Introduction
The launch of oral direct-acting antivirals (DAAs) to treat chronic hepatitis C virus (HCV) infection represents a significant shift in the HCV treatment paradigm. With DAAs, the sustained virologic response (SVR), i.e. efficacy of treatment has increased to more than 90%, treatment duration has decreased to as few as 8 weeks, and these regimens have no major side effects. Coupled with the updates in HCV screening guidelines, use of new DAAs could make HCV a rare disease in the next 20 years in the United States (US).1
However, the high price of DAAs is a barrier, and has drawn criticism from patients and payers.2–4 Challenged with the budget needed to treat all HCV patients, Medicaid in at least 30 US states has restricted these treatments to patients with advanced fibrosis stage.5 With more than a million patients needing HCV treatment in the next 3–5 years in the US, the high price of DAAs could impact the budget of private payers and government. On the other hand, several recent studies have shown that these drugs provide a good value for money. Furthermore, the price of DAAs has come down since their first availability. For example, the average discounts on sofosbuvir-based regimens in 2015 are 46%.6 As additional antiviral drugs become available in the near future, drug prices may drop even further.
Here we discuss the value of HCV treatment with oral DAAs considering new discounts, the importance of treating all HCV patients, and how HCV treatment costs and value compare to that of human immunodeficiency treatment (HIV) treatment.
Value of HCV Treatment
Recently published cost-effectiveness studies showed that HCV regimens based on sofosbuvir, ledipasvir, and simeprevir are cost-effective for most patients.7–12 The incremental cost-effectiveness ratios (ICERs) of these regimens (when compared to the old standard of care) ranged from $10,000 to $284,000 per QALY depending on the patient’s status with respect to treatment history, HCV genotype, and cirrhosis status. The average ICER for all HCV patients was $55,400 per QALY.7 The ICERs of treatment with older therapies based on first-generation protease inhibitors, boceprevir and telaprevir, were between $17,000 and $103,000 per QALY, depending on disease stage.13–17 The ICERs of peginterferon-ribavirin (in comparison with peginterferon) were between $26,000–$64,000 per QALY. In general, the ICERs were higher in patients with early stages of liver fibrosis than in those with advanced fibrosis. Collectively, these data show that throughout its history, compared to the previous standard, overall the “new” HCV treatment cost an additional ~50,000 to 100,000 for one additional QALY gained and the DAAs are no exception.
HCV Treatment is Now Cost-Saving
With recent rebates on drug prices, sofosbuvir-based treatment in 2015 on an average costs 54% of the wholesale acquisition cost.6 Applying these discounted drug prices to our previously published simulation model,7 we evaluated the cost-effectiveness of DAAs. We found that compared to treatment with telaprevir / boceprevir or peginterferon-based therapies, treatment with sofosbuvir-ledipasvir regimens is cost-saving in all patients, i.e., these regimens increased QALYs and saved healthcare costs (Figure 1). This effect was most prominent in patients with genotype 1 infection. Treatment was not cost-saving, although it was cost-effective, in patients with other genotypes.
Figure 1.
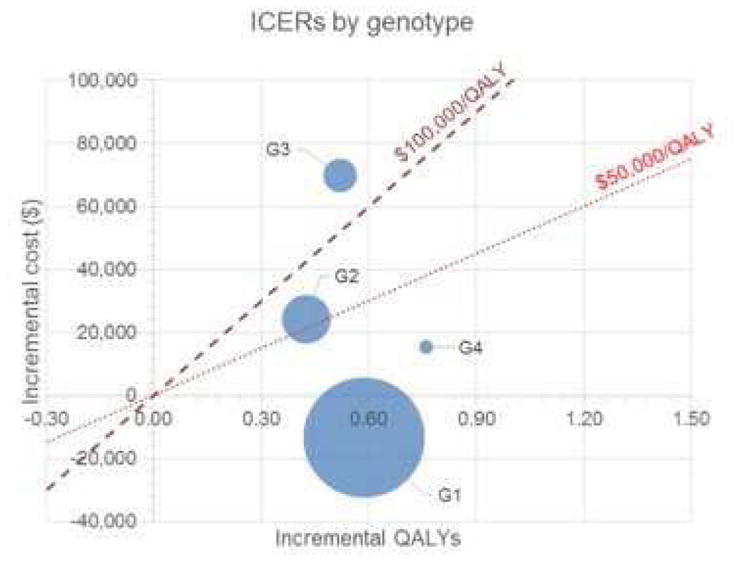
Incremental cost and effectiveness of new antiviral regimens in comparison with old standard-of-care by HCV genotype
The size of each bubble represents the relative population size needing treatment. The center of the bubble represent the incremental costs and QALYs of sofosbuvir/ledipasvir-based therapies in comparison with the old standard of care. Bubbles below the red line are cost-effective at that threshold, and bubbles below the green line are cost-saving strategies. For instance, treatment of genotype 1 patients with sofosbuvir-ledipasvir in comparison with telaprevir/boceprevir will increase QALYs and decease costs, i.e., cost-saving strategy. Treatment of genotype 4 patients will increase both QALYs and costs, but is still cost-effective at $100,000 willingness to pay threshold. The weighted average of the results across all genotypes is cost-saving.
Decreased Cost Per SVR
Although the cost of antiviral treatment increased with the availability of new therapies, the cost-per-SVR has decreased. As shown in Figure 2, the cost of treating HCV genotype 1 with peginterferon-ribavirin, first-generation protease inhibitors, and sofosbuvir-ledipasvir (at wholesale acquisition cost) increased from $43,000 to $103,000 per patient. However, the corresponding costs-per-SVR decreased from $213,000 to $108,000. After applying the recent discounts (46%), the cost of treatment decreased to $56,000, which is less expensive than boceprevir- and telaprevir-based therapies, and the cost-per-SVR fell to $58,000.
Figure 2.
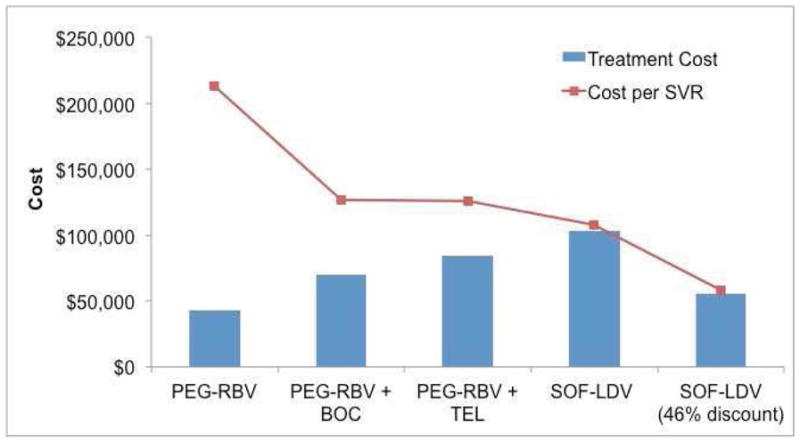
Cost and cost-per-SVR of different antiviral regimens to treat patients with hepatitis C virus genotype 1
The cost of treatment increased from peginterferon-ribavirin (PEG-RBV) to sofosbuvir-ledipasvir (SOF-LDV) in genotype 1 patients, however the corresponding costs-per-SVR decreased at the same time. Furthermore, at 46% discounts, both costs and cost-per-SVR of SOF-LDV were lower than boceprevir (BOC)- and telaprevir (TEL)-based therapies, the old standard of care.
Health Economics of HCV versus HIV Treatment
HCV has superseded HIV as a cause of death in the US since 2007.18 Therefore, to put the health economics of HCV into perspective, we can compare the cost of HCV treatment with DAAs to the cost of treating HIV. The discounted lifetime cost of treating one person with HIV in the US is $315,000 in 2014 US Dollar.19 The corresponding cost of curing HCV with oral DAAs is $58,000--which is only 18% of the total HIV treatment cost. HIV antiretroviral treatment is cost-effective in the US;20 HCV treatment is cost-saving.
The total federal budget requested for HIV and AIDS in 2015 is $24.2 billion, of which $17.5 billion is allocated to HIV treatment and care.21 Ryan White’s AIDS Drug Assistance Program, which provides access to HIV-related medications to people with HIV, is funded at $900 million. The federal spending on HCV treatment is unknown. However, using a simulation model, we predicted that the maximum 5-year budget needed to treat all patients (by private as well as government payers) who are candidates for HCV treatment is $37 billion, ie, $7.4 billion per year.7 Of note, unlike HIV, HCV treatment offers a cure; therefore annual spending on HCV treatment would reduce sharply in subsequent years.
Why we should be willing to pay for HCV treatment
The cost of HCV treatment with the available oral DAAs has decreased substantially since their first availability in 2014. Furthermore, we anticipate more discounts with increased competition from other manufacturers in the near future. The overall budget needed to treat HCV is not huge and is reasonable when comparable to that of HIV. Therefore, HCV treatment should be not be restricted to only those in advanced fibrosis stages. We have an opportunity to eliminate hepatitis C by taking appropriate and timely steps. We as a society should be willing to pay for the current HCV therapies by providing additional resources and giving attention to hepatitis C that it deserves.
Acknowledgments
Funding/Support: This study was supported in parts by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000146. Dr. Kanwal’s effort was supported in part by the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413).
Footnotes
Author contribution:
Study concept and design: Chhatwal, Kanwal
Drafting of manuscript: Chhatwal
Critical revision of the manuscript for important intellectual content: Chhatwal, Chen, Kanwal
Statistical analysis: Chhatwal, Chen
Interpretation of data: Chhatwal, Chen, Kanwal
Conflict of interest disclosures: Chhatwal received a consulting fee from Merck, Gilead and Complete HEOR Solutions, and none to report for Chen and Kanwal.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kabiri M, Jazwinski AB, Roberts MS, et al. The changing burden of hepatitis C in the United States: Model-based predictions. Annals of Internal Medicine. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sussman NL, Remien CH, Kanwal F. The end of hepatitis C. Clin Gastroenterol Hepatol. 2014;12:533–6. doi: 10.1016/j.cgh.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Sherker AH. Therapy for hepatitis C—The costs of success. N Engl J Med. 2014;370:1552–1553. doi: 10.1056/NEJMe1401508. [DOI] [PubMed] [Google Scholar]
- 4.Knox R. NPR. NPR; Dec 30, 2014. [Accessed March 21, 2014]. $1,000 pill for hepatitis C spurs debate over drug prices. http://www.npr.org/blogs/health/2013/12/30/256885858/-1-000-pill-for-hepatitis-c-spurs-debate-over-drug-prices. NPR 2013. [Google Scholar]
- 5.Japsen B. As Pricey Hepatitis Pill Harvoni Joins Sovaldi, States Erect Medicaid Hurdles. Forbes; 2014. Retrieved from: http://www.forbes.com/sites/brucejapsen/2014/10/10/as-hepatitis-pill-harvoni-joins-sovaldi-states-erect-medicaid-hurdles/ [Google Scholar]
- 6.Silverman E. What the ‘Shocking’ Gilead Discounts on its Hepatitis C Drugs Will Mean. [last accessed February 4, 2015];The Wall Street Journal. 2015 Retrieved from: http://blogs.wsj.com/pharmalot/2015/02/04/what-the-shocking-gilead-discounts-on-its-hepatitis-c-drugs-will-mean/
- 7.Chhatwal J, Kanwal F, Roberts MS, et al. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Annals of Internal Medicine. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linas BP, Barter DM, Morgan JR, et al. The Cost-Effectiveness of Sofosbuvir-Based Regimens for Treatment of Hepatitis C Virus Genotype 2 or 3 Infection. Ann Intern Med. 2015 doi: 10.7326/M14-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407–19. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 10.Rein DB, Wittenborn JS, Smith BD, et al. The Cost-effectiveness, Health Benefits, and Financial Costs of New Antiviral Treatments for Hepatitis C Virus. Clin Infect Dis. 2015 doi: 10.1093/cid/civ220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwabara H, Westerhout K, Treur M, et al. Cost-effectiveness analysis of simeprevir in combination with peginterferon and ribavirin for treatment-naive chronic hepatitis C genotype 1 patients in Japan. J Med Econ. 2015:1–10. doi: 10.3111/13696998.2015.1029492. [DOI] [PubMed] [Google Scholar]
- 12.Younossi ZM, Park H, Saab S, et al. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2015;41:544–63. doi: 10.1111/apt.13081. [DOI] [PubMed] [Google Scholar]
- 13.Brogan AJ, Talbird SE, Thompson JR, et al. Cost-effectiveness of Telaprevir combination therapy for chronic hepatitis C. PLoS One. 2014;9:e90295. doi: 10.1371/journal.pone.0090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhatwal J, Ferrante SA, Brass C, et al. Cost-Effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 Infection in the United States. Value in Health. 2013;16:973–986. doi: 10.1016/j.jval.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrante SA, Chhatwal J, Brass CA, et al. Boceprevir for previously untreated patients with chronic hepatitis C Genotype 1 infection: a US-based cost-effectiveness modeling study. BMC infectious diseases. 2013;13:190. doi: 10.1186/1471-2334-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Cipriano LE, Holodniy M, et al. New protease inhibitors for the treatment of chronic hepatitis C: A cost-effectiveness analysis. Annals of internal medicine. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11:1503–10. doi: 10.1016/j.cgh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Ly KN, Xing J, Klevens RM, et al. The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007. Annals of internal medicine. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 19.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Medical care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 20.Walensky RP, Freedberg KA, Weinstein MC, et al. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis. 2007;45 (Suppl 4):S248–54. doi: 10.1086/522546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser Family Foundation. [last accessed: April 8, 2015];US Federal Funding for HIV/AIDS: The President’s FY 2015 Budget Request. Retreieved from: http://kff.org/global-health-policy/fact-sheet/u-s-federal-funding-for-hivaids-the-presidents-fy-2015-budget-request/


