SUMMARY
Dietary restriction (DR) attenuates many detrimental effects of aging and consequently promotes health and increases longevity across organisms. While over the last 15 years extensive research has been devoted towards understanding the biology of aging, the precise mechanistic aspects of DR are yet to be settled. Abundant experimental evidence indicates that the DR effect on stimulating health impinges several metabolic and stress-resistance pathways. Downstream effects of these pathways include a reduction in cellular damage induced by oxidative stress, enhanced efficiency of mitochondrial functions and maintenance of mitochondrial dynamics and quality control, thereby attenuating age-related declines in mitochondrial function. However, the literature also accumulates conflicting evidence regarding how DR ameliorates mitochondrial performance and whether that is enough to slow age-dependent cellular and organismal deterioration. Here, we will summarize the current knowledge about how and to which extent the influence of different DR regimes on mitochondrial biogenesis and function contribute to postpone the detrimental effects of aging on healthspan and lifespan.
Keywords: Dietary restriction, Caloric restriction, Carbohydrate restriction, Protein restriction, Mitochondrial function, OXPHOS, Mitochondrial biogenesis, Mitochondrial uncoupling, TORC1, Aging, Longevity, Healthspan, Lifespan
INTRODUCTION
Altered mitochondrial metabolism is a hallmark of aging [1]. Reduced respiratory capacity and increased oxidative stress associated with age have been reported in an array of tissues, including muscle and several brain regions [1]. The mitochondrial/free radical theory of aging explains many aspects of cell and organismal aging. This theory proposes that mitochondrial energy production decline and mitochondrial reactive oxygen species (ROS)-induced damage are the major causes of aging [2]. Although this is a longstanding theory, evidence supporting and contradicting its postulates, gathered from studies on diverse model organisms, accumulate in the literature [3-6]. Overall, these studies suggest that although oxidative damage is an important contributor, there is more to aging than damage mediated by ROS. On the other hand, preservation of mitochondrial functions and enhancement of mitochondrial biogenesis are critical components of several mechanisms promoting health and lifespan extension that are conserved from yeast to mammals [7-9].
Research efforts have been devoted to strategies for longevity extension, regarded as a retardation of biological aging. Although longevity extension strategies will not eliminate aging-related diseases, they are expected to postpone their age of onset, thus contributing to the objective of extending healthspan [10]. Calorie restriction (CR), which usually refers to a 20–40% reduction in calorie intake without malnutrition, is the most robust environmental intervention that slows aging and extends lifespan in yeast, worms, fruit flies, rodents, and perhaps also in primates [10-13], through largely conserved mechanisms.
Studies to understand the molecular mechanisms of CR-mediated longevity in simple research models, such as the yeast Saccharomyces cerevisiae, have allowed for the identification of several longevity genes and pathways. In yeast, CR down-regulates the conserved signaling pathways Ras/cAMP/PKA, TOR (target of rapamycin), and its major target the serine/threonine protein kinase Sch9, all of which integrate nutrient and other environmental cues to regulate cell growth, division, and lifespan [12]. Deletion of RAS2, TOR1 or SCH9 enhances cellular protection against thermal and oxidative stresses and extends yeast chronological lifespan (CLS) [14]. Inhibition of these pathways converges on the activation of stress resistance transcription factors that positively regulate the expression of cell protection systems; e.g. chaperones to combat protein misfolding, free radical scavengers such as catalase and mitochondrial superoxide dismutase (SOD2), and accumulation of store nutrients (trehalose and glycogen in yeast cells). The key components of these pathways also regulate stress resistance and lifespan in higher eukaryotes [15]. For example, both serine/threonine-specific protein kinases Akt and S6K (ribosomal protein S6 kinase), homologues of yeast SCH9, regulate lifespan in higher eukaryotes and inhibition of TOR/S6K signaling extends lifespan in worms, flies, and mice [16, 17]. Also, mice deficient in elements of the RAS pathway have extended health- and lifespan [18]. Together, these studies have highlighted an important role of nutrient-sensing pathways in lifespan modulation and CR-induced lifespan extension, suggesting a common evolutionary origin of aging regulation [14].
The longevity pathways play an important role in the regulation of mitochondrial biogenesis and/or function in yeast and higher eukaryotes including mammals [19, 20]. For example, deletion of the TOR1 gene extends yeast CLS in part by increasing mitochondrial mass and respiration [19] and by promoting adaptive mitochondrial ROS signaling [21]. The Ras/cAMP/PKA pathway senses excessive ROS to signal to the Hap2,3,4,5 transcriptional system and down-regulates mitochondrial biogenesis [22]. Also, in mammals, modulation of mitochondrial biogenesis and metabolism through the TOR, Akt1, and Ras pathways involves the peroxisome proliferator activated receptor gamma transcriptional coactivator-1α (PGC-1α) [20]. PGC-1α is a master regulator of mitochondrial biogenesis that coactivates the nuclear respiratory factors (NRF-1 and NRF-2), which induce the transcription of genes involved in mitochondrial biogenesis.
Numerous recent studies on model organisms and even in humans suggest that the health-promoting effect of CR interventions can be achieved by decreases in specific dietary components. Interventions such as protein restriction (PR), methionine restriction, and alternate day fasting while maintaining overall calorie intake, have outcomes similar to that observed following a CR diet. It is also becoming evident that the balance of nutrients such as carbohydrates, proteins, amino acids, fats, and minerals may play an essential role in regulating both lifespan and healthspan. Dietary restriction (DR) studies in yeast, fruit flies, rodents, and primates are beginning to clarify the dietary factor/s that cause/s the beneficial changes and the role of mitochondrial function in the mechanism/s involved.
The effects of DR on aging and healthspan have been covered by many recent reviews, focusing on simple model organisms or mammals [15, 23, 24], mostly on calorie restriction (CR) and less frequently on protein and amino acid restriction [15, 25]. In this manuscript, we will cover the information gained from exploiting yeast models of aging and will subsequently focus on studies performed in higher organisms to summarize the current knowledge about the influence of different DR regimes on mitochondrial biogenesis and function, the pathways involved, and the extent to which maintaining mitochondrial health through DR contributes to postpone the detrimental effects of aging on health- and lifespan.
1. DIETARY RESTRICTION, MITOCHONDRIA, AGING AND LONGEVITY IN THE YEAST SACCHAROMYCES CEREVISIAE
1.1. Mitochondrial determinants of yeast longevity
The facultative aerobe/anaerobe metabolism of the yeast Saccharomyces cerevisiae makes this organism a good model for mitochondrial studies. Over the last three decades, S. cerevisiae models of aging have contributed to the discovery of conserved longevity factors that modulate aging in mammals [26, 27]. Two models of aging, the replicative lifespan (RLS) model and the chronological lifespan (CLS) model, have been established in this organism. RLS is defined as the number of asymmetric mitotic divisions a mother cell can undergo prior to cell cycle arrest. This yeast mother-cell-specific aging constitutes a model of replicative aging as it occurs in fibroblasts, lymphocytes, or stem cell populations of higher eukaryotes [26-28]. CLS, defined as the capacity of postdiauxic, stationary (G0) cultures to maintain viability over time, is, on the other hand, a model for the aging process of postmitotic cells such as mature neurons and muscle cells [27, 29].
In yeast, mitochondrial function is important for both replicative and chronological lifespan, as mitochondrial oxidative phosphorylation (OXPHOS) function deteriorates and mitochondrial ROS generation amplifies with increasing age [29, 30]. During replicative aging, the changes the mother cell undergoes include an increased generation time, increased size, declined mating ability, nucleolar and mitochondrial fragmentation, and the accumulation of both extra-chromosomal rDNA circles (ERCs) and oxidatively damaged proteins [31, 32]. The effect of mitochondrial dysfunction on RLS may be not fully dependent of the OXPHOS capacity of the organelle, since the literature accumulates examples of mutations limiting respiration that either curtail or prolong RLS [30]. However, RLS is extended by enhancement of mitochondrial biogenesis [33], and mitochondrial function links RLS and CLS [34]. Additional parameters, such as proper mitochondrial segregation and inheritance [33], prevention of mitochondrial proteotoxic stress [35, 36], and maintenance of proper nuclear-mitochondrial communication through activation of mitochondrial retrograde signaling pathways [37] are important regulators of RLS. For example, a mechanism exists for retaining “less fit” mitochondria within the mother cell, thus preventing their transmission into the daughter cell [38]. Thanks to asymmetrical protein inheritance, daughter cells are able to eliminate ROS after completion of cytokinesis [39], ensuring that the new cell is born with a full replicative potential. Additionally, special mention also go to mitochondrial retrograde responses that have emerged as important players in aging and longevity [40], further emphasizing the importance of metabolic control in determining these processes. In yeast, the first described mitochondria-to-nucleus retrograde (RTG) pathway senses decline in mitochondrial membrane potential and initiates a transcriptional response to compensate for mitochondrial dysfunction by reconfiguring metabolism [37, 41, 42]. The RTG response increases the expression of genes involved in anaplerotic pathways that supply acetyl-CoA and citrate to mitochondria, including genes involved in peroxisomal biogenesis and fatty acid β-oxidation [37]. The TCA cycle is disrupted in respiratory-deficient yeast, and peroxisomal anaplerotic contributions become critical to maintenance of an adequate pool of α-ketoglutarate, essential for cellular redox maintenance and the generation of glutamate, a basic source of nitrogen [37]. Three proteins, Rtg1, Rtg2, and Rtg3, are required for the retrograde response in yeast [43, 44]. Rtg2 transduces the mitochondrial dysfunction signal, specifically the loss of mitochondrial membrane potential [42], and promotes the formation of the active heterodimeric Rtg1-Rtg3 transcription factor that translocates to the nucleus and binds to the retrograde response element in the promoters of retrograde response genes [37, 43, 44]. Activation of the RTG pathway has been shown to extend RLS in certain conditions [42]. Other organelles also influence mitochondrial fitness and overall replicative aging [45]. Vacuolar acidification decreases with replicative age and this parameter correlates with loss of mitochondrial membrane potential [45]. Thus, multiple factors influence mitochondrial quality control, ultimately affecting RLS.
Chronologically aging cells require mitochondrial respiration along their lifespan [46]. For CLS studies, yeast cells are usually aged in media containing 2% glucose. Under these conditions, cells divide exponentially producing energy preferentially by fermentation while respiration is repressed in a glucose-concentration-dependent manner. As glucose is being consumed, growth slows down and the diauxic shift occurs, which involves a shift from fermentation to respiration, the activation of stress resistance mechanisms, and the accumulation of nutrient stores (glycogen and trehalose) to be used later in the stationary phase in which the metabolic rate is significantly reduced. Mitochondrial respiration during exponential growth is essential for strains to achieve a standard wild-type CLS [47-49]. However, we have shown that yeast cells have a large reserve respiratory capacity to sustain CLS, as respiration only limits CLS when depleted below ~40% of wild-type threshold [7]. Strains that respire below the threshold during growth have extremely poor respiratory capacity in the stationary phase, rapidly consume their nutrient stores, since they produce energy by the inefficient fermentation pathway, and have very short CLS [7]. Respiratory defects are less detrimental when produced only in the stationary phase, once yeast have accumulated nutrient stores during growth and undergone their diauxic shift metabolic remodeling [7]. Several cell-intrinsic factors are involved in the requirement for a certain level of respiratory capacity to maintain wild-type CLS. First, aerobic energy production is required for the efficient function of anti-oxidative stress systems and for proper synthesis and accumulation of reserve carbohydrates, particularly trehalose. In fact, trehalose supplementation to the growth media enhances the stress-resistance capacity of respiratory deficient strains and significantly extends their CLS [7]. Consistently, a recent report has shown that deletions of genes in the trehalose biosynthetic pathway, including tsl1, tps3, and ath1 that increase intracellular trehalose concentration during growth and postdiauxic shift, extend CLS even in CR conditions by modulating cellular proteostasis throughout lifespan [50]. Mitochondrial respiration also controls aging and longevity through maintenance of mitochondrial membrane potential since activation of the RTG pathway is necessary for wild-type CLS [51]. Furthermore, mitochondrial respiration controls aging and longevity through generation of ROS, which represents another kind of mitochondrial retrograde response. Although mitochondrial ROS are known to limit the long-term survival of yeast cells during CLS and can have a detrimental effect during aging in higher eukaryotes, they can also act as signaling molecules with hormetic effects on longevity [5, 21]. For example, we have shown that, in addition to increased coupled respiration, TOR1 mutant strains also have enhanced ROS production during exponential growth, which provides an adaptive signal that contributes to increase cell protection systems and extends CLS [21]. The CLS of yeast strains can be curtailed by increased mitochondrial SOD2 levels, or can be significantly extended by additional exogenous ROS exclusively during growth, since ROS are harmful during stationary phase [7, 21].
1.2. Mitochondria-mediated effects of CR in yeast longevity
Although the determinants of CLS and RLS in yeast seem to be both overlapping and distinct [27], one of the features that are shared between the two aging models is a robust lifespan extension in response to dietary restriction. The most popular form of DR is calorie restriction, usually mimicked in yeast by reducing the glucose concentration in the media from 2% to 0.5% or lower, although restriction of one or several amino acids also extends both lifespans. The mechanisms responsible for the lifespan extension induced by CR are not yet fully understood but all models include a relevant role for mitochondrial functions.
Replicative lifespan
Early studies suggested that CR extends replicative lifespan due to enhanced mitochondrial respiration under low glucose levels [33]. Supporting this hypothesis, overexpression of HAP4, the catalytic subunit of the Hap2,3,4,5 transcriptional system that positively controls mitochondrial biogenesis and consequently shifts metabolism towards respiration, extends RLS [33]. Both interventions, CR and HAP4 overexpression, induce an increase in NAD+/NADH ratio, which serves to activate the NAD-dependent histone deacetylase Sir2 [52], which mediates rDNA silencing, reducing the generation of toxic rDNA circles. Consistent with this model, Sir2-dependent RLS extension can be achieved by overexpression of two components of the mitochondrial malate-aspartate NADH shuttle, malate dehydrogenase (Mdh1) and aspartate amino transferase (Aat1) [53]. Since this effect does not synergize with CR-induced lifespan extension, it was suggested that CR may extend lifespan by activating components of the mitochondrial NADH shuttles [53]. An alternative model suggested that CR may act through inhibition of the TOR pathway and the transcription factors Msn2/4, to increase the levels of the nicotinamidase Pnc1, thus activating Sir2 by decreasing the levels of its inhibitor, nicotinamide [54, 55]. However, the importance of Sir2 in lifespan extension by CR has been questioned since it is not reproducible in all yeast backgrounds [56]. Furthermore, comparison of CR effects on different yeast strains has indicated that respiration or Sir2 are not absolutely required for at least some the replicative longevity benefits of CR and that nicotinamide inhibits RLS extension by DR through a Sir2-independent mechanism [57]. Some of these experiments used either CR of 0.5% glucose, or extreme CR of 0.05% glucose, which could explain some of the differences in the results obtained [58]. However, more recent studies showed that the levels of rDNA silencing are not affected by CR; rather, CR reduces the level of internal DNA recombination at rDNA loci, in a Sir2-independent manner [59]. Although the CR mechanism is not fully clarified, there is at least general agreement that respiration confers full extension capacity by CR, and that, as explained in the previous section, some respiration-independent mitochondrial functions related to organelle quality control are essential for RLS extension by CR.
Chronological lifespan
CR modeled by growing yeast cells in 0.5% glucose, extends yeast CLS through a mechanism that involves: (i) inhibition of nutrient-responsive kinases such as the target of rapamycin (TOR-Sch9) and the Ras2–cAMP–PKA pathway [12, 15], (ii) general enhancement of stress-resistance mechanisms, (iii) maximization of ROS adaptive signaling, (iv) increased respiration during growth, (v) metabolic remodeling following the diauxic shift, and (vi) dramatic metabolic rate reduction in the stationary phase allowing for slow consumption of stored nutrients. Genetic interventions, such as deletion of tor1 (homolog of the mechanistic target of rapamycin -mTOR), sch9 (homolog of the ribosomal S6 kinase), or ras2, act as DR mimetics [56, 60]. These mutations seem to work through the same mechanisms as CR, but their effect on CLS is not as robust as CR itself. All of these interventions induce an increase in mitochondrial respiration and ROS adaptive signaling during growth, involving increased superoxide and hydrogen peroxide levels [61, 62]. However, while CR induces a Hap2,3,4,5 complex-mediated enhancement of mitochondrial biogenesis, TOR1 signaling-deficient strains have a higher density of mitochondrial respiratory-chain enzymes per organelle without global enhancement of mitochondrial biogenesis [19, 21]. Independently, mitochondrial respiration is essential for CLS extension by CR or tor1Δ, since their effect is abrogated by genetic and pharmacological interventions that eliminate respiration during growth [7]. CR leads to a 30% higher respiratory rate during growth [7, 63]. However, although CR-treated strains reach the stationary phase at a time similar to non-restricted cells, they barely respire once they reach this point [7]. This observation suggests that the metabolic remodeling program that begins with the entry of CR cells into the stationary phase alters the requirement of respiration during chronological aging. Consistent with this effect, pharmacological inhibition of respiration in CR cultures during the stationary phase does not affect CLS [7]. While both CR and tor1Δ enhance mitochondrial respiration during growth, their effect on stationary phase respiration is different. Contrary to what is seen during CR-treatment, tor1Δ strains are sensitive to OXPHOS inhibitors when supplemented in the stationary phase. Thus, CR-treated cells are less dependent on mitochondrial respiration during the stationary phase than are tor1Δ cells [7], consistent with the notion that inhibition of TOR1 is just one of the mechanisms contributing to the CR effect on CLS.
Notably, the requirement of respiration for CR-induced CLS follows a threshold pattern. Yeast that respire above a ~40%-of-wild-type threshold during growth can benefit from either CR- or tor1Δ-induced CLS extension. However, contrary to the effect on RLS, increased respiration during growth (e.g. by HAP4-overexpression) is not sufficient to extend CLS in strains that have maximized the CLS extension achievable by ROS signaling [7]. The relationship between respiration, ROS, and CLS is, however, complex. A comparison of yeast strains with different genetic backgrounds suggested a correlation between respiratory capacity, mitochondrial ROS generation, and CLS [7]. In general, strains with high respiration and ROS production have a longer CLS, which is curtailed by increased SOD2 levels, whereas strains with low respiration and ROS production have a shorter CLS and can significantly benefit from additional exogenous ROS or tor1Δ-induced endogenous ROS [21]. Our data support a model in which ROS signaling and the respiratory thresholds are complementary aging modulators that utilize two distinct mechanisms to achieve the same adaptive endpoint: increased stress resistance, efficient use of energy stores, and probably other beneficial effects in the stationary phase, all of which extend CLS.
1.3. Mitochondrial function links replicative and chronological lifespan and their extension by CR
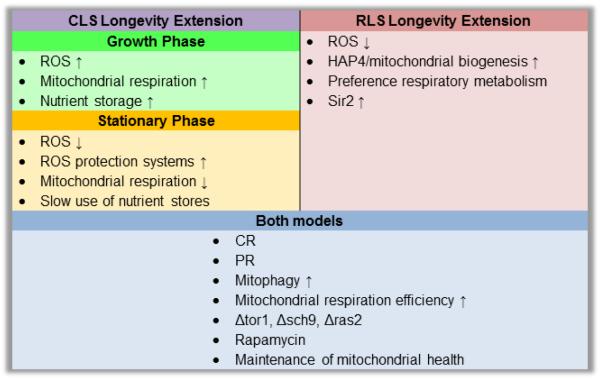
Yeast RLS and CLS are different in nature. However, some common basic mechanisms influence the aging process generally (Figure 1) since chronological aging has been shown to reduce RLS [34]. As explained earlier, both aging paradigms are associated with increased accumulation of mitochondrial damage and oxidatively damaged/aggregated proteins [26]. A recent study showed that DR during CLS maintains phenotypically “younger” cells and delays the reduction in subsequent RLS up to 23 days of chronological age [34]. Looking for a mechanism, the authors found that among the viable portion of the control population aged 26 days, individual cells with the longest subsequent RLS had the lowest mitochondrial membrane potential. These observations are in agreement with our data on the barely detectable respiration of CR-treated cells during stationary phase [7]. Additional pathways seem to converge at or depend on mitochondrial functions. For example, CR also induces autophagy via inhibition of mTOR [64], which would be expected to facilitate degradation of damaged mitochondria and other organelles, maintaining healthier mother cells at the moment when vegetative growth resumes. Another essential parameter is the accumulation of stored nutrients, whose increase during CR is dependent on mitochondrial respiration, particularly trehalose, as mentioned earlier. Relevant to the connection between CLS and RLS, trehalose is not only essential in enabling yeast cells to survive starvation conditions, but also in facilitating their rapid proliferation upon their return to favorable growth conditions by fueling cell-cycle progression [65].
Figure 1. Interventions that beneficially affect longevity within the two yeast models of aging.
Studies in S. cerevisiae have identified conserved longevity pathways, and may be important in elucidating how CR may differentially effect specific tissues through the CLS model, which mirrors the aging process of post-mitotic cells such as neurons and muscle cells, and the RLS model, which mirrors the aging of dividing cells in higher eukaryotes such as fibroblasts, lymphocytes, and stem cells. In this figure, the specific, as well as shared, interventions that lead to longevity extension in these two pathways are outlined. Interventions that affect yeast longevity in the CLS model are further divided by the phase of CLS in which they must be present to have beneficial effect.
Together, the data available demonstrate that CR modulates a common molecular mechanism linking mitotic and post-mitotic aging in yeast. Notably, mitochondrial function plays a critical role in this common aging mechanism.
1.4. Protein and amino acid restriction stimulate specific nutrient signaling pathways to increase longevity
Basic research with the goal of understanding the effects of individual dietary components on cellular and organismal response has shown that restriction of proteins (PR) or certain amino acids is the most effective single macromolecule restriction in the extension of longevity in cellular and animal models and reduced incidence and/or progression of multiple age-related diseases in mammals [25].
In the yeast S. cerevisiae, several nutrient-sensing pathways control growth and aging, as mentioned earlier [15]. Whereas the TOR1–Sch9 pathway is primarily activated by amino acids, the Ras2–cAMP–PKA pathway is stimulated predominantly by glucose [15]. Dietary restriction, either CR or PR inhibits these pathways, resulting in enhanced mitochondrial respiration, activation of the serine/threonine kinase Rim15 and consequently the activity of stress-sensitive transcription factors Msn2/Msn4 and Gis1, which control the expression of stress resistance systems that postpone the effects of aging and extend lifespan.
Like CR, amino acid restriction is sufficient to extend yeast lifespan [66, 67]. Shortage of amino acids results in accumulation of uncharged tRNAs and subsequent activation of the protein kinase GCN2, which facilitates metabolic adaptation leading to slow growth [66], and inhibition of the TOR1–Sch9 pathway, leading to enhanced stress resistance. Recent investigations have focused on identifying those specific amino acids that particularly affect lifespan. Methionine restriction and increased glutamic acid have both been shown to promote yeast longevity. The effect of methionine restriction has been proposed to be mediated by transsulfuration pathway (TSP)–dependent hydrogen sulfide (H2S) production, which induces stress resistance and extends longevity through a recently discovered mechanism involving mitochondrial functions [68]. In a different screen, supplementation of serine (through the Pkh1/2-Sch9 pathway), or threonine and valine (through the Tor1-Sch9 pathway), caused a strong sensitization of cells to oxidative stress in glucose-containing media, and promoted aging in these conditions [67]. Finally, leucine catabolism generates acetic acid, which can be converted to acetyl-CoA in mitochondria or the nucleus, thus affecting both mitochondrial function and the acetylation state of histones, and as a consequence, significantly impacting cellular longevity [67].
2. DIETARY RESTRICTION, MITOCHONDRIA, AGING AND LONGEVITY IN MULTICELLULAR MODEL ORGANISMS
2.1. Conservation of dietary restriction-induced pathways in higher eukaryotes
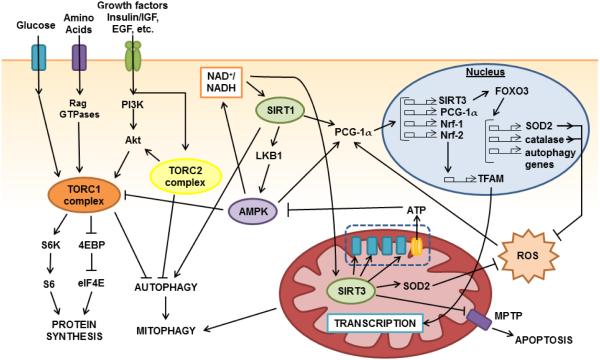
Studies in nematodes, flies, and rodents have established the evolutionary conservation of longevity factors and nutrient-sensing pathways identified in yeast. The molecular signaling pathways mediating the pro-health and anti-aging effect of DR in higher organisms include the insulin and insulin-like growth factor-1 (IGF-1), TOR, NAD+-dependent sirtuin deacetylase, AMP-activated protein kinase (AMPK), PGC-1α and mitochondrial retrograde pathways, which form an extensive, interacting network [15] (partially depicted in Figure 2). These pathways and their effect on regulating healthspan and lifespan will be summarized in this section.
Figure 2. Mammalian energy-sensing pathways implicated in dietary interventions.
A complex crosstalk exists between the energy-sensing pathways in mammals. The complexes of the mTOR pathway, TORC1 and TORC2 are activated by signals of high nutrient availability. TORC1 is stimulated by the presence of amino acids, growth factors including insulin, ATP, and glycolysis metabolites. TORC2 is also activated by growth factor signaling including insulin and EGF and further activates TORC1 through Akt. Together, TORC1 and TORC2 stimulate cell growth and proliferation through the inhibition of cell maintenance pathways such as autophagy, and the stimulation of global protein synthesis. Conversely, AMPK inhibits TORC1 and is activated by a low energy status in the cell. AMPK also contributes activate the sirtuins, including SIRT1 and SIRT3, and is in turn further activated by SIRT1. SIRT1 leads to the activation of PGC-1α. PGC-1α activation induces the transcription of many important genes involved in mitochondrial biogenesis and regulation, including Nrf-1, and Nrf-2, which induce expression of TFAM, and also further transcriptionally activates itself and SIRT3. Meanwhile, SIRT3 activates FoxO3, which induces the expression of key ROS antioxidants such as SOD2 and catalase as well as key genes involved in autophagy. SIRT1 further activates autophagic machinery by deacetylation of key atophagic components. Activation of autophagy can consequently activate mitophagy, leading to selective clearance of damaged or unfit mitochondria. In the mitochondria, SIRT3 also deacetylates many proteins including complexes of the electron transport chain, MPTP component cyclohpilin D, and SOD2, resulting in greater respiratory efficiency, apoptotic resistance, and ROS protection. Arrows indicate activation. T bars indicate suppression.
Insulin/IGF-1 signaling (IIS)
Different nutrients can activate different pathways directly or indirectly. In yeast, glucose stimulates the Ras2-cAMP-PKA pathway, whose pro-aging effect is neutralized by CR [15]. In higher eukaryotes, nutrients increase the level of insulin and IGF-1, which, in turn, activate pro-aging pathways and play contributory roles in diseases of aging, whereas CR counteracts their effects. The partial conservation and differences of the glucose or insulin/IGF-I–like pathways from yeast to mammals has been extensively reviewed [14]. In C. elegans, mutations that inactivate the insulin/IGF-I pathway result in increased thermotolerance and antioxidant defenses as well as longevity. These effects require activation of a stress resistance factor, the forkhead transcription factor Daf-16, which regulates the expression of a large number of genes involved in xenobiotic metabolism and stress resistance [69]. Overexpression of Daf-16 in worms or an ortholog in flies extends lifespan [69, 70]. In mammals, insulin and IGF-I bind to their respective receptors triggering a cascade of events including activation of phosphatidylinositol 3-kinase (PI3K) and serine–threonine protein kinases (Akt-1/Akt-2/protein kinase B [PKB]). FoxO family proteins are the mammalian homologs of Daf-16. When nutrients are available, binding of insulin or insulin-like molecules to their respective receptors leads to activation of PI3K and Akt and phosphorylation of Daf-16 in C. elegans and FoxOs in mammals. Phosphorylated FoxO factors are transported out of the nucleus. When insulin signaling is decreased (such as during CR), FoxO expression and nuclear compartmentalization are both increased, thus ensuring the buildup of robust anti-stress defenses. Mutations in mammalian genes that downregulate IIS increase longevity, such as in the case of dwarf mice, which harbor mutations in the pituitary growth hormone (GH) receptor, involved in regulating IGF-I release from the liver (reviewed in [14]). This lifespan extension effect has been linked to that induced by CR, with partially overlapping mechanisms [71]. Similar to TOR, IIS activates the downstream effector S6K, which plays a key role in the regulation of aging in worms and mammals [17]. S6K1 knockout mice display ameliorated age-related pathology and life span extension similar to those observed under CR. In addition, the loss of S6K1 increases AMPK activity, which further regulates the TOR pathway [17]. As discussed below, TOR kinase and AMPK are major upstream regulators of mitochondrial metabolism, a regulation that offers an essential contribution to mediate CR-induced longevity [72]. Interestingly, dwarf mice have increased mitochondrial respiration and increased metabolism per body weight, indicating that decreased GH signaling may beneficially affect mitochondrial flexibility by increasing the capacity for fat oxidation [73, 74]. Similarly, fat-specific insulin receptor knockout mice, which also have extended lifespan, have increased expression of PGC-1α and PGC-1β, enhanced mitochondrial gene expression, and boosted oxidative metabolism in white adipose tissue [75].
TOR signaling
First studied in yeast, the amino acid sensing TOR kinase pathway also mediates DR-induced longevity in worms, flies, and mammals. Lifespan extension by inhibition of the TOR pathway is nutrient dependent in both Drosophila [76] and yeast [56], establishing the role of TOR in mediating the effects of DR. In yeast, as well as in mammalian cells, TOR kinase exists in two separate multi-protein complexes, designated TORC1 and TORC2, which have different biological functions. In mammals, however, there is a single TOR gene that functions in both the TORC1 and TORC2 complexes, while in S. cerevisiae there are two distinct TOR kinase genes [77]. TORC1 induces protein synthesis and becomes active only when an adequate supply of amino acids is available within the cell, particularly leucine [78]. TORC1 activity is stimulated by signals of energy availability including ATP, insulin, and growth factor signaling and is inhibited by signals of cellular stress including ROS, ER stress, and hypoxia [77]. Dysregulation of TORC1 signaling plays a major role in many cancers as well as many diseases associated with aging [79]. Like TORC1, TORC2 is stimulated by the availability of nutrients and growth factors, although through lesser-known mechanisms. TORC2 functions as a key regulator of pathways involved in cytoskeletal maintenance and growth as well as cellular metabolism [77]. Both TORC1 and TORC2 inhibit autophagy, with TORC1 known to specifically interact with the autophagy initiation complex [77, 79]. Thus, it may be assumed TOR signaling may also inhibit the selective degradation of damaged mitochondria through mitophagy since both processes share the same initiation mechanisms. Upstream activators of TORC1 include the insulin/PI3K/Akt-signaling pathway, stimulated when circulating insulin increases in response to high glucose availability. Activation of the pathway leads to the phosphorylation and activation of Akt, which ultimately leads to an increase in TORC1 through a release of inhibition. Additionally, TORC1 can be activated by other growth factors, such as epidermal growth factor (EGF), which signal through the Ras/MAPK/ERK pathway [77]. Downstream targets of TORC1 include eukaryotic translation initiation factor 4E-binding protein (4E-BP) and S6K, both of which function to increase global protein synthesis. TORC2 activates Akt, further stimulating TORC1 activity and inhibiting the activity of FoxO3 [79]. The intricacies of TOR signaling have been extensively reviewed [77, 79].
Accumulating evidence suggests that TOR kinase can also regulate mitochondrial mass and function in higher eukaryotes as it has been observed in yeast, although the effects may be different. In Drosophila, 4EBP1 extends lifespan upon dietary restriction by translationally upregulating mitochondrial OXPHOS components specifically, therefore enhancing mitochondrial activity, as measured in whole animal homogenates [76]. Similar to what has been observed in yeast tor1 knockouts [19], increased mitochondrial respiration upon TORC1 ablation (raptor KO in adipose tissue) has also been seen in mice [80]. However, TORC1 inhibition in mammalian cells appears to reduce mitochondrial function, suggesting that TORC1 is important for maintenance of mitochondrial OXPHOS. Furthermore, mammalian TORC1 (mTORC1) appears to play a role at multiple levels to regulate mitochondrial function. mTORC1 is involved in controlling mitochondrial biogenesis through a yin-yang 1 (YY1)/PGC-1α transcriptional complex to balance energy metabolism through transcriptional control of mitochondrial oxidative function [81]. mTORC1 is also involved in direct regulation of mitochondrial activity via phosphorylation of specific proteins, regulation of uptake and utilization of carbohydrates to balance glycolytic flux with mitochondrial respiration, and regulation of mitophagy [82]. Maintenance of mitochondrial homeostasis involves feedback of mitochondrial function on TORC1, in order to adjust mitochondrial biogenesis to nutrient availability. In this respect, TORC1 activity is regulated by ROS. Although many effects of ROS on TORC1 activity are cell-type specific, they seem to be concentration dependent: TORC1 is induced by low levels of ROS, while mid-range and high levels of ROS inhibit TORC1 activity [82].
TORC2 could also play a distinct role linked to mitochondrial function. Activated TORC2 has recently been found to localize to specialized areas of mitochondria-associated endoplasmic reticulum (ER) membranes (MAMs), contributing to mitochondrial and ER calcium release/uptake regulation, and therefore affecting apoptosis resistance [83].
Sirtuins
Sirtuins are directly linked to nutrient and metabolic signaling through their requirement of NAD+ as a cofactor. Sirtuins function as crucial regulators of many protein post-translational modifications within numerous cellular pathways, suggesting that sirtuins may be capable of conveying widespread functional changes with altered nutrient availability [84]. Support for sirtuins as possible mediators of the benefits of CR first came from yeast, in which knockout of SIR2, the lone yeast sirtuin gene, was found to eliminate lifespan extension by CR [85]. Additionally, overexpression of SIR2 orthologs has been shown to convey lifespan extension in yeast, worms, flies, and mice [84, 86]. Mammals possess seven sirtuin proteins (SIRT1-7) each with distinct cellular localizations and functions. Of these, nuclear- and cytoplasmic-localized SIRT1 and mitochondrial-localized SIRT3 deacetylases have been most extensively studied for their possible roles in the benefits of CR [84]. For example, SIRT1 expression increases during CR in both mice and humans and declines under high fat diet or obesity [86]. Studies in mice have also shown that SIRT1 overexpression conveys similar beneficial health effects as CR and protects various markers of health upon diet-, injury-, and disease-related stressors [84]. Protein pathways activated by SIRT1 deacetylation include PGC-1α-regulated mitochondrial biogenesis, AMPK signaling, and autophagy, discussed in more detail below and reviewed in [84]. Studies of SIRT1 overexpression have failed to find any significant lifespan extension, suggesting that SIRT1 activity may not be the major mediator of this beneficial effect of CR. However, a recent report has proposed that the age-dependent mitochondrial OXPHOS activity decline observed in mouse skeletal muscle is due to reduced SIRT1 activity, which induces alterations in expression of nuclear genes including mitochondrial transcription factor A (TFAM) [87]. Intriguingly, although TFAM expression is known to be stimulated by PGC-1α, the SIRT1-PGC-1α pathway is not involved in the mechanism described. Rather, the mechanism involves SIRT1-dependent downregulation of the von Hippel-Landau protein (VHL), a negative regulator of the hypoxia inducing factor alpha (HIF-1α), which is stabilized and in turn inhibits the ability of c-Myc to activate the TFAM promoter [87]. Further verification of this model is warranted. Additionally, the same report showed that the age-dependent mitochondrial OXPHOS dysfunction, and several features of muscle pathology, could be reversed by activating SIRT1 enzymatic activity via increasing NAD+ levels by treatment with nicotinamide mononucleotide (NMN).
The mitochondrial deacetylase SIRT3 has been also shown to be relevant to aging. Like SIRT1, increased activation of SIRT3 is seen after multiple DR regimens and decreased SIRT3 activation occurs under high fat diets [88]. Loss of SIRT3 results in robust mitochondrial protein hyperacetylation [89], while CR induces deacetylation of a wide range of mitochondrial proteins [89]. Importantly, most of the mitochondrial proteins with decreased acetylation after CR show hyperacetylation in SIRT3 knockouts [89], suggesting that SIRT3 is a chief mediator of this post-translational modification in mitochondria. Furthermore, changes in mitochondrial protein acetylation following CR were much more robust than changes in the expression levels of mitochondrial proteins [89], further supporting post-translational modification through SIRT3 as a possible key mediator of CR effects. SIRT3-mediated protein deacetylation has numerous effects on mitochondria. For example, SIRT3 directly deacetylates complex I and II of the mitochondrial electron transport chain (ETC), enhancing the activity of the complexes and increasing ATP production [90]. Complex IV activity is also reduced in the absence of SIRT3 [91]. Additionally, cellular ROS levels are regulated by SIRT3 with increased ROS levels upon SIRT3 inhibition, and reduced ROS levels with SIRT3 overexpression [91]. One mechanism through which SIRT3 has been shown to reduce intracellular ROS is through the deacetylation and activation of the forkhead transcription factor FoxO3, which leads to the increased translation of antioxidant proteins including SOD2, catalase, and peroxiredoxin III [92, 93]. SIRT3 also deacetylates SOD2 directly leading to enhanced SOD2 enzymatic activity and decreased intracellular ROS [94, 95]. Additionally, mitochondrial apoptosis induction is inhibited by SIRT3 through the SIRT3-mediated deacetylation of cychlophilin D, a key component of the mitochondrial permeability transition pore (MPTP) [96]. Interest in the role of SIRT3 in aging, DR, and mitochondrial function is likely to grow in the future, since, in addition to the many known targets of SIRT3 [97], many more targets are likely to exist as the majority of mitochondrial proteins have recently been predicted to have acetylation sites likely acted upon by SIRT3 [89].
AMPK
The AMPK energy-sensing pathway is highly conserved within eukaryotes. The yeast AMPK homolog is Snf1 kinase, found as part of a transcriptional complex (SNF1 complex) required for the transcription of glucose-repressed genes. Essentially, yeast cells attain energy homeostasis through complex regulatory events that are predominantly controlled by Snf1 kinase. This major regulator senses stress and energy starvation and responds by activating metabolic processes to produce ATP and by inhibiting biosynthesis, thereby controlling the switch between catabolism and anabolism [98]. Sfn1 plays an important role in lifespan regulation. For example, constitutive genetic coactivation of the mitochondrial RTG response and the Snf1 pathway reprograms energy metabolism to increase the supply of acetyl-CoA to lysine acetyltransferases and extend the CLS of cells devoid of mitochondrial DNA (ρ0 cells) [99]. Furthermore, enhanced Snf1 kinase activity by either overexpression of SNF1 or through double-deletion mutants of the SNF1 repressors Mig1 and Hxk2, significantly curtails CLS. Similarly, increased Snf1 activity, through deletion of repressor subunit SIP2 or overexpression of the SNF1 gene, also decreases yeast RLS [100].
In C. elegans and Drosophila, overexpression of wild-type or constitutively active AMPK as well as AMPK upstream activators has been seen to consistently extend lifespan [101]. AMPK promotes healthy aging in part from its ability to integrate multiple signaling and transcriptional pathways known to promote longevity. However, frequent feedback regulation within the network creates a challenge in defining important linear pathway components. In contrast to the TOR signaling pathway, AMPK is activated when cellular energy stores become depleted, sensed through minute increases in the AMP/ADP:ATP ratio. AMPK activation then acts to restore cellular energy through the inhibition of energy-consuming processes such as the synthesis of proteins, glycogen, and fatty acids combined with the activation of energy-producing pathways such as glucose uptake, glycolysis, and fatty acid β-oxidation [101]. In accordance with the contrasting roles of AMPK and TOR activation, there is considerable crosstalk between the two pathways. Activated AMPK acts to inhibit TORC1 at multiple levels through direct as well as upstream inhibition [77]. Additionally, S6K, a downstream target of TORC1 has recently been found to phosphorylate and inactivate AMPK [101] Therefore, inhibition of TORC1 can further enhance the activation of AMPK. Furthermore, AMPK activation also leads to an increase in autophagy, while TORC1 and TORC2 are implicated in reduced autophagy. On the other hand, AMPK has been shown to act in a positive feedback loop with both SIRT1 and SIRT3 by increasing the NAD+:NADH ratio [102], leading to sirtuin activation. Activation of both SIRT1 and SIRT3 then leads to the deacetylation and activation of the upstream activator of AMPK, LKB1, resulting in increased AMPK activation [88]. SIRT1 and AMPK further act together to activate PGC-1α through respective deacetylation and phosphorylation [102, 103], therefore regulating mitochondrial biogenesis and cellular bioenergetics as explained below. For a review of AMPK signaling, see [101].
PGC-1α
In metazoans, PGC-1α, considered the master regulator of mitochondrial biogenesis [24], belongs to a family of co-activators that are activated by fundamental cellular signals that control energy and nutrient homeostasis, such as cAMP and cytokine pathways [104].
PGC-1s are not found in yeast, where mitochondrial biogenesis requires two heme-regulated transcriptional activators, the Hap1 protein and the HAP complex (Hap2/3/4/5) [105, 106]. Whereas Hap1 mediates only the oxygen regulation of aerobic genes and its activity is modulated by heme binding [107], the HAP complex mediates the regulation in response to both, oxygen concentration and carbon source availability, and senses heme through its catalytic subunit (Hap4)[108]. The genes regulated by the HAP complex are repressed by glucose even in the presence of oxygen/heme [108]. During the diauxic shift, nuclear genes involved in oxidative metabolism are de-repressed, resulting in enhanced mitochondrial biogenesis. As explained earlier, HAP4 overexpression extends yeast RLS. Additionally, the HAP complex is also activated during CR, thereby contributing to inducing RLS and CLS extension. Mutations in hap1 also negatively affect CLS [7] .
In metazoans, once PGC-1α is activated, it induces and coordinates gene expression regulating many metabolic and cell fate decisions, including the stimulation of mitochondrial oxidative metabolism in many tissues, fiber-type switching in skeletal muscle, and multiple aspects of the fasting response in liver, among other functions [104]. PGC-1α achieves regulation through specific interaction with a variety of transcription factors, including nuclear respiratory factors NRF1 and NRf2, which stimulate genes associated with oxidative metabolic pathways [109] including those genes involved in mitochondrial respiration and mitochondrial DNA expression. The control of mitochondrial biogenesis by PGC-1α, initially observed in brown fat has been observed in most tissues, including skeletal muscle and brain [109]. PGC-1α and β are induced by oxidative stress and the ability of ROS to induce a ROS scavenging program (including mitochondrial SOD2 as well as cytoplasmic proteins such as catalase and GPX1) depends on the PGC-1s. Cells lacking PGC-1α are hypersensitive to oxidative stress. Mice deficient in PGC-1α are viable but have a marked reduction in the mRNA levels of genes coding for mitochondrial proteins in all tissues examined. These mice undergo excessive neurodegeneration when given pharmacological agents that cause Parkinsonism. These data show that the PGC-1s are not only key modulators of mitochondrial biology but also important protective molecules against ROS generation and damage [109].
The role of PGC-1α in CR and other models of longevity has been recently reviewed [110] and was discussed as a target of the different pathways mentioned earlier. An important question is whether enhancement of mitochondrial biogenesis in a PGC-1α-mediated manner is sufficient to extend healthspan and lifespan. In this regard, enhanced mitochondrial homeostasis mediated by tissue-specific overexpression of Drosophila PGC-1α is sufficient to extend lifespan in flies [111]. Importantly, while ubiquitous PGC-1α overexpression enhanced mitochondrial biogenesis and induced accumulation of glycogen, it also moderately shortened lifespan, suggesting that these outcomes might have adverse effects in select tissues. On the contrary, enhancing PGC-1α specifically in intestinal stem cells enhanced coupled mitochondrial OXPHOS and reduced ROS generation in cells from this high-turnover tissue, leading to improved tissue maintenance and promoting organismal longevity [111]. These studies are reminiscent of the observations made in yeast, where HAP4 overexpression extends RLS lifespan but not CLS. In mammals, PGC-1α levels have been shown to decline with age, and to be restored by CR [112]. Overexpression of PGC-1α has proven to be beneficial in some mouse models of Huntington’s disease and other neuromuscular degenerative diseases [113]. However, although moderate enhancement of mitochondrial biogenesis can be beneficial in some disease scenarios, it has not been shown to extend longevity. Furthermore, excessive PGC-1α levels can be deleterious in some tissues such as skeletal muscle or heart, indicating that mitochondrial biogenesis needs to be carefully adjusted, perhaps as it occurs during CR, to achieve the desired health benefits [114].
Mitochondrial retrograde pathways
The originally described yeast mitochondrial-to-nucleus RTG signaling pathway does not seem to be exactly conserved in higher eukaryotes, although it has been demonstrated in yeast crosstalk between the retrograde response and the conserved TOR1 and RAS-cAMP-PKA signaling pathways, both of which are key players in yeast RLS and CLS and also in metazoan aging [40].
Furthermore, a variety of mitochondrial retrograde signaling processes (broadly defined as signaling from the mitochondria to the rest of the cell) have been identified in yeast and higher eukaryotes (reviewed in [115]), some of which will be briefly discussed here. CR, inhibition of IGF1 signaling, and inhibition of TOR signaling promote longevity at least in part by eliciting mitochondrial retrograde signaling processes. From yeast to human, these prolongevity interventions enhance mitochondrial respiration and consequently mitochondrial ROS production. ROS can act as adaptive signaling molecules by activating pathways that promote cellular resistance to various stresses, as explained earlier for yeast tor1 mutants [21]. These observations align with the concept of “mitohormesis”, in which low-levels of stressors, for example during DR, (i.e. reactive oxygen and nitrogen species due to enhanced overall mitochondrial OXPHOS activity and/or mitochondrial fatty acid oxidation) promote adaptive changes resulting in stress resistance [5]. The reader is directed to a full article in this BBA Special Issue, dedicated to “mitohormesis” (REF).
Mitochondrial stress, mitonuclear protein imbalance, and general loss of mitochondrial fitness also trigger the mitochondria-specific unfolded protein response (UPRmt) [116, 117], a mitochondria-nuclear signaling pathway that induces the expression of mitochondria-protective molecular chaperones and other regulators of mitochondria homeostasis. In C. elegans, the UPRmt significantly contributes to the transduction of mitochondrial respiratory chain alteration based extension of lifespan [118]. Interventions such as CR and inhibition of TOR signaling generate mitonuclear imbalance and therefore also elicit the UPRmt, which contributes to longevity mechanisms [119]. A full article in this BBA special issue is dedicated to the UPRmt, how it is activated, and crosstalks with other anti-stress pathways to afford cytoprotection in the context of organismal aging [120].
The effects of mitochondrial retrograde signaling may even spread to other cells/tissues in a cell-non-autonomous manner by yet unidentified signaling mediators that have been termed “mitokines” [118]. These mitokines could then, in theory, help coordinate the cellular metabolic state and longevity of the entire organism. UPRmt has additionally been implicated in this signaling mechanism in C. elegans, as mitochondrial functional deficits in neuronal cells have been seen to activate UPRmt in distal intestinal tissue [118].
An emerging new class of mitochondrial signals capable of regulating aging and lifespan are the mitochondrial-derived peptides (MDPs), encoded by functional short open reading frames (sORFs) in the mtDNA [121]. Among them, Humanin (HN) is a recently identified neuroprotective and antiapoptotic peptide derived from a portion of the mitochondrial 16S ribosomal RNA, MT-RNR2 gene [122]. HN is found in both tissues and plasma and has been seen to decline with age in both rats in humans, suggesting that this small peptide may play a role in longevity [123, 124]. Similarly, MOTS-c is a MDP derived from the open reading frame of the mitochondrial 12S RNA that has been found to be able to systemically alter metabolism through downstream effects on the AMPK signaling pathway [125]. However, despite these recent advances, the mechanisms of mitochondrial retrograde signaling in multicellular organisms remains to be fully understood.
2.2. DR mimetics, aging, and mitochondria
Studies on the molecular mechanisms underlying the beneficial effects of DR have identified targets for natural or pharmacological interventions that could reproduce the beneficial effects in humans without the necessity to comply with a rigorous dietary program, particularly late in life. Likely candidates act on the same signaling pathways as DR, and include resveratrol and other polyphenols, rapamycin, insulin and AMPK pathway activators, autophagy stimulators, alpha-lipoic acid, and perhaps other antioxidants. Studies on some of these interventions are discussed below.
Sirtuin activators
Produced by grapes, berries, and peanuts and found in relatively high concentrations in red wine, the natural polyphenol resveratrol gained widespread interest as a possible mediator of numerous beneficial health effects including a reduced risk for cancer and cardiovascular disease and improvements in metabolic fitness in mice and monkeys fed a high-fat diet (reviewed in [126]). Resveratrol also increases lifespan in C. elegans and Drosophila, although the robustness of these effects has been questioned [126]. Resveratrol was first found, however, to be able to extend RLS in yeast. It was proposed to act through the same mechanism as CR, by activating Sir2 [127]. However, the involvement of Sir2 in the mechanism of action of resveratrol has been challenged [128] and it has been suggested that the physiological effects of resveratrol could be mediated by alternative targets. In mice, recent data suggests that the mechanism of action of resveratrol could be dose-dependent. Accordingly, resveratrol may act through SIRT1 only at low doses but may also activate AMPK across concentrations to produce beneficial effects on mitochondrial function though activation of PGC-1α and subsequent enhancement of mitochondrial biogenesis, oxidative phosphorylation, and fatty acid oxidation [129]. In addition, frequent feedback loops exist among these pathways, with SIRT1 being able to activate AMPK and therefore activate the same downstream effects [129].
TOR inhibitors
Rapamycin, an inhibitor of TOR signaling across species, is a robust DR mimetic. Rapamycin has been shown to consistently increase lifespan in many model organisms from yeast to mammals (reviewed in [130]). In yeast, rapamycin induces comparable effects on mitochondrial function and longevity as the tor1 null mutation. While rapamycin does not seem to enhance mitochondrial biogenesis, rapamycin increases the density of OXPHOS complexes and increases coupled respiration and ROS during growth, preconditioning the cell and ultimately leading to CLS extension. In Drosophila, rapamycin increases mitochondrial oxidative capacity while simultaneously reducing mitochondrial ROS production, similar to what is seen in CR [131]. In mammals, rapamycin is a potent inhibitor of TORC1, and also of TORC2 at high doses or chronic treatments [132]. Rapamycin treatment drastically attenuates the detrimental effects of both high protein and high fat diets in mice [133]. Specifically, in mice a significant decrease in the amount and function of muscle mitochondria was seen following a life-long high-protein diet, which was reverted by chronic treatment with rapamycin [133]. Inhibition of mTOR by rapamycin also activates selective degradation of unfit mitochondria through mitophagy, a process critical for mitochondrial quality control. Mitophagy is essential for healthy aging since it has the potential of eliminating mitochondrial genomes carrying OXPHOS-disabling mutations [134].
AMPK agonists
Many classical agonists activate AMPK indirectly by altering the AMP/ADP:ATP balance in the cell and act as DR mimetics. The list includes 5-aminoimidazole-4-carboxamide-1-D-ribo-furanoside (AICAR), which cells convert to an AMP analog (ZMP), and metformin, a mitochondrial respiratory chain complex I inhibitor. Although these compounds can promote longevity in some settings, particularly in C. elegans and mice, conflicting results have been reported in other model organisms. In mice, AICAR induces a PGC-1α-mediated increase in mitochondrial biogenesis and a 4-week treatment of AICAR can confer comparable benefits as endurance training to sedentary mice in terms of both muscle mitochondrial profiles and actual treadmill endurance (reviewed in [101]). Regarding metformin, several reports suggest similarities between its actions and the effects of CR. As such, microarray analyses have shown that metformin induces a gene expression profile that overlaps more than 85% with that of DR [135]. In mammals, the beneficial effect of metformin on health (diabetes and cancer in human) is more established than its effects on longevity [101]. However, although research is advancing on the physiological benefits of metformin, its exact molecular target/s remain to be fully identified. The proposed targets include activation of mitochondrial respiratory chain complex I, AMPK, and SIRT1. In addition, metformin has recently been reported to inhibit mTOR, thereby suggesting a potential overlap in the signaling pathways induced by both metformin and rapamycin [101].
2.3. Conservation of mitochondrial mechanisms of dietary restriction-induced extension of healthspan and lifespan
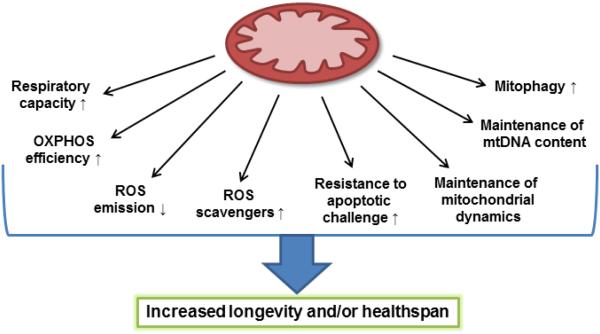
As in yeast, mitochondrial function declines during aging in higher eukaryotes from worms to mammals, including humans (reviewed in [72]). The mitochondrial alterations affect morphology, mitochondrial mass, mitochondrial DNA (mtDNA) copy number, and OXPHOS enzyme function (Figure 3), sometimes in a tissue-dependent manner. Most of these alterations are prevented by DR regimes. In this section we will briefly discuss the mitochondria-mediated effects of DR on health, aging, and longevity of higher organisms.
Figure 3. DR-induced alterations in mammalian mitochondrial function implicated in longevity extension.
Representation of mitochondrial-functional alterations that are most conserved across dietary interventions in mammals in which increased health or longevity has been reported.
Mitochondrial biogenesis
As explained in the previous section, several of the pathways believed to be responsible for the beneficial effects of DR lead to the activation of PGC-1α and the consequent enhancement of mitochondrial biogenesis. However, it remains a very controversial issue whether or not DR itself leads to increased mitochondrial biogenesis through the activation of PGC-1α, particularly in mammals. A report indicated that 30% calorie restriction (food provided in alternate days) for either 3 or 12 months in 8-month-old male mice raised nitric oxide (NO) levels in liver, muscle, and brain. This increase in NO levels was found to be the mediator of enhanced SIRT1 expression and PGC-1α activation resulting in increased mitochondrial biogenesis, oxygen consumption, and ATP production in these tissues. [9]. In the same line, primary hepatocytes from 12-month-old rats fed 40% CR since weaning also had increased SIRT1 leading to boosted stress resistance, and enhanced PGC-1α activation leading to increased mitochondrial biogenesis and improved bioenergetics [112]. In this study, CR was found to produce very efficient mitochondrial electron transport based on low-potential mitochondria that sustain reduced oxygen consumption while maintaining cellular ATP levels and reducing ROS production [112]. Furthermore, a decline in mtDNA content and mitochondrial transcription factor A (TFAM) protein levels with age has been observed in several mice tissues, in most of which the depletion is completely prevented by CR [136], although it was not reported whether overall mitochondrial biogenesis had been enhanced. However, new reports question whether CR truly increases mitochondrial biogenesis. The increased mitochondrial mass observed in response to 30% CR in mice was not detected in similar studies in rats [137], nor in 40% CR mice in which PGC-1α mRNA levels increased without a concordant increase in PGC-1α protein levels or mitochondrial protein synthesis [138]. Adding to this line of observations, it was reported that chronic CR in mice preserves mitochondrial function in senescence without increasing mitochondrial biogenesis, as shown by transcriptome and functional analyses, but by enhanced mitochondrial efficiency, decreased mitochondrial oxidant generation, and upregulated antioxidant defenses [139]. Combined, these studies indicate that independently of the mechanism involved, CR preserves mitochondrial content and function. To reconcile the different results on mitochondrial biogenesis, the possibility exists that CR-activated PGC-1α signaling may be tissue-specific. In fact, a recent report proposed that PGC-1α does not necessarily regulate basal mitochondrial biogenesis, but rather is involved in increasing OXPHOS enzyme density and mitochondrial function when required, by activating the expression of certain nuclear genes in a tissue-dependent manner [140].
Respiratory efficiency and ROS generation
Despite the controversy regarding CR-mediated enhancement of mitochondrial biogenesis, it seems widely accepted that CR improves OXPHOS efficiency and reduces ROS, favoring healthy aging. Additionally, several studies [141] have shown that long term CR decreases metabolic rate in rodents as it does during yeast CLS, thus further minimizing ROS generation. Accordingly, CR mice have lower levels of oxidized and deamidated proteins and of oxidatively damaged DNA [139]. Upregulation of antioxidant defenses [139], as part of the overall upregulation of cellular and molecular defense systems during CR, also accounts for CR-induced reduction of intracellular ROS. In yeast and flies, CR enhances the levels of catalase and SOD2. Also in mice, CR prevents the decline of these enzymes and peroxiredoxin III with age, although some tissue-dependent variations have been observed [136, 139]. Interestingly, a study evaluating the effects of 20% CR in humans found significantly reduced oxidative DNA and RNA damage in white blood cells, indicating that ROS levels may also decrease in humans under CR [142].
Respiratory thresholds
Energy and respiratory thresholds have been described in human mitochondrial physiology and disease as respiratory enzymes can be inhibited to varying degrees before observing an OXPHOS phenotype [143]. Respiratory thresholds to support cellular life probably vary for every tissue, most likely altering the importance of other factors, like ROS signaling, in a tissue-specific manner. Respiratory deficiencies are associated with aging and age-related disorders [72]. Thus it is tempting to speculate that interventions mimicking CR (or modulating nutrient sensing pathways) might lower the energetic thresholds of critical, affected tissues which, if accompanied by an enhancement of cellular protection systems, could extend healthspan in normal and diseased individuals suffering from age-associated disorders.
Nutrient stores
Accumulation and efficient mobilization of nutrient stores, one of the hallmarks of yeast CLS, and dependent on mitochondrial functions as explained earlier, is also relevant to stress responses across species. Throughout the course of evolution, factors such as limiting resource availability were most likely instrumental in driving adaptations that, among other effects, were capable of enhancing survival during periods of environmental pressure by impinging upon behavior and metabolic changes that improved survivability. While yeast cells accumulate carbohydrates (glycogen/trehalose), mammals can accumulate glycogen and increase fat mass as a stress response to the uncertainty of food availability. Therefore, a conserved response to starvation stress is to accumulate carbon sources that would maximize long-term survival and to utilize them efficiently later in life [6]. Worth mentioning, one of the effects of CR in mammals is the elevation of liver insulin sensitivity by the hormone adiponectin, which lowers endogenous glucose production, diminishes reliance on glucose and its metabolites for energy, and raises fatty acid oxidation [144]. This switch in substrate utilization can reduce ROS generation at mitochondrial respiratory chain complex I and therefore, minimize oxidative stress. Although it is tempting to speculate that stimulating fatty acid utilization could increase longevity, this may be true only under CR. In fact, high fat diets and dependence on fatty acid oxidation for production of energy by the mitochondria drives insulin resistance, diabetes, and cardiovascular disease [144].
2.4. Conservation of longevity pathway selectivity to respond to different dietary restriction regimens
Accumulating data in animal models as well as in humans suggest that, as in yeast, not only CR but also restriction or supplementation of individual dietary nutrients may have beneficial effects on health- and lifespan (reviewed in [23, 25]). As an example, in this section we will summarize the current knowledge on the contribution of protein and amino-acid restriction to the beneficial effects of DR regimes and will highlight the emerging significance of mitochondrial functions on the mechanisms involved.
Studies in worms have linked protein and amino acid intake to activation of pro-aging and disease-promoting pathways and have highlighted the beneficial effects of diets restricting these nutrients. For example, as in yeast, GCN2, the evolutionarily conserved kinase responsive to amino acid deficiency, mediates lifespan extension under DR conditions by converging on TOR/S6K signaling via FoxA transcription factors and downstream target genes [145]. In another example, AMPK/Aak-2 and the forkhead transcription factor FoxO/Daf-16 are necessary for longevity induced by a particular low-protein PR regimen (dilution of peptone, a protein source) [146]. Studies in Drosophila have highlighted the relevance of the ratio between proteins and carbohydrates (P/C) on determining longevity, with lower P/C ratios (1/16) extending lifespan [147] in a TOR signaling-dependent manner. As a result of TORC1 inhibition, amino acid restriction in flies resulted in overall reduction in relative intracellular translation rate but an increase in the translation of genes involved in important mitochondrial processes, such as OXPHOS enzyme subunits and mitoribosomal proteins, which led to an overall increase in OXPHOS activity [76]. These changes were proposed to be dependent on an up-regulation of the translational repressor, 4EBP as the changes in mitochondrial protein synthesis occurred post-transcriptionally [76]. These results on a TORC1-4EBP axis are consistent with previous studies in flies [77] and are reminiscent of the observations made in yeast tor1 knockouts which have decreased global translation but increased mitochondrial respiration. As in flies, a study that examined 25 different diets with various ratios of protein, fat, carbohydrate, and energy contents found that mice on diets with the lowest P/C ratio achieved the longest median lifespan independent of calorie intake [148].
However, not all amino acids exert the same effect. In flies, restriction of essential amino acids, and in particular methionine, confers most DR longevity benefits [149], similar to what it has been observed in yeast. In rodents, diets lacking the nonessential amino acid cysteine and restricted for methionine also extend longevity by lowering serum levels of IGF-I, insulin, and glucose and promoting resistance to oxidative stress [150]. Methionine restriction in mice has been reported to increase mitochondrial ATP production efficiency, to reduce electron leak through respiratory chain complex I, and to decrease mtDNA damage and protein oxidation [151]. Based on the observations that restriction of sulfur amino acids (methionine and cysteine) is common to numerous DR regimens across model organisms, that the transsulfuration pathway (TSP) controls the conversion of methionine into cysteine and is required for DR-mediated lifespan extension in flies, and that hydrogen sulfide (H2S) supplementation extends the lifespan of worms, it was recently hypothesized that H2S could mediate the physiological benefits of methione/cysteine restriction, including stress resistance and extended longevity [68]. This study demonstrated that in mice, DR stimulates endogenous H2S production via repression of mTORC1 and activation of the TSP pathway and that H2S Is necessary and sufficient to confer DR-mediated stress resistance in vivo, which was abrogated by administration of the antioxidant N-acetyl-cysteine [68]. TSP-dependent H2S production was observed in yeast, worm, fruit fly, and rodent models of DR-mediated longevity. Further studies in vitro showed that cytoprotective effects of H2S during ischemia require sulfhydration of mitochondrial sulfide-quinone-reductase (SQR), which allows transfer of electrons from H2S to coenzyme Q and the mitochondrial respiratory chain [68], thus establishing a new link between mitochondrial functions and the health and longevity benefits exerted by DR interventions.
These studies on the beneficial effects of mild increase of H2S bring about the concept of “mitohormesis” [5] described earlier.
2.5. DR, mitochondria and aging in humans
To gain insight into the ability of CR to delay aging in humans, several studies have been conducted on non-human primates. However, the conclusions from these studies, performed in rhesus monkeys, were conflicting, most likely due to differing control group diets. In one study, CR increased the average age of mortality, and was associated with the prevention of hyperinsulinemia and the mitigation of age-related diseases in comparison with ad-libitum fed animals [152]. In a parallel study, improvements in the health of CR animals were apparent but did not reach significance (P=0.06), and survival was not significantly different between control-fed and CR monkeys [153]. Looking back, a comparison of control animal body weight from both studies suggests that the diet imposed to the control monkeys in the second study was effectively already CR. The combination of these data indicate that the benefits of CR on health and aging are actually conserved in primates [154], but moderate CR may not be enough to confer longevity benefits compared to more severe CR.
The effects of CR in human health have also been investigated. The CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Energy Intake) multicenter study focused on the effects of short-term CR. The results obtained so far are promising. CR implemented for 6–12 months on moderately overweight individuals resulted not only in weight loss, but also enhanced glucose tolerance and insulin sensitivity, decreased metabolic rate, and improved serum indicators of disease risk [155]. These studies also showed that short term, 6-month CR (10% or 30%) induced increased levels of plasma glutathione peroxidase activity (p=0.04) and decreased plasma protein carbonyl levels (p=0.02), although no significant change was observed in other plasma antioxidants such as SOD and catalase. These findings indicate that CR can modulate antioxidant defense and oxidative stress in humans [156]. Beneficial effects of long-term CR on disease risk in humans have also been reported in studies involving members of the Caloric Restriction Society who voluntarily engage in the practice of CR. In these individuals, insulin sensitivity is enhanced and levels of adiponectin are increased [157], suggesting an increase in mitochondrial fatty acid oxidation as reported in mouse models.
The currently available data indicates that a lifestyle modification in the form of CR, even if started during adulthood, can help to minimize the risk of age-associated disorders and promote healthy aging in humans, probably through molecular mechanisms largely conserved along evolution.
3. CONCLUSIONS AND FUTURE PERSPECTIVES
Despite the discoveries made in the field of dietary research, many questions remain. Studies of the yeast S. cerevisiae and other model organisms have allowed for the identification of conserved pathways that control healthspan and lifespan in response to nutrient availability. These studies have identified mitochondria as essential organelles, contributors and targets of these pathways, orchestrating health-promoting and longevity-defining processes beyond the postulates defined by the free radical theory of aging. The DR-mediated effects leading to mitohormesis, enhanced mitochondrial biogenesis, increased OXPHOS efficiency, and improved mitochondrial quality control, to name a few, as well as the involvement of mitochondrial retrograde pathways, will require further mechanistic investigations. Although further investigations in human populations are required, overall, however, it is becoming apparent that DR-mediated preservation of mitochondrial functions is fundamental to delay the onset of age-related diseases and perhaps extend the average rate of mortality in humans as it does across evolutionary boundaries, from yeast to non-human primates.
Highlights.
DR-mediated health stimulation affects several metabolic and anti-stress pathways.
DR-effects include attenuation of age-related declines in mitochondrial function.
DR-effects on mitochondrial function are largely conserved from yeast to humans.
ACKNOWLEDGMENTS
We apologize to those not cited due to space limitations. A.B. is supported by grants from the National Institutes of Health (NIH) RO1 GM071775, GM105781 and GM112179.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- [2].Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- [3].Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J. Intern. Med. 2008;263:167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- [4].Bonawitz ND, Shadel GS. Rethinking the mitochondrial theory of aging: the role of mitochondrial gene expression in lifespan determination. Cell Cycle. 2007;6:1574–1578. doi: 10.4161/cc.6.13.4457. Epub 2007 May 1522. [DOI] [PubMed] [Google Scholar]
- [5].Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- [6].Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol. Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ocampo A, Liu J, Schroeder EA, Shadel GS, Barrientos A. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 2012;16:55–67. doi: 10.1016/j.cmet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dillon LM, Williams SL, Hida A, Peacock JD, Prolla TA, Lincoln J, Moraes CT. Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Hum. Mol. Genet. 2012;21:2288–2297. doi: 10.1093/hmg/dds049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- [10].Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- [12].Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- [15].Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr.. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Enns LC, Morton JF, Treuting PR, Emond MJ, Wolf NS, Dai DF, McKnight GS, Rabinovitch PS, Ladiges WC. Disruption of protein kinase A in mice enhances healthy aging. PLoS ONE. 2009;4:e5963. doi: 10.1371/journal.pone.0005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Anderson R, Prolla T. PGC-1alpha in aging and anti-aging interventions. Biochim. Biophys. Acta. 2009;1790:1059–1066. doi: 10.1016/j.bbagen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chevtzoff C, Yoboue ED, Galinier A, Casteilla L, Daignan-Fornier B, Rigoulet M, Devin A. Reactive oxygen species mediated regulation of mitochondrial biogenesis in the yeast Saccharomyces cerevisiae. J Biol Chem. 2009;285:1733–1742. doi: 10.1074/jbc.M109.019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Taormina G, Mirisola MG. Calorie restriction in mammals and simple model organisms. Biomed. Res. Int. 2014 doi: 10.1155/2014/308690. 308690. (2014) 10.1155/2014/308690. Epub 302014 May 308696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martin-Montalvo A, de Cabo R. Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid. Redox Signal. 2013;19:310–320. doi: 10.1089/ars.2012.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464:513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and Chronological Aging in Saccharomyces cerevisiae. Cell. 2012;16:18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schmidt M, Kennedy BK. Aging: one thing leads to another. Curr. Biol. 2012;22:R1048–1051. doi: 10.1016/j.cub.2012.11.016. [DOI] [PubMed] [Google Scholar]
- [29].Goldberg AA, Bourque SD, Kyryakov P, Gregg C, Boukh-Viner T, Beach A, Burstein MT, Machkalyan G, Richard V, Rampersad S, Cyr D, Milijevic S, Titorenko VI. Effect of calorie restriction on the metabolic history of chronologically aging yeast. Exp. Gerontol. 2009;44:555–571. doi: 10.1016/j.exger.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [30].Ugidos A, Nystrom T, Caballero A. Perspectives on the mitochondrial etiology of replicative aging in yeast. Exp. Gerontol. 2010;45:512–515. doi: 10.1016/j.exger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [31].Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- [32].Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- [33].Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- [34].Delaney JR, Murakami C, Chou A, Carr D, Schleit J, Sutphin GL, An EH, Castanza AS, Fletcher M, Goswami S, Higgins S, Holmberg M, Hui J, Jelic M, Jeong KS, Kim JR, Klum S, Liao E, Lin MS, Lo W, Miller H, Moller R, Peng ZJ, Pollard T, Pradeep P, Pruett D, Rai D, Ros V, Schuster A, Singh M, Spector BL, Wende HV, Wang AM, Wasko BM, Olsen B, Kaeberlein M. Dietary restriction and mitochondrial function link replicative and chronological aging in Saccharomyces cerevisiae. Exp. Gerontol. 2013;48:1006–1013. doi: 10.1016/j.exger.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Heeren G, Rinnerthaler M, Laun P, von Seyerl P, Kossler S, Klinger H, Hager M, Bogengruber E, Jarolim S, Simon-Nobbe B, Schuller C, Carmona-Gutierrez D, Breitenbach-Koller L, Muck C, Jansen-Durr P, Criollo A, Kroemer G, Madeo F, Breitenbach M. The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1. Aging (Albany NY) 2009;1:622–636. doi: 10.18632/aging.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Caballero A, Ugidos A, Liu B, Oling D, Kvint K, Hao X, Mignat C, Nachin L, Molin M, Nystrom T. Absence of mitochondrial translation control proteins extends life span by activating sirtuin-dependent silencing. Mol. Cell. 2011;42:390–400. doi: 10.1016/j.molcel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- [37].Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu. Rev. Genet. 2006;40:159–185. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- [38].Vevea JD, Swayne TC, Boldogh IR, Pon LA. Inheritance of the fittest mitochondria in yeast. Trends Cell Biol. 2014;24:53–60. doi: 10.1016/j.tcb.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Erjavec N, Nystrom T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10877–10881. doi: 10.1073/pnas.0701634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jazwinski SM. Mitochondria to nucleus signaling and the role of ceramide in its integration into the suite of cell quality control processes during aging. Ageing Res. Rev. 2014;31:00142–00141. doi: 10.1016/j.arr.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol. Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- [42].Miceli MV, Jiang JC, Tiwari A, Rodriguez-Quinones JF, Jazwinski SM. Loss of mitochondrial membrane potential triggers the retrograde response extending yeast replicative lifespan. Front. Genet. 2011;10:102. doi: 10.3389/fgene.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liao X, Butow RA. RTG1 and RTG2: two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell. 1993;72:61–71. doi: 10.1016/0092-8674(93)90050-z. [DOI] [PubMed] [Google Scholar]
- [44].Jia Y, Rothermel B, Thornton J, Butow RA. A basic helix-loop-helix-leucine zipper transcription complex in yeast functions in a signaling pathway from mitochondria to the nucleus. Mol. Cell Biol. 1997;17:1110–1117. doi: 10.1128/mcb.17.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ocampo A, Liu J, Barrientos A. NAD+ salvage pathway proteins suppress proteotoxicity in yeast models of neurodegeneration by promoting the clearance of misfolded/oligomerized proteins. Hum. Mo.l Genet. 2013;22:1699–1708. doi: 10.1093/hmg/ddt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Smith DL, Jr., McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- [48].Aerts AM, Zabrocki P, Govaert G, Mathys J, Carmona-Gutierrez D, Madeo F, Winderickx J, Cammue BP, Thevissen K. Mitochondrial dysfunction leads to reduced chronological lifespan and increased apoptosis in yeast. FEBS Lett. 2009;583:113–117. doi: 10.1016/j.febslet.2008.11.028. [DOI] [PubMed] [Google Scholar]
- [49].Bonawitz ND, Rodeheffer MS, Shadel GS. Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span. Mol. Cell Biol. 2006;26:4818–4829. doi: 10.1128/MCB.02360-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kyryakov P, Beach A, Richard VR, Burstein MT, Leonov A, Levy S, Titorenko VI. Caloric restriction extends yeast chronological lifespan by altering a pattern of age-related changes in trehalose concentration. Front. Physiol. 2012;3:256. doi: 10.3389/fphys.2012.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- [52].Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Easlon E, Tsang F, Skinner C, Wang C, Lin SJ. The malate-aspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes Dev. 2008;22:931–944. doi: 10.1101/gad.1648308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- [57].Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005;1:e69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lin SJ, Guarente L. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2006;2:e33. doi: 10.1371/journal.pgen.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Riesen M, Morgan A. Calorie restriction reduces rDNA recombination independently of rDNA silencing. Aging Cell. 2009;8:624–632. doi: 10.1111/j.1474-9726.2009.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- [61].Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leao C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Acad. Sci. U. S. A. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pan Y. Mitochondria, reactive oxygen species, and chronological aging: a message from yeast. Exp Gerontol. 2011;46:847–852. doi: 10.1016/j.exger.2011.08.007. [DOI] [PubMed] [Google Scholar]
- [63].Oliveira GA, Tahara EB, Gombert AK, Barros MH, Kowaltowski AJ. Increased aerobic metabolism is essential for the beneficial effects of caloric restriction on yeast life span. J. Bioenerg. Biomembr. 2008;40:381–388. doi: 10.1007/s10863-008-9159-5. [DOI] [PubMed] [Google Scholar]
- [64].Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim. Biophys. Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shi L, Sutter BM, Ye X, Tu BP. Trehalose is a key determinant of the quiescent metabolic state that fuels cell cycle progression upon return to growth. Mol. Biol. Cell. 2010;21:1982–1990. doi: 10.1091/mbc.E10-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zaborske JM, Wu X, Wek RC, Pan T. Selective control of amino acid metabolism by the GCN2 eIF2 kinase pathway in Saccharomyces cerevisiae. BMC Biochem. 2010;11:29. doi: 10.1186/1471-2091-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mirisola MG, Taormina G, Fabrizio P, Wei M, Hu J, Longo VD. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet. 2014;10:e1004113. doi: 10.1371/journal.pgen.1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, Wang R, Gladyshev VN, Madeo F, Mair WB, Mitchell JR. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- [70].Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- [71].Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bratic A, Larsson NG. The role of mitochondria in aging. J. Clin. Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Westbrook R, Bonkowski MS, Arum O, Strader AD, Bartke A. Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2014;69:25–33. doi: 10.1093/gerona/glt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6:827–839. doi: 10.1111/j.1474-9726.2007.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin. Cell Dev. Biol. 2014;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. American journal of physiology. Endocrinology and metabolism. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [81].Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- [82].Groenewoud MJ, Zwartkruis FJ. Rheb and mammalian target of rapamycin in mitochondrial homoeostasis. Open Biol. 2013;3:130185. doi: 10.1098/rsob.130185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nature reviews. Drug discovery. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- [86].Chang HW, Shtessel L, Lee SS. Collaboration between mitochondria and the nucleus is key to long life in Caenorhabditis elegans. Free radical biology & medicine. 2015;78:168–178. doi: 10.1016/j.freeradbiomed.2014.10.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, Deng CX, Spitz DR, Gius D. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 2008;4:291–299. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- [95].Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hafner AV, Dai J, A.P. G, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013;48:634–639. doi: 10.1016/j.exger.2012.08.007. [DOI] [PubMed] [Google Scholar]
- [98].Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408–2420. doi: 10.2741/2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Friis RM, Glaves JP, Huan T, Li L, Sykes BD, Schultz MC. Rewiring AMPK and mitochondrial retrograde signaling for metabolic control of aging and histone acetylation in respiratory-defective cells. Cell Rep. 2014;7:565–574. doi: 10.1016/j.celrep.2014.03.029. [DOI] [PubMed] [Google Scholar]
- [100].Ashrafi K, Lin SS, Manchester JK, Gordon JI. Sip2p and its partner snf1p kinase affect aging in S. cerevisiae. Genes Dev. 2000;14:1872–1885. [PMC free article] [PubMed] [Google Scholar]
- [101].Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- [105].Kwast KE, Burke PV, Poyton RO. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J. Exp. Biol. 1998;201:1177–1195. doi: 10.1242/jeb.201.8.1177. [DOI] [PubMed] [Google Scholar]
- [106].Zitomer RS, Lowry CV. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol. Rev. 1992;56:1–11. doi: 10.1128/mr.56.1.1-11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang L, Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 1995;14:313–320. doi: 10.1002/j.1460-2075.1995.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Forsburg SL, Guarente L. Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev. 1989;3:1166–1178. doi: 10.1101/gad.3.8.1166. [DOI] [PubMed] [Google Scholar]
- [109].Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–69. [PubMed] [Google Scholar]
- [110].Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- [111].Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr., Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- [114].Wenz T. PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB Life. 2009;61:1051–1062. doi: 10.1002/iub.261. [DOI] [PubMed] [Google Scholar]
- [115].Long YC, Tan TM, Takao I, Tang BL. The biochemistry and cell biology of aging: metabolic regulation through mitochondrial signaling. American journal of physiology. Endocrinology and metabolism. 2014;306:E581–591. doi: 10.1152/ajpendo.00665.2013. [DOI] [PubMed] [Google Scholar]
- [116].Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim. Biophys Acta. 2013;1833:410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Haynes CM, Fiorese CJ, Lin YF. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013;23:311–318. doi: 10.1016/j.tcb.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schulz AM, Haynes CM. UPR-mediated cytoprotection and organismal aging. Biochim. Biophys. Acta. 2015;7:00058–00054. doi: 10.1016/j.bbabio.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Alis R, Lucia A, Blesa JR, Sanchis-Gomar F. The Role of Mitochondrial Derived Peptides (MDPs) in Metabolism. J. Cell Physiol. 2015;20:25023. doi: 10.1002/jcp.25023. [DOI] [PubMed] [Google Scholar]
- [122].Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50:R11–19. doi: 10.1530/JME-12-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends in endocrinology and metabolism: TEM. 2013;24:222–228. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- [128].Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- [129].Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Villa-Cuesta E, Holmbeck MA, Rand DM. Rapamycin increases mitochondrial efficiency by mtDNA-dependent reprogramming of mitochondrial metabolism in Drosophila. J. Cell Sci. 2014;127:2282–2290. doi: 10.1242/jcs.142026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- [133].Mitsuishi M, Miyashita K, Muraki A, Tamaki M, Tanaka K, Itoh H. Dietary protein decreases exercise endurance through rapamycin-sensitive suppression of muscle mitochondria. American journal of physiology. Endocrinology and metabolism. 2013;305:E776–784. doi: 10.1152/ajpendo.00145.2013. [DOI] [PubMed] [Google Scholar]
- [134].Dai Y, Zheng K, Clark J, Swerdlow RH, Pulst SM, Sutton JP, Shinobu LA, Simon DK. Rapamycin drives selection against a pathogenic heteroplasmic mitochondrial DNA mutation. Hum. Mol. Genet. 2014;23:637–647. doi: 10.1093/hmg/ddt450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol. Genomics. 2005;23:343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- [136].Picca A, Pesce V Fau - Fracasso F, Fracasso F Fau - Joseph A-M, Joseph Am Fau - Leeuwenburgh C, Leeuwenburgh C Fau - Lezza AMS, Lezza AM. Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. Plos One. 2013;8:e74644. doi: 10.1371/journal.pone.0074644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012;11:150–161. doi: 10.1111/j.1474-9726.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, 3rd, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z, Nair KS. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16:777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Knight WD, Witte MM, Parsons AD, Gierach M, Overton JM. Long-term caloric restriction reduces metabolic rate and heart rate under cool and thermoneutral conditions in FBNF1 rats. Mech. Ageing Dev. 2011;132:220–229. doi: 10.1016/j.mad.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11:793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rossignol R, Faustin B, Rocher C, Malgat M, Mazat JP, Letellier T. Mitochondrial threshold effects. Biochem. J. 2003;370:751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].McKee Alderman J, DePetrillo MA, Gluesenkamp AM, Hartley AC, Verhoff SV, Zavodni KL, Combs TP. Calorie restriction and dwarf mice in gerontological research. Gerontology. 2010;56:404–409. doi: 10.1159/000235720. [DOI] [PubMed] [Google Scholar]
- [145].Rousakis A, Vlassis A, Vlanti A, Patera S, Thireos G, Syntichaki P. The general control nonderepressible-2 kinase mediates stress response and longevity induced by target of rapamycin inactivation in Caenorhabditis elegans. Aging Cell. 2013;12:742–751. doi: 10.1111/acel.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Grandison RC, Wong R, Bass TM, Partridge L, Piper MD. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS One. 2009;4:e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sanchez-Roman I, Gomez A, Gomez J, Suarez H, Sanchez C, Naudi A, Ayala V, Portero-Otin M, Lopez-Torres M, Pamplona R, Barja G. Forty percent methionine restriction lowers DNA methylation, complex I ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J. Bioenerg. Biomembr. 2011;43:699–708. doi: 10.1007/s10863-011-9389-9. [DOI] [PubMed] [Google Scholar]
- [152].Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- [153].Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Meydani M, Das S, Band M, Epstein S, Roberts S. The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE Trial of Human Caloric Restriction. J. Nutr. Health. Aging. 2011;15:456–460. doi: 10.1007/s12603-011-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Hamdy O. Lifestyle modification and endothelial function in obese subjects. Expert. Rev. Cardiovasc. Ther. 2005;3:231–241. doi: 10.1586/14779072.3.2.231. [DOI] [PubMed] [Google Scholar]