |
| Race/ethnicity, n (%) | ||||
| White | 77 (68.1) | 27 (62.8) | 50 (71.4) | 0.41 |
| Hispanic | 17 (15.0) | 7 (16.3) | 10 (14.3) | 0.72 |
| Black | 11 (9.7) | 6 (14.0) | 5 (7.1) | 0.32 |
| Asian | 8 (7.1) | 3 (7.0) | 5 (7.1) | 1.0 |
| 147 pregnancies | Total, n=147 |
Any organ-specific activity during pregnancy, n=54 |
No organ-specific activity during pregnancy, n=93 |
|
| Median age at conception [IQR], years | 31.3 [27.3, 35.0] | 30.9 [26.8, 34.0] | 31.3 [27.8, 35.4] | 0.42 |
| Planned pregnancy, n (%) | 55 (37.4) | 16 (29.6) | 39 (41.9) | 0.16 |
| Serologies prior to conception, n (%) | ||||
| ANA >1:40 | 147 (100.0) | 54 (100.0) | 93 (100.0) | 1.0 |
| Anti-dsDNA elevated | 98 (66.7) | 42 (77.8) | 56 (60.2) | 0.03 |
| APLAb positive | 43 (29.3) | 17 (31.5) | 26 (28.0) | 0.71 |
| Lupus anticoagulant | 12 (8.2) | 2 (3.7) | 10 (10.8) | 0.21 |
| Anticardiolipin IgM | 10 (6.8) | 3 (5.6) | 7 (7.5) | 0.75 |
| Anticardiolipin IgG | 29 (19.7) | 13 (24.1) | 16 (17.2) | 0.39 |
| Anti-La | 25 (17.0) | 10 (18.5) | 15 (16.1) | 0.82 |
| Anti-Ro | 41 (27.9) | 16 (29.6) | 25 (26.9) | 0.85 |
IQR = interquartile range
p-values from Fisher’s exact testing for proportions and Wilcoxon rank-sum testing for continuous variables.
Thirty percent of women had a history of lupus nephritis prior to conception, 47% (16/34) of whom had been treated with cyclophosphamide, with a median interval of 51.1 months [IQR 21.8 – 84.8 months] between the last dose and the date of conception. All women had a positive ANA, and 67% had elevated dsDNA. Antiphospholipid antibodies were present in 29% of women, with anticardiolipin IgG most frequently observed. Five women had a history of antiphospholipid antibody syndrome, two of whom had a history of recurrent miscarriage. Neither of these women had three miscarriages as a result of failed assisted reproductive therapy. During 73% of pregnancies, women visited the clinic at least twice during gestation. Lupus-related prescriptions during the six months prior to conception and during pregnancy is presented in Table 2. Fifty-four percent of women were prescribed HCQ within six months prior to conception, which decreased to 41% during pregnancy. Among the twenty-eight women who discontinued HCQ during pregnancy, 12 had been instructed to stop taking HCQ by their physician and 11 made a personal decision to stop taking it while pregnant; the reason for discontinuation was unclear in five cases. Eleven of the 12 cases in which the physician advised stopping HCQ occurred before the year 2006. Similar proportions of women (37%) were prescribed corticosteroids before conception and during pregnancy, with a median prednisone dose of 10 mg before conception and 20 mg during pregnancy. Azathioprine was prescribed to approximately 10% of women both six months before and during pregnancy. The other lupus-related medications were prescribed infrequently. The proportion of women prescribed anticoagulation or aspirin doubled between the six months before conception and during pregnancy.
Table 2.
Medications six months before conception and during 147 pregnancies
| Medication* | Six months before conception |
During pregnancy |
|---|---|---|
| Hydroxychloroquine | 80 (54.4) | 60 (40.8)+ |
| Corticosteroid | 54 (36.7) | 55 (37.4) |
| Median prednisone equivalent [IQR], mg | 10 [5, 12.5] | 20 [10, 35] |
| Azathioprine | 14 (9.5) | 16 (10.9) |
| Sulfasalazine | 1 (0.7) | 0 |
| Cyclosporine | 0 | 3 (2.0) |
| Mycophenolate | 4 (2.7) | 0 |
| Rituximab | 2 (1.4) | 0 |
| Methotrexate | 1 (0.7) | 0 |
| Cyclophosphamide | 1 (0.7) | 0 |
| Anticoagulant | 7 (4.8) | 17 (11.6) |
| Aspirin | 21 (14.3) | 36 (24.4) |
n (%) unless otherwise indicated
Among these 60 women, eight initiated HCQ while pregnant
Organ-specific lupus activity during 147 pregnancies
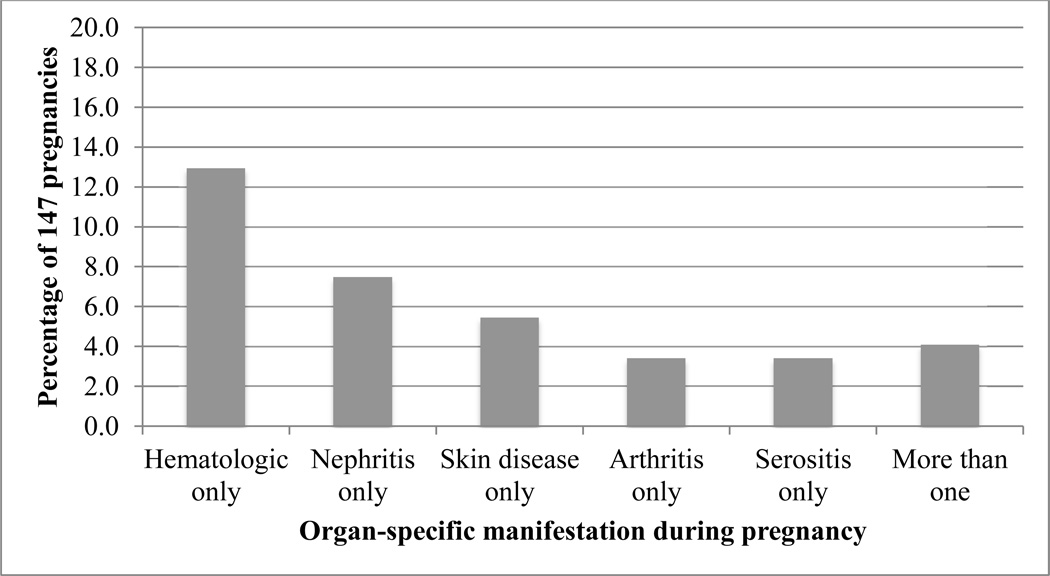
Organ-specific lupus activity occurred during 54 of 147 pregnancies and is depicted in Figure 1. The vast majority of these pregnancies were characterized by activity in one organ system only, depicted by a solid-colored box. The remaining six pregnancies, clustered in the center of the figure, were characterized by activity in multiple organ systems and are shaded by the colors corresponding to specific organ systems. Hematologic activity was the most common manifestation, followed by nephritis, skin disease, arthritis, and serositis.
Figure 1.
Organ-specific lupus activity occurred in 54 of 147 pregnancies
Hematologic disorder
Hematologic disorder occurred in 23 pregnancies (15.6%), carried by 18 unique women. Several pregnancies were characterized by more than one type of hematologic disorder, but were counted only once when calculating crude odds ratios.
Leukopenia occurred in 15 pregnancies, 10 of which also had leukopenia six months prior to conception. Of these 10, leukopenia was stable in nine and worsened in one; this worsening did not lead to an increase in corticosteroid therapy.
Thrombocytopenia occurred during nine pregnancies, four of which were characterized by thrombocytopenia in the six months before conception. Among these four pregnancies, thrombocytopenia was stable in one and worsened in three, leading to an increased corticosteroid dose. Among these nine pregnancies, the median platelet count was 67,000 (range 11,000 to 96,000). HELLP syndrome did not occur in any woman with thrombocytopenia. One woman had TTP during the first trimester, which manifested as thrombocytopenia and hemolysis without elevated transaminases.
Hemolytic anemia developed during two pregnancies, in both of which the woman had a remote history of hemolysis, but did not have hemolytic anemia during the six months before conception. One was treated with corticosteroids alone, while the other was treated with corticosteroids and plasmapheresis due to concern for thrombotic thrombocytopenia purpura.
The median increase in prednisone dose during pregnancy that was attributable to hematologic disorder alone was 7.5 mg [IQR 0 mg, 20 mg].
Nephritis
Lupus nephritis was present during 14 pregnancies (9.5%), each of which occurred in a unique woman. We identified four pregnancies in which pre-eclampsia, rather than lupus nephritis, accounted for proteinuria. Thus, lupus nephritis occurred during 14 of the 18 pregnancies with proteinuria >0.5g/24h. During pregnancy, two women (14% of those with active lupus nephritis) had renal biopsies. One biopsy revealed WHO Class III lupus nephritis, and the other revealed both Class II and Class V nephritis in a single biopsy specimen.
Six women had active nephritis during the six months before conception. During pregnancy, nephritis was stable in two and worsened in four women who developed moderate-to-severe nephritis. All four with moderate-to-severe nephritis were treated with increased doses of corticosteroids and/or azathioprine; additionally, one underwent Caesarean section at 33 weeks gestation in order to start cyclophosphamide treatment.
Another six women with lupus nephritis during pregnancy had a remote history of lupus nephritis that was inactive during the six months before conception. Of these six women with recurrent nephritis, three had mild nephritis and three had moderate-to-severe nephritis.
Two women had no prior history of lupus nephritis and developed nephritis for the first time while pregnant. One of these had mild nephritis, and one had moderate-to-severe nephritis.
The median increase in prednisone dose, attributable to nephritis alone during pregnancy, compared to the prednisone dose before conception was 20 mg [IQR 5 mg, 25 mg].
Skin disease
Skin disease was present in 12 pregnancies (8.2%) carried by eleven women. Several pregnancies were characterized by both malar rash and discoid lesions; however, these incidences were counted only once when calculating crude odds ratios.
Malar rash was more common than discoid lesions. Three of nine pregnancies with malar rash also had malar rash within the six months before conception. All three had unchanged malar rashes during pregnancy.
Three of four pregnancies with discoid lesions also had discoid lesions in the six months before conception. Of these three, one had stable discoid lesions during pregnancy while the other two had worsened lesions.
The median increase in prednisone dose, attributable to skin disease alone during pregnancy, compared to the prednisone dose before conception was 0 mg [IQR 0 mg, 7.5 mg].
Arthritis
Arthritis occurred in eight pregnancies (5.4%) among eight unique women. In three of these, arthritis had also been active in the six months before conception, and was stable during pregnancy. Of the five pregnancies in which arthritis was not active in the six months before conception, two of the women had mild arthritis during pregnancy, and three had moderate-to-severe arthritis involving multiple joints and requiring an increase in prednisone.
The median increase in prednisone dose, attributable to arthritis alone during pregnancy, compared to the prednisone dose before conception was 4 mg [IQR 0 mg, 5 mg].
Serositis
Serositis occurred in seven pregnancies (4.8%) among seven women. Pleural involvement was the only type of serositis observed during pregnancy. Two of these seven women had active pleuritis in the six months before conception, one of which had stable pleuritis during pregnancy, and one of which had worsened pleuritis. Of the other five pregnancies with pleuritis, four were mild and one was moderate-to-severe (with a pleural effusion and requiring a large increase in prednisone dose).
The median change in prednisone dose, attributable to pleuritis alone during pregnancy, was 0 mg [IQR −7.5 mg, 5 mg].
Odds of organ-specific lupus activity during pregnancy
The crude risk of having organ-specific lupus activity during pregnancy is shown in Table 3. The crude risk of each organ-specific manifestation was calculated among 147 pregnancies, based on the presence or absence of the same type of activity during the six months before conception. For example, hematologic disorder was observed in 71% of pregnancies with hematologic activity during the six months prior to conception, compared to 9% of pregnancies without hematologic activity in the six months before conception.
Table 3.
Risks and odds ratios for organ-specific lupus activity during pregnancy based on the presence versus absence of the same type of disease activity six months prior to conception
| N=147 pregnancies | N=113 pregnancies* | |||||
|---|---|---|---|---|---|---|
| Active During Pregnancy | Active During Pregnancy | |||||
| Percentage of those active 6 months prior |
Percentage of those inactive 6 months prior (ref) |
Crude odds ratio (95%CI) |
Percentage of those active 6 months prior |
Percentage of those inactive 6 months prior (ref) |
Crude odds ratio (95%CI) |
|
| Hematologic | 12/17 (70.6%) | 11/130 (8.5%) | 26.0 (7.7, 87.3) | 6/10 (60.0%) | 11/103 (10.7%) | 14.0 (3.4, 58.0) |
| Nephritis | 6/9 (66.7%) | 8/138 (5.8%) | 32.5 (6.8, 154.5) | 4/6 (66.7%) | 8/107 (7.5%) | 24.7 (3.9, 156.4) |
| Skin disease | 6/15 (40.0%) | 6/132 (4.5%) | 14.0 (3.7, 52.3) | 5/12 (41.7%) | 4/101 (4.0%) | 17.3 (3.8, 79.4) |
| Arthritis | 3/13 (23.1%) | 5/134 (3.7%) | 7.7 (1.6, 37.2) | 2/11 (18.2%) | 5/102 (4.9%) | 4.3 (0.7, 25.5) |
| Serositis | 2/5 (40.0%) | 5/142 (3.5%) | 18.2 (2.4, 134.9) | 2/4 (50.0%) | 4/109 (3.7%) | 26.2 (2.9, 236.9) |
Analysis restricted to each woman’s first pregnancy during the study period.
The highest crude odds ratio for organ-specific activity during pregnancy was observed for nephritis. Women with active nephritis in the six months prior to conception had 33-fold odds for nephritis during pregnancy, compared to women without active nephritis in the six months before conception. The odds of each of the five types of organ-specific activity during pregnancy were significantly higher among women with the same type of organ-specific activity during the six months preceding conception (p<0.05).
We repeated the analysis, including only the first pregnancy for each of the 113 women during the study period. The highest crude odds ratios for organ-specific activity in pregnancy were observed for nephritis and serositis. Hematologic disorder, skin disease, and serositis during pregnancy remained significantly associated with organ-specific activity six months before conception (p<0.05), but arthritis activity during pregnancy was no longer associated with arthritis activity in the months preceding conception.
The impact of organ-specific activity six months before conception on the odds of organ-specific activity during pregnancy was then adjusted for age, race, history of anti-dsDNA elevation, corticosteroid and/or azathioprine prescription in the six months before conception, and hydroxychloroquine prescription in the six months before conception (Table 4). After adjustment, the odds of hematologic disorder during pregnancy were 28-fold higher if the pregnancy had been preceded by hematologic activity within six months of conception, versus if it had not been recently preceded by hematologic disorder (95% CI 7.3,107). The adjusted odds ratios for nephritis, skin disease, arthritis, and serositis also remained significantly higher if that type of activity had been present within six months of conception, rather than absent immediately before conception. The other covariates included in each model were not significantly associated with any of the types of organ-specific activity during pregnancy. In a sensitivity analysis, we observed no change in the significance of associations between organ-specific disease activity before and during pregnancy, based on whether the pregnancy had been conceived before 2006 or during/after 2006 (data not shown).
Table 4.
Multivariable-adjusted odds ratios for organ-specific activity during 147 pregnancies*
| Activity six months prior to conception |
Adjusted odds ratio (95% CI) |
|---|---|
| Hematologic disorder | 27.9 (7.3, 107) |
| Nephritis | 80.0 (8.9, 723) |
| Skin disease | 17.4 (3.9, 78.8) |
| Arthritis | 11.7 (1.8, 76.7) |
| Serositis | 81.1 (3.1, infinity) |
Adjusted for age at conception, race, history of elevated anti-dsDNA, corticosteroid and/or azathioprine prescription in the six months before conception, and hydroxychloroquine prescription in the six months before conception.
We also repeated the analysis, adjusting for use of corticosteroid, methotrexate, rituximab, mycophenolate, and/or cyclophosphamide in the six months before conception (instead of corticosteroid and/or azathioprine), and obtained similar results to those presented in Table 4. Upon including HCQ cessation during pregnancy into the multivariable model, we found that cessation was not significantly associated with any of the types of organ-specific lupus activity.
Discussion
In our population of 113 women with 147 pregnancies, women with organ-specific lupus activity during the six months prior to conception were likely to have persistence of, or increase in, the same type of activity during pregnancy. Conversely, women without a particular type of organ-specific activity in the six months prior to conception were unlikely to develop that organ specific manifestation during pregnancy. Thus, the crude risk of organ-specific activity during pregnancy ranged from 23% to 71% in women with a specific type of activity during the six-month period before conception, compared to 3.5% to 8.5% in women without such activity in the six months before conception.
Hematologic disorder was the most common organ-specific lupus manifestation during pregnancy in our study (15.6% of pregnancies), followed by nephritis (9.5%); skin disease, arthritis, and serositis were each observed in less than 10% of pregnancies. While other studies have not specifically examined these five categories of lupus symptoms in pregnant verses non-pregnant women, they have similarly reported that the most common lupus symptoms during pregnancy include skin disease, joint symptoms, constitutional symptoms, and thrombocytopenia.1, 19, 20
Our cohort was comprised of mostly White women; approximately one-third had a prior history of lupus nephritis, and half of those had been treated with cyclophosphamide. All patients were ANA positive, and two-thirds were dsDNA positive. Hydroxychloroquine was only prescribed to 54% of patients in our study within six months of conception, and the percentage prescribed HCQ during pregnancy declined to 41%. This is explained by the long duration of our study period, and regional and institutional trends in the use of HCQ for management of lupus during pregnancy. By the start of the twenty-first century, a mounting body of literature supported the safety and efficacy of hydroxychloroquine during lupus pregnancy.17, 21, 22 We chose 2006 as a cutoff year to analyze historical pregnancy data, given that eleven of 12 cases in which the physician had advised discontinuation of HCQ during pregnancy occurred before 2006. In the years after 2006, HCQ was prescribed during the first trimester to seven of 16 women that had not been taking HCQ during the six months before conception. We did not observe any difference in the relationship between pre-conception lupus organ activity, and the same type of activity during pregnancy, based on whether women conceived before or after 2006. The percentage of women using corticosteroids did not appreciably increase between the months before conception and during pregnancy, suggesting generally low lupus disease activity in the study population. Interestingly, the use of corticosteroids and/or azathioprine, and the use of hydroxychloroquine, during the six months before conception did not appear to mitigate the risk of organ-specific disease activity among women with organ-specific activity during that timeframe. This finding must be interpreted cautiously, however, as we were not able ascertain medication adherence, which would influence the effectiveness of lupus-related medications.
Our multivariable analysis, which accounted for medication use, found that organ-specific activity within six months of conception was the only significant predictor of organ-specific activity during pregnancy. Given that the number of pregnancies with each type of organ-specific activity was low, the 95% confidence intervals were wide and thus should be interpreted with caution.
In a sensitivity analysis, we ran the multivariable model including a term for prior history (ever) of any of the five types of organ-specific activity, instead of organ-specific activity within six months of conception. The odds ratios for hematologic activity, nephritis, skin disease and serositis were each similar to but slightly lower than the odds ratios in our initial multivariable model, with overlapping confidence intervals. By contrast, a prior history of arthritis activity did not result in a significantly higher odds of arthritis activity during pregnancy (data not shown).
In Petri et al.’s prospective cohort study of lupus flares among 40 lupus pregnancies in Baltimore between 1987–1991,1 the most common components of flares included constitutional symptoms (57%), followed by skin disease (52%), renal involvement (43%), hematologic disorder (38%), musculoskeletal symptoms (35%), and serositis (17%). The women in the study were of similar age and lupus duration compared to the women in our study, but there was a higher proportion of Black patients (46%), and slightly higher mean prednisone dose during pregnancy (25 mg daily, compared to a mean of 23.6 mg daily in our study) than we observed. Cortes-Hernandez et al. reported disease activity during 103 lupus pregnancies in Spain, 12% of which had active lupus in any organ system at the time of conception.19 Maternal flare (defined as “any clinical event attributable to disease activity that required a change in therapy”) occurred during 33% of pregnancies; among these, arthritis was most common (69%), followed by skin disease in 33%, and lupus nephritis in 10%; however, some of these flares occurred during the post-partum period, rather than during pregnancy. Lupus disease traits prior to conception were relatively similar to those in our population, although a history of nephritis was less frequent (20%) in the Spanish group.
Georgiou et al. reported on 59 lupus pregnancies in a prospective cohort study, and defined an exacerbation or flare as signs of disease activity in two or more of six organ systems (including fever) that had been quiescent before pregnancy.20 Eight pregnancies underwent a flare by this definition, most often with skin lesions (7/8 pregnancies), fever (7/8), nephritis (3/8), serositis (5/8), and hematologic involvement (3/8). Musculoskeletal symptoms were not reported in these eight pregnancies. While the clinical manifestations during pregnancy were similar to what was found in our study, all eight patients had been in remission during the period preceding conception. More recent data from a retrospective cohort study by Al Arfaj et al. reported on the “onset of new signs of lupus disease activity during pregnancies in patients previously in remission” among 118 lupus pregnancies in Saudi Arabia, with malar/discoid lesions found in 29.7%, renal flare in 29.7%, arthralgia and arthritis in 22.9%, and hematologic disorder in 9.3% of subjects.16 Lupus manifestations prior to pregnancy, and management during pregnancy were different than in our population; the Saudi women had a higher prevalence of anti-dsDNA (80%) and more nephritis (41.5%) prior to conception. Fifty-eight percent were treated with corticosteroid monotherapy during pregnancy, and only 18% with a combination of HCQ and corticosteroids. Moreover, their analysis did not interpret the presence of organ-specific disease activity during pregnancy in the context of its presence or absence prior to pregnancy.
These past reports of lupus activity during pregnancy have defined lupus activity differently, namely as an aggregate of lupus disease activity. Our study suggests another way to conceptualize lupus activity before and during pregnancy. Notably, 89% (48/54) of women in our study with organ-specific lupus activity during pregnancy had only one type of organ system activity during pregnancy. Generally, that one organ system was a site of lupus activity during the period immediately preceding pregnancy. We found that hematologic involvement, in particular thrombocytopenia, during the six months prior to conception was strongly related to ongoing hematologic manifestations in pregnancy. In this study and in clinical practice, it is difficult to distinguish between gestational thrombocytopenia, gestational ITP, and thrombocytopenia secondary to lupus flare. Despite these limitations, we were able to identify that none of the women in our study had HELLP syndrome and one woman did have TTP during pregnancy. Although leukopenia was the most common type of hematologic activity, it is notable that leukopenia did not result in a change in lupus therapy, whereas thrombocytopenia and hemolytic anemia did tend to lead to increased corticosteroid dose. Thus, leukopenia may be interpreted as evidence of lupus activity, but in our cohort it did not have the same clinical impact as did other manifestations. Given these observations, we believe that approaching lupus based on the specific organ systems involved prior to conception and during pregnancy is a useful way for women and their physicians to consider the potential risk for disease activity during pregnancy.
Due to the protean manifestations of lupus, quantifying and predicting lupus disease activity during pregnancy is complex. Our findings are important for women with lupus and their providers who want to better understand the potential risks of a pregnancy including disease activity and the need for medication. Women who have disease activity in a specific organ within six months of conception should be aware that they have a significant chance of continued or worsened organ-specific activity of the same type when pregnant. Our findings also suggest that if a woman enters into pregnancy without a particular type of lupus activity, she has a low but not nonexistent risk of developing this activity during pregnancy.
These data must be interpreted within the context of our mostly White lupus patient population that was evaluated and followed at a large urban academic medical center. The data were recorded prospectively in the medical record, but were collected retrospectively for the purpose of analysis. Clinic visits occurred at varying intervals, and therefore data were available according to routine clinical practice, rather than based on protocol.
We were not able to evaluate whether a particular type of organ-specific activity prior to conception was associated with multiple different types of organ-specific activity during pregnancy, due to small numbers of women with each type of activity during pregnancy. While this is a limitation of our study, we believe that dissecting lupus into specific types of organ-specific activity allows for women and their physicians to consider a more individualized risk assessment prior to conception.
Conclusions and future avenues
Our findings need to be replicated in different lupus populations, particularly those with higher proportions of Black patients. Even larger populations would also allow the simultaneous assessment of a number of predictors of organ-specific lupus flare. Such analyses will enhance rheumatologists’ and obstetricians’ abilities to counsel women about the risk of specific types of lupus disease activity during pregnancy.
Acknowledgements
Our work was supported by the U.S. Department of Health and Human Services-National Institutes of Health-National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K24AR066109, P60AR047782, and T32 AR007530.
References
- 1.Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis and rheumatism. 1991;34:1538–1545. doi: 10.1002/art.1780341210. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Irastorza G, Lima F, Alves J, et al. Increased rate of lupus flare during pregnancy and the puerperium: a prospective study of 78 pregnancies. British journal of rheumatology. 1996;35:133–138. doi: 10.1093/rheumatology/35.2.133. [DOI] [PubMed] [Google Scholar]
- 3.Urowitz MB, Gladman DD, Farewell VT, Stewart J, McDonald J. Lupus and pregnancy studies. Arthritis and rheumatism. 1993;36:1392–1397. doi: 10.1002/art.1780361011. [DOI] [PubMed] [Google Scholar]
- 4.Tandon A, Ibanez D, Gladman DD, Urowitz MB. The effect of pregnancy on lupus nephritis. Arthritis and rheumatism. 2004;50:3941–3946. doi: 10.1002/art.20638. [DOI] [PubMed] [Google Scholar]
- 5.Lockshin MD, Reinitz E, Druzin ML, Murrman M, Estes D. Lupus pregnancy. Case-control prospective study demonstrating absence of lupus exacerbation during or after pregnancy. The American journal of medicine. 1984;77:893–898. doi: 10.1016/0002-9343(84)90538-2. [DOI] [PubMed] [Google Scholar]
- 6.Buyon JP, Kalunian KC, Ramsey-Goldman R, et al. Assessing disease activity in SLE patients during pregnancy. Lupus. 1999;8:677–684. doi: 10.1191/096120399680411272. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Irastorza G, Khamashta MA, Gordon C, et al. Measuring systemic lupus erythematosus activity during pregnancy: validation of the lupus activity index in pregnancy scale. Arthritis and rheumatism. 2004;51:78–82. doi: 10.1002/art.20081. [DOI] [PubMed] [Google Scholar]
- 8.Yee CS, Akil M, Khamashta M, et al. The BILAG2004-Pregnancy index is reliable for assessment of disease activity in pregnant SLE patients. Rheumatology. 2012;51:1877–1880. doi: 10.1093/rheumatology/kes158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey-Goldman RMS, Schilling EM, et al. Lupus activity in pregnant and non-pregnant women. Arthritis and rheumatism. 1995;38(Suppl):S219. (abstract). [Google Scholar]
- 10.Clowse ME, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis and rheumatism. 2005;52:514–521. doi: 10.1002/art.20864. [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, Ponticelli C. The risk of pregnancy in patients with lupus nephritis. Journal of nephrology. 2003;16:161–167. [PubMed] [Google Scholar]
- 12.Imbasciati E, Tincani A, Gregorini G, et al. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:519–525. doi: 10.1093/ndt/gfn348. [DOI] [PubMed] [Google Scholar]
- 13.Rheumatology ACo. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. 1997 [Google Scholar]
- 14.Dooley MA, Aranow C, Ginzler EM. Review of ACR renal criteria in systemic lupus erythematosus. Lupus. 2004;13:857–860. doi: 10.1191/0961203304lu2023oa. [DOI] [PubMed] [Google Scholar]
- 15.Branch DWWL. Normal pregnancy, pregnancy complications and obstetric management. In: Sammaritano LBB, editor. Pregnancy and Rheumatic Diseases. New York: Springer; 2014. pp. 31–62. [Google Scholar]
- 16.Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus. 2010;19:1665–1673. doi: 10.1177/0961203310378669. [DOI] [PubMed] [Google Scholar]
- 17.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis and rheumatism. 2006;54:3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 18.A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. The New England journal of medicine. 1991;324:150–154. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 19.Cortes-Hernandez J, Ordi-Ros J, Paredes F, Casellas M, Castillo F, Vilardell-Tarres M. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology. 2002;41:643–650. doi: 10.1093/rheumatology/41.6.643. [DOI] [PubMed] [Google Scholar]
- 20.Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA. Outcome of lupus pregnancy: a controlled study. Rheumatology. 2000;39:1014–1019. doi: 10.1093/rheumatology/39.9.1014. [DOI] [PubMed] [Google Scholar]
- 21.Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmunity reviews. 2005;4:111–115. doi: 10.1016/j.autrev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Ostensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis research & therapy. 2006;8:209. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]