Abstract
Duchenne muscular dystrophy (DMD), an X-linked disorder caused by the lack of dystrophin, is characterized by the progressive wasting of skeletal muscles. To date, what is known about dystrophin function is derived from studies of dystrophin-deficient animals, with the most common model being the mdx mouse. Most studies on patients with DMD and in mdx mice have focused on skeletal muscle and the development of therapies to reverse, or at least slow, the severe muscle wasting and progressive degeneration. However, dystrophin is also expressed in the CNS. Both mdx mice and patients with DMD can have cognitive and behavioral changes, but studies in the dystrophic brain are limited. We examined the brain structure and metabolites of mature wild type (WT) and mdx mice using magnetic resonance imaging and spectroscopy (MRI/MRS). Both structural and metabolic alterations were observed in the mdx brain. Enlarged lateral ventricles were detected in mdx mice when compared to WT. Diffusion tensor imaging revealed elevations in diffusion diffusivities in the prefrontal cortex and a reduction of fractional anisotropy in the hippocampus. Metabolic changes included elevations in phosphocholine and glutathione, and a reduction in γ-aminobutyric acid in the hippocampus. In addition, an elevation in taurine was observed in the prefrontal cortex. Such findings indicate a regional structural change, altered cellular antioxidant defenses, a dysfunction of GABAergic neurotransmission, and a perturbed osmoregulation in the brain lacking dystrophin.
Keywords: skeletal muscle, dystrophy, DMD, magnetic resonance spectroscopy, magnetic resonance imaging, diffusion tensor imaging
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked muscle disorder characterized clinically by severe, progressive and irreversible muscle wasting and loss of muscular function [1–3]. This devastating and fatal disease is due to the lack of dystrophin, a large membrane-associated protein expressed in striated muscle and localized to the inner face of the sarcolemma [4;5]. The mdx mouse, the most common animal model for DMD, also lacks dystrophin and shares much of the muscle pathology found in DMD patients. Despite the wealth of studies describing the role of dystrophin in muscle, there has been relatively little progress in developing disease therapies.
In addition to the well-described muscle wasting phenomenon, it is generally accepted that some patients with DMD have cognitive impairments. In the original description of the disease circa 1861, Duchenne reported 5 patients with some degree of cognitive defects [6]. Since then, an overwhelming amount of evidence has shown that there can be a significant cognitive impairment in DMD patients [7;8]; mental retardation affects around 30% of boys with DMD [9]. Impaired memory function, especially restricted verbal short term memory, seems to characterize the neuropsychological profile of many patients with DMD [10;11]. Language impairments, long-term memory problems, and limited executive functions have also been reported [9;12–14]. For mdx mice, behavioral studies have also shown impairments in learning as tested in several paradigms [15–17].
The presence of the cognitive dysfunction raises the question of neuropathology. Both CT and in vivo 1H MRS studies [18–20] suggest a slow, progressive cerebral degeneration in the DMD patients. Clear signs of cortical atrophy and ventricular dilation were observed in CT images in older patients, and patients with large amounts of physical disability [18]. Using 1H MRS, Rae et al. [20] showed significant increases in ratios of choline-containing compounds to N-acetylaspartate (tCho/NAA) and tCho/creatine (Cr) in the cerebellum in DMD boys compared to age-matched normal boys. Within the DMD group, the abnormal tCho/NAA group was significantly older (mean age: 12 years) than the normal tCho/NAA group (mean age: 8 years), which reflects the progressive nature of the disease. In contrast, a localized in vivo 1H MRS study by Kreis et al. [21] showed a significant decrease in absolute tCho levels in both the cerebellum and the temporo-parietal cortex the DMD patients (mean age: 12 years). Very few studies have explored in vivo 1H MRS on mdx mice. In an early non-localized in vivo 1H MRS study, there were significantly low ratios of Cr/tCho and NAA/tCho in mdx brains compared to controls at the age between 2 and 5 months [22].
Dystrophin, normally expressed in the post-synaptic neuronal terminals of the cortex, hippocampus and cerebellum areas, is involved with reasoning and learning [23] and is completely or partially absent in DMD patients, as well as in the genetically homologous mdx mice [24;25]. This suggests that a cognitive impairment may be the result of the missing protein; however the precise function of dystrophin in brain is still unknown. In the muscle, it has been suggested that dystrophin plays both functional and structural roles such as: supporting the sarcolemma against mechanical stress and stabilizing it in the course of contraction–relaxation cycles [26]; taking part in the regulation of intracellular calcium and the cascade of calcium-related events [27;28]; working in force and signal transduction [29]; influencing the aggregation of neurotransmitter receptors [30;31]; and preventing excessive generation of reactive oxygen free radical species [32;33]. Some of these mechanisms may also be applicable in the brain. Identifying the role of dystrophin in the brain would be extremely helpful as effective therapies are developed for DMD.
In this study, we used in vivo magnetic resonance imaging (MRI), high resolution localized magnetic resonance spectroscopy (1H MRS), and ex vivo diffusion tensor imaging (DTI) to investigate alterations in brain structure (MRI and DTI) and metabolites (MRS) from mature mdx mouse brains. The use of in vivo MRI will not only be helpful in elucidating dystrophin function in the brain, but also for continuing development of non-invasive tools for use in diagnosis, disease progression and even drug therapy. As non-invasive technology continues to improve and imaging such as MRI/MRS becomes more commonplace, these tools will play a greater role in diagnosis, prognosis, and in rehabilitation planning.
Materials and Methods
Eight male dystrophic mice (mdx, C57BLScSn-DMDmdx, approximately 9 months old) and seven male aged-matched wild type mice (WT, C57BL/10ScSn) were obtained from Jackson Laboratory, Bar Harbor, ME. The MRI and MRS acquisitions were performed on mice close to one year of age. The experiments were performed on a Bruker BioSpec 70/30USR Avance III 7T scanner. A Bruker four-element 1H surface coil array was used as the receiver and a Bruker 72 mm linear-volume coil as the transmitter. Each mouse was anesthetized in an animal chamber using a gas mixture of O2 (1 L/min) and isoflurane (3 %) then later maintained at 1–1.5% isoflurane during scanning. An MR compatible small-animal monitoring system was used to monitor the animal respiration rate and body temperature. The animal body temperature was maintained at 36–37°C using warm water circulation.
Both proton-density-weighted and T2-weighted images were obtained using a 2D rapid acquisition with relaxation enhancement (RARE) sequence in the axial plane (repetition time (TR)/echo time (TE)eff1/TEeff2 = 5500/19/58 msec, RARE factor = 4, field of view (FOV) = 20 × 20 mm2, slice thickness = 1 mm, in-plane resolution = 114 × 114 μm2, number of averages (NA) = 1). A fluid-attenuated inversion recovery (FLAIR) pulse sequence (TR/Inversion time/TE = 10000/1600/48 ms, in-plane resolution = 78 × 78 μm2, other parameters were the same as T2-weighted images) was performed on 3 WT and 5 mdx mice to confirm the dilution of the ventricles, which was found in the T2-weighted MRI examination, and to check the potential periventricular hyperintense lesions in mdx mice. Volumetric analysis was measured by hand-drawing a region of interest with the Medical Image Processing, Analysis and Visualization tool (MIPAV v5.3.1, CIT; NIH, Bethesda, MD, USA). An atlas of mouse brain anatomy [34] was used for identifying structural boundaries. Nine consecutive slices were used for the volumetric analysis of the brain. The first slice was set at the beginning of the cerebrum (interaural 7.36 mm and bregma 3.56), which did not include the olfactory bulb. The slice prior to the opening of the cerebellum was (interaural −1.04 mm and bregma −4.84 mm) defined as the last slice. Brain ventricles were included in measuring brain tissue volume.
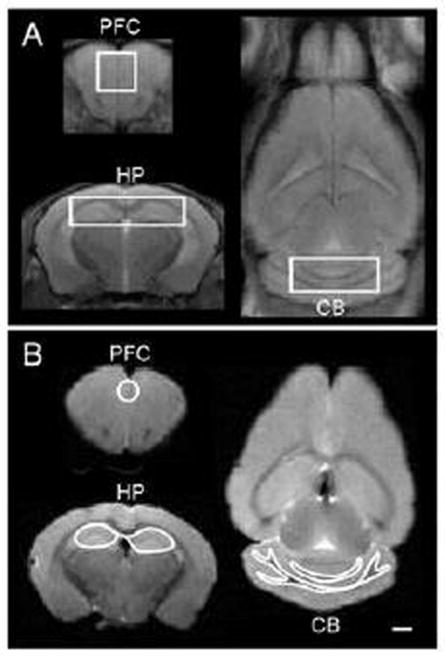
A short-TE Point-RESolved Spectroscopy (PRESS) pulse sequence [35] (TR/TE = 2500/10 ms, NA = 600) was used for MRS data acquisition with the voxel centered on the prefrontal cortex (PFC, 1.8 × 1.8 × 1.8 mm3), hippocampus (HP, 1.5 × 6.0 × 1.5 mm3), and cerebellum (CB, 1.4 × 4 × 1.5 mm3), respectively (Figure 1A). The unsuppressed water signal from each of the prescribed voxels was obtained to serve as a reference for determining the specific metabolite concentrations. Quantification of the MRS was based on frequency domain analysis using a “Linear Combination of Model spectra” (LCModel)[36]. Absolute concentrations were estimated with the LCModel automatic procedure.
Figure 1.
A: In vivo proton-density-weighted MR images showing regions of interest (ROI) for in vivo 1H MRS experiments. B: Ex vivo proton-density-weighted MR images showing the placement of the brain regions of interest of DTI experiments. PFC = prefrontal cortex; HP = hippocampus; CB = cerebellum. Scale bar = 1 mm
After all the in vivo MR procedures, mice were anesthetized with 4% isoflurane and then perfused through the left ventricle with 1 × phosphate buffered saline (PBS) and followed by 4% paraformaldehyde. The whole brain was removed from the skull and stored in PBS. The fixed brain was placed in a customized conical tube filled with Fluorinert (3M, St. Paul, MN) that decreased background during ex vivo MRI experiments. The anatomical images were taken from a 3-dimentional (3D) RARE T2-weighted sequence (TR/TEeff = 2500/33 ms, RARE factor = 8, FOV = 12 × 18 × 14 mm3, isotopically spatial resolution = 150 μm3, NA=2). DTI images were acquired with a 3D multiple shots, spin-echo echo-planar imaging (EPI) sequence (TR/TE = 500/30.95 ms, number of segments = 4, gradient directions = 64, diffusion gradient on time = 4 ms, temporal spacing time = 20 ms, NA=1, the FOV and spatial resolution were the same as T2-weighted images). Two b-values (2000 and 4000 s/mm2) were acquired for each direction following the acquisition of 5 images acquired with b = 0 s/mm2. DTI reconstruction was performed on each voxel using in-house MATLAB program (Mathworks, Natick, MA), as described [37]. The regions of interest for the DTI experiments were drawn manually with FSLview (Analysis Group, FMRIB, Oxford, UK) including PFC, HP, cerebellum white matter, and cerebellum grey matter (Figure 1B). Maps of mean diffusivity (MD) which measures the average water diffusion within the brain tissue, axial diffusivity (AD) which measures the water diffusion along the neuronal axons, radial diffusivity (RD) which measures the water diffusion perpendicular to the axons, and fractional anisotropy (FA) which measures the degree of diffusion anisotropy of the brain tissue were generated.
After imaging, several brains were then processed for histology (WT and mdx, N=2 each). Samples were dehydrated with 30% sucrose for 1 week. Samples were placed in a cryostat at −19°C for 5 min, mounted using OCT mounting medium, and sectioned at 40 μm from anterior to posterior, through the hippocampus. Sections were stored at −20 °C in cryo-protectant until processed for histology. Qualitative histological comparison of brain sections to MRI were made with sections selected based on similar anatomical landmarks such as the corpus callosum and the caudate putamen using a mouse brain atlas [38]. Sections were mounted in PBS, coversliped and imaged using a Nikon Eclipse 50i microscope and Nikon’s NIS-Elements Basic Research software to create a stitched image (10× objective). Volumetric analysis was not performed as was done for the imaging.
All experimental procedures were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. MRI and MRS results from mdx and WT mice were compared using independent student’s t-test.
Results
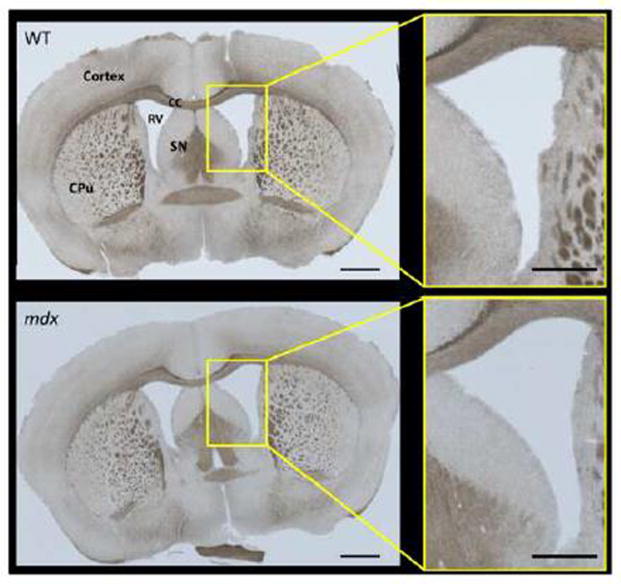
The mdx mice showed obviously enlarged lateral ventricles on both T2-weighted (Fig. 2A) and FLAIR images (Fig. 2B) and this finding was confirmed by volumetric measurements (Fig. 2C); (despite no differences in total brain tissue volume, lateral ventricle volume/brain tissue volume ratios were: WT [0.034 ± 0.003] vs. mdx [0.066 ± 0.005], p = 0.0001). The enlarged ventricles are likely caused by grey matter atrophy, although we cannot rule out compression from excess cerebrospinal fluid (CSF), which was displayed by the high signal intensity (bright white) on the coronal T2-weighed images (Fig. 2A) and low signal intensity (dark black) on the coronal FLAIR images (Fig. 2B). No obvious periventricular hyper-intense lesions were detected on the mdx mice by the FLAIR images. When comparing brain tissue volume, there was no significant difference between the whole brain tissue volume (mm3)/body weight (g) ratios between WT (8.861 ± 0.196) and mdx (9.287 ± 0.397, p=0.380). Representative histology confirmed enlarged ventricles in the mdx brain when compared to WT (Fig. 3).
Figure 2.
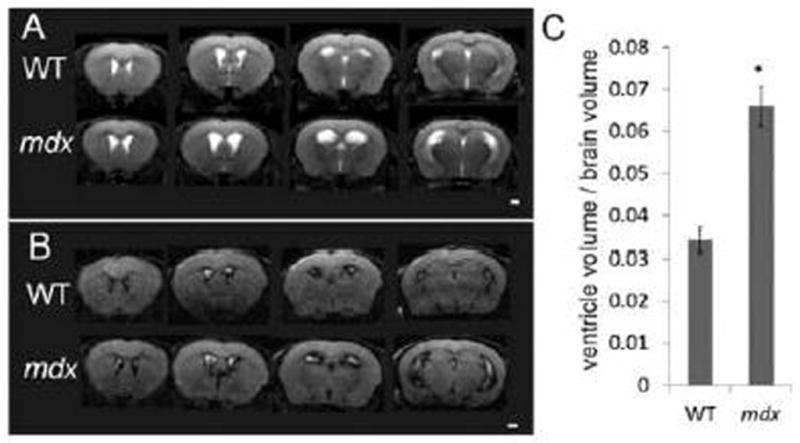
A: Representative in vivo T2-weighted coronal MR images from a WT mouse (top row) and an mdx (bottom row) mouse. B: Representative in vivo fluid-attenuated inversion recovery (FLAIR) coronal MR images from a WT mouse (top row) and an mdx mouse (bottom row). Excess cerebrospinal fluid displayed by the high signal intensity (bright white) on the T2-weighed images (A) and low signal intensity (dark black) on the FLAIR images (B). C: Comparison of the ventricle/whole brain tissue volume from the T2-weighted MRI images between WT and mdx mice. Data are expressed as mean ± standard error, * p < 0.05 (p = 0.0001). Scale bar = 1 mm
Figure 3.
Representative sections of brain from WT and mdx mice. Gross morphology was similar in both groups (N = 2 per group), but the changes in ventricle size seen with imaging (Fig. 2) were also apparent with histological imaging. CC = corpus callosum, CPu = caudate putamen, RV = right ventricle, SN = septal nucleus. Image scale bars = 1000 μm. Panel inset scale bars = 500 μm.
DTI results demonstrated significant elevations in MD, AD, and RD in the PFC of mdx mice when compared to WT (Fig. 4, p < 0.05). These data indicate a more continuous movement of water molecules with less restrictions and boundaries. There was also a significant decrease in FA in HP (p < 0.05), indicating a low density of the ordered tissue in mdx mice. There were no statistical differences in DTI measurements for both white and grey matter in the CB (data not shown).
Figure 4.
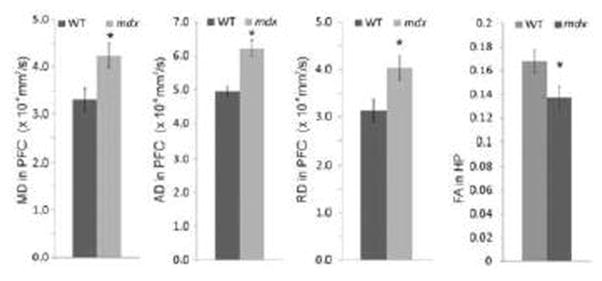
Comparison of the water diffusion characters between WT and mdx mice in prefrontal cortex (PFC) and hippocampus (HP). MD = mean diffusivity; RD = radial diffusivity; AD = axial diffusivity; FA = fractional anisotropy (relative units). Data are derived from brains ex vivo and expressed as mean ± standard error, * p < 0.05
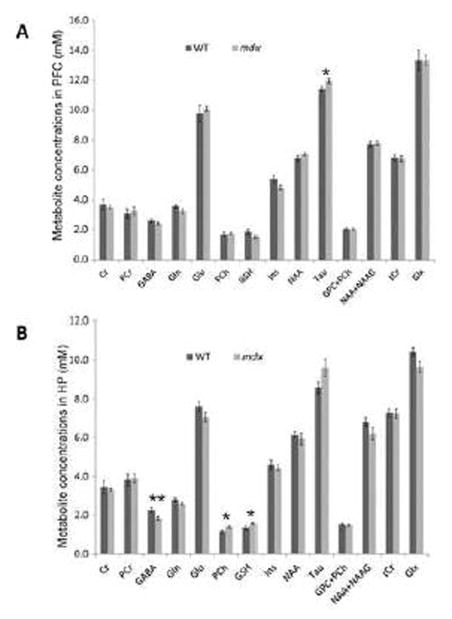
Representative high resolution localized in vivo MRS spectra from the HP of the two groups of mice are shown in Fig. 5. The following neurochemicals were observed: Creatine (Cr), γ-aminobutyric acid (GABA), glutamate (Glu), glutamine (Gln), glycerophosphocholine (GPC), glutathione (GSH), myo-inositol (Ins), N-acetyl-aspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocholine (PCh), phosphocreatine (PCr), taurine (Tau), total Creatine (tCr) = Cr+ PCr, total Choline (tCho) = GPC + PCh, and glutamate/glutamine complex (Glx =Glu +Gln). Compared to WT mice, mdx mice demonstrated a significant elevation in Tau (p < 0.05) in the PFC, and a decrease in GABA (p < 0.01) as well as significant elevations in PCh (p < 0.05) and GSH (p < 0.05) in the HP (Fig. 6). No differences were observed in other metabolites from these two regions. There were no significant alterations of the metabolites in the CB (data not shown).
Figure 5.
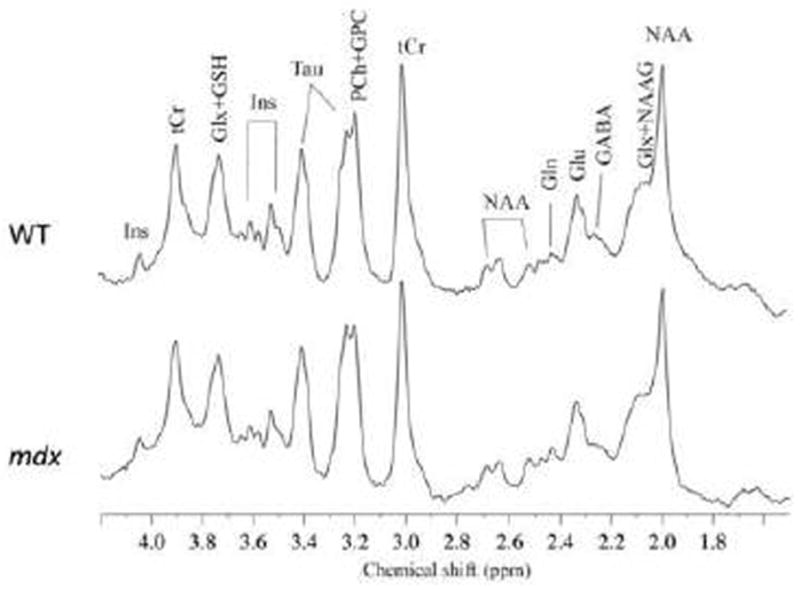
Representative in vivo 1H MR spectra at 7 Tesla from the hippocampus from a WT mouse and an mdx mouse. γ-aminobutyric acid (GABA), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (Ins), N-acetylaspartate (NAA), N-acetylaspartateglutamate (NAAG), taurine (Tau), total creatine (tCr), phosphocholine (PCh), glycerophosphocholine (GPC), and glutamate/glutamine complex (Glx).
Figure 6.
Comparison of the neurometabolic concentration (mM) between WT and mdx mice in prefrontal cortex (PFC, A) and hippocampus (HP, B). Data are expressed as mean ± standard error. * p < 0.05; ** p < 0.01.
Discussion
To the best of our knowledge, this is the first study to demonstrate that the combination of MRI and 1H MRS can detect alterations in structure (MRI and DTI) and metabolism (MRS) in mature mdx mouse brains. The structural alterations seen in mdx mice included a macro level change (ventricular dilation) and micro level changes (increased water diffusivity and decreased anisotropy in cells). The alterations in metabolite concentrations in mdx mouse brains occurred in the HP and PFC regions, where dystrophin is typically expressed in WT mice. Significant elevations in Tau (in PFC), GSH (in HP), PCh (in HP) and a significant reduction in GABA (in HP) were observed in mdx mice. An imbalance in these metabolites has implications for dystrophin functions such as osmoregulation, oxidative stress, membrane phospholipid synthesis, and GABAergic neurotransmission in the brain.
In addition to its mechanical function, the dystrophin-associated protein complex (DAPC) could act as a transmembrane signaling complex [39;40]. Further complicating matters, the dystrophin gene also has several internal promoters throughout the gene that give rise to shorter dystrophin proteins; some of these specific isoforms of dystrophin are exclusively, or predominantly, expressed in the brain [41;42]. The function(s) of dystrophin in the brain remains to be elucidated. Recent studies suggest a possible relationship between exists between certain mutations or loss of specific isoforms and cognitive impairment in DMD patients [43–46], but this is difficult to prove until all brain dystrophin isoforms are fully understood [47].
Dystrophin is found in the soma and dendrites of cortical and hippocampal pyramidal neurons, and in cerebellar Purkinje cells [48]. Little if any dystrophin reactivity is detected in striatum, thalamus, hypothalamus and brain stem [48]. Dystrophin is associated with the post-synaptic membrane of neurons and is present at high levels in post-synaptic dendrites, suggesting that it might have a role in synapse structure or/and interneuronal transmission [48;49]. In muscle, the uniform distribution of dystrophin beneath the plasma membrane is thought to provide structural support to the membrane. In the mdx mouse brain, the blood-brain barrier (BBB) is markedly altered due to disruption of tight junctions, leading to increased permeability in the BBB [50;51]. Although its role in the brain is still being defined, the absence of dystrophin may enable water in the cell to diffuse more easily and less anisotropically, as indicated by DTI results of the current study (i.e. increased MD, AD, RD, and a decreased FA).
Tau is an atypical amino acid that is required for proper development and function of skeletal muscle [52;53]. Tau is a hydrolyte attributed to efficiently transporting potassium, sodium, and calcium (Ca2+) in and out of the cell [54]. It is also known to play a significant role in many physiological activities, including maintaining a stable, constant condition of Ca2+ in the brain tissue. Studies have shown that Ca2+ homeostasis is perturbed in mdx muscle [33;55–57], elevations are seen in skeletal muscle at all ages in mdx mice. The high level of Tau in the mature mdx brain may indicate a perturbed Ca2+ homeostasis in the PFC. Such a role would be tantamount to the altered calcium handling observed in skeletal muscle when dystrophin is missing [28;33;55;58–61].
GSH is an antioxidant, and antioxidants play a role in preventing damage to cells caused by reactive oxygen species (ROS). The high level of GSH we observed in mdx mice may be compensatory due to high levels of glutathione reductase, glutathione peroxidase, and glutathione S-transferases, as reported in both patients with muscular dystrophy [62] and in homologous animal models such as dystrophic chickens [63;64]. The increase in GSH might indicate altered cellular antioxidant defenses in the HP in the mature mdx mice.
Elevated concentrations of choline-containing compounds has previously been reported in extracts of whole brain (60–150 days old) [22] and in the HP and CB (> 6 months old) in mdx mice [65]. More specifically, our current in vivo study demonstrated an elevated concentration of PCh in HP. The alteration of the PCh level may be a result of oxidative modification in the mdx mouse brains. It has been reported that there is a significant decrease in the level of phosphocholine-hydrolyzing activity, which decreases the conversion of phosphocholine into choline in brains of Alzheimer’s disease patients [66]. An elevated phosphocholine level was found early in Alzheimer’s pathogenesis [67]. The decrease in the level of phosphocholine-hydrolyzing activity has been suggested to be caused by the oxidative inactivation of the related enzymes through oxidant radicals [68]. One of the hypothesized functions of dystrophin in muscle is to prevent excessive generation of reactive oxygen free radical species [33;69]. The ultimate effect from the lack of dystrophin and resulting differences in the levels of GSH and PCh between the WT and mdx mice in the HP is still not clear.
The reduction in GABA in the HP may indicate a dysfunction of GABAergic neurotransmission in the mdx mice. Knuesel et al. [70] found co-localization of the GABAA channel with dystrophin in the mouse HP and CB. The number of GABAA clusters in mdx mice was reduced by 50% in these regions. However, in the cerebral cortex, where dystrophin clusters and GABAA clusters are normally separated, no reduction of GABAA clusters was detected. The reduction in GABAA in the HP adds evidence showing that dystrophin influences the aggregation of neurotransmitter receptors [30;31;70].
In our current study, no structural and metabolic alterations were detected in the CB of mdx mice, a region normally expressing dystrophin. The reason for this is unknown and warrants further investigation. Indeed, dystrophin is present in neurons in a very restricted distribution, both in terms of cell type and subcellular location. It is possible that dystrophin may play different roles in different regions. As such, it may not be surprising that despite the consistent lack of dystrophin our findings are restricted to the PFC and HP. Interestingly, these are the brain regions most strongly associated with cognitive function. Given the limited distribution of dystrophin, it is also not surprising that only mild cognitive dysfunction characterizes DMD and mdx. The major clinical issue in DMD is the skeletal muscle dysfunction, and this has understandably received the greatest attention. There has been very little clinical investigation of the role of dystrophin in the CNS in DMD. In the current study, we report enlarged lateral ventricles, changes in DTI parameters, elevations in GSH and PCh, and a reduction in GABA in the HP corresponding to an elevation in Tau in the PFC. Such findings indicate a regional structural change, altered cellular antioxidant defenses, a dysfunction of GABAergic neurotransmission, and a perturbed osmoregulation in the brain lacking dystrophin.
The use of in vivo MRI/MRS has shown to be helpful in elucidating dystrophin’s function in the brain in the current study. These technologies could be expanded for continuing development of non-invasive tools for use in diagnosis, monitoring disease progression, and even drug therapy outcomes. Restoration of dystrophin expression in the brain is the ideal therapeutic goal. A recently developed class of antisense oligonucleotides made of tricyclo-DNA (tcDNA-AONs) is showing promise in restoring dystrophin to levels necessary for a life-changing therapy for patients with DMD. A recent study has shown that systemic delivery of tcDNA-AONs promotes a high degree of rescue of dystrophin expression in skeletal muscles, the heart and, to a lesser extent, the brain in mdx mice [71]. The neuroimaging methods used in the current study can be used to noninvasively monitor the efficiency of this potential therapeutic agent in both patient and animals. It is now clear that therapeutic studies in mdx mice should not simply focus on muscle, but should include other tissues such as brain.
The current study extends our understanding the dystrophin function in brain, but has several limitations. First, we are using an animal model of DMD. Much of what is known about dystrophin structure-function in muscles is derived from studies of dystrophin-deficient animals, with the most common model being the mdx mouse. The DMD and the mdx conditions are similar in that dystrophin is missing from all tissues, however, the absence of dystrophin is not equally damaging to muscles of patients with DMD and mdx mice; the mdx phenotype is much less severe than that seen with DMD. Second, the data we generated represent only one time point. A “critical period” has been described for the mdx mouse, during which there is a peak in muscle weakness, myofiber necrosis and regeneration between the 2nd and 5th weeks of life [72–76]. It is not known if such changes in severity also occur in the brain over time. Third, it is not clear if patients with DMD have consistent gross or histological abnormalities of the brain. Available studies show conflicting results, from no change in brain weight or gross histology [20;77;78] to marked brain abnormalities [79–82], including ventricular dilation [18;83;84]. Fourth, we have a relatively small sample size for all the experiments and the corresponding histological sections were not quantified. Finally, the enzyme glutamic acid decarboxylase responsible for GABA levels (Gad) is known to be deficient in the background of mice used (C57BLScSn) [85], which alters synaptic clustering of GABA receptors [65;86] in this strain. Despite the differences measured between mice of the same strain, it is not known if mice with healthy Gad/GABA activity show the same results as found here. One way to address this question would be to perform these same experiments in mdx mice of a different background, such as the exon 52–deleted mdx (mdx52) mouse.
In summary, we studied the structure and metabolites of healthy and dystrophic mouse brains with MRI/MRS and found in dystrophic brains: enlarged lateral ventricles, alterations in diffusion of the prefrontal cortex and hippocampus, and several metabolic alterations.
Highlights.
Structure/metabolites of healthy and dystrophic (mdx) mouse brains were studied with MRI/MRS.
MRI revealed enlarged lateral ventricles in mdx brains.
DTI showed alterations in diffusion in the mdx prefrontal cortex and hippocampus.
MRS indicated several metabolic alterations for mdx brains.
The brain lacking dystrophin has structural changes and multiple brain dysfunctions.
Acknowledgments
Grants: This work was supported by grants to RML (R01AR059179) from National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70–S92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 3.Lovering RM, Porter NC, Bloch RJ. The muscular dystrophies: from genes to therapies. Phys Ther. 2005;85:1372–1388. [PMC free article] [PubMed] [Google Scholar]
- 4.Tinsley JM, Blake DJ, Zuellig RA, Davies KE. Increasing complexity of the dystrophin-associated protein complex. Proc Natl Acad Sci U S A. 1994;91:8307–8313. doi: 10.1073/pnas.91.18.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake DJ, Tinsley JM, Davies KE. Utrophin: a structural and functional comparison to dystrophin. Brain Pathol. 1996;6:37–47. doi: 10.1111/j.1750-3639.1996.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 6.Duchenne G. I’Electrisation Localisee at de son Application a la Pathologie at a la Therapeutique. Paris: Bailliere et Fils; 1861. [Google Scholar]
- 7.Anderson JL, Head SI, Rae C, Morley JW. Brain function in Duchenne muscular dystrophy. Brain. 2002;125:4–13. doi: 10.1093/brain/awf012. [DOI] [PubMed] [Google Scholar]
- 8.Nardes F, Araujo AP, Ribeiro MG. Mental retardation in Duchenne muscular dystrophy. J Pediatr (Rio J ) 2012;88:6–16. doi: 10.2223/JPED.2148. [DOI] [PubMed] [Google Scholar]
- 9.Cotton SM, Voudouris NJ, Greenwood KM. Association between intellectual functioning and age in children and young adults with Duchenne muscular dystrophy: further results from a meta-analysis. Dev Med Child Neurol. 2005;47:257–265. doi: 10.1017/s0012162205000496. [DOI] [PubMed] [Google Scholar]
- 10.Hinton VJ, Fee RJ, Goldstein EM, De Vivo DC. Verbal and memory skills in males with Duchenne muscular dystrophy. Dev Med Child Neurol. 2007;49:123–128. doi: 10.1111/j.1469-8749.2007.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingeier K, Giger E, Strozzi S, et al. Neuropsychological impairments and the impact of dystrophin mutations on general cognitive functioning of patients with Duchenne muscular dystrophy. J Clin Neurosci. 2011;18:90–95. doi: 10.1016/j.jocn.2010.07.118. [DOI] [PubMed] [Google Scholar]
- 12.Cotton S, Voudouris NJ, Greenwood KM. Intelligence and Duchenne muscular dystrophy: full-scale, verbal, and performance intelligence quotients. Dev Med Child Neurol. 2001;43:497–501. doi: 10.1017/s0012162201000913. [DOI] [PubMed] [Google Scholar]
- 13.Wicksell RK, Kihlgren M, Melin L, Eeg-Olofsson O. Specific cognitive deficits are common in children with Duchenne muscular dystrophy. Dev Med Child Neurol. 2004;46:154–159. doi: 10.1017/s0012162204000283. [DOI] [PubMed] [Google Scholar]
- 14.Marini A, Lorusso ML, D’Angelo MG, et al. Evaluation of narrative abilities in patients suffering from Duchenne Muscular Dystrophy. Brain Lang. 2007;102:1–12. doi: 10.1016/j.bandl.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Muntoni F, Mateddu A, Serra G. Passive avoidance behaviour deficit in the mdx mouse. Neuromuscul Disord. 1991;1:121–123. doi: 10.1016/0960-8966(91)90059-2. [DOI] [PubMed] [Google Scholar]
- 16.Perronnet C, Chagneau C, Le BP, et al. Upregulation of brain utrophin does not rescue behavioral alterations in dystrophin-deficient mice. Hum Mol Genet. 2012;21:2263–2276. doi: 10.1093/hmg/dds047. [DOI] [PubMed] [Google Scholar]
- 17.Vaillend C, Rendon A, Misslin R, Ungerer A. Influence of dystrophin-gene mutation on mdx mouse behavior. I. Retention deficits at long delays in spontaneous alternation and bar-pressing tasks. Behav Genet. 1995;25:569–579. doi: 10.1007/BF02327580. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka M, Okuno T, Honda Y, Nakano Y. Central nervous system involvement in progressive muscular dystrophy. Arch Dis Child. 1980;55:589–594. doi: 10.1136/adc.55.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen DH, Takeshima Y, Ishikawa Y, Ishikawa Y, Minami R, Matsuo M. A novel deletion of the dystrophin S-promoter region cosegregating with mental retardation. Neurology. 1999;52:638–640. doi: 10.1212/wnl.52.3.638. [DOI] [PubMed] [Google Scholar]
- 20.Rae C, Scott RB, Thompson CH, et al. Brain biochemistry in Duchenne muscular dystrophy: a 1H magnetic resonance and neuropsychological study. J Neurol Sci. 1998;160:148–157. doi: 10.1016/s0022-510x(98)00190-7. [DOI] [PubMed] [Google Scholar]
- 21.Kreis R, Wingeier K, Vermathen P, et al. Brain metabolite composition in relation to cognitive function and dystrophin mutations in boys with Duchenne muscular dystrophy. NMR Biomed. 2011;24:253–262. doi: 10.1002/nbm.1582. [DOI] [PubMed] [Google Scholar]
- 22.Tracey I, Dunn JF, Parkes HG, Radda GK. An in vivo and in vitro H-magnetic resonance spectroscopy study of mdx mouse brain: abnormal development or neural necrosis? J Neurol Sci. 1996;141:13–18. doi: 10.1016/0022-510x(96)00135-9. [DOI] [PubMed] [Google Scholar]
- 23.Cyrulnik SE, Hinton VJ. Duchenne muscular dystrophy: a cerebellar disorder? Neurosci Biobehav Rev. 2008;32:486–496. doi: 10.1016/j.neubiorev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Tracey I, Dunn JF, Radda GK. Brain metabolism is abnormal in the mdx model of Duchenne muscular dystrophy. Brain. 1996;119 (Pt 3):1039–1044. doi: 10.1093/brain/119.3.1039. [DOI] [PubMed] [Google Scholar]
- 25.Uchino M, Yoshioka K, Miike T, et al. Dystrophin and dystrophin-related protein in the brains of normal and mdx mice. Muscle Nerve. 1994;17:533–538. doi: 10.1002/mus.880170511. [DOI] [PubMed] [Google Scholar]
- 26.Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 27.Lansman JB, Franco A., Jr What does dystrophin do in normal muscle? J Muscle Res Cell Motil. 1991;12:409–411. doi: 10.1007/BF01738325. [DOI] [PubMed] [Google Scholar]
- 28.Lovering RM, Michaelson L, Ward CW. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol Cell Physiol. 2009;297:C571–C580. doi: 10.1152/ajpcell.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci. 1998;18:128–137. doi: 10.1523/JNEUROSCI.18-01-00128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong J, Anderson JE. Dystrophin is required for organizing large acetylcholine receptor aggregates. Brain Res. 1999;839:298–304. doi: 10.1016/s0006-8993(99)01737-0. [DOI] [PubMed] [Google Scholar]
- 31.Pratt SJ, Shah SB, Ward CW, Inacio MP, Stains JP, Lovering RM. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol. 2013;591:559–570. doi: 10.1113/jphysiol.2012.241679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khairallah RJ, Shi G, Sbrana F, et al. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal. 2012;5:ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 34.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3 2007. [Google Scholar]
- 35.Xu S, Ji Y, Chen X, Yang Y, Gullapalli RP, Masri R. In vivo high-resolution localized (1) H MR spectroscopy in the awake rat brain at 7 T. Magn Reson Med. 2013;69:937–943. doi: 10.1002/mrm.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 37.Zhuo J, Xu S, Proctor JL, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59:467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golmohammadi MG, Blackmore DG, Large B, et al. Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells. 2008;26:979–987. doi: 10.1634/stemcells.2007-0919. [DOI] [PubMed] [Google Scholar]
- 39.Petrof BJ. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am J Phys Med Rehabil. 2002;81:S162–S174. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- 40.Rando TA. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 41.Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2:731–740. doi: 10.1016/s1474-4422(03)00585-4. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Tovar CG, Perez A, Luna J, et al. Biochemical and histochemical analysis of 71 kDa dystrophin isoform (Dp71f) in rat brain. Acta Histochem. 2001;103:209–224. doi: 10.1078/0065-1281-00591. [DOI] [PubMed] [Google Scholar]
- 43.Daoud F, Angeard N, Demerre B, et al. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum Mol Genet. 2009;18:3779–3794. doi: 10.1093/hmg/ddp320. [DOI] [PubMed] [Google Scholar]
- 44.Moizard MP, Toutain A, Fournier D, et al. Severe cognitive impairment in DMD: obvious clinical indication for Dp71 isoform point mutation screening. Eur J Hum Genet. 2000;8:552–556. doi: 10.1038/sj.ejhg.5200488. [DOI] [PubMed] [Google Scholar]
- 45.Taylor PJ, Betts GA, Maroulis S, et al. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One. 2010;5:e8803. doi: 10.1371/journal.pone.0008803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenk U, Hanke R, Thiele H, Speer A. Point mutations at the carboxy terminus of the human dystrophin gene: implications for an association with mental retardation in DMD patients. Hum Mol Genet. 1993;2:1877–1881. doi: 10.1093/hmg/2.11.1877. [DOI] [PubMed] [Google Scholar]
- 47.Culligan K, Ohlendieck K. Diversity of the Brain Dystrophin-Glycoprotein Complex. J Biomed Biotechnol. 2002;2:31–36. doi: 10.1155/S1110724302000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lidov HG, Byers TJ, Watkins SC, Kunkel LM. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990;348:725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- 49.Kim TW, Wu K, Xu JL, Black IB. Detection of dystrophin in the postsynaptic density of rat brain and deficiency in a mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1992;89:11642–11644. doi: 10.1073/pnas.89.23.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nico B, Frigeri A, Nicchia GP, et al. Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice. Glia. 2003;42:235–251. doi: 10.1002/glia.10216. [DOI] [PubMed] [Google Scholar]
- 51.Goodnough CL, Gao Y, Li X, et al. Lack of dystrophin results in abnormal cerebral diffusion and perfusion in vivo. Neuroimage. 2014;102(Pt 2):809–816. doi: 10.1016/j.neuroimage.2014.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warskulat U, Flogel U, Jacoby C, et al. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004;18:577–579. doi: 10.1096/fj.03-0496fje. [DOI] [PubMed] [Google Scholar]
- 53.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 54.Conte CD, Tricarico D, Pierno S, et al. Taurine and skeletal muscle disorders. Neurochem Res. 2004;29:135–142. doi: 10.1023/b:nere.0000010442.89826.9c. [DOI] [PubMed] [Google Scholar]
- 55.Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol. 2008;586:5063–5075. doi: 10.1113/jphysiol.2008.160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol. 2004;557:59–75. doi: 10.1113/jphysiol.2004.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung EW, Whitehead NP, Suchyna TM, Gottleib PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2004 doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernandez-Ochoa EO, Pratt SJ, Garcia-Pelagio KP, Schneider MF, Lovering RM. Disruption of action potential and calcium signaling properties in malformed myofibers from dystrophin-deficient mice. Physiol Rep. 2015;3 doi: 10.14814/phy2.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woods CE, Novo D, DiFranco M, Capote J, Vergara JL. Propagation in the transverse tubular system and voltage dependence of calcium release in normal and mdx muscle fibres. J Physiol. 2005 doi: 10.1113/jphysiol.2005.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedrich O, von WF, Chamberlain JS, Fink RH, Rohrbach P. L-type Ca2+ channel function is linked to dystrophin expression in mammalian muscle. PLoS One. 2008;3:e1762. doi: 10.1371/journal.pone.0001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kar NC, Pearson CM. Catalase, superoxide dismutase, glutathione reductase and thiobarbituric acid-reactive products in normal and dystrophic human muscle. Clin Chim Acta. 1979;94:277–280. doi: 10.1016/0009-8981(79)90076-7. [DOI] [PubMed] [Google Scholar]
- 63.Mizuno Y. Changes in superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activities and thiobarbituric acid-reactive products levels in early stages of development in dystrophic chickens. Exp Neurol. 1984;84:58–73. doi: 10.1016/0014-4886(84)90006-2. [DOI] [PubMed] [Google Scholar]
- 64.Murphy ME, Kehrer JP. Activities of antioxidant enzymes in muscle, liver and lung of chickens with inherited muscular dystrophy. Biochem Biophys Res Commun. 1986;134:550–556. doi: 10.1016/s0006-291x(86)80455-7. [DOI] [PubMed] [Google Scholar]
- 65.Rae C, Griffin JL, Blair DH, et al. Abnormalities in brain biochemistry associated with lack of dystrophin: studies of the mdx mouse. Neuromuscul Disord. 2002;12:121–129. doi: 10.1016/s0960-8966(01)00253-x. [DOI] [PubMed] [Google Scholar]
- 66.Kanfer JN, McCartney DG. Reduced phosphorylcholine hydrolysis by homogenates of temporal regions of Alzheimer’s brain. Biochem Biophys Res Commun. 1986;139:315–319. doi: 10.1016/s0006-291x(86)80115-2. [DOI] [PubMed] [Google Scholar]
- 67.Pettegrew JW, Moossy J, Withers G, McKeag D, Panchalingam K. 31P nuclear magnetic resonance study of the brain in Alzheimer’s disease. J Neuropathol Exp Neurol. 1988;47:235–248. doi: 10.1097/00005072-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Sok DE. Oxidative inactivation of brain alkaline phosphatase responsible for hydrolysis of phosphocholine. J Neurochem. 1999;72:355–362. doi: 10.1046/j.1471-4159.1999.0720355.x. [DOI] [PubMed] [Google Scholar]
- 69.Reggiani C. Between channels and tears: aim at ROS to save the membrane of dystrophic fibres. J Physiol. 2008;586:1779. doi: 10.1113/jphysiol.2008.152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 71.Goyenvalle A, Griffith G, Babbs A, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med. 2015;21:270–275. doi: 10.1038/nm.3765. [DOI] [PubMed] [Google Scholar]
- 72.Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- 73.Glesby MJ, Rosenmann E, Nylen EG, Wrogemann K. Serum CK, calcium, magnesium, and oxidative phosphorylation in mdx mouse muscular dystrophy. Muscle Nerve. 1988;11:852–856. doi: 10.1002/mus.880110809. [DOI] [PubMed] [Google Scholar]
- 74.Muntoni F, Mateddu A, Marchei F, Clerk A, Serra G. Muscular weakness in the mdx mouse. J Neurol Sci. 1993;120:71–77. doi: 10.1016/0022-510x(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 75.De la PS, Morin S, Koenig J. Characteristics of skeletal muscle in mdx mutant mice. Int Rev Cytol. 1999;191:99–148. doi: 10.1016/s0074-7696(08)60158-8. [DOI] [PubMed] [Google Scholar]
- 76.Dangain J, Vrbova G. Muscle development in mdx mutant mice. Muscle Nerve. 1984;7:700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- 77.Dubowitz V, Crome L. The central nervous system in Duchenne muscular dystrophy. Brain. 1969;92:805–808. doi: 10.1093/brain/92.4.805. [DOI] [PubMed] [Google Scholar]
- 78.Bresolin N, Castelli E, Comi GP, et al. Cognitive impairment in Duchenne muscular dystrophy. Neuromuscul Disord. 1994;4:359–369. doi: 10.1016/0960-8966(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt B, Watters GV, Rosenblatt B, Silver K. Increased head circumference in patients with Duchenne muscular dystrophy. Ann Neurol. 1985;17:620–621. doi: 10.1002/ana.410170622. [DOI] [PubMed] [Google Scholar]
- 80.Appleton RE, Bushby K, Gardner-Medwin D, Welch J, Kelly PJ. Head circumference and intellectual performance of patients with Duchenne muscular dystrophy. Dev Med Child Neurol. 1991;33:884–890. doi: 10.1111/j.1469-8749.1991.tb14797.x. [DOI] [PubMed] [Google Scholar]
- 81.Rosman NP, Kakulas BA. Mental deficiency associated with muscular dystrophy. A neuropathological study. Brain. 1966;89:769–788. doi: 10.1093/brain/89.4.769. [DOI] [PubMed] [Google Scholar]
- 82.Rosman NP. The cerebral defect and myopathy in Duchenne muscular dystrophy. A comparative clinicopathological study. Neurology. 1970;20:329–335. doi: 10.1212/wnl.20.4.329. [DOI] [PubMed] [Google Scholar]
- 83.Septien L, Gras P, Borsotti JP, Giroud M, Nivelon JL, Dumas R. Mental development in Duchenne muscular dystrophy. Correlation of data of the brain scanner. Pediatrie. 1991;46:817–819. [PubMed] [Google Scholar]
- 84.al-Qudah AA, Kobayashi J, Chuang S, Dennis M, Ray P. Etiology of intellectual impairment in Duchenne muscular dystrophy. Pediatr Neurol. 1990;6:57–59. doi: 10.1016/0887-8994(90)90081-b. [DOI] [PubMed] [Google Scholar]
- 85.Ortiz JG, Negron AE, Garcia MT, Rosado JE, Maldonaldo CS. The C57BL/10Bg sps/sps mouse: a mutant with absence-like seizures; neurochemical and behavioral correlates. Neurosci Lett. 1990;114:231–236. doi: 10.1016/0304-3940(90)90077-m. [DOI] [PubMed] [Google Scholar]
- 86.Kueh SL, Dempster J, Head SI, Morley JW. Reduced postsynaptic GABAA receptor number and enhanced gaboxadol induced change in holding currents in Purkinje cells of the dystrophin-deficient mdx mouse. Neurobiol Dis. 2011;43:558–564. doi: 10.1016/j.nbd.2011.05.002. [DOI] [PubMed] [Google Scholar]