Abstract
Background
Longitudinal studies of upper extremity aging in humans include logistical concerns that animal models can overcome. The vervet is a promising species with which to study aging related processes. However, age-related changes in upper extremity muscle structure have not been quantified in this species. This study measured age-related changes to muscle structure, examined relationships between muscle structure and measures of physical performance, and evaluated the presence of rotator cuff tears.
Methods
Muscle structure: volume, optimal fiber length, physiological cross-sectional area (PCSA), of 10 upper extremity muscles was quantified from the right upper limb of 5 middle aged and 6 older adult female vervets.
Results
Total measured PCSA was smaller (p=0.001) in the older adult vervets than the middle aged vervets. Muscle volume reduction predominate the age-related reductions in PCSA. Total measured PCSA was not correlated to any measures of physical performance. No rotator cuff tears were observed. Supraspinatus volume was relatively larger and deltoid volume relatively smaller in the vervet compared to a human.
Conclusion
The vervet is an appropriate translational model for age-related upper extremity muscle volume loss. Functional measures were not correlated to PCSA, suggesting the vervets may have enough strength for normal function despite loss of muscle tissue. Reduced relative demand on the supraspinatus may be responsible for the lack of naturally occurring rotator cuff tears.
Keywords: aging, muscle, volume loss, function, primate
Introduction
Age-related changes to muscle structure and function in the upper limb are thought to be associated with progression to disability in humans, and thus quantifying these features is important for providing context for both healthy aging and other musculoskeletal disorders of the upper limb when they occur in an older patient group. Age-related changes to skeletal muscle are well known, but have largely been evaluated in cross-sectional studies.28 In the upper limb specifically, declines in muscle volume1,36,43 and increased levels of fat and connective tissue36 have been reported. Further, relationships between structural and functional changes are limited; longitudinal studies of the upper limb have primarily measured either grip8,16,26,35,41 or elbow strength3,10,15,34, but not more complex tasks and corresponding measurements of skeletal muscle mass were not consistently measured or were not specific to the upper limb.15,26
Understanding longitudinal changes to muscle structure, function, and the interplay between the two would lend insight into early predictive factors for future disability that are difficult to assess in a cross-sectional study design. However high cost, long life span, and the need for invasive measurements to fully characterize skeletal muscle make longitudinal studies of the musculoskeletal system difficult to perform in humans. An animal model of upper extremity aging would more easily allow for longitudinal studies by limiting many of the logistical concerns associated with human subjects. Unfortunately, data regarding musculoskeletal degeneration in animals resulting strictly from normal aging are limited7,12,24,31,32 and many of the animal models are small, quadrupedal, or have bony geometry that is substantially different from that of a human.9,22,31 A non-human primate model may offer a solution that mitigates many of these problems.31
Previous data suggest that the African vervet monkey (Chlorocebus pygerythrus) may be a promising species to use as a human surrogate to study age-related changes in physical function and in the upper extremity. Reductions in muscle fiber force in the vastus lateralis were present in older vervets,7 although it is unclear if the upper extremity musculature experiences the same decline. Older vervets demonstrated age-related deteriorations of the shoulder similar to reports in older adult humans including; degeneration of the glenoid, increased glenoid retroversion, and decreased supraspinatus superficial cross-sectional area..31 Similarly, measures of physical performance that incorporate the upper extremity were diminished in older vervets.7,31,39 However, within this vervet species, age-related changes to the upper extremity musculature have not been evaluated and degenerative rotator cuff tears, a common age-related injury affecting older adult humans,44 have not been described.31
Quantifying age-related changes in the upper extremity musculature is necessary before the vervet can be appropriately evaluated as a human surrogate for longitudinal studies on upper extremity aging. To this end, we sought to expand upon the findings of age-related bony degeneration and decreased fiber cross sectional area in the superficial portion of the supraspinatus in this vervet species by measuring the physiological cross sectional area (PCSA) of 10 upper extremity muscles important for upper limb function. PCSA combines measurements of volume and fiber length and is proportional to force-generating capacity. We also sought to determine how PCSA affects physical performance and the presence of rotator cuff tears.
Materials and Methods
The right upper limb of 5 middle aged (MA) and 6 older adult (OA) female African vervets (Chlorocebus pygerythrus) were obtained from a previously-studied population of vervets (Table I). Current reports list vervets above the age of 20 as elderly, with the 26.4 year old in the current study the oldest known female vervet in captivity originating from the original colony.31 Briefly, all animals were housed in social groups at the Wake Forest Primate Center and were allowed to traverse the inside/outside pens at their own leisure. Feeding was ad libitum. Physical performance (walking speed, % time hanging, and % time climbing) was measured for the animals as described previously.7,31,39 Euthanasia was performed as part of a larger experiment exploring immunological and physiological parameters and their relationship to aging. Following sacrifice, the right upper extremity was removed from the torso and frozen.
Table I.
Characteristics of vervet specimens
| Age | Age Group |
Body Mass |
Arm Length |
Forearm Length |
|---|---|---|---|---|
| (years) | (MA/OA) | (kg) | (mm) | (mm) |
| 11.76 | M | 6.57 | 119.55 | 108.80 |
| 9.43 | M | 4.68 | 112.15 | 100.69 |
| 11.53 | M | 5.87 | 114.01 | 105.99 |
| 11.63 | M | 4.92 | 108.18 | 100.17 |
| 11.53 | M | 7.08 | 113.99 | 109.99 |
| 21.48 | O | 6.14 | 119.42 | 109.21 |
| 25.75 | O | 4.00 | 107.67 | 103.71 |
| 26.41 | O | 4.93 | 127.48 | 114.22 |
| 23.73 | O | 5.51 | 111.17 | 108.51 |
| 19.86 | O | 4.63 | 113.80 | 107.99 |
| 21.55 | O | 5.12 | 111.46 | 106.15 |
Each limb was thawed over a period of 24 hours, skinned, and fixed in 10% phosphate buffered formalin for 24 hours. Prior to fixation, the limb was placed in a neutral shoulder and wrist posture with 90° of elbow flexion and attached to an aluminum plate via the spine. Each limb was then removed from the formalin and placed in a 70% ethanol solution for a minimum of 24 hours to preserve the fixation and rinse any excess formalin. Measurements of arm length (acromion to lateral epicondyle) and forearm length (lateral epicondyle to ulnar styloid) were made using digital calipers.
The muscle-tendon unit of the four rotator cuff muscles (subscapularis, infraspinatus, supraspinatus, and teres minor), teres major, deltoid, biceps, triceps, coracobrachialis, and brachialis were dissected from the skeleton. Care was taken to ensure the entire muscle- tendon unit was removed from the skeleton. Prior to dissection, rotator cuff tendons were visually inspected for tears. Subscapularis, infraspinatus, deltoid, triceps, and biceps were all divided into subsections according to previous descriptions of muscle architecture13,42 for a total of 18 muscle-tendon units (Table II). Following dissection, excess connective tissue and fat was removed from the muscle-tendon unit and each muscle-tendon unit was stored in a 70% ethanol solution.
Table II.
Mean (standard deviation) of anatomic parameters.
| PCSA (cm2) | Volume (cm3) | Optimal fiber length (mm) | Optimal muscle length (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OA | MA | OA | MA | OA | MA | OA | MA | |||||
| Superior subscapularisⱡ | 2.14 (0.35) | * | 2.66 (0.29) | 4.32 (1.15) | * | 5.83 (0.65) | 20.37 (3.29) | 22.30 (1.86) | 62.05 (9.19) | 64.74 (11.55) | ||
| Middle subscapularisaⱡ | 2.62 (0.65) | 3.20 (0.33) | 4.19 (1.28) | * | 5.87 (0.99) | 16.17 (2.84) | 18.44 (2.45) | 67.53(15.32) | 75.95 (8.06) | |||
| Inferior subscapularisⱡ | 1.10 (0.32) | 1.35 (0.23) | 2.16 (0.65) | 2.62 (0.17) | 19.79 (2.07) | 20.02 (2.65) | 71.80 (7.78) | 65.13 (3.27) | ||||
| Subscapularisaⱡ | 5.87 (0.60) | * | 7.21 (0.42) | 10.67 (2.17) | * | 14.32 (1.59) | 18.36 (2.91) | 20.07 (1.67) | 65.84 (9.66) | 68.37 (5.71) | ||
| Superior infraspinatusⱡ | 1.74 (0.27) | 2.01 (0.24) | 4.47 (0.82) | 5.32 (0.61) | 26.15 (3.66) | 26.97 (2.05) | 78.70 (9.65) | 82.10 (7.91) | ||||
| Inferior infraspinatusⱡ | 1.47 (0.30) | 1.81 (0.39) | 3.82 (0.92) | 4.91 (0.98) | 26.40 (2.90) | 27.49 (2.97) | 81.12 (9.52) | 78.07 (13.14) | ||||
| Infraspinatusaⱡ | 3.20 (0.41) | 3.82 (0.59) | 8.30 (1.44) | 10.23 (1.40) | 26.22 (3.28) | 27.23 (2.36) | 79.86 (9.63) | 79.66 (9.84) | ||||
| Supraspinatusⱡ | 2.42 (0.47) | 2.77 (0.55) | 6.47 (1.65) | 8.12 (1.45) | 26.94 (3.36) | 29.80 (3.07) | 74.22 (4.98) | 78.11 (9.96) | ||||
| Teres minor | 0.76 (0.12) | * | 0.98 (0.14) | 1.28 (0.14) | * | 1.63 (0.23) | 17.27 (2.11) | 16.77 (1.40) | 44.70 (6.15) | 45.89 (3.22) | ||
| Anterior deltoid | 0.85 (0.18) | * | 1.25 (0.28) | 4.70 (0.96) | * | 7.31 (1.26) | 56.06 (3.06) | 59.86 (5.14) | 67.37 (5.39) | * | 77.17 (4.90) | |
| Middle deltoid | 1.97 (0.63) | * | 2.91 (0.52) | 5.08 (1.50) | * | 7.50 (1.50) | 26.45 (2.95) | 26.29 (2.09) | 50.62 (3.07) | 55.22 (6.82) | ||
| Posterior deltoid | 0.89 (0.20) | 1.12 (0.20) | 3.02 (0.61) | * | 4.74 (1.36) | 34.55 (2.25) | * | 42.65 (8.13) | 53.91 (5.54) | 62.53 (8.49) | ||
| Deltoida | 3.71 (0.90) | * | 5.28 (0.76) | 12.79 (2.89) | * | 19.55 (3.62) | 35.13 (2.29) | 37.48 (1.81) | 56.36 (1.66) | * | 63.53 (3.97) | |
| Teres major | 1.64 (0.37) | * | 2.15 (0.21) | 6.29 (1.11) | * | 8.98 (1.80) | 39.10 (3.96) | 42.41 (6.88) | 74.50 (6.67) | 79.00 (12.19) | ||
| Long triceps | 5.59 (0.71) | * | 8.66 (0.97) | 16.88 (1.99) | * | 24.72 (3.73) | 30.68 (1.35) | 29.14 (2.83) | 120.85 (8.52) | * | 109.39 (4.48) | |
| Lateral triceps | 2.83 (0.43) | * | 4.63 (0.89) | 11.96 (1.92) | * | 17.30 (2.59) | 42.91 (3.47) | 38.32 (4.03) | 112.79 (8.10) | 104.50 (7.68) | ||
| Medial triceps | 2.18 (0.42) | 2.71 (0.77) | 7.52 (1.14) | 9.05 (2.10) | 35.25 (1.68) | 34.41 (4.58) | 108.82 (3.33) | 108.55 (10.65) | ||||
| Tricepsa | 10.59 (1.39) | * | 16.00 (2.35) | 36.36 (4.55) | * | 51.06 (8.29) | 34.85 (1.66) | 32.52 (2.93) | 115.35 (6.01) | * | 107.26 (4.45) | |
| Long biceps | 1.92 (0.42) | * | 2.46 (0.46) | 9.03 (2.01) | 11.61 (2.10) | 47.34 (2.41) | 47.41 (4.59) | 90.08 (3.73) | 93.00 (11.75) | |||
| Short biceps | 0.59 (0.05) | * | 0.88 (0.19) | 3.12 (0.36) | * | 4.81 (1.14) | 53.22 (3.91) | 54.86 (6.13) | 90.81 (3.01) | 93.47 (12.85) | ||
| Bicepsa | 2.52 (0.44) | * | 3.34 (0.35) | 12.15 (2.27) | * | 16.42 (2.87) | 48.68 (2.43) | 49.36 (4.88) | 90.25 (3.43) | 93.17 (11.40) | ||
| Coracobrachialis | 0.36 (0.12) | 0.47 (0.14) | 0.60 (0.20) | 0.80 (0.23) | 17.32 (2.31) | 17.35 (1.14) | 54.36 (10.55) | 61.86 (11.15) | ||||
| Brachialis | 1.47 (0.25) | * | 1.81 (0.25) | 4.82 (1.02) | 5.86 (0.95) | 33.17 (2.29) | 32.78 (3.32) | 71.74 (2.79) | 69.35 (9.11) | |||
: PCSA and volume were calculated from the summation of PCSA and volume from the subsections.
Optimal fiber length and optimal muscle length were calculated from equations 4 and 5.
: Statistically significant differences (p<0.05) between groups.
: Indicates a rotator cuff muscle.
Measurements of muscle length and volume were made for each of the 18 muscle-tendon units. Tendon was removed at the muscle-tendon junction and muscle belly length was measured using digital calipers and defined as the distance from the most proximal point to the most distal point. The 70% ethanol solution was placed in a graduated cylinder and muscle volume was determined as the difference in volume with and without the muscle. Location of the meniscus was determined from high resolution photos (57.37 pixels/ml) of each cylinder and calculated using Image J.38
Following fixation and gross muscle measurements, thin strips of muscle fibers were dissected from the interior of the muscle using fine sharp dissection18 and fiber length (lf) was measured using digital calipers. Fibers were dissected from different locations within the muscle belly to ensure the entire muscle belly was represented. A microscope was used to dissect the fibers into smaller bundles and these bundles were mounted on a microscope slide for sarcomere length measurements and a cover slip was added.
A custom built laser diffractometer (Thor Labs, HRP050-1, 5.0mW@633nm, Newton, NJ, USA) was used to determine the sarcomere lengths for each dissected fiber.45 Digital calipers were used to measure the width of the 1st order diffraction band (y) which was used to calculate the sarcomere length (ls) (eq. 1, eq. 2): where λ is the laser wave-length (633nm) and L is the distance from the microscope slide to the diffraction surface.
| Equation 1 |
| Equation 2 |
Repeatability of the caliper measurements was ±0.32mm, corresponding to ±0.048µm for sarcomere lengths. Optimal fiber length was calculated for each sarcomere measurement by normalizing the measured sarcomere length by the optimal sarcomere length (2.8µm) determined for mammalian muscle (eq. 3).21 Optimal muscle length () was determined in the same manner. Optimal fiber length (eq. 4) and optimal muscle length (eq. 5) were also calculated for the whole muscle: where t equals the number of subsections (subscapularis=3, infraspinatus=2, deltoid=3, triceps=3, biceps=2). Relative length (eq. 6) of the rotator cuff muscles was calculated to facilitate comparisons between vervet and other animal species.
| Equation 3 |
| Equation 4 |
| Equation 5 |
| Equation 6 |
PCSA was calculated by dividing the muscle volume by optimal fiber length.27 A representative cross-sectional area (CSA) was calculated by dividing muscle volume by optimal muscle length. For muscles with multiple subsections (subscapularis, infraspinatus, deltoid, triceps, and biceps) whole muscle PCSA was calculated by adding the PCSA from each section.27 Total measured PCSA was calculated by adding the PCSA for every muscle.
The primary outcome of this study was to evaluate whether there are differences between old and middle aged animals in total measured PCSA; a Student t-test was used for this purpose. Effect of total measured PCSA on measures of physical performance previously measured in the same animals was quantified for all animals and each age group using linear regression models. T-tests were used to compare the components of PCSA (volume and optimal fiber length) for each muscle between the groups. Total rotator cuff PCSA was compared between groups using a t-test. PCSA fraction for each rotator cuff muscle (subscapularis, infraspinatus, supraspinatus, teres minor) and the deltoid was calculated by dividing the PCSA of the muscle by the total rotator cuff PCSA and compared between groups using Student t-tests. Volume fraction was calculated and compared in the same way. All statistical tests were performed in SAS (Cary, NC, USA) and statistical significance was defined as p<0.05. Due to the exploratory nature of this study, corrections for multiple comparisons were not performed.
Results
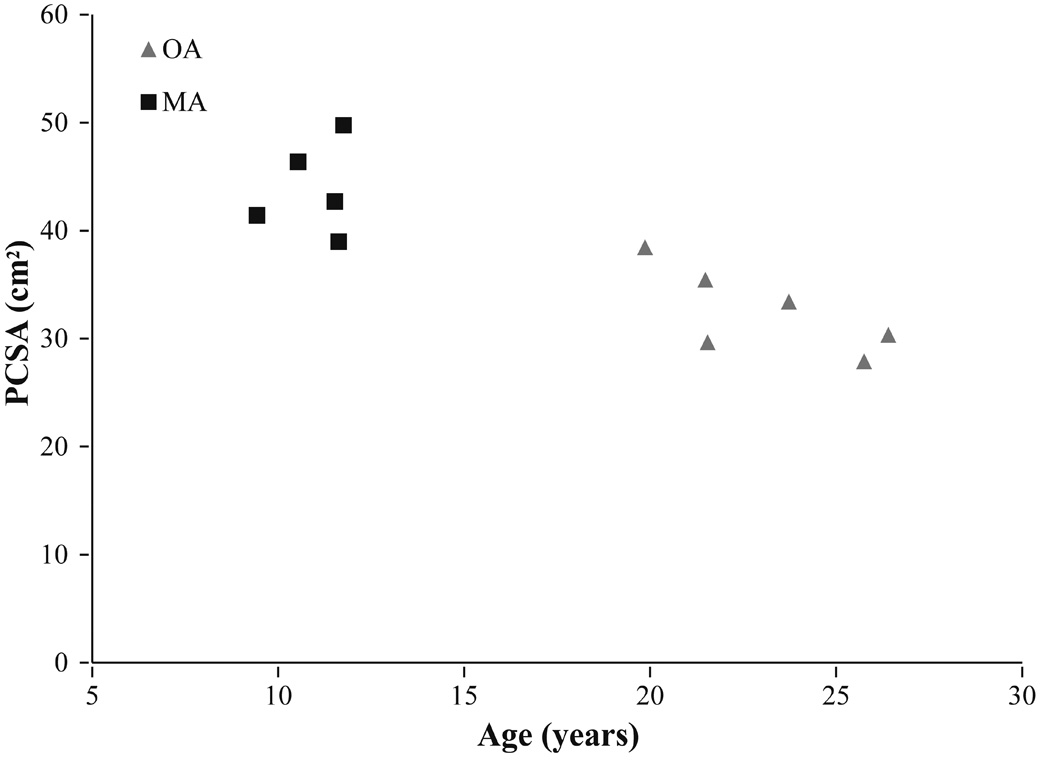
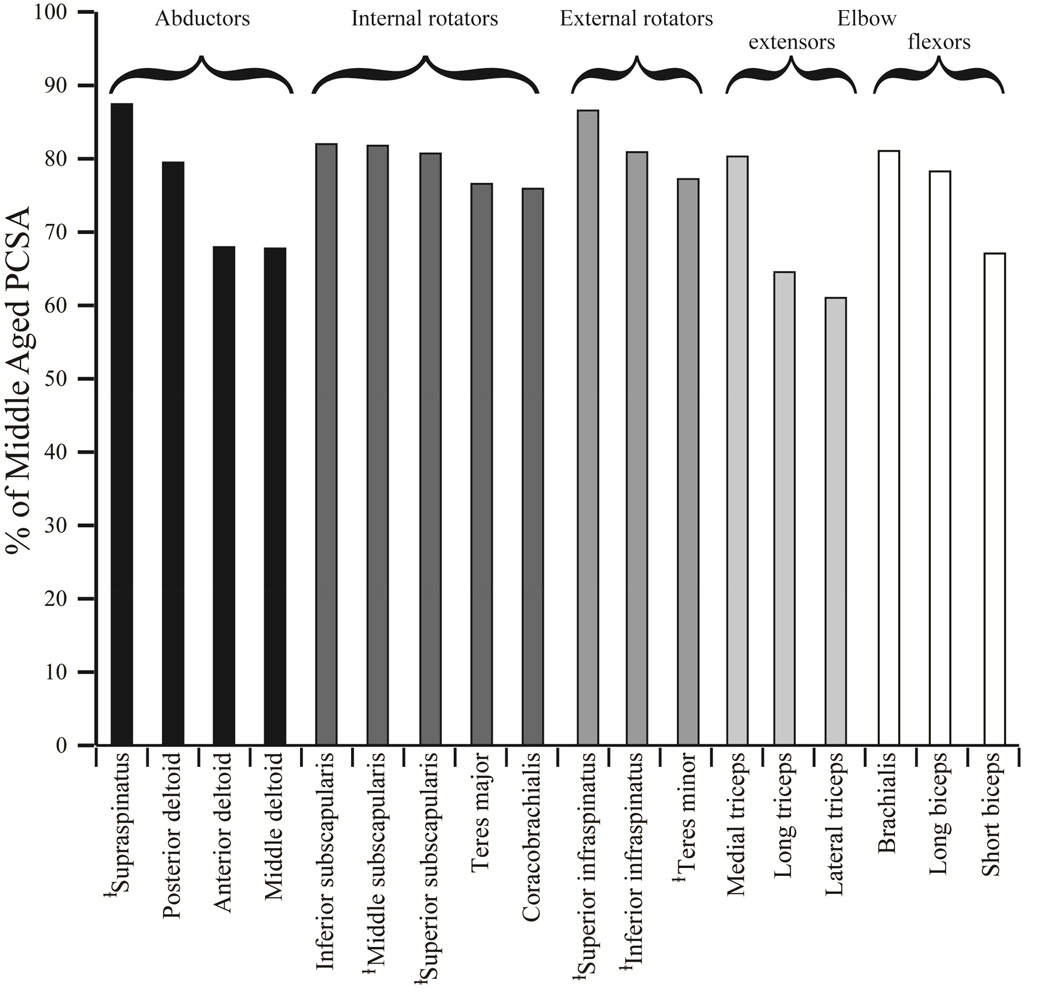
Despite no differences in body mass, upper arm length, and forearm length between the middle age and older vervets (Table I), the older vervets had significantly smaller total measured PCSA than the middle age vervets (p=0.001) (Table II, Figure 1). Older PCSA was smaller for every individual muscle studied and 10 of the 18 muscles exhibited significant decreases. The lateral head of the triceps exhibited the largest reduction in PCSA between groups (38.97%), and the supraspinatus exhibited the smallest reduction in PCSA (12.55%) (Figure 2). Total measured PCSA was not significantly correlated to any of the physical performance measures when the middle aged and older adults were considered together for statistical analysis walking speed (r2 = 0.0843, p = 0.3863), % time hanging (r2 = 0.0247, p=0.644), % time climbing (r2 = 0.0736, p = 0.420). Similarly, when the same analysis was repeated for each age group separately, PCSA was not correlated to any physical performance measure for either age group.
Figure 1.
Total measured PCSA vs age for the older adult (OA) and middle aged (MA) vervets.
Figure 2.
Older adult individual muscle PCSA as a percentage of middle aged PCSA. There was not a preferential decrease in PCSA of any muscle group in the older adults. Of the rotator cuff muscles, teres minor had the largest decrease in PCSA (22.7%) and supraspinatus had smallest (12.5%). ⱡ: indicates a rotator cuff muscle.
Mean muscle volume in the older adults was numerically smaller for every single muscle studied and was statistically different in 10 of the 18 muscles (Table II). The posterior portion of the deltoid (36.25%) and the superior portion of the infraspinatus (15.88%) exhibited the largest and smallest volumes reductions, respectively. Optimal fiber length for the posterior portion of the deltoid was significantly shorter for the older vervets due to a decrease in measured fiber length; no other muscles exhibited a significant difference in optimal fiber length.
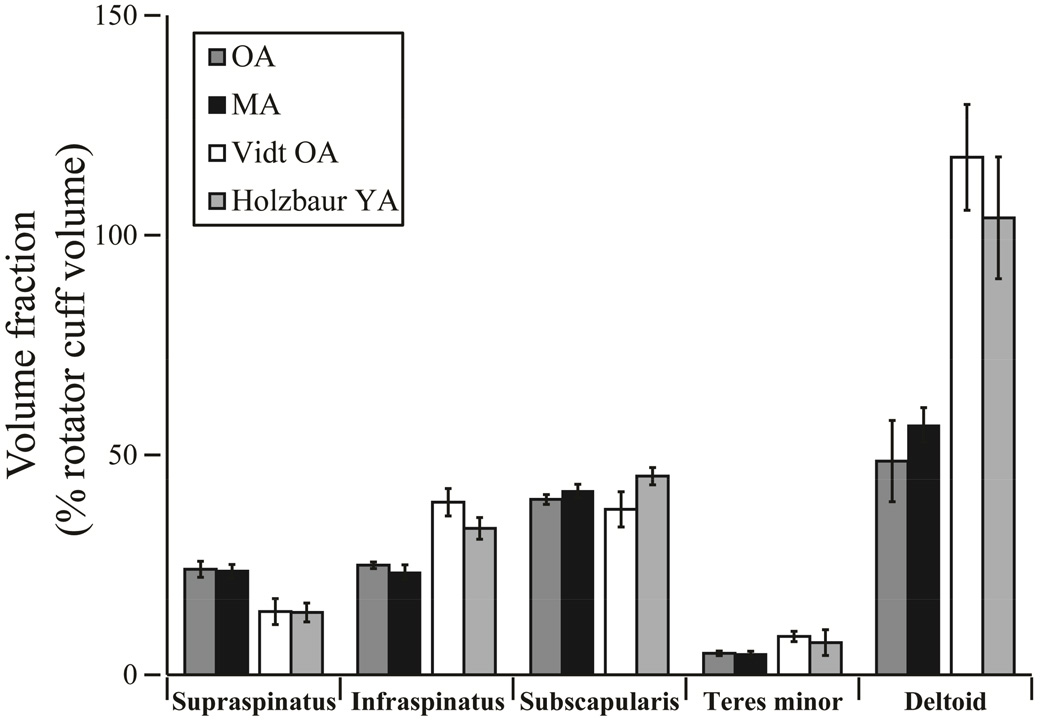
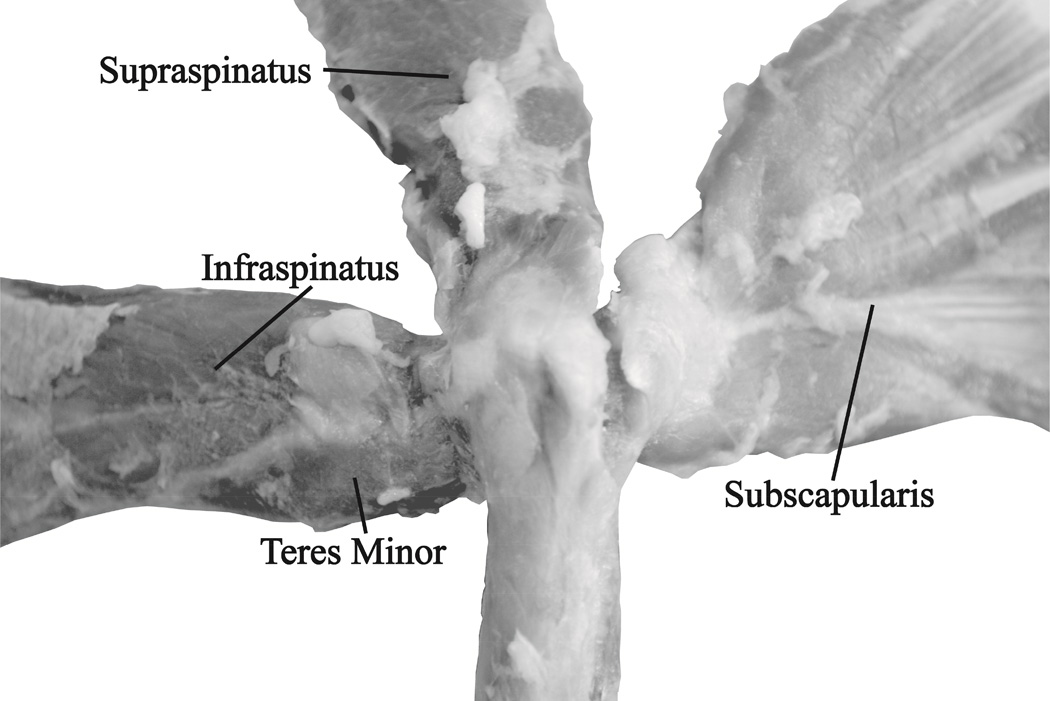
Despite significant differences in total rotator cuff (supraspinatus, infraspinatus, subscapularis, and teres minor) PCSA (p=0.008), there were no significant differences in the rotator cuff PCSA fraction between the older and middle aged vervets for any muscle. The same was true for volume fraction (Figure 3). Subscapularis made up the largest proportion of the rotator cuff PCSA for both older (48.03±3.75%) and middle aged vervets (48.96±3.39%) and teres minor made up the smallest (older = 6.22±0.84% and middle = 6.70± 1.04%) (Table III). The deltoid measured 30.11±4.94% and 35.64±2.34% of the rotator cuff PCSA for the older and middle aged vervets respectively. Upon visual inspection of both the articular and bursal side (Figure 4) there were no rotator cuff tears present. Unlike hominoids, such as humans, chimpanzees, and orangutans, the vervets did not have a “true” rotator cuff in which the supraspinatus, infraspinatus, subscapularis, and teres minor share a common insertion site (Figure 4).40
Figure 3.
Volume fraction for vervet and female young adult (Holzbaur YA) and older adult (Vidt OA) humans as determined by a percentage of the total rotator cuff volume.14,43 There were no statistical differences between the older adult (OA) and middle aged (MA) vervet volume fractions for any muscle. When combined, the vervet supraspinatus volume fraction (24%) was larger than the humans (14%) and the vervet deltoid volume fraction (52%) was smaller than the humans (112%).
Table III.
Comparison of vervet, capuchin, and chimpanzee
| % Rotator cuff PCSA | Relative length | |||||
|---|---|---|---|---|---|---|
| Vervet | Capuchin* | Chimpanzee* | Vervet | Capuchin* | Chimpanzee* | |
| Subscapularis | 48.45 | 49.15 | 47.23 | 0.29 | 0.25 | 0.39 |
| Infraspinatus | 25.94 | 21.57 | 26.62 | 0.34 | 0.26 | 0.28 |
| Supraspinatus | 19.16 | 23.18 | 21.12 | 0.37 | 0.38 | 0.30 |
| Teres minor | 6.44 | 6.09 | 5.04 | 0.38 | 0.31 | 0.45 |
: data adapted from Mathewson et. 2014
Figure 4.
Representative bursal-surface view of rotator cuff muscle-tendon units and their anatomical insertion sites. Unlike hominoids, such as humans, chimpanzees, and orangutans, the vervets did not have a “true” rotator cuff which is indicated by a lack of distinction between the tendon insertion sites of the supraspinatus, infraspinatus, subscapularis, and teres minor.40 No tears were discovered during dissection.
Discussion
We examined age-related differences in the physiological cross sectional area (PCSA) of 18 muscles of the right upper extremity in a cohort of 5 middle aged and 6 older adult female vervet monkeys. We observed that, despite similarities in body mass and arm length, total measured PCSA was significantly smaller in the older vervets. Reduced PCSA was driven by an overall loss of muscle volume, rather than changes in optimal fiber length, which were not observed. Multiple cross-sectional studies in human volunteers have demonstrated reduced upper extremity muscle volume in older adults.1,36,43 While age-related muscle volume loss has been established in lower limb of animal models,12,24 to our knowledge the current study is the first to observe age-related upper extremity muscle volume loss in an animal model. This observation provides support for the vervet monkey as an animal model for age-related upper extremity muscle volume loss.
Prevalence of rotator cuff tears in humans increases with advancing age, with the incidence reaching 50% in adults over age 70.44 Older vervets presented with no grossly detectable rotator cuff tears. This extends previous findings of the left upper limb of the same vervets.31 Vervet monkeys employ the supraspinatus, the most commonly torn cuff muscle-tendon unit,37 as a dynamic stablizer similar to humans.19 However, our results indicate that the vervet supraspinatus represents a much larger proportion of the total rotator cuff volume (24%) than in a human (14%).14,43 Additionally, the vervet deltoid volume is only 52% of the total rotator cuff volume while the human deltoid is 112% (Figure 3). Similarly to a previous study in knuckle walking non-human primates (Pan troglodytes),33 these two findings suggest a reduced relative demand on the supraspinatus in this vervet model. In humans, a lifetime of increased demand on a proportionally smaller supraspinatus from the action of a proportionally larger deltoid may predispose the supraspinatus to age-related tearing. Due to the lack of naturally occurring rotator cuff tears, it would be necessary to inflict a tear in a vervet model, as is done in other animal models used to study rotator cuff injury. However, the vervet may be a useful model of atrophy and fatty infiltration, common muscle changes associated with rotator cuff tears,25 as these changes may be exaggerated and occur more quickly in the supraspinatus due to its large PCSA and subsequently increased role in movement.
Previous architectural analyses of other primate species suggest that chimpanzee (Pan troglodytes) and capuchin (cebus apella) have rotator cuff muscle architecture more similar to human rotator cuff than large and small quadrupedal animals.23 Specifically the volume fraction of the muscles of the rotator cuff and the relative length of the muscles (eq 6) were similar to that of humans. The same parameters for the vervet rotator cuff muscles in this study are similar (Table III). However, the relative deltoid PCSA was not determined for the capuchin or the chimpanzee in the previous study, which may be an important factor for establishing the appropriateness of a rotator cuff model with regard to tear propensity. Further, it should be noted that, while the vervet is an old world monkey, the capuchin and chimpanzee are considered new world monkeys and advanced primates, respectively. New world monkeys and advanced primates exhibit tendinous connection between rotator cuff tendons, while the vervet (Figure 4) and other older monkeys do not.40 This suggests that other factors beyond architectural parameters should be taken into consideration when considering an animal model for the rotator cuff.
In humans, the relationship between joint strength and overall function is important for understanding upper extremity disability and associated therapies. Because PCSA is proportional to muscle force-generating capacity20, we expected that reduced PCSA would be associated with a corresponding reduction in functional performance. Contrary to this hypothesis, total measured PCSA was not significantly correlated with walking speed, % time climbing, or % time hanging within either age group or when all vervets were considered together. Any strength capacity that exists above the strength requirements of a task is referred to as reserve strength.5 In humans, it has been reported that small reductions in strength result in large functional declines only when strength falls below the minimum required strength to perform a task and reserve strength is zero.5 We hypothesize that the older vervets, despite the substantial reduction in PCSA, retained reserve strength above the strength requirements of the measured functional tasks. Retaining reserve strength would indicate that in order to successfully perform the tasks, it is unnecessary for all of the available motor units to be recruited and/or unnecessary for all of the recruited motor units to be fully activated. In a previous study of these vervets, it was demonstrated that these functional measures are significantly correlated to degenerative bony changes,31 suggesting that discomfort due to age-related degenerative changes in the joint, rather than reduced strength, may impair function in these animals. Accurate measurements of strength and functional measures that include fatigue may prove more sensitive to reductions in PCSA and should be explored. Inclusion of other upper extremity musculature, including the pectoralis and latissimus dorsi, in the total PCSA calculation is prudent in determining the full extent of the effect of age-related PCSA reductions on functional performance.
There are limitations to the current study that should be considered. Architectural parameters were not measured for all of the upper extremity muscles due to method of upper extremity removal from the torso following sacrifice. While the inclusion of more muscles is not expected to significantly alter the weak relationships between total PCSA and functional performance, future characterization of these architectural parameters would provide a more complete picture of age-related PCSA loss and would allow for additional comparison of the vervet muscle structure to those obtained for other non-human primates.2,6,11,17,29,30,33 Only female vervets were included in this study. Although it is unclear if hormonal imbalances due to aging or menstrual cycle phase affect the results of this study, previous work found that lean body mass in adult human women was approximately 64% of that in men from regardless of decade of life indicating that women and men lose muscle mass at approximately the same rate.28 Further, functional performance in macque monkeys and human women do not vary based on menstrual cycle phase.4 However, future work should include male vervets to ensure any gender differences in the age-related decline of PCSA are captured.
Conclusion
The results of this study suggest that the vervet monkey provides an appropriate translational model for studying longitudinal age-related muscle volume loss in the upper extremity. Future work relating the muscle volume changes to functional performance should consider additional measurements of strength or functional measures that include fatigue. Although naturally occurring age-related rotator cuff tears have not been observed in the vervets, the large size of the supraspinatus may make it an adequate muscle-tendon unit in which to study fatty infiltration and atrophy associated with naturally occurring rotator cuff tears. Furthermore, when considering an appropriate rotator cuff tear translational model, the relative size of the deltoid should be taken into consideration. The similarities to humans in both the anatomical features and age-related musculoskeletal changes suggest that there may be a benefit for the use of the vervet in translational research involving healthy aging of the upper extremity.
Acknowledgments
This study was supported by grants from the National Institute of Health (RR019963/OD010965), the Department of Veterans Affairs (VA 247-P-0447), Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the Wake Forest Primate Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commerical entity related to the subject of this article.
References
- 1.Akagi R, Takai Y, Ohta M, Kanehisa H, Kawakami Y, Fukunaga T. Muscle volume compared to cross-sectional area is more appropriate for evaluating muscle strength in young and elderly individuals. Age Ageing. 2009;38(5):564–569. doi: 10.1093/ageing/afp122. [DOI] [PubMed] [Google Scholar]
- 2.Anapol F, Gray JP. Fiber architecture of the intrinsic muscles of the shoulder and arm in semiterrestrial and arboreal guenons. Am J Phys Anthropol. 2003;122(1):51–65. doi: 10.1002/ajpa.10269. [DOI] [PubMed] [Google Scholar]
- 3.Aniansson A, Sperling L, Rundgren A, Lehnberg E. Muscle function in 75-year-old men and women. A longitudinal study. Scand J Rehabil Med Suppl. 1983;9:92–102. [PubMed] [Google Scholar]
- 4.Barger LK, Hoban-Higgins TM, Fuller CA. Assessment of circadian rhythms throughout the menstrual cycle of female rhesus monkeys. Am J Primatol. 2008;70(1):19–25. doi: 10.1002/ajp.20451. [DOI] [PubMed] [Google Scholar]
- 5.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25(5):386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 6.Cheng EJ, Scott SH. Morphometry of Macaca mulatta forelimb. I. Shoulder and elbow muscles and segment inertial parameters. J Morphol. 2000;245(3):206–224. doi: 10.1002/1097-4687(200009)245:3<206::AID-JMOR3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Choi SJ, Shively CA, Register TC, Feng X, Stehle J, High K, et al. Force-generation capacity of single vastus lateralis muscle fibers and physical function decline with age in African green vervet monkeys. J Gerontol A Biol Sci Med Sci. 2013;68(3):258–267. doi: 10.1093/gerona/gls143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC Geriatr. 2013;13(1):71. doi: 10.1186/1471-2318-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16(1):21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. (1985) [DOI] [PubMed] [Google Scholar]
- 11.Graham KM, Scott SH. Morphometry of Macaca mulatta forelimb. III. Moment arm of shoulder and elbow muscles. J Morphol. 2003;255(3):301–314. doi: 10.1002/jmor.10064. [DOI] [PubMed] [Google Scholar]
- 12.Hagen JL, Krause DJ, Baker DJ, Fu MH, Tarnopolsky MA, Hepple RT. Skeletal muscle aging in F344BN F1-hybrid rats: I. Mitochondrial dysfunction contributes to the age-associated reduction in VO2max. J Gerontol A Biol Sci Med Sci. 2004;59(11):1099–1110. doi: 10.1093/gerona/59.11.1099. [DOI] [PubMed] [Google Scholar]
- 13.Hogfors C, Sigholm G, Herberts P. Biomechanical model of the human shoulder--I. Elements. J Biomech. 1987;20(2):157–166. doi: 10.1016/0021-9290(87)90307-1. [DOI] [PubMed] [Google Scholar]
- 14.Holzbaur KR, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. J Biomech. 2007;40(4):742–749. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56(5):B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 16.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–M88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi Y. Comparative analysis of muscle architecture in primate arm and forearm. Anat Histol Embryol. 2010;39(2):93–106. doi: 10.1111/j.1439-0264.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 18.Langenderfer J, Jerabek SA, Thangamani VB, Kuhn JE, Hughes RE. Musculoskeletal parameters of muscles crossing the shoulder and elbow and the effect of sarcomere length sample size on estimation of optimal muscle length. Clin Biomech (Bristol, Avon) 2004;19(7):664–670. doi: 10.1016/j.clinbiomech.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Larson SG, Stern JT., Jr Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): implications for interpretation of humeral morphology. Am J Phys Anthropol. 1989;79(3):369–377. doi: 10.1002/ajpa.1330790313. [DOI] [PubMed] [Google Scholar]
- 20.Lieber RL. Skeletal muscle structure, function & plasticity : the physiological basis of rehabilitation. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 369. Edited, xii. [Google Scholar]
- 21.Lieber RL, Loren GJ, Friden J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol. 1994;71(3):874–881. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- 22.Longo UG, Forriol F, Campi S, Maffulli N, Denaro V. Animal models for translational research on shoulder pathologies: from bench to bedside. Sports Med Arthrosc. 2011;19(3):184–193. doi: 10.1097/JSA.0b013e318205470e. [DOI] [PubMed] [Google Scholar]
- 23.Mathewson MA, Kwan A, Eng CM, Lieber RL, Ward SR. Comparison of rotator cuff muscle architecture between humans and other selected vertebrate species. J Exp Biol. 2014;217(Pt 2):261–273. doi: 10.1242/jeb.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, et al. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2011;46(1):23–29. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009;95(5):319–324. doi: 10.1016/j.otsr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57(10):B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 27.Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. J Biomech. 2000;33(8):943–952. doi: 10.1016/s0021-9290(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 28.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 29.Ogihara N, Makishima H, Aoi S, Sugimoto Y, Tsuchiya K, Nakatsukasa M. Development of an anatomically based whole-body musculoskeletal model of the Japanese macaque (Macaca fuscata) Am J Phys Anthropol. 2009;139(3):323–338. doi: 10.1002/ajpa.20986. [DOI] [PubMed] [Google Scholar]
- 30.Oishi M, Ogihara N, Endo H, Asari M. Muscle architecture of the upper limb in the orangutan. Primates. 2008;49(3):204–209. doi: 10.1007/s10329-008-0082-5. [DOI] [PubMed] [Google Scholar]
- 31.Plate JF, Bates CM, Mannava S, Smith TL, Jorgensen MJ, Register TC, et al. Age-related degenerative functional, radiographic, and histological changes of the shoulder in nonhuman primates. J Shoulder Elbow Surg. 2013 Aug;22(8):1019–1029. doi: 10.1016/j.jse.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plate JF, Pace LA, Seyler TM, Moreno RJ, Smith TL, Tuohy CJ, et al. Age-related changes affect rat rotator cuff muscle function. J Shoulder Elbow Surg. 2013 doi: 10.1016/j.jse.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Potau JM, X B, Ciurana N, Camprubi D, Pastor JF, de Paz F, et al. Quantitative Analysis of the Deltoid and Rotator Cuff Muscles in Humans and Great Apes. Internal Journal of Primatology. 2009;30(5):697–708. [Google Scholar]
- 34.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45(12):1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 35.Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85(6):2047–2053. doi: 10.1152/jappl.1998.85.6.2047. (1985) [DOI] [PubMed] [Google Scholar]
- 36.Rice CL, Cunningham DA, Paterson DH, Lefcoe MS. Arm and leg composition determined by computed tomography in young and elderly men. Clin Physiol. 1989;9(3):207–220. doi: 10.1111/j.1475-097x.1989.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 37.Sano H, Ishii H, Trudel G, Uhthoff HK. Histologic evidence of degeneration at the insertion of 3 rotator cuff tendons: a comparative study with human cadaveric shoulders. J Shoulder Elbow Surg. 1999;8(6):574–579. doi: 10.1016/s1058-2746(99)90092-7. [DOI] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shively CA, Willard SL, Register TC, Bennett AJ, Pierre PJ, Laudenslager ML, et al. Aging and physical mobility in group-housed Old World monkeys. Age (Dordr) 2012;34(5):1123–1131. doi: 10.1007/s11357-011-9350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnabend DH, Young AA. Comparative anatomy of the rotator cuff. J Bone Joint Surg Br. 2009;91(12):1632–1637. doi: 10.1302/0301-620X.91B12.22370. [DOI] [PubMed] [Google Scholar]
- 41.Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliovaara M, Impivaara O, et al. Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J Am Geriatr Soc. 2012;60(1):77–85. doi: 10.1111/j.1532-5415.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- 42.Van der Helm FC, Veenbaas R. Modelling the mechanical effect of muscles with large attachment sites: application to the shoulder mechanism. J Biomech. 1991;24:1151–1163. doi: 10.1016/0021-9290(91)90007-a. [DOI] [PubMed] [Google Scholar]
- 43.Vidt ME, Daly M, Miller ME, Davis CC, Marsh AP, Saul KR. Characterizing upper limb muscle volume and strength in older adults: a comparison with young adults. J Biomech. 2012;45(2):334–341. doi: 10.1016/j.jbiomech.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19(1):116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Yeh Y, Baskin RJ, Lieber RL, Roos KP. Theory of light diffraction by single skeletal muscle fibers. Biophys J. 1980;29(3):509–522. doi: 10.1016/S0006-3495(80)85149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]