Abstract
Background
Isolation-based practices in nursing homes (NHs) differ from those in acute care. NHs must promote quality of life while preventing infection transmission. Practices used in NHs to reconcile these goals of care have not been characterized.
Purpose
To explore decision-making regarding isolation-based infection prevention and control practices in NHs.
Methods
A qualitative study was conducted with staff (e.g., staff nurses, infection prevention directors and directors of nursing) employed in purposefully sampled U.S. NHs. Semi-structured, role-specific interview guides were developed and interviews were digitally recorded, transcribed verbatim and analyzed using directed content analysis. The research team discussed emerging themes in weekly meetings to confirm consensus.
Results
We inferred from 73 interviews in 10 NHs that there was variation between NHs in practices regarding who was isolated, when isolation-based practices took place, how they were implemented, and how they were tailored for each resident. Interviewees’ decision-making depended on staff perceptions of acceptable transmission risk and resident quality of life. NH resources also influenced decision-making, including availability of private rooms, extent to which staff can devote time to isolation-based practices and communication tools. A lack of understanding of key infection prevention and control concepts was also revealed.
Conclusions and Implications
Current clinical guidelines are not specific enough to ensure consistent practice that meets care goals and resource constraints in NHs. However, new epidemiological research regarding effectiveness of varying isolation practices in this setting is needed to inform clinical practice. Further, additional infection prevention and control education for NH staff may be required.
INTRODUCTION
Infections are a leading cause of morbidity and mortality among nursing home (NH) residents.[1] In the U.S. alone, an estimated 1.6 to 3.8 million infections occur in NHs annually.[2] Because NH residents are at high risk for infection,[3] prevalence will likely continue to rise given the global aging population[4] that will increase demand for NH services (1.5 million U.S. residents today[5] compared with an estimated 5.3 million by 2030[2]). Therefore, identifying effective practices to reduce infection transmission is necessary to manage health outcomes and costs.[3]
Isolation precautions are recommended to prevent the spread of pathogens associated with high morbidity and mortality, such as multidrug-resistant organisms (MDROs).[6–8] This practice includes confining an MDRO-infected resident to a private room or cohorting if no private rooms are available (i.e., grouping together patients colonized or infected with the same organism by location during all activities to prevent organism transmission to unaffected patients).[6–10] Infection prevention guidelines also suggest using standard precautions for contact with the MDRO-infected resident (i.e., hand hygiene, use of gowns, gloves and other personal protective equipment depending on the anticipated exposure).[7] Further, it is recommended that infected residents should have dedicated disposable patient care equipment,[9] such as private commodes for patients with a diarrheal disease, if private bathrooms are not available.[10] Studies concerning the effectiveness of isolation precautions have had mixed results and have been deemed to be of moderate or poor quality.[11, 12]
Infection prevention and control guidelines are based on evidence collected in acute care settings, and therefore are not always practical or appropriate in NHs where resources are more constrained and the healthcare facility is often the residents’ home.[6, 7] Further, isolation has well-established negative psychological effects,[13, 14] both for semi-private and private room isolation.[14] These adverse effects may be of greater concern in a NH facility since it is also a primary residence. A qualitative description of isolation-based infection control practices in this setting has not been conducted. Therefore, it is important to understand how NH staff balance benefits and drawbacks of isolation in order to establish best practices that can be implemented across facilities.[15]
A gap in the literature exists regarding how it is decided when and how to implement isolation of infected residents in this setting. In a previous survey of 331 NHs in Iowa, most facilities reported use of isolation precautions for methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococcus infections. The majority also reported cohorting some residents infected with these organisms. Staff in approximately a third of the NHs reported that the need for private room placement depended on the particular resident. However the survey did not capture how it was determined that isolation or cohorting was appropriate,[16] thus providing limited insight into factors that may influence isolation practices versus cohorting. Therefore, the objective of this study was to explore decision-making in isolation-based infection prevention and control practices in U.S. NHs. Understanding variations in practice is necessary to ensure that NH residents receive consistent, high-quality care in this setting.
METHODS
A qualitative study was conducted. This study was a secondary data analysis of a larger study regarding infection control and prevention resources in NHs (R01NR013687), which is described in detail elsewhere.[17] Each NH was purposively selected with the goal of obtaining variation in geographical region, size, ownership status and 3-year infection control deficiency citation performance. The deficiency citation score is derived from infection control-related evaluation criteria found in annual, unscheduled inspections by the state that are required for Medicare and Medicaid certification and reimbursement (deficiency citations indicate poor performance).
NHs were recruited through informational mailings, follow-up phone calls and emails. At each facility, a site contact was identified who then recruited individual interviewees based on our guidelines for inclusion.[17] We aimed to recruit interviewees who were familiar with the facility based on tenure and who would provide a range of perspectives based on role (e.g., infection prevention directors, directors of nursing, assistant directors of nursing, medical directors, environmental service workers and staff nurses). Recruitment concluded when theoretical saturation across the entire NH sample was achieved for all infection control-related topics covered by the interview guides.[18]
Members of our study team (three male, five female) conducted in-depth, semi-structured interviews from May through September 2013. Each interviewee was interviewed once, one-on-one, with an interview guide informed by Donabedian’s healthcare quality theoretical framework[19] and tailored for each personnel type.[17] All interviewers were trained on in-depth qualitative interviewing techniques and encouraged to manually record field notes regarding observations not captured in the interview. Interviews were digitally recorded and transcribed verbatim. The Institutional Review Board of Columbia University Medical Center approved this study. All interviewees were informed of study goals and provided written informed consent.
A directed content analysis of all transcripts was performed (see Appendix A). This analytical technique helps to determine the initial coding scheme and is useful when existing theory or prior research insufficiently describes a particular phenomenon.[20] A keyword search of all transcripts was conducted in NVivo 10 (QSR International)[21] software using “isolation” and related terms (e.g., isolate, contact precaution, contact isolation, isolation precaution, cohort, quarantine, outbreak, cart, special precautions, single room, private room, signs, mask, gown, roommate) to highlight passages of text pertaining to the phenomena of interest. A keyword search is beneficial in content analysis when a large volume of text is available as it allows researchers to target passages with pertinent content to focus in-depth analysis.[22] Using Microsoft Excel[23] software to facilitate coding and analysis, CCC and MPM reviewed the extracted passages, generated a comprehensive set of primary and secondary codes and drafted definitions for each. Emerging themes were discussed weekly with all authors to ensure a shared understanding. The authors followed the Consolidated Criteria for Reporting Qualitative Research checklist in writing this manuscript (see Appendix B).[24]
RESULTS
In total, 10 NHs were visited and 73 interviews were conducted, with 6–8 interviewees per facility. On average, interviews lasted approximately 45 minutes. Characteristics of the sample are described in detail elsewhere.[17] A total of 1533 references in 75 passages (representing 72 of 73 transcripts) were identified in the keyword search.
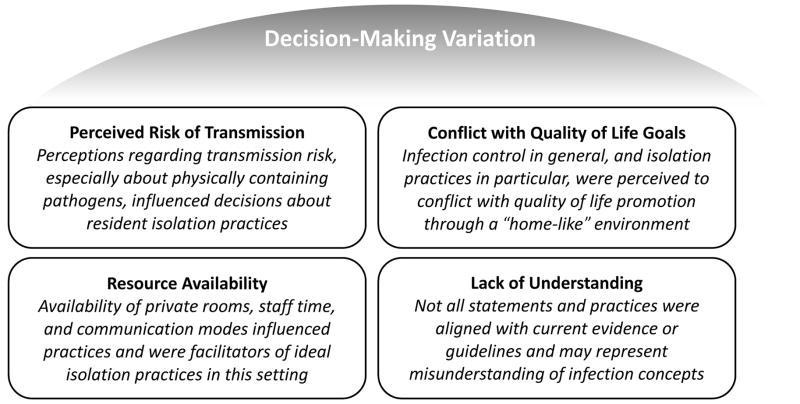
We found that isolation-based practices differed between NHs. The residents who received these interventions and the way they were implemented varied by facility. For example, some facilities automatically used isolation practices for residents with new respiratory or gastrointestinal symptoms, positive laboratory cultures and/or all residents admitted from a hospital setting. Other NHs rarely isolated residents. There was also variation with regard to whether isolation practices were discontinued based on laboratory cultures or upon resolution of symptoms. One exception to the variation between facilities existed: colonization (i.e., asymptomatic carriage) was not mentioned as a consideration for isolation practices in any NH. Further, none of the interviewees reported routine screening of residents. As one interviewee stated, lack of routine surveillance was part of a “don’t look, don’t tell” approach to managing colonization (Participant 27: Medical Director, NH 4). Throughout the narratives we found that decision-making to use isolation practices was complex and this could be attributed to four emergent themes: (1) perceived risk of transmission; (2) conflict with quality of life goals; (3) resource availability; and (4) lack of understanding regarding infection prevention and control. Each of these themes are outlined in Figure 1 and described in-depth below.
Figure 1.
Emergent themes from qualitative directed content analysis regarding isolation-based infection control and prevention practices in nursing homes.
Perceived Risk of Transmission
Interviewees discussed practice decisions in the context of organism transmission risk in specific situations and among individual residents. Most NHs’ isolation practices incorporated the concept of organism ‘containment’, that is, low perceived transmission risk. This was a factor when staff decided the degree to which an infected resident would be limited in social and environmental contact.
“Anything that can be contained, like MRSA [methicillin-resistant Staphylococcus aureus], or VRE [vancomycin-resistant enterococcus] in a wound. Or if they have it in the urine, it’s in a bag so it’s contained. [...] so if it’s contained, they can be cohorted.” (Participant 57: Infection Prevention Director, NH 8)
There appeared to be variation regarding the emphasis on perceived organism containment, resident compliance, and surrounding residents’ health when deciding to initiate or discontinue isolation-based practices and the nature of these practices. Additionally, the concept of effective containment varied, but generally applied to scenarios in which infectious secretions or drainage stayed within a colostomy bag or catheter, or were covered by personal protective equipment, a dressing or clothing. As one interviewee stated,
“If it was contained, [...] you didn’t have to isolate [...] a catheter bag is closed… whereas if [there is …] no catheter, no coverage; then you know they’re at risk.” (Participant 35: Minimum Data Set Coordinator, NH 5)
In contrast, interviewees mentioned Clostridium difficile most often as an example of an infection with high transmission risk because it is “uncontrollable” (Participant 17: Director of Nursing/Infection Prevention Director, NH 3). A resident’s ability and willingness to use appropriate personal hygiene, standard precautions and potentially personal protective equipment outside of his/her room was also important. As explained by an administrator,
“If [a resident with diarrhea is] sharing the toilet with multiple people, then we [...] have to determine are they cognitively with it enough to know to use a bedside toilet? Or do we need to look at moving them to not risk contaminating the other residents?” (Participant 47: Assistant Director of Nursing, NH 7)
Additionally, the overall health condition of a resident’s existing roommate(s) was also a key factor in decision-making as explained below;
“We carefully monitor […] if [a resident is] placed on isolation, does their roommate have any open sores?” (Participant 73: Infection Prevention Director, NH 10)
Variations in isolation-based practices included leaving a resident in a shared room, cohorting the infected resident with other infected resident(s) or transmission-based precautions in a private room. Additionally, practices varied as to whether an infected resident was allowed to leave his/her room, or was encouraged to participate in activities outside the room. As one interviewee stated,
“If [residents] are on isolation we do put an isolation gown on them and gloves, but they’re free to come out of their room […] We try to get them to socialize, too.” (Participant 41: Director of Nursing/Infection Prevention Director, NH 6)
Interviewees in almost all facilities believed that isolation precautions were necessary when an infectious organism could not be contained or controlled, though this was not ideal.
Conflict with Quality of Life Goals
The importance of resident quality of life and concerns that isolation practices conflicted with resident quality of life was pervasive throughout the interviews. As explained by one administrator,
“If you have to isolate somebody or you have to put restrictions on them because of an infection [...] you have to balance the quality of life aspect.” (Participant 9: Administrator, NH 2)
When discussing this balance, interviewees regarded isolation as “horrible” (Participant 15: Administrator, NH 3). This is further described in the quotes below:
“We’d love to never have anybody on isolation.” (Participant 3: Quality Improvement Coordinator, NH 1)
“It’s almost like holding a person prisoner.” (Participant 47: Assistant Director of Nursing, NH 7)
However, interviewees felt that isolation-based practices are an important aspect of preventing and controlling infection. One administrator elaborated on this sentiment:
“We have a mission statement and the promise is to keep our residents safe and secure [...] that includes keeping them infection free as best as we can.” (Participant 1: Administrator, NH 1)
However, ways in which staff attempted to balance the NH environment as both a home and medical facility differed based on perceptions of resident needs. For example, at one facility socialization among residents was encouraged and the interviewee referred to isolation as allowing residents to leave their rooms while donning personal protective equipment (see the previous section); staff in another NH did not want to violate a resident’s privacy by placing a sign on the resident’s door, let alone encourage personal protective equipment use outside a private room. As an administrator explained,
“We do not put signs up [for isolation] because that’s… considered a violation of their rights. So, you have [a] whole set of new issues in this home setting.” (Participant 47: Assistant Director of Nursing, NH 7)
In this way, differences in perception of what maximizes quality of life led to variation in practice.
Resource Availability
Interviewees mentioned that the NH resources influenced isolation-based infection control practices; specifically, the availability of private rooms. For example,
“If it’s [...] respiratory isolation, we can’t handle that unless we can put them in a private room and usually our private rooms are full.” (Participant 24: Director of Nursing, NH 4)
It was advantageous, therefore, if a NH had all private rooms, as explained by one medical director,
“One good thing about this facility is that every room is a private room. [... the] need to isolate [an infected resident] from one resident or bulk of residents doesn’t arise” (Participant 20: Medical Director, NH 3)
The extent to which staff were pressed for time in daily practice was also a factor leading to variation as being “in a hurry” could result in forgetfulness or lack of awareness of appropriate isolation practices (Participant 43: Licensed Practical Nurse, NH 6). Having more time and other resources that enabled communication through multiple channels (e.g., email, formal in-person meetings, and/or headset intercoms) raised awareness of recent infections and/or changes in practice and were facilitators to appropriate isolation practice. As described by an infection prevention director,
“[NH staff] can page me, they can stop me in the hallway. I receive phone calls at home with questions [...] it’s very important to have that communication because they help me arrange private rooms, room changes.” (Participant 12: Infection Prevention Director, NH 2)
However, there was high variation across facilities in the modes of communication.
Lack of Understanding
In the majority of NHs, at least one interviewee offered information that conflicted with commonly accepted infection-related terminology. These statements may indicate a lack of understanding regarding key infection prevention and control concepts. Of note, three of those interviewees were in charge of infection prevention and control at his or her facility.
The terms isolation and cohorting were used inconsistently among interviewees. Isolation was used to refer both to processes to isolate organisms (e.g., personal protective equipment use by the resident outside of his/her room) as well as physically limiting interaction between residents and the surrounding environment. Isolation was used by some as an umbrella term that also encompassed the concept of cohorting. Interviewees used the term cohorting for various scenarios, some of which did not match the definition of cohorting given by the Centers for Disease Control and Prevention.[7] For example, one interviewee described placing healthy (low infection risk) residents with infectious residents as cohorting and referenced these same guidelines, as long as the non-infected roommate was “alert” and had no “open orifices” through which pathogens may be transferred (Participant 32: Director of Nursing, NH 5). Another discussed that cohorting might include placing residents with active infections caused by different drug-resistant organisms together in the same room provided that the infections of each were “contained” and the residents’ provider(s) or families did not object to this action (Participant 41: Infection Prevention Director/Director of Nursing, NH 6).
For some interviewees, there were misunderstandings about bacterial colonization and the infection risk it poses. For example in discussing this topic, one interviewee stated that it is “safe” to place a methicillin-resistant Staphylococcus aureus-colonized resident with a roommate (Participant 50: Director of Nursing, NH 7) and another stated that asymptomatic residents are “not infectious” (Participant 53: Administrator, NH 8).
Interviewees also noted fears of spreading infection not only among the residents but also to themselves, and to their families.
“We had someone that was just admitted not too long ago that had just a skin breakout [… staff members] were all very scared. They were gowning and gloving and masking to go in the room. But [the resident] wasn’t infectious… we had to call another in-service and say look, [personal protective equipment] isn’t needed.” (Participant 48: Assistant Director of Nursing/Infection Prevention Director, NH 7)
Appropriate use of personal protective equipment was important to interviewees as observed inappropriate use during a mandatory annual state inspection of the facility may result in a deficiency citation and a costly fine. Interviewees noted that education might be key to alleviating fear of infection among staff as well as fear, frustration and intentional non-compliance among residents and their families in response to the resident’s restricted location and/or activities.
DISCUSSION
We inferred from these rich data that differences existed in isolation-based practices between facilities. This study confirmed that a lack of private rooms and other resources are barriers to isolation practices, as demonstrated in previous work.[16] We found that current practice to maintain a ‘home-like’ environment was informed by perceptions of transmission risk and resident quality of life. However, there were clear misunderstandings among some interviewees about current infection control terminology, recommendations and concepts.
Variation in practice between NHs was conspicuous and not surprising. According to clinical guidelines for this setting, contact precautions and other isolation-based infection prevention and control practices may be applied on a case by case basis to adapt practice to the needs of the individual facility and resident.[25] We infer from our data that these practices in NHs appear to be aligned with the clinical guidelines in this way. Our findings also suggest that variation is likely driven by a combination of factors including quality of life perception and prioritization, limited availability of private rooms, and lack of routine laboratory services and other resources. In particular, the desire among interviewees to balance resident quality of life and infection prevention and control practices was striking and represents a specific challenge to infection reduction in this setting.[26] However, the degree to which NH staff are adjusting practice based on perception rather than evidence highlights ambiguity in published infection prevention and control guidelines and an overall lack of infection intervention effectiveness data specific to this setting.
A salient example of how care for residents may be improved with new evidence is greater understanding of transmission risk from residents colonized with MDROs in NHs. Contact precautions are not required for all MDRO carriers in this setting, but MDRO colonization should be a consideration for isolation when the risk is high that the resident will infect others.[25] Our interviewees either did not mention colonization in discussion of decision-making factors or stated specifically that their NH lacked colonization care protocols. This is consistent with a previous survey in which 36% of NH staff would not change their practices if they knew a resident was colonized or infected with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococcus.[27] That survey did not provide data about why resident colonization status would not affect interviewee practices. While current guidelines advise NH staff to make isolation decisions on a case-by-case basis,[3, 6, 25], removing colonization status from the decision-making process entirely does not seem congruent with current clinical guidelines.[3, 25]
Guidelines and the evidence supporting them should specifically address the relative transmission risk posed by certain residents and practices. The American Medical Directors Association, Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) guidelines encourage covering draining wounds with dry dressings[6, 25] but the extent to which transmission risk is lower when secretions, colonization, or infection are contained under a dressing, within a device (i.e., urinary catheter drainage bag), or under clothing is not known.[3, 6–8] Further, limited evidence exists that the use of a bedside commode effectively reduces infection transmission risk when no private bathrooms are available.[10] The relative safety and benefits of allowing infected individuals to attend activities in shared spaces while donning personal protective equipment is not known. Therefore, practices based on perceived containment of the infection described here may not in fact be effective in preventing transmission of pathogens between residents. As mentioned above, isolation precautions have been primarily studied in acute care settings where the quality of data produced has been poor.[11, 12, 28] More evidence regarding processes for precaution discontinuation as well as isolating residents when private rooms are not available (e.g., cohorting) would be beneficial for informed decision-making. This new evidence may help ensure consistent, high quality care for residents across NHs. Further, more standard, and perhaps simplified, guidelines may be warranted as new setting-specific evidence becomes available.
Given the inconsistent use of terminology and misunderstandings of infection concepts among NH staff, there may be a need to increase and/or reinforce understanding of existing guidelines. For example, although we cannot determine if interviewees’ descriptions of cohorting an infected resident with a healthy resident in the same room represented an ineffective infection control practice, use of the term cohorting was inconsistent with the definition of cohorting provided in the Society of Healthcare Epidemiology of America/ISDA long-term care infection prevention and control guidelines (i.e., grouping together patients colonized or infected with the same organism by location during all activities to prevent organism transmission to unaffected patients).[6] It is doubtful that NH staff can apply the guidelines appropriately if the terminology is not understood. Inconsistent use of terminology and other misunderstandings revealed in these data may be due to the fact that infection prevention directors in this setting typically have minimal training for this role and multiple responsibilities.[17] However, training and education would presumably have a greater impact to reduce healthcare associated infections with the availability of new evidence regarding infection prevention and control practice effectiveness in this setting.
Limitations
While our sample was purposefully geographically dispersed and sampled for diversity, high heterogeneity between NH facilities and resident populations[29] as well as state laws and initiatives[30] purposeful sampling may limit the transferability of study findings. Although these data represent U.S. NHs, themes may be more broadly applicable. As interviews were semi-structured to capture unanticipated and relevant content, there was variation in specific follow-up questions asked by each interviewer. Unless explicitly stated by the interviewee, we cannot conclude that certain decision-making factors, resources or practices were either present or absent at a particular NH, nor can we make conclusions about the relative importance of specific factors at a given facility or how frequently they were implemented. While we were not able to have each interviewee review transcripts, in an effort to conduct member-checking, each NH was sent a summary of the findings from their facility and no corrections were offered. Use of a keyword search to identify passages of interest for our directed content analysis may have limited this study if a relevant passage was not identified. However, we are confident this was not the case as two randomly-selected, full transcripts were reviewed to ensure the search results highlighted all relevant sections. The keyword search was therefore timesaving and helped to identify passages with content of interest.
Conclusion
There is wide variation in isolation-based infection prevention and control practices in NHs. Additional training may help staff better understand key infection prevention and control concepts and definitions. However, efforts to improve care in this setting should focus on generating new effectiveness research, which is necessary to understand which isolation-based infection prevention and control practices are associated with the lowest infection risk among NH residents. Results of those studies can better inform clinicians’ decision-making regarding transmission risk and appropriate practices for individual residents, especially in cases of colonization, cohorting and other organism containment practices. New evidence on these topics is required to ensure high-quality, consistent care for this vulnerable population.
Supplementary Material
Acknowledgments
We thank Nicholas Castle, Laurie Conway, Andrew Dick, John Engberg and May Uchida for their assistance in data collection and Victoria Raveis for her expert guidance regarding qualitative analysis. We also thank Elaine Larson and the advisory board of the Prevention of Nosocomial Infections and Cost Effectiveness in Nursing Homes (PNICE-NH) study for their contributions. We are especially grateful to the staff of the NHs that participated in the PNICE-NH study.
FUNDING
Funding for this study was generously provided by the National Institute of Nursing Research (NINR R01 NR013687). CCC was also supported by NINR (F31 NR015176-01 and T32 NR013454). EC received financial support from the NINR over the course of the study (F31 NR014599) and JT is supported by the Jonas Center for Nursing and Veterans Healthcare
Footnotes
COMPETING INTERESTS
MPM has served as a consultant to Becton, Dickinson and Company. This consulting work was not related to the research presented in this article. The other authors have no potential conflicts of interest to report.
References
- 1.Richards C. Infections in residents of long-term care facilities: an agenda for research report of an expert panel. J Am Geriatr Soc. 2002;20:570–6. doi: 10.1046/j.1532-5415.2002.50128.x. [DOI] [PubMed] [Google Scholar]
- 2.Strausbaugh LJ, Joseph CL. The Burden of Infection in Long_Term Care. Infection Control and Hospital Epidemiology. 2000;21(10):674–9. doi: 10.1086/501712. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JD, Rhinehart E, Jackson M, et al. Management of multidrug-resistant organisms in health care settings, 2006. American Journal of Infection Control. 2006;35(10):S165–S93. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. “Ageing well” must be a global priority. Geneva, Switzerland: 2014. [accessed January 9 2015]. updated November 6 2014. Available from: http://www.who.int/ageing/en/ [Google Scholar]
- 5.U.S. Department of Health and Human Services. National action plan to prevent health care-associated infections: Road map to elimination. Washington D.C: 2013. pp. 194–239. [Google Scholar]
- 6.Smith PW, Bennett G, Bradley S, et al. SHEA/APIC guideline: infection prevention and control in the long-term care facility, July 2008. Infection Control and Hospital Epidemiology. 2008 Sep;29(9):785–814. doi: 10.1086/592416. Epub 2008/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel JD, Rhinehart E, Jackson M, et al. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. 2007;2007 doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regional Office for Western Pacific, Regional Office for South-East Asia. Practical Guidelines for Infection Control in Health Care Facilities. India: World Health Organization; 2004. [Google Scholar]
- 9.Association for Professionals in Infection Control and Epidemiology. Guide to elimination of methicillin-resistant staphylococcus aureus (MRSA) transmission in hospital settings. 2. Washington, D.C: 2010. [Google Scholar]
- 10.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infection Control and Hospital Epidemiology. 2010 May;31(5):431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 11.Aboelela SW, Saiman L, Stone P, et al. Effectiveness of barrier precautions and surveillance cultures to control transmission of multidrug-resistant organisms: a systematic review of the literature. American Journal of Infection Control. 2006 Oct;34(8):484–94. doi: 10.1016/j.ajic.2006.03.008. Epub 2006/10/04. [DOI] [PubMed] [Google Scholar]
- 12.De Angelis G, Cataldo MA, De Waure C, et al. Infection control and prevention measures to reduce the spread of vancomycin-resistant enterococci in hospitalized patients: a systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy. 2014 May;69(5):1185–92. doi: 10.1093/jac/dkt525. [DOI] [PubMed] [Google Scholar]
- 13.Morgan DJ, Diekema DJ, Sepkowitz K, et al. Adverse outcomes associated with Contact Precautions: a review of the literature. American Journal of Infection Control. 2009 Mar;37(2):85–93. doi: 10.1016/j.ajic.2008.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann C. How do patients experience isolation due to an infection or colonisation with MRSA? Pflege Z. 2006 Oct;59(10 suppl):2–8. Wie erleben Patienten die Isolierung wegen einer Infektion oder Kolonisierung mit MRSA? [PubMed] [Google Scholar]
- 15.Brooks E, Medina-Walpole A, Gillespie S, et al. Elimination of Contact Precautions for Nursing Home Residents Colonized With Multi-Drug Resistant Organisms: Substantial Cost Reduction and Improved Quality of Life. Journal of the American Medical Directors Association. 2014;15(3):B19-B. doi: 10.1016/j.jamda.2013.12.050. [DOI] [Google Scholar]
- 16.Kreman T, Hu J, Pottinger J, et al. Survey of long-term-care facilities in Iowa for policies and practices regarding residents with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2005 Oct;26(10):811–5. doi: 10.1086/502498. [DOI] [PubMed] [Google Scholar]
- 17.Stone PW, Herzig CT, Pogorzelska-Maziarz M, et al. Understanding infection prevention and control in nursing homes: A qualitative study. Geriatr Nurs. 2015 Mar 17; doi: 10.1016/j.gerinurse.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guest G. How Many Interviews Are Enough?: An Experiment with Data Saturation and Variability. Field Methods. 2006;18(1):59–82. doi: 10.1177/1525822x05279903. [DOI] [Google Scholar]
- 19.Donabedian A. Evaluating the quality of medical care. Milbank Quarterly. 1966 Jul;44(3 Suppl):166–206. [PubMed] [Google Scholar]
- 20.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005 Nov;15(9):1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 21.NVivo qualitative data analysis software. 10. QSR International Pty Ltd; 2012. [Google Scholar]
- 22.Seale C, Charteris-Black J. 27. Keyword Analysis: A New Tool for Qualitative Research. The SAGE Handbook Qualitative Methods Health Research; 2010. [Internet]. Available from: http://srmo.sagepub.com/view/sage-hdbk-qualitative-methods-in-health-research/n28.xml. [Google Scholar]
- 23.Microsoft. Microsoft Excel 14.4.4 ed. Redmond, Washington: Microsoft; 2011. [Google Scholar]
- 24.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007 Dec;19(6):349–57. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 25.American Medical Directors Association. Common Infections in the Long-Term Care Setting Clinical Practice Guidelines. Columbia, MD: AMDA; 2011. [Google Scholar]
- 26.Schora DM, Boehm S, Das S, et al. Impact of Detection, Education, Research and Decolonization without Isolation in Long-term care (DERAIL) on methicillin-resistant Staphylococcus aureus colonization and transmission at 3 long-term care facilities. American Journal of Infection Control. 2014 Oct;42(10 Suppl):S269–73. doi: 10.1016/j.ajic.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Furuno JP, Krein S, Lansing B, et al. Health care worker opinions on use of isolation precautions in long-term care facilities. American Journal of Infection Control. 2012 Apr;40(3):263–6. doi: 10.1016/j.ajic.2011.03.019. http://dx.doi.org/10.1016/j.ajic.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landelle C, Pagani L, Harbarth S. Is patient isolation the single most important measure to prevent the spread of multidrug-resistant pathogens? Virulence. 2013 Feb 15;4(2):163–71. doi: 10.4161/viru.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mor V, Caswell C, Littlehale S, et al. Changes in the quality of nursing homes in the US: A review and data update. Vol. 2009. American Health Care Association; 2009. Aug 15, Contract No. [Google Scholar]
- 30.Cohen CC, Herzig CT, Carter EJ, et al. State focus on health care-associated infection prevention in nursing homes. American Journal of Infection Control. 2014 Apr;42(4):360–5. doi: 10.1016/j.ajic.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.