Abstract
Background & Aims
Mechanisms that regulate regeneration of injured livers are complex. YAP, a stem-cell associated factor, controls liver growth in healthy adult mice. Increasing nuclear localization of YAP triggers accumulation of reactive-appearing ductular cells (YAP+RDC) with liver progenitor capabilities. The significance of YAP activation, and mechanisms involved, are unknown in diseased livers. We evaluated the hypothesis that YAP is more activated in injured livers that are scarring than in those that are regenerating effectively.
Methods
Immunohistochemistry and qRT-PCR analysis were used to localize and quantify changes in YAP and RDC in 52 patients with nonalcoholic fatty liver disease (NAFLD) and two mouse models of diet-induced nonalcoholic steatohepatitis (NASH). Results were correlated with liver disease severity, metabolic risk factors, and factors proven to control NAFLD progression.
Results
YAP increased in NAFLD where it mainly localized in nuclei of RDC that expressed progenitor markers. Accumulation of YAP+RDC paralleled the severity of hepatocyte injury and accumulation of Sonic hedgehog (Shh), but not steatosis or metabolic risk factors. YAP+RDC expressed osteopontin, a Shh-regulated fibrogenic factor. Myofibroblast accumulation, fibrosis, and numbers of YAP+RDC strongly correlated. In murine NASH models, atrophic fibrotic livers contained significantly more YAP+RDC than livers with less severe NASH.
Conclusion
YAP+RDC promote scarring, rather than effective regeneration, during NASH.
Keywords: YAP, ductular reaction, regeneration, fibrosis, nonalcoholic steatohepatitis
Introduction
Hepatomegaly is common in many chronic liver diseases, but the mechanisms that control liver mass in this context remain confusing. The size of healthy adult liver is controlled by a tonic inhibitory process which involves constraining activation of transcriptional regulators that promote multi-potency and progenitor growth [1]. Signals that suppress nuclear localization of YAP, a stem cell-associated transcription co-factor, are particularly important for controlling adult liver size in healthy mice [2]. Simply increasing nuclear YAP in liver cells experimentally causes progressive hepatomegaly [2]. Interestingly, experimental YAP activation triggers accumulation of reactive-appearing duct-like cells (RDC). These YAP(+) RDC are stem-like cells [1]. YAP(+) RDC are beneficial when transplanted into recipient mice with fatal liver injury: they engraft, eventually repopulate the livers with healthy mature hepatocytes, and rescue recipients from liver failure [1].
The significance of YAP(+) RDC in diseased human livers is unknown. Hence, the goal of the present studies is to clarify this issue. Like the livers of healthy adult mice, healthy adult human livers are known to express barely-detectable levels of YAP. Rare YAP-positive cells localize along hepatic sinusoids and within small ductules immediately adjacent to portal tracts [3]. These latter structures are presumably canals of Herring, a putative progenitor compartment in adult livers. Ductular-appearing cells with nuclear YAP have been reported to accumulate peri-portally in several types of pediatric and adult cholestatic liver disease [3, 4]. Although the mechanisms driving such increases in YAP(+) RDC are uncertain, toxic levels of certain bile acids were recently shown to mediate the process in bile duct-ligated rodents [3]. In children with biliary atresia, fibrosis was noted in areas of YAP(+) RDC accumulation [3]. Liver fibrosis has not been reported to result from YAP activation in mouse models. However, the mice in those studies were generally healthy at the time of YAP induction. Further, in many studies, constitutively-active YAP constructs were inserted into liver cell types that do not normally express YAP [1]. Thus, research is needed to fill current gaps in knowledge about YAP’s role in human liver disease.
Obesity-related liver disease (also known as nonalcoholic fatty liver disease, NAFLD) provides an opportunity to investigate the significance of endogenous YAP. Although no studies have reported on YAP in this condition, NAFLD patients often exhibit hepatomegaly, and it is well-established that RDC accumulation can occur in NAFLD [5]. However, RDC accumulation is a poor prognostic indicator in NAFLD, strongly correlating with the severity of liver fibrosis and thus, risk for cirrhosis and liver-related death [5]. The association between RDC accumulation and bad NAFLD outcomes raises intriguing questions. Is YAP activated in RDC during NAFLD? If so, are the YAP-positive RDC helping the injured livers to regenerate, or promoting defective, fibrogenic repair? Epidemiologic studies suggest that at least 25% of American adults have some form of NAFLD [6], with about 2% of the general adult population having NAFLD-related cirrhosis [7]. Thus, defining mechanisms that control regression, as opposed to progression, of NAFLD is highly relevant, and abundant human liver samples are on hand for cross-sectional analysis. Different animal models that mimic relatively mild NAFLD or more severe NAFLD are also available, permitting prospective studies to determine if/how endogenous YAP expression changes as liver damage progresses. These models can also be used to identify signals that regulate endogenous YAP during liver injury. Progress is likely to be expedited by earlier work, which demonstrated mechanisms that control RDC accumulation in human and murine NAFLD [8, 9]. Some of these signaling pathways (e.g., Hedgehog) have been linked to YAP activation in other tissues and certain cancers [10–12].
Herein we evaluate the hypothesis that YAP is deregulated in NAFLD patients who are at high risk for cirrhosis. Scaring (e.g., cirrhosis) is characterized by defective regeneration of mature cells despite an exuberant wound healing response. Therefore, we reasoned that futile regeneration that results in cirrhosis would manifest excessive nuclear localization of YAP in RDC, hepatic repopulation with liver cells that are unable to fully mature, and stromal enrichment with myofibroblasts that produce type 1 collagen and other stiff matrix proteins that promote growth of immature cells. Analysis of liver samples obtained from 52 morbidly obese subjects at the time of bariatric surgery, and complementary studies of two different animal NASH models, strongly support this hypothesis. Further, our findings suggest a positive feedback loop that maintains YAP activation in RDC during NASH, thereby implying a novel pathogenic mechanism for NAFLD cirrhosis and identifying YAP as a diagnostic and therapeutic target in this disease.
Material and Methods
Human Samples
We evaluated fifty-two morbidly obese adult patients with biopsy-proven NAFLD, who were referred for bariatric surgery at Hospital Santa Maria, Lisbon, Portugal. The study was approved by Hospital Santa Maria’ Human Ethics Committee and written informed consent was obtained from all participants.
Diabetes mellitus was defined as fasting blood glucose >126 mg/dL, or regular use of hypoglycemic medications [13]. Metabolic syndrome was defined as the presence of at least three of the following: waist circumference >102 cm in men and 88 cm in women, hypertriglyceridemia (>150 mg/dL), low HDL-cholesterol (<40 mg/dL in men, 50 mg/dL in women), fasting glucose >110mg/dL or use of hypoglycemic drugs and high blood pressure (>130/85 mmHg or use of antihypertensive drugs) [14]. Adiponectin and leptin serum levels were assessed by ELISA.
Animal Studies
Male wild-type mice C57Bl/6 (Jackson Laboratory) were fed either standard rodent food, chow diet (Picolab® Rodent diet 20, #5053; n=7 mice); methionine choline-deficient (MCD) diet (MP Biomedicals, #960439; n=6 mice) for 8 weeks, or Western diet (TD.120330 22% HVO+0.2% cholesterol diet, Teklad Research, supplemented with high-corn fructose syrup-equivalents in the drinking water; n=8 mice) for 16 weeks. Diets are summarized in Supplemental Table 1. Animal care and procedures were approved by the Duke University Institutional Animal Care and fulfilled National Institutes for Health and Duke University IACUC requirements for humane animal care.
Histopathological analysis
All patients were submitted to wedge liver biopsy during surgery. Formalin-fixed, paraffin-embedded liver biopsies were cut into 5 μm serial sections. A trained pathologist evaluated the severity of NAFLD by examining H&E, Masson trichrome and reticulin stained sections according to criteria described by Brunt et al. [15]. NAFLD was defined as presence of >5% of steatosis [15] and NASH was defined as any degree of steatosis along with lobular inflammation and centrilobular ballooning and/or Mallory-Denk bodies or any degree of steatosis along with centrilobular pericellular/perisinusoidal fibrosis or bridging fibrosis in the absence of another identifiable cause [16]. Isolated portal fibrosis (F1c according to Brunt’s classification) was not considered sufficient for diagnosing NASH.
Immunohistochemistry (IHC) was done as previously described [17]. Morphometric analysis was done with Metamorph Software (Molecular Devices Corporation) in 20x magnification, 20 fields/sample, and expressed as percent of section stained. Immunohistochemistry antibodies are specified in Supplemental Table 2. Liver fibrosis was assessed by Picrosirius red (#365548, Sigma) staining [17]. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (11684817910, Roche) was performed according to manufacturer’s protocol. Hydroxyproline content was quantified colorimetrically in flash frozen liver samples as previously described [18].
Molecular Studies
mRNA quantification by Real-time Reverse Transcription-PCR (RT-PCR)
Total RNA was extracted from livers using TRIzol (Invitrogen). RNA was reverse transcribed to cDNA templates using random primer and Super Script RNAse H-Reverse Transcriptase (Invitrogen) and amplified. Semiquantitative qRT-PCR was performed using iQ-SYBR Green Supermix (Bio-Rad) and StepOne Plus Real-Time PCR Platform (ABI/Life Technologies), as previously described [18]. For primers, see Supplemental Table 3.
Western Blotting
Total proteins were extracted from liver using RIPA buffer (Sigma) and nuclear and cytoplasmic extracts were prepared with kit from Thermo Fisher Scientific, catalog #78833. Equal amounts of protein were separated by electrophoresis on 4%-20% Criterion gels (BioRad), transblotted into polyvinylidene difluoride membranes. Primary antibodies are specified in Supplemental Table 4.
Statistics
Results were expressed as mean±SEM. Quantitative variables were compared by unpaired Student’s t-test and one-way ANOVA with Bonferroni’s correction for multiple comparisons, and correlations by Spearman correlation coefficient. The independence of the associations of variables with YAP IHC (dependable variable) was assessed by multivariable logistic regression analysis. Analyzes were run using IBM SPSS software version 20. Two-tailed P values less than 0.05 were considered statistically significant.
Results
Reactive-appearing ductular cells (RDC) with nuclear YAP accumulate in human NASH
Hepatomegaly is common in NAFLD patients, but progressive liver fibrosis occurs infrequently [19]. Severity of liver fibrosis predicts liver-related morbidity and mortality [19], and strongly correlates with RDC accumulation, with both processes being more intense in nonalcoholic steatohepatitis (NASH) than in less severe liver injury (i.e., non-NASH/simple steatosis) [5]. Although nuclear localization of YAP drives RDC accumulation in healthy rodents [2], it is not known if YAP activation is involved when that process occurs during NAFLD. To address this question, we analyzed liver biopsies from 52 morbidly obese patients undergoing bariatric surgery. Half fulfilled standard histologic criteria for NASH and half had non-NASH/simple steatosis. Main characteristics of the patient population are shown in Table 1. Liver immunohistochemistry showed that NASH patients accumulated significantly more YAP-positive cells than patients without NASH (Figure 1. A and 1. B). Furthermore, YAP expression strongly correlated with markers of liver injury, including hepatocellular ballooning, lobular inflammation and NAFLD Activity Score (NAS – R=0.339, P=0.014) (Supplemental Figure 1. A). In contrast, YAP expression did not correlate with severity of hepatic steatosis or steatosis-associated metabolic disorders (Supplemental Figure 1. B). YAP staining localized mainly in ductular structures, either within small or large (presumably more mature) cholangiocytes (Figure 1. A). Total YAP was typically demonstrated in both cell nuclei and cytoplasm, but staining was generally stronger in the former. Only rare cholangiocytes exhibited cytoplasmic staining for total YAP without nuclear staining (Figure 1. C). Phosphorylation of YAP at position Ser127 causes YAP to translocate from the nucleus into the cytoplasm for eventual degradation. We found that phospho-YAP localizes in the cytoplasm and cannot be demonstrated in the nuclei of ductular cells (Figure 1. C). The most important YAP-expressing cell-type is the ductular cell. Small number of “isolated cells” in the lobule and portal tract also express YAP. To better identify YAP-expressing cells we performed double-immunohistochemistry with YAP and either α-smooth muscle actin (α-SMA, marker of activated myofibroblasts), CD68 (marker of macrophages/Kupffer cells) and CD31 (marker of activated endothelial cells) (Supplemental Figure 2). Most “isolated” YAP-expressing cells in liver lobules did not co-express any of the above markers, although we were able to detect a few α-SMA and CD31-expressing cells that also expressed YAP. (In contrast, we could not find co-localization of CD68 and YAP). After accounting for cellular morphology and localization, we believe that most “isolated” YAP-expressing cells in the lobules are actually neo-ductular cells that appear in the hepatic parenchyma as the ductular reaction spills out of the portal tracts.
Table 1.
Population characteristics
| No NASH (n=26) | NASH (n=26) | P | |
|---|---|---|---|
| Gender (% male) | 8 | 23 | 0.124 |
| Age (years) | 40±2 | 44±2 | 0.329 |
| BMI (kg/m2) | 43±1 | 50±2 | 0.001 |
| Ethnicity (% black/white) | 12/88 | 0/100 | 0.233 |
| Type 2 diabetes mellitus (%) | 17 | 42 | 0.047 |
| HOMA-IR | 2.7±0.3 | 6.9±1.6 | 0.016 |
| Hypertension (%) | 46 | 61 | 0.204 |
| Dyslipidemia (%) | 29 | 61 | 0.022 |
| Metabolic syndrome (%) | 39 | 79 | 0.006 |
| ALT/AST (IU/L) | 23±2/25±2 | 42±6/51±8 | 0.003/0.003 |
| Adiponectin/Leptin (ng/mL) | 49±4/19±1 | 35±5/22±2 | 0.042/0.279 |
| Histology | |||
| - Steatosis 1/2/3 (%) | 58/34/8 | 11/27/62 | <0.0001 |
| - Lobular inflam. 0/1/2/3 (%) | 88/4/8/0 | 8/23/42/27 | <0.0001 |
| - Ballooning 0/1/2 (%) | 100/0/0 | 54/31/15 | <0.0001 |
| - Fibrosis 0/1/2/3/4 (%) | 42/58/0/0/0 | 4/35/31/19/11 | <0.0001 |
| - Portal Inflam. 0/1 (%) | 31/69 | 8/92 | 0.038 |
| - Portal fibrosis 0/1 (%) | 42/58 | 8/92 | 0.005 |
Figure 1. Reactive-appearing ductular cells (RDC) with nuclear YAP accumulate in human NASH.
A. YAP-immunohistochemistry (brown) in normal human liver and representative sections from patients with simple steatosis (n=26) and NASH (n=26). B. Morphometric analysis as a function of presence of NASH. Mean±SEM are graphed. C. Representative liver sections stained for YAP and phospho-YAP.
YAP staining strongly correlated with serum levels of ductular cell markers, such as alkaline-phosphatase (R=0.511, P<0.001) and γ-glutamyltranspeptidase (R=0.423, P=0.002), as well as bilirubin levels (R=0.422, P=0.002). Despite evidence that YAP accumulation and histologic features of liver injury/inflammation were strongly associated, we were unable to demonstrate correlations between liver YAP expression and serum levels of aminotransferases, traditional markers of hepatocyte injury (Supplemental Figure 3). This may reflect the known poor performance of aminotransferases as “biomarkers” of liver damage. Indeed, a study of more than 600 liver biopsies from morbidly obese patients submitted to bariatric surgery demonstrated that aminotransferases levels predicted neither liver fibrosis nor hepatocyte injury (namely acidophilic bodies and Malory-Denk bodies) [20].
YAP(+) RDC accumulate when expression of Sonic hedgehog (Shh) is increased in human NAFLD
NASH and other types of liver injury trigger local production of Sonic hedgehog (Shh) ligands that activate Hedgehog signaling which stimulates the growth of cells involved in liver repair, including RDC and liver myofibroblasts [8, 21, 22]. The number of Shh-expressing hepatocytes (Figure 2A) correlated with severity of NASH-related liver injury and inflammation (assessed by the NAS score), and intensity of RDC accumulation (assessed by immunostaining for the RDC marker keratin-19, K19, Figure 2B). Double-immunostaining showed that YAP and K19 staining were strongly co-localized. Morphometry confirmed that accumulation of YAP(+) cells strongly correlated with numbers of K19(+) cells, and showed that the latter correlated with numbers of Shh(+) cells (Figure 2C). The aggregate data, therefore, suggest that injured hepatocytes release Shh ligands that promote expansion/activation of the YAP(+) RDC population.
Figure 2. YAP(+) RDC accumulate when expression of Shh is increased in human NAFLD.
A. Shh and K19 immunohistochemistry (brown) in the same patients as figure 1. Number of Shh(+) hepatocytes and K19 positive ductular cells analyzed as a function of presence of NASH. Mean±SEM are graphed. B. Correlation between Shh and K19 immunostaining. C. Left panel, double-immunohistochemistry for YAP and K19 in human NAFLD. Right panels, YAP morphometry in function of K19 and Shh immunostaining.
Fibrosis markers increase with NASH-related accumulation of YAP(+) RDC in humans
RDC accumulation tightly parallels myofibroblast accumulation and fibrosis in NASH [5]. In liver, as in most organs, tissue-resident myofibroblasts are the major sources of fibrous matrix [23]. Such myofibroblasts derive from local populations of Hedgehog-responsive cells that retain mesenchymal stem cell-like plasticity [24]. Although YAP is an accepted regulator of stem cell fate [1], and activating YAP has been shown to drive RDC accumulation in rodents [2], the relationship between YAP, myofibroblasts, and organ fibrosis is unclear. Immunohistochemistry of our NAFLD cohort revealed that expansion of YAP-expressing RDC cells paralleled accumulation of cells that expressed the myofibroblast marker, α-SMA (Figure 3. A). Furthermore, YAP and K19 immunostaining correlated with fibrosis stage (Figure 3. B). Fibrosis distribution generally matched that of the expanded RDC. Both RDC and fibrosis were present in portal areas of more than two thirds of the patients. Pericellular fibrosis also localized near expanded ductular reactions that spilled out into the lobule. Multivariate analysis that considered multiple variables (e.g., presence of NASH, steatosis, fibrosis, lobular and portal inflammation, portal fibrosis, hepatocellular ballooning, as well as α-SMA, K19, osteopontin, and Shh immunohistochemistry) showed that α-SMA was the only histochemical marker that independently correlated with YAP morphometry (P<0.001). This finding is noteworthy because it establishes a strong, but previously unrecognized, link between YAP activation and liver fibrosis, the single most important predictor of liver-related mortality in human NAFLD [19].
Figure 3. Fibrosis markers increase with NASH-related accumulation of YAP(+)in humans.
A. YAP and aSMA immunohistochemistry (brown) in normal human liver and representative sections from NAFLD patients with no/mild (n=36) versus severe fibrosis and NASH (n=16). Correlation between aSMA staining and accumulation of ductular cells, expressing K19/YAP. B. Evaluation of YAP, K19 and aSMA staining as a function of fibrosis.
YAP(+) RDC produce pro-fibrogenic factors in NASH patients
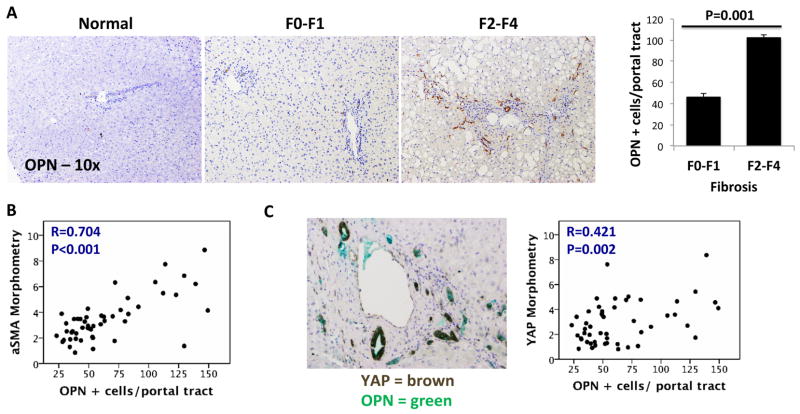
RDC are known to produce various factors that stimulate accumulation of fibrogenic MF. The Hedgehog-regulated factor, osteopontin (OPN), is one such RDC-derived factor [25] that is particularly important in NASH. Hepatic OPN expression increases in parallel with Hedgehog pathway activation and correlates with the severity of fibrosis in NASH patients [26]. Double-immunostaining in our NAFLD cohort co-localized OPN and YAP expression, demonstrating, for the first time, that both factors are strongly expressed in RDC during NASH. Accordingly, OPN immunostaining correlated with YAP staining, α-SMA accumulation, and liver fibrosis severity (Figure 4. A, B, C). These results suggest a mechanism by which YAP(+) RDC promote fibrogenic repair, rather than effective liver regeneration, during liver injury.
Figure 4. Accumulation of Osteopontin(+) and YAP(+)RDC in human NASH.
A. Osteopontin (OPN) immunohistochemistry (brown) in liver sections from the same patients. B. Correlation between aSMA and osteopontin immunostaining. C. Left panel, double immunohistochemistry for YAP and OPN. Right panel, correlation between YAP and OPN immunostaining. Mean±SEM are graphed.
YAP(+) RDC accumulate in mouse models of NASH
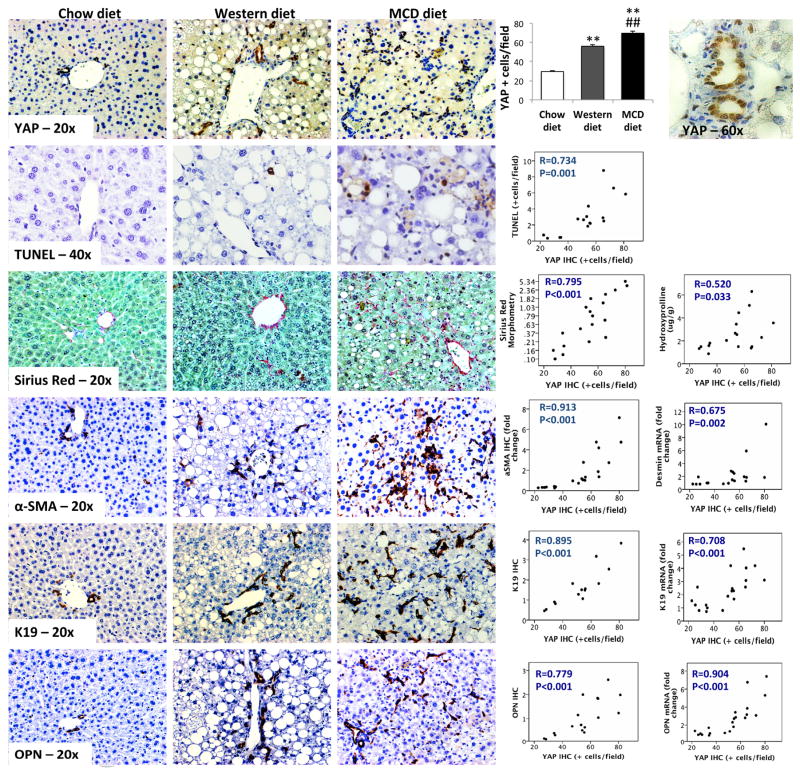
We evaluated YAP induction in two mouse dietary models of NASH to overcome some of the limitations inherent to our cross-sectional analysis of human liver samples. The Western diet models relatively mild NASH in humans because Western diet-fed mice develop very little liver fibrosis even after months of dietary challenge [27]. The MCD diet models more severe human NASH. In fact, MCD diet reproducibly induces progressive liver fibrosis in mice [27]. Compared to age- and gender-matched chow-fed controls, mice fed either diet demonstrated an expansion of YAP-expressing cells (Figure 5). To verify these immunohistochemistry findings, we performed Western blot analysis of cytoplasmic and nuclear extracts from whole liver tissue of the NASH mouse models (Supplemental Figure 4). We found that total YAP was increased in the nuclear extracts and decreased in the cytoplasmic extracts (resulting in an increase in the ratio nuclear/cytoplasmic YAP, i.e., YAP activation) in MCD diet-fed mice, which model fibrosing NASH in humans.
Figure 5. YAP(+) RDC accumulate in mouse models of NASH.
Immunostaining for YAP, apoptosis marker (TUNEL), fibrosis markers (sirius red and aSMA), progenitors (K19) and osteopontin, from representative liver sections from wild-type mice fed chow diet (n=7), Western diet (n=8) or methionine-choline deficient (MCD)-diet (n=6). YAP staining is graphed as Mean±SEM. **P<0.01, chow versus NASH-inducing diets, ##P<0.01, Western versus MCD diet. Correlations between YAP immunostaining and the above immunohistochemistries as well as mRNA expression.
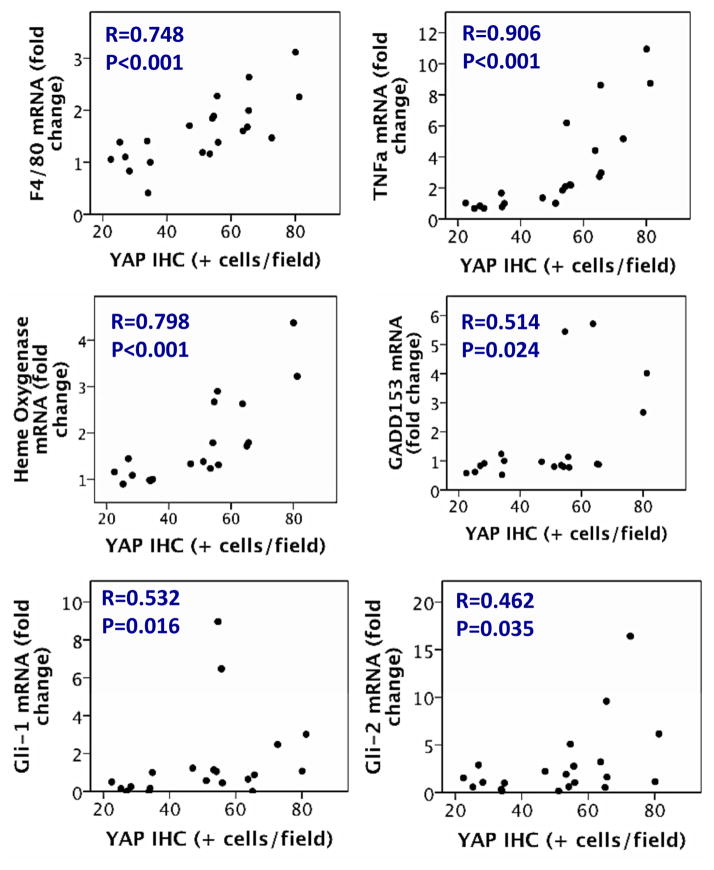
As in human NAFLD, YAP immunostaining localized mainly in RDC. Both YAP immunostaining and YAP nuclear protein content as assessed by Western blot strongly correlated with morphometric assessment of RDC markers, such as K19/K7 and OPN. Further, qRT-PCR analysis of the rodent livers established that mRNA expression of these RDC markers also strongly associated with YAP immunostaining and YAP nuclear protein content. As in humans, nuclear YAP accumulation correlated with hepatocyte injury/cell death in mice, paralleling TUNEL staining (Figure 5 and Supplemental Figure 6) and mRNA markers of oxidative stress (Heme oxygenase), endoplasmic reticulum stress (GADD153), macrophage activation (F4/80), and inflammatory cytokines (TNF-α) (Figure 6 and Supplemental Figure 6). Also, as occurred in diseased human livers, YAP activation accompanied injury-related activation of the Hedgehog pathway (demonstrated by increased expression of Gli1/Gli2 mRNA, Figure 6 and Supplemental Figure 6), accumulation of Hedgehog-responsive MF (demonstrated by α-SMA immunohistochemistry and qRT-PCR analysis of desmin mRNA expression, Figure 5 and Supplemental Figure 6), and liver fibrosis (shown by increased Sirius red staining and hydroxyproline content, Figure 5 and Supplemental Figure 6). In mice with NASH, accumulation of YAP(+) RDC did not associate with increased liver mass (R=0.038, P=0.88). Coupled with the other evidence that YAP(+) RDC produce potent pro-fibrogenic factors, this finding supports the concept that persistence of RDC with nuclear YAP contributes to fibrogenic repair, rather than effective regeneration, of injured livers.
Figure 6. In mouse models of NASH, accumulation of YAP(+) RDC correlates with liver injury.
Correlation between YAP immunostaining in mouse models of NASH and mRNA expression by qRT-PCR from whole liver for markers of inflammation, oxidative and endoplasmic reticulum stress, and hedgehog target genes.
To further clarify the association between YAP expression and fibrosis, we performed qRT-PCR and Western blot analysis of the pro-fibrogenic cytokine transforming growth factor (TGF)-β1 in whole liver lysates from mice with diet-induced NASH. TGF-β1 mRNA and protein were increased in mice with diet-induced NASH. In the most aggressive NASH model, MCD diet-fed mice, downstream components of TGF-β1 signaling, i.e., SMAD2 and phospho-SMAD2 were also increased (Supplemental Figure 5). Moreover, TGF-β1 expression and phospho-SMAD2 positively correlated with YAP immunostaining and nuclear YAP content (Supplemental Figure 5 and 6).
Comparison of YAP target gene expression in the mouse models of NASH supports the concept that YAP transcriptional activity promotes fibrosis in NASH. Messenger RNA levels of two well-accepted YAP target genes that have been proven to play critical roles in liver fibrosis, i.e., amphiregulin [28] and connective tissue growth factor, CTGF [29] were significantly increased in MCD diet-fed mice. Expression of these pro-fibrogenic YAP-target genes correlated with liver nuclear enrichement with YAP protein. Further, both of these parameters correlated significantly with various other measures of liver fibrosis, including hepatic hydroxyproline content, Sirius red staining, accumulation of TGFβ1, OPN, desmin, and α-SMA (Supplemental Figure 6).
Discussion
Our work provides novel insight into the role of endogenous YAP during chronic liver injury. Analysis of both people and mice with chronically injured fatty livers showed that native YAP strongly localizes within nuclei of RDC that produce potent pro-fibrogenic factors, such as osteopontin. Further, expansion of the YAP(+) RDC population was found to correlate positively with liver injury and fibrosis. Indeed, numbers of YAP(+) RDC were significantly increased in the mouse model of NASH that developed liver atrophy. These new results prove that activating YAP in RDC of injured livers is not sufficient to assure re-growth of healthy adult liver tissue. They also confirm an earlier study, which showed that liver growth was not enhanced by activating YAP in ductular cells of healthy livers [1]. It is important to consider both findings in light of recent evidence that selectively over-expressing constitutively-active YAP transgenes in healthy hepatocytes caused those cells to de-differentiate into ductular-appearing cells that later re-differentiated into hepatocytes when transplanted into recipients with injured livers [1]. Although the latter results revealed the regenerative potential of YAP-expressing cells, that work did not identify endogenous signals that control native YAP expression when injury triggers liver regeneration. It also did not demonstrate which cells “naturally” accumulate endogenous YAP during that process, or determine the significance YAP-expressing cells for liver repair. The present study addressed those gaps in knowledge. Our analysis of many humans and mice with mild liver damage consistently demonstrated increased numbers of YAP(+) ductular cells but rarely identified YAP in hepatocyte nuclei. In contrast, “sicker”, scarred livers were extremely enriched with YAP(+) ductular cells. Hence, our findings show that chronic liver injury progressively and predominantly activates YAP in ductular cells, making it extremely unlikely that defective liver repair reflects a primary failure of hepatocytes to activate endogenous YAP.
It has long been known that effective liver regeneration is precisely proportional to hepatocyte loss [30]. The mechanisms that assure tight coupling of liver cell replacement with liver cell loss are complex, but seem to be modulated, at least in part, by signals from damaged hepatocytes and cells involved in the regenerative process. Hepatocyte lipotoxicity and death are increased in NASH, distinguishing that potentially progressive form of NAFLD from simple hepatic steatosis, which has a benign prognosis [31]. It is important to emphasize, however, that natural history studies of humans with NASH demonstrate that liver damage remains stable or regresses more often than it progresses [19, 32]. This suggests that liver regeneration is effective (i.e., able to keep pace with liver cell death) in most NASH patients. Deficient regeneration of injured epithelia results in progressive fibrosis (i.e., scarring) that can culminate in cirrhosis. Indeed, natural history studies of NASH patients indicate that fibrosis severity is the sole independent predictor of ultimate liver outcomes [19]. Given that regenerative efficiency (i.e., the ability to recover from lipotoxicity) determines the prognosis of NASH, treatments that optimize regeneration might be beneficial. Developing such interventions will require a better understanding of the mechanisms that de-rail effective repair
Our results demonstrate that livers which are scarring (i.e., not regenerating efficiently) are marked by progressive accumulation of YAP(+) RDC. Moreover, our findings suggest that these YAP(+) RDC actively suppress effective reconstitution of damaged hepatic epithelia by producing various pro-fibrogenic factors, such as amphiregulin, CTGF, and osteopontin. YAP is known to activate transcription of amphiregulin and CTGF, and previous studies in knock-out mice proved that liver fibrosis depends upon these factors [28, 29]. Osteopontin accumulation and fibrogenic repair of liver damage occur when the Hedgehog pathway becomes excessively activated. Genetic approaches that reduce osteopontin [33] or disrupt Hedgehog signaling [18] have also established that these factors are necessary for liver fibrosis. New evidence that numbers of YAP(+) RDC strongly correlate with levels of Shh expression in NASH patients suggests a novel model that may explain the outgrowth of YAP(+) RDC (Figure 7). The new model is grounded in earlier work showing that lipotoxicity stimulates hepatocytes to produce Shh [17]. Hepatocyte-derived Shh is released into the microenvironment where it activates cells, such as RDC, that express receptors/co-receptors for Shh. Shh promotes the viability, growth, and migration of RDC. It also stimulates RDC to generate Shh, osteopontin, and various other pro-fibrogenic factors that direct hepatic stellate cells to become and remain myofibroblasts [34]. These liver myofibroblasts, in turn, generate Shh and other factors that sustain the outgrowth of YAP(+) RDC. Thus, we propose that accumulation of YAP(+) RDC is initiated by paracrine signals released from dying hepatocytes and maintained by autocrine/paracrine signaling among cells involved in the ensuing wound healing response, including RDC and myofibroblasts. Recently, it was shown that phospholipids may induce YAP activation [35]. This has particularly noteworthy implications for our model because hepatocytes injured by the phospholipid lysophosphatidylcholine induce Shh expression [36]. It is conceivable, therefore, that phospholipid-dependent activation of YAP may be explained by induction of Shh. Further research is needed to investigate this intriguing possibility.
Figure 7. YAP(+) RDC promote fibrogenic repair of liver lipotoxicity.
Lipotoxic hepatocytes release Sonic hedgehog (Shh) ligands. Shh stimulates the growth, viability and migration of reactive-appearing ductular cells that have activated YAP (YAP+ RDC). These YAP(+) RDC generate soluble factors, such as Shh and osteopontin, that stimulate the growth, viability and migration of myofibroblasts (MF) derived from hepatic stellate cells (HSC). MF-HSC generate fibrous matrix and produce Shh and osteopontin, further enriching the microenvironment with factors that promote accumulation of both YAP(+) RDC and fibrogenic MF-HSC. These autocrine/paracrine mechanisms sustain accumulation of MF and YAP(+) RDC and promote fibrogenic repair of NASH-related liver injury.
In any case, it is difficult to imagine that outgrowth of YAP(+) RDC is a stereotypical response to liver injury and yet, inherently anti-regenerative. Hence, we propose that such cells emerge transiently during effective regeneration, but persist when processes that normally modulate the inherent plasticity of YAP(+) RDC become subverted. RDC are very sensitive to reprogramming signals that stimulate multipotent cells to become more proliferative and migratory (i.e., less epithelial and more mesenchymal). Shh stimulates RDC to acquire more mesenchymal characteristics. Conversely, inhibiting hedgehog signaling in RDC permits them to revert back to the less mesenchymal/more epithelial state that is characteristic of most duct cells in healthy livers [34]. YAP activation exerts similar pro-mesenchymal actions in multi-potent cells of other tissues. Conversely, inactivating YAP in those cells drives them to become less migratory and proliferative, and more adherent and quiescent [1, 4]. Emerging evidence suggests that Hedgehog-initiated signals regulate YAP activity. Shh has been reported to promote nuclear localization (i.e., activation) of YAP [11, 37]. Activated YAP, on the other hand, induces transcription of CTGF, which increases TGF-β production and stimulates myofibroblasts accumulation [38]. Liver myofibroblasts, in turn, produce Shh and TGF-β. Either factor could maintain YAP activation in RDC [11, 37, 39]. In mouse models of NASH, we observed significant correlations between Shh pathway, nuclear YAP content, mRNA levels of the YAP target gene CTGF, TGF-β1, TGF-β1 signaling, osteopontin, accumulation of aSMA(+) cells, and fibrosis. Thus, our experimental evidence support the concept that RDC may be unable to inactivate YAP when immersed in a MF-enriched, pro-fibrogenic microenvironment. Current knowledge predicts that RDC would be incapable of regenerating hepatic epithelia while harboring activated YAP. Indeed, YAP is typically inactive in healthy mature hepatocytes [1]. Moreover, forming/maintaining epithelial structures typically requires inactivation of YAP [40]. Finally, artificially activating YAP in hepatocytes enabled them to disassociate and convert into RDC [1]. Results from the present study provide additional strong support for the concept that persistence of activated YAP in RDC impairs liver regeneration. We observed more liver scarring in NASH patients and mouse models of NASH with greater numbers of YAP(+) RDC. Moreover, liver size actually declined as YAP(+)RDC increased during diet-induced NASH in mice. The aggregate data, therefore, support our hypothesis that YAP is deregulated in NAFLD patients who are at high risk for cirrhosis, and justify further research to delineate specific mechanisms involved.
Supplementary Material
Acknowledgments
Financial support: This research is supported by NIH DK0077794, DK053792 and R37 AA010154 (Diehl AM), and Duke Endowment: The Florence McAlister Professorship (Diehl AM). MVM is a receiver of a PhD grant from Fundação para a Ciência e Tecnologia, FCT, Portugal.
Abbreviations
- YAP
yes-associated protein
- RDC
reactive-appearing ductular cells
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- Shh
Sonic hedgehog
- WT
wild type
- MCD
methionine choline deficient
- IHC
immunohistochemistry
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- MF
myofibroblast
- K19
keratin 19
- α-SMA
α-smooth muscle actin
- OPN
osteopontin
- TNF-α
tumor necrosis factor α
- TGF
transforming growth factor
- CTGF
connective tissue growth factor
Footnotes
Conflict of interest: There are no conflicts of interest to state.
Authors contributions:
Machado MV – study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis.
Michelotti GA – study concept and design, acquisition of data, analysis and interpretation of data.
Pereira de Almeida T – acquisition of data, analysis and interpretation of data.
Xie G – acquisition of data, analysis and interpretation of data.
Premont R – analysis and interpretation of data
Cortez-Pinto H – collection of samples, study design
Diehl AM – study concept and design, analysis and interpretation of data, manuscript writing, obtained funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. Bile acids activate YAP to promote liver carcinogenesis. Cell Reports. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, et al. YAP regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in NASH: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of NAFLD in the US: Third NHANES, 1988–1994. American Journal Epidemiology. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nature Reviews Gastroenterology Hepatology. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 8.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human NAFLD. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung Y, Diehl AM. NASH pathogenesis: role of repair in regulating the disease progression. Digestive Diseases. 2010;28:225–228. doi: 10.1159/000282092. [DOI] [PubMed] [Google Scholar]
- 10.Tariki M, Dhanyamraju PK, Fendrich V, Borggrefe T, Feldmann G, Lauth M. The YAP controls the cell density regulation of Hedgehog signaling. Oncogenesis. 2014;3:e112. doi: 10.1038/oncsis.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857–4866. doi: 10.1038/onc.2013.433. [DOI] [PubMed] [Google Scholar]
- 12.Lin YT, Ding JY, Li MY, Yeh TS, Wang TW, Yu JY. YAP regulates neuronal differentiation through Shh signaling pathway. Experimental Cell Research. 2012;318:1877–1888. doi: 10.1016/j.yexcr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for NAFLD. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for NASH: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 17.Machado MV, Michelotti GA, Pereira TD, Boursier J, Kruger L, Swiderska-Syn M, et al. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with NASH. Gut. 2014 doi: 10.1136/gutjnl-2014-307362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, et al. Smoothened is a master regulator of adult liver repair. Journal Clinical Investigation. 2013;123:2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1647–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Berk PD, Hsu JY, Courcoulas AP, Flum D, Khandelwal S, et al. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: observations and a perspective from the longitudinal assessment of bariatric surgery study. Seminars Liver Disease. 2014;34:98–107. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, et al. Hedgehog pathway activation and EMT during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. American Journal Physiology Gastrointestinal Liver Physiology. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleig SV, Choi SS, Yang L, Jung Y, Omenetti A, VanDongen HM, et al. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Laboratory Investigation. 2007;87:1227–1239. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- 23.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Comprehensive Physiology. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 24.Xie G, Diehl AM. Evidence for and against EMT in the liver. American Journal Physiology Gastrointestinal Liver Physiology. 2013;305:G881–890. doi: 10.1152/ajpgi.00289.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in NASH. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in NAFLD. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado MV, Michelotti GA, Xie G, de Pereira TA, Boursier J, Bohinc B, et al. Mouse Models of Diet-induced Nonalcoholic Steatohepatitis Reproduce the Heterogeneity of the Human Disease. PloS One. 2015 doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Castillo J, Goni S, et al. The EGFR-ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2008;48:1251–1261. doi: 10.1002/hep.22437. [DOI] [PubMed] [Google Scholar]
- 29.Pi L, Robinson PM, Jorgensen M, Oh SH, Brown AR, Weinreb PH, et al. CTGF and integrin alphavbeta6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology. 2015;61:678–691. doi: 10.1002/hep.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia C. Advances in the regulation of liver regeneration. Expert Review Gastroenterology Hepatology. 2011;5:105–121. doi: 10.1586/egh.10.87. [DOI] [PubMed] [Google Scholar]
- 31.Machado MV, Cortez-Pinto H. NAFLD: what the clinician needs to know. World Journal Gastroenterology. 2014;20:12956–12980. doi: 10.3748/wjg.v20.i36.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis Progression in Nonalcoholic Fatty Liver vs NASH: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clinical Gastroenterology Hepatology. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancha A, Rodriguez A, Catalan V, Becerril S, Sainz N, Ramirez B, et al. Osteopontin deletion prevents the development of obesity and hepatic steatosis via impaired adipose tissue matrix remodeling and reduced inflammation and fibrosis in adipose tissue and liver in mice. PloS One. 2014;9:e98398. doi: 10.1371/journal.pone.0098398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, et al. Hedgehog signaling regulates EMT during biliary fibrosis in rodents and humans. Journal Clinical Investigation. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakisaka K, Cazanave SC, Werneburg NW, Razumilava N, Mertens JC, Bronk SF, et al. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. Journal of Hepatology. 2012;57:844–851. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Shh-driven neural precursor proliferation. Genes Development. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct TGFβ-induced tumorigenic phenotypes in breast cancer cells. Journal Biological Chemistry. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Developmental Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.