Abstract
Single nucleotide polymorphisms (SNPs) in the promoter region of FAS and FASLG may alter their transcriptional activity. Thus, we determined the associations between four FAS and FASLG promoter variants (FAS1377G>A, rs2234767; 670A>G, rs1800682; FASLG844T>C, rs763110; and 124A>G, rs5030772) and the risk of recurrence of squamous cell carcinoma of the oropharynx (SCCOP). We evaluated the associations between FAS and FASLG genetic variants and the risk of recurrence in a cohort of 1008 patients. The log-rank test and multivariate Cox models were used to evaluate the associations. Compared with patients with common homozygous genotypes of FAS670 and FASLG844 polymorphisms, patients with variant genotypes had lower disease-free survival rates (log-rank P < 0.0001 and P < 0.0001, respectively) and an approximately 3-fold higher risk of SCCOP recurrence (HR, 3.2;95% CI, 2.2–4.6; and HR, 3.1; 95% CI, 2.2–4.4, respectively) after multivariate adjustment. Furthermore, among patients with HPV16-positive tumors, those with variant genotypes of these two polymorphisms had lower disease-free survival rates (log-rank, P < 0.0001 and P < 0.0001, respectively) and a higher recurrence risk than did patients with common homozygous genotypes (HR, 12.9; 95% CI, 3.8–43.6; and HR, 8.1; 95% CI, 3.6–18.6, respectively), whereas no significant associations were found for FAS1377 and FASLG124 polymorphisms. Our findings suggest that FAS670 and FASLG844 polymorphisms modulate the risk of recurrence of SCCOP, particularly in patients with HPV16-positive tumors. Larger studies are needed to validate these results.
Keywords: FAS and FASLG, recurrence, genetic variants, biomarkers, apoptosis, human papillomavirus, head and neck cancer, oropharyngeal cancer
Introduction
Despite declining smoking rates in the United States, the incidence of squamous cell carcinoma of the oropharynx (SCCOP), a subset of squamous cell carcinomas of the head and neck (SCCHN), is increasing1. This increase can be mainly attributed to the increasing prevalence of infection with high-risk types of human papillomavirus (HPV), particularly HPV16. Such infections have been established as risk factors for SCCOP, in addition to smoking and alcohol use2–4. Surgery, radiotherapy, and chemotherapy are successful in the treatment of SCCOP, but the long-term survival rates have improved only moderately. SCCOP has a high rate of recurrence (approximately 15%–30% of cases)5. The overall 5-year relative survival rate is approximately 50%, while patients who develop disease recurrence after multimodal treatment have a 5-year survival rate of less than 5%6.
Although clinical (e.g., index tumor site and disease stage), lifestyle (e.g., history of smoking and alcohol consumption), and other risk factors are used to assess the risk of recurrence of SCCOP, they do not account for all recurrences; genetic factors may also contribute to recurrent SCCOP. SCCOP recurrence rates differ among patients with similar clinical characteristics (e.g., tumor grade, stage, and therapeutic approach); thus, understanding and modifying patients’ genetic risk factors for recurrence may help us identify predictive markers on the basis of individual patients’ germ-line genetic variations, leading to improved survival and better quality of life.
Apoptosis plays an important role in tumorigenesis and cancer treatment7,8. The acquired ability to resist apoptotic stimuli is one of the primary characteristics of a malignant cell, and abnormal regulation of apoptosis is a key factor in the development and prognosis of cancer9. FAS is a cell surface receptor that can interact with the FAS ligand (FASLG) to trigger apoptosis10–12. Therefore, the FAS/FASLG pathway plays an important role in regulating apoptosis and maintaining cellular homeostasis. Genetic alterations in the FAS/FASLG signaling pathway may affect these genes’ expression and apoptotic efficacy, affecting cancer development and prognosis. Furthermore, since apoptosis-related genes are implicated in the apoptotic cascade, genetic variants of apoptotic variants may affect response to radiotherapy in SCCOP patients, particularly tumor HPV-positive SCCOP cases with p53-induced apoptotic pathways and fewer somatic mutations.
Functional single nucleotide polymorphisms (SNPs) in the promoters of FAS and FASLG genes have been found to affect the differential expression of the two genes13–15; such alterations of expression may affect the risk of SCCOP recurrence. For example, the FAS1377 and FAS670 polymorphisms have been shown to interfere with the SP1 and STAT1 transcription factor binding sites, respectively, decreasing promoter activity and in turn, FAS gene expression13,14; the C allele of the FASLG844 polymorphism creates a binding site for the CAAT/enhancer binding protein β transcription factor, resulting in higher basal expression of the FASLG gene15.
The results of previous studies suggest that polymorphisms of FAS/FASLG are associated with increased susceptibility to a variety of cancers16–23. In our previous study, we showed that the FAS670, FAS1377, and FASLG844 polymorphisms were significantly associated with the risk of primary SCCHN or second primary tumors after index SCCHN23,24, but no such risk was associated with FASLG124 polymorphisms. To date, no associations have been reported between FAS and FASLG polymorphisms and the risk of SCCOP recurrence. Given the roles of the FAS and FASLG genes in regulating cell death and the abnormal expression of FAS and FASLG in various types of tumors, we hypothesized that FAS and FASLG polymorphisms affect their transcription levels, cause interindividual variations in apoptotic efficacy, and lead to different treatment responses. In the current study, we tested the hypothesis that the variant genotypes of these apoptotic promoter variants were predictive of an increased risk of recurrence, particularly in HPV16-positive [HPV16(+)] tumors, in a cohort of 1008 SCCOP patients.
Patients and methods
Study subjects
Patients with SCCOP were consecutively enrolled from May 1995 through April 2010, as described previously25. In brief, all patients had newly diagnosed, histopathologically confirmed, untreated SCCOP; patients of all ages, sexes, ethnicities, and clinical stages were recruited. Patients with distant metastases at presentation were excluded. Approximately 95% of contacted patients consented to enrollment in the study. Patients were excluded if they 1) had known distant metastases; 2) had any prior cancers, except nonmelanoma skin cancer; 3) had a primary sinonasal tumor, a salivary gland tumor, cervical metastases of unknown origin, or a tumor outside the upper aerodigestive tract; 4) had no blood samples available for genotyping; 5) had undergone treatment outside of our institution; or 6) had undergone only palliative treatment. All subjects signed an informed consent form that had been approved by the institutional review board of The University of Texas MD Anderson Cancer Center (Houston, Texas).
Patients were monitored during and after treatment with scheduled regular clinical and radiographic examinations. Patients were considered disease free if absence of disease was documented at the date of the last visit with the head and neck surgeon, head and neck radiation oncologist, or head and neck medical oncologist. There were no universal standards for imaging. Typically, patients underwent either routine serial imaging or follow-up imaging on the basis of symptoms or physical examination findings.
Patients were considered to have recurrent disease if they developed a new lesion of the same histologic type, as verified by biopsy (incisional, excisional, or needle), and any lesions that had disappeared. Clinical data, such as stage at presentation of the index tumor, treatment, comorbidity, and recurrence, were obtained from a review of the medical records. The sixth edition of the American Joint Committee on Cancer TNM staging system was used to determine disease stage at the time of presentation for all study patients. Definitive radiotherapy was defined as radiotherapy with or without other therapeutic modalities. Medical comorbidities were classified according to a modification of the Kaplan-Feinstein comorbidity index (Adult Comorbidity Evaluation 27), which categorizes related comorbidities as none to mild, moderate, or severe26. The ACE-27 grades specific diseases and conditions as 1 of 3 levels of comorbidity: grade 1 (mild), grade 2 (moderate), or grade 3 (severe), according to the severity of individual organ decompensation and the prognostic effect. Once the patient’s individual diseases or comorbid conditions have been classified, an overall comorbidity score (none, mild, moderate, or severe) is assigned on the basis of the highest ranked single ailment. In cases in which 2 or more moderate ailments occur in different organ systems or disease groups, the overall comorbidity score is designated as severe.
“Ever drinkers” were defined as patients who had drunk at least one alcoholic beverage per week for at least 1 year during their lifetime; patients who had no such pattern of drinking were considered “never drinkers.” Patients who had smoked at least 100 cigarettes in their lifetime were defined as “ever smokers,” and patients who had smoked fewer than 100 cigarettes in their lifetime were categorized as “never smokers.”
Genotyping
DNA was extracted from 1 ml of blood with the Qiagen DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. We genotyped the four SNPs of FAS and FASLG genes: FAS1377, FAS 670, FASLG844, and FASLG124 using the polymerase chain reaction-restriction fragment length polymorphism (PCR) assay23. In brief, the following primers were used to amplify the target fragments containing these four polymorphisms (mismatch bases are underlined): 5′-TGTGTGCACAAGGCTGGCGC-3′ (forward) and 5′-TGCATCTGTCACTGCACTTACCACCA-3′ (reverse) for FAS 1377 G>A, 5′-ATAGCTGGGGCTATGCGATT-3′ (forward) and 5′-CATTTGACTGGGCTGTCCAT-3′ (reverse) for FAS 670 A>G, 5′-CAATGAAAATGAACACATTG-3′ (forward) and 5′-CCCACTTTAGAAATTAGATC-3′ (reverse) for FASLG 844 C>T, and 5′-GCAGTTCAGACCTACATGATTAGGAT-3′ (forward) and 5′-CCAGATACAGACCTGTTAAATGGGC-3 for FASLG 124 A>G. The amplified PCR products were 122, 193, 85, and 230 bp for 1377 G>A, 670 A>G, 844 C>T, and 124 A>G polymorphisms, respectively. The BstUI, ScrFI, DraIII, and FokI restriction enzymes (New England Biolabs, Beverly, MA) were used to distinguish 1377 G>A, 670 A>G, 844 C>T, and 124 A>G polymorphisms, respectively, which resulted in 104- and 18-bp fragments in the 1377 G allele; 136- and 57-bp fragments in the 670 G allele; 66- and 19-bp fragments in the 844 T allele; and 180- and 50-bp fragments in the 124 G allele. Approximately 10% of samples were retested, demonstrating 100% concordance.
Determination of tumor HPV16 status
Paraffin-embedded tissue biopsies or specimens from study patients with tissues available were used to extract DNA for tumor HPV16 detection using PCR and in situ hybridization methods, described previously27. For quality control, a subset of samples was re-assayed for tumor HPV16 status. The results of the re-run samples were 100% concordant with the original results.
Statistical analysis
For all analyses, statistical significance was set at P < 0.05, and all tests were two-sided. SAS software (version 9.2.3; SAS Institute) was used to perform all statistical analyses. The primary endpoint of the study was recurrence. The time to event was calculated from the date of diagnosis of the index SCCOP to the date of clinically detectable recurrence (local, regional, or distant). Patients who were not known to have had an event at the date of last contact and patients who were lost to follow-up or died of other or unknown causes were censored. We first used Student’s t test to compare the mean age and follow-up time between patients with and without recurrence. The associations between individual epidemiologic risk factors, clinical characteristics (including stage, comorbidity, and treatment variables), and time to recurrence were initially assessed using univariate Cox proportional hazards regression models. An examination of Kaplan-Meier survival curves and log-minus-log survival plots indicated that the data were consistent with the assumptions of the Cox proportional hazard regression models. The associations between variables and disease-free survival (DFS) were evaluated using the log-rank test. We assessed the associations between individual epidemiologic risk factors, clinical characteristics (including stage, comorbidity, and treatment variables), and time to recurrence using both univariate and multivariate Cox proportional hazards regression models. Associations between genotypes and risk of recurrence were quantified by calculating the hazard ratios (HRs) and their 95% CIs. The Cox model included adjustment for potential confounders, including age, sex, ethnicity, smoking status, alcohol use status, tumor stage, comorbidity, and treatment.
Results
From May 1995 to April 2010, 1226 patients with SCCOP were enrolled in this study. Of these patients, 218 were excluded because insufficient information was available about follow-up and treatment or no blood samples were available for genotyping. Therefore, our final analysis included 1008 patients with previously untreated incidental SCCOP. These patients were followed up from May 1995 to October 2013, with an overall median follow-up time of 44.7 months (range, 1.7 to 170.9 months); 181 patients experienced disease recurrence. The median follow-up durations for patients without and with recurrence were 50.9 and 11.6 months, respectively. Of the 181 patients with recurrence, 70 (38.7%) had distant recurrence, 49 (27.1%) had local recurrence, 20 (11.0%) had regional recurrence, and 42 (23.2%) had recurrence of more than one type.
The mean ages at diagnosis for the overall cohort, patients who developed recurrence, and patients without recurrence were 55.8, 58.6, and 55.2 years, respectively. Table 1 shows patients’ demographic, risk, and clinical factors and the corresponding 5-year actuarial recurrence rates. Patients in the overall group were predominantly male (86.5%) and non-Hispanic white (90.6%). The univariate Kaplan-Meier analyses showed that age, ethnicity, smoking, alcohol use, comorbidity, and treatment were significantly associated with DFS (all P < 0.05), whereas significant associations were not found for sex and index cancer stage (all P > 0.05).
Table 1.
Characteristics of patients with SCCOP (N = 1008)
| Variable | No. (%) of patients | No. of patients with recurrence | 5-year recurrence rate (%) | Pa value |
|---|---|---|---|---|
| No. of patients | 1008 (100) | 181 | 0.20 | |
| Age | ||||
| ≤ 57 years | 621 (61.6) | 85 | 0.15 | < 0.0001 |
| > 57 years | 387 (38.4) | 96 | 0.27 | |
| Sex | ||||
| Male | 872 (86.5) | 161 | 0.20 | 0.3110 |
| Female | 136 (13.5) | 20 | 0.19 | |
| Ethnicity | ||||
| Non-Hispanic white | 913 (90.6) | 146 | 0.17 | < 0.0001 |
| Other | 95 (9.4) | 35 | 0.41 | |
| Smoking | ||||
| Never | 388 (38.5) | 51 | 0.14 | 0.0004 |
| Ever | 620 (61.5) | 130 | 0.23 | |
| Alcohol use | ||||
| Never | 247 (24.5) | 26 | 0.10 | 0.0005 |
| Ever | 761 (75.5) | 155 | 0.23 | |
| Comorbidity | ||||
| None or mild | 913 (90.6) | 157 | 0.19 | 0.0370 |
| Moderate to severe | 95 (9.4) | 24 | 0.27 | |
| Index cancer stage | ||||
| 1 or 2 | 72 (7.1) | 11 | 0.19 | 0.5280 |
| 3 or 4 | 936 (92.9) | 170 | 0.20 | |
| Treatment | ||||
| X/XC/XS/S | 947 (93.9) | 166 | 0.19 | 0.0030 |
| SXC | 61 (6.1) | 15 | 0.32 | |
P: Log-rank test for DFS between the two groups.
X, radiotherapy; C, chemotherapy; and S, surgery.
The DFS was significantly poorer in patients with SCCOP with the FAS670 AG+GG and FASLG844 CT+TT variant genotypes than in patients with the corresponding common homozygous genotypes, respectively (log-rank, P < 0.0001 and P < 0.0001) (Figure 1), whereas no significant differences in DFS were observed between different genotypes of the FAS1377 (log-rank P = 0.211) or FASLG124 polymorphism (log-rank P = 0.121). To evaluate the associations between the four SNPs and recurrence risk in patients with SCCOP, we performed a multivariate Cox proportional hazards regression analysis with adjustment for several major confounders, including age, sex, ethnicity, smoking status, alcohol status, comorbidity, stage, and treatment. As shown in Table 2, compared with the FAS670 AA and FASLG844 CC common homozygous genotypes, patients with the FAS670 AG+GG variant and FASLG844 CT+TT variant genotypes had an approximately 3-fold significantly higher risk of disease recurrence (HR, 3.2; 95% CI, 2.2–4.6; and HR, 3.1; 95% CI, 2.2–4.4, respectively); no such significant associations were observed in patients with FAS1377 and FASLG124 polymorphisms.
Figure 1.
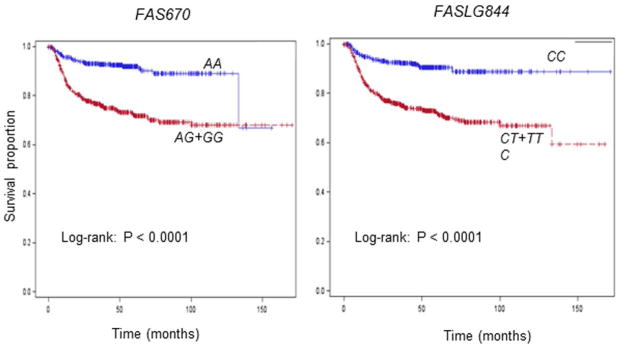
Kaplan-Meier estimates of the cumulative recurrence rates in all patients according to the FAS670 and FASLG844 genotypes (N = 1008).
Table 2.
Association between FAS and FASLG genotypes and SCCOP recurrence in patients with SCCOP (N = 1008)
| Genotype | No. of recurrences/no. of patients | 5-year recurrence rate | Log-rank P value | aHR*, 95% CI |
|---|---|---|---|---|
| FAS1377G>A | ||||
|
| ||||
| GG (ref.)a | 152/872 | 0.19 | 0.323 | 1.0 |
| GA | 28/128 | 0.22 | 1.1 (0.4–1.4) | |
| AA | 1/8 | 0.18 | 0.9 (0.3–1.2) | |
| Ptrend | 0.662 | |||
|
| ||||
| GG (ref.) | 152/872 | 0.19 | 0.211 | 1.0 |
| GA+AA | 29/136 | 0.22 | 1.1 (0.8–2.0) | |
|
| ||||
| FAS670A>G | ||||
|
| ||||
| AA (ref.)a | 36/449 | 0.10 | 0.0021 | 1.0 |
| AG | 106/430 | 0.28 | 2.7 (2.1–9.2) | |
| GG | 39/129 | 0.21 | 4.2 (0.8–10.3) | |
| Ptrend | 0.024 | |||
|
| ||||
| AA (ref.) | 36/449 | 0.10 | < 0.0001 | 1.0 |
| AG+GG | 145/559 | 0.28 | 3.2 (2.2–4.6) | |
|
| ||||
| FASLG844C>T | ||||
|
| ||||
| CC (ref.)a | 42/491 | 0.11 | 0.0011 | 1.0 |
| CT | 111/420 | 0.27 | 3.3 (1.7–9.8) | |
| TT | 28/97 | 0.29 | 3.8 (0.7–11.4) | |
| Ptrend | 0.132 | |||
|
| ||||
| CC (ref.) | 42/491 | 0.11 | < 0.0001 | 1.0 |
| CT+TT | 139/517 | 0.28 | 3.1 (2.2–4.4) | |
|
| ||||
| FASLG124A>G | ||||
|
| ||||
| AA (ref.)a | 119/715 | 0.19 | 0.226 | 1.0 |
| AG | 56/265 | 0.23 | 1.5 (0.9–2.4) | |
| GG | 6/28 | 0.20 | 1.1 (0.4–2.8) | |
| Ptrend | 0.743 | |||
|
| ||||
| AA (ref.) | 119/715 | 0.19 | 0.121 | 1.0 |
| AG+GG | 62/293 | 0.22 | 1.4 (0.9–2.0) | |
HR, hazard ratio.
Adjusted for age, sex, ethnicity, smoking status, alcohol use status, stage, comorbidity, and treatment.
The observed genotype frequencies were in agreement with the Hardy-Weinberg equilibrium (p2 + 2pq + q2 = 1). (HWE by χ2 tests: P = 0.175 for FAS1377, P = 0.104 for FAS670, P = 0.603 for FASLG844, and P = 0.565 for FASLG124, respectively).
We further explored the associations between the four polymorphisms and risk of recurrence among 233 HPV16(+) patients with SCCOP since both HPV and apoptosis play important roles in SCCOP carcinogenesis and prognosis. As shown in Figure 2, a significantly better DFS was found in patients with the FAS670 AA and FASLG844 CC genotypes than in those with the corresponding AG+GG (log-rank P < 0.0001) and CT+TT variant genotypes (log-rank P < 0.0001), respectively. However, no significant differences in DFS were observed for the FAS1377 and FASLG124 polymorphisms. Furthermore, after adjustment for several major confounders, patients with the FAS670 AG+GG and FASLG844 CT+TT variant genotypes had approximately 13- and 8-fold significantly increased risks of recurrence compared with those with the FAS670 AA and FASLG844 CC common homozygous genotypes, respectively (HR, 12.9; 95% CI, 3.8–43.6 for FAS670; and HR, 8.1; 95% CI, 3.6–18.6 for FASLG844) (Table 3); no significant associations were observed for the FAS1377 or FASLG124 polymorphisms. In addition, we observed no similar associations between the four polymorphisms and recurrence risk among patients with HPV16(−) SCCOP; these polymorphisms might not affect the risk of recurrence in HPV16(−) SCCOP, or it is possible that we did not have enough samples or outcome events in this study (only 78 patients had HPV16(−) SCCOP).
Figure 2.
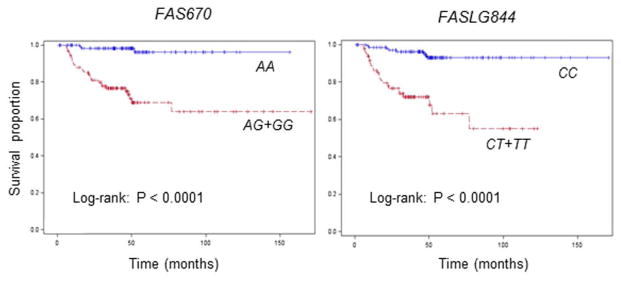
Kaplan-Meier estimates of the cumulative recurrence rates in HPV-positive SCCOP patients according to the FAS670 and FASLG844 genotypes (N = 233).
Table 3.
Association between FAS and FASLG genotypes and HPV-positive SCCOP recurrence in patients with SCCOP
| Genotype | No. of recurrences/no. of patients | 5-year recurrence rate | Log-rank P value | aHR*, 95% CI |
|---|---|---|---|---|
| FAS1377G>A | 0.662 | |||
|
| ||||
| GG (ref.) | 30/213 | 0.17 | 1.0 | |
| GA+AA | 2/20 | 0.19 | 0.8 (0.2–3.3) | |
|
| ||||
| FAS670A>G | < 0.0001 | |||
|
| ||||
| AA (ref.) | 3/125 | 0.10 | 1.0 | |
| AG+GG | 29/108 | 0.36 | 12.9 (3.8–43.6) | |
|
| ||||
| FASLG844C>T | < 0.0001 | |||
|
| ||||
| CC (ref.) | 8/151 | 0.12 | 1.0 | |
| CT+TT | 24/82 | 0.40 | 8.1 (3.6–18.6) | |
|
| ||||
| FASLG124A>G | 0.100 | |||
|
| ||||
| AA (ref.) | 21/185 | 0.17 | 1.0 | |
| AG+GG | 11/48 | 0.21 | 1.6 (0.8–3.3) | |
HR, hazard ratio.
Adjusted for age, sex, ethnicity, smoking status, alcohol use, stage, comorbidity, and treatment.
Reference group.
Discussion
In this large cohort study, we found that FAS670 and FASLG844 polymorphisms modified the recurrence risk of SCCOP, particularly in patients with HPV16(+) tumors. The SNPs were reported to alter the expression of FAS and FASLG genes19, resulting in non-physiological levels of FAS/FASL, which may disrupt cellular homeostasis. However, the underlying mechanisms that govern the effects of FAS and FALG on cell death are not understood. It has been shown that downregulation of FAS and FASLG may protect tumor cells from elimination by inducing a reduced apoptotic response; up-regulation of FAS/FASLG has the opposite effect28–31. The results of most studies indicate that low expression of FAS/FASLG is a common feature of malignant transformation and an early event that is associated with the development of most human cancers16–20,23,32–34. Given the important roles of FAS and FASLG in apoptosis, it is biologically plausible that genetic variants in the promoters of the FAS and FASLG genes affect the expression levels of these genes, thus affecting the regulation of apoptotic efficacy and the responses of cancer patients to treatment, such as radiotherapy. Therefore, FAS and FASLG polymorphisms may serve as predictive biomarkers of clinical outcome in SCCOP patients and help physicians individualize treatment, leading to an improved prognosis and better quality of life for patients with this disease.
The associations between FAS/FASLG polymorphisms and the risk of developing many types of human cancer have been previously reported14,17–20,23,24,32,33. However, few studies have investigated the associations between these polymorphisms and recurrence risk in SCCOP and HPV-associated SCCOP. For example, the association between the FASLG844 polymorphism and the risk of some cancers has been reported in previous studies,15,17–20,34 but not for FASLG124. Previously, we also reported that both FAS670 AG+GG and FASLG844 CT+TT variant genotypes were associated with a significantly higher risk of second primary malignancies in patients with index SCCHN compared with the corresponding FAS670 AA and FASLG844 CC genotype24, although the results of our previous case-control study indicated that these polymorphisms were not significantly associated with risk of SCCHN23. In the current study, however, we observed an association between the FAS 670 AG+GG and FASLG844 CT+TT variant genotypes and a high risk of recurrence compared with that of their corresponding common homozygous genotypes; no such significant associations were found for the FAS1377 and FASLG124 polymorphisms. Although the exact mechanisms by which these polymorphisms affect disease recurrence are unclear, it is expected that FAS670 and FASLG844 in the promoters of these genes affect FAS and FASLG expression, which subsequently affects treatment response and cancer prognosis. In vitro studies have shown that the FAS670 G allele and FASLG844 T allele result in reduced promoter activity, leading to lower expression of FAS/FASLG genes13–15. The low expression of both genes might result in a lower apoptotic capacity in cancer cells, leading to an increased risk of disease recurrence after radiotherapy. In the current study, we observed significant associations between the variant genotypes (AG+GG for FAS670 and CT+TT for FASLG844) and an increased risk of recurrence in SCCOP patients, which is consistent with our previous findings that patients with the FAS670 and FASLG844 variant genotypes had a significantly increased risk of second primary tumors after index SCCHN24. Nevertheless, future studies of the underlying mechanisms are needed.
Although many factors, such as genetic, clinical, and lifestyle factors, affect recurrence risk in patients with SCCOP, this risk can also be influenced by tumor HPV status. Since HPV(+) and HPV(−) SCCOP are distinct groups with different molecular, pathologic, and clinical characteristics, HPV(+) SCCOP patients experience a better response to radiotherapy and have a better prognosis than do HPV(−) SCCOP patients. HPV(+) SCCOP patients generally lack somatic genetic changes (e.g., intact p53), whereas patients with HPV(−) SCCOP, which is mostly driven by smoking, have the most common p53 mutations. Such p53 mutations seem to be correlated with a poor response to radiotherapy, partially due to inactivation of the p53-mediated apoptotic pathway. Therefore, HPV status is highly relevant to SCCOP prognosis, suggesting that a stratified analysis by tumor HPV status should be considered in future studies of the prognosis of SCCOP patients, such as studies of recurrence.
We further explored the roles of FAS/FASLG polymorphisms in recurrence risk in SCCOP patients stratified by tumor HPV16 status. The association between FAS670 and FASLG844 variant genotypes and recurrence risk of SCCOP was higher in patients with HPV16(+) tumors, suggesting that the modifying effect of FAS670 and FASLG844 variant genotypes on the risk of SCCOP recurrence was more pronounced in these patients. The mechanism behind these results in patients with SCCOP is not fully understood. When HPV-positive SCCOP patients undergo radiotherapy or chemoradiotherapy, tumor cells harboring intact p53 may induce apoptosis. We expect that HPV-positive SCCOP patients with variant genotypes will have lower apoptotic efficacy than will patients with the corresponding common homozygous genotypes; thus, these patients are at higher risk of disease recurrence or progression through apoptotic escape. Genetic variants of these genes may lead to interindividual differences in apoptotic response, resulting in different susceptibilities to the genotoxic effects of radiation and different clinical outcomes. However, these hypotheses need to be verified in future studies.
Our study has limitations. First, we did not collect information on the exact dosage and duration of radiotherapy for each patient. Therefore, well-designed studies with detailed information on radiotherapy and a uniform treatment plan are needed. Second, due to the relatively small sample size and small number of outcome events in patients with HPV16(+) tumors, our findings could be due to chance. Third, our cases were recruited at a single cancer center and the population was primarily non-Hispanic whites; thus, our results are not generalizable to other racial and ethnic groups. Finally, the disease recurrence outcomes were collected retrospectively, with no strictly defined screening or follow-up regimen. Despite these limitations, the current investigation supports a significant role for FAS670 and FASLG844 polymorphisms in individual variation in disease recurrence susceptibility after definitive radiotherapy in SCCOP patients. Large and prospective studies are needed to validate our results and further explore the molecular mechanisms that underlie the observed associations, which may have future utility as clinical prognostic biomarkers.
Novelty and impact statements.
Apoptotic variants in FAS and FASLG promoters modify the risk of SCCOP recurrence and may be markers of genetic susceptibility to SCCOP recurrence, particularly in HPV-positive patients.
Acknowledgments
We thank Margaret Lung, Jenny Vo, and Jessica Fiske for their assistance in recruiting the subjects and Chong Zhao and Yingdong Li for laboratory assistance.
Grant support
This work was partly supported by NIH grants R01 ES011740 (Q.W.), CA128110-01A1 (E.M.S.), CA135679 (G.L.), and CA133099 (G.L.).
Abbreviations
- SCCHN
squamous cell carcinoma of the head and neck
- SCCOP
squamous cell carcinoma of the oropharynx
- HR
hazard ratio
- CI
confidence interval
- PCR
polymerase chain reaction
- SNPs
single nucleotide polymorphisms
Footnotes
Conflict of interest statement
None declared.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 6.Amar A, Chedid HM, Rapoport A, Dedivitis RA, Cernea CR, Brandão LG, et al. Update of assessment of survival in head and neck cancer after regional recurrence. J Oncol. 2012;154303 doi: 10.1155/2012/154303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zörnig M, Hueber A, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 8.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 9.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 10.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–89. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 11.Itoh N, Yonehara S, Ishii A. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 12.Oehm A, Behrmann I, Falk W. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–15. [PubMed] [Google Scholar]
- 13.Huang QR, Morris D, Manolios N. Identification and characterization of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol. 1997;34:577–82. doi: 10.1016/s0161-5890(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 14.Sibley K, Rollinson S, Allan JM. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003;63:4327–30. [PubMed] [Google Scholar]
- 15.Wu J, Metz C, Xu X. A novel polymorphic CAAT/enhancer-binding protein h element in the FasL gene promoter alters Fas ligand expression: a candidate background gene in African American systemic lupus erythematosus patients. J Immunol. 2003;170:132–8. doi: 10.4049/jimmunol.170.1.132. [DOI] [PubMed] [Google Scholar]
- 16.Lai HC, Lin WY, Lin YW. Genetic polymorphisms of FAS and FASL (CD95/CD95L) genes in cervical carcinogenesis: an analysis of haplotype and gene-gene interaction. Gynecol Oncol. 2005;99:113–8. doi: 10.1016/j.ygyno.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Miao X, Sun T. Functional polymorphisms in cell death pathway genes FAS and FASL contribute to risk of lung cancer. J Med Genet. 2005;2:479–84. doi: 10.1136/jmg.2004.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, Sun T, Xue L. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067–73. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 19.Sun T, Miao X, Zhang X, Tan W, Xiong P, Lin D. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1030–6. doi: 10.1093/jnci/djh187. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Miao XP, Zhang XM. Genetic polymorphisms of apoptosis-associated genes FAS and FASL and risk of colorectal cancer. Zhonghua Yi Xue Za Zhi. 2005;85:2132–5. (in Chinese) [PubMed] [Google Scholar]
- 21.Muraki Y, Yoshioka C, Tateishi A, Fukuda J, Haneji T, Kobayashi N. Localization of Fas antigen in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 1999;37:37–40. doi: 10.1054/bjom.1998.0298. [DOI] [PubMed] [Google Scholar]
- 22.Sundelin K, Jadner M, Norberg-Spaak L, Davidsson A, Hellquist HB. Metallothionein and Fas (CD95) are expressed in squamous cell carcinoma of the tongue. Eur J Cancer. 1997;33:1860– 4. doi: 10.1016/s0959-8049(97)00216-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Wang LE, Sturgis EM. Polymorphisms of FAS and FAS ligand genes involved in the death pathway and risk and progression of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2006;12:5596–602. doi: 10.1158/1078-0432.CCR-05-1739. [DOI] [PubMed] [Google Scholar]
- 24.Lei D, Sturgis EM, Wang LE, Liu Z, Zafereo ME, Wei Q, et al. FAS and FASLG genetic variants and risk for second primary malignancy in patients with squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 2010;19:1484–91. doi: 10.1158/1055-9965.EPI-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan X, Sturgis EM, Lei D, Liu Z, Dahlstrom KR, Wei Q, et al. Association of TGF-1 genetic variants with HPV16-positive oropharyngeal cancer. Clin Cancer Res. 2010;16:1416–22. doi: 10.1158/1078-0432.CCR-09-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 27.Ji X, Sturgis EM, Zhao C, Etzel CJ, Wei Q, Li G. Association of p73 G4C14-to-A4T14 polymorphism with human papillomavirus type 16 status in squamous cell carcinoma of the head and neck in non-Hispanic whites. Cancer. 2009;115:1660–68. doi: 10.1002/cncr.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas-ligand induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 29.Strand S, Hofmann WJ. Lymphocyte apoptosis induced by CD95 (APO-1/CD95) ligand expressing tumor cells Ma mechanism of immune evasion? Nat Med. 1996;2:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 30.Muschen M, Warskulat U, Beckmann MW. Defining CD95 as a tumor suppressor gene. J Mol Med. 2000;78:312–25. doi: 10.1007/s001090000112. [DOI] [PubMed] [Google Scholar]
- 31.Reichman E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol. 2002;12:309–15. doi: 10.1016/s1044-579x(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 32.Kripple P, Langsenlehner U, Renner W, Koppel H, Samonigg H. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1478–9. doi: 10.1093/jnci/djh289. [DOI] [PubMed] [Google Scholar]
- 33.Ueda M, Terai Y, Kanda K, Kanemura M, Takehara M, Yamaguchi H, et al. Fas gene promoter 670 polymorphism in gynecological cancer. Int J Gynecol Cancer. 2006;16(Suppl 1):179–82. doi: 10.1111/j.1525-1438.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 34.Sun T, Zhou Y, Li H, Han X, Shi Y, Wang L, et al. FASL 844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med. 2005;202:967–74. doi: 10.1084/jem.20050707. [DOI] [PMC free article] [PubMed] [Google Scholar]


