Abstract
Impaired T cell responses are a defining characteristic of HIV infection but the extent to which altered mononuclear phagocyte function contributes to this defect is unclear. We show that mononuclear phagocytes enriched from rhesus macaque lymph nodes have suppressed ability to stimulate CD4 T cell proliferation and IFN-γ release after acute SIV infection. When individual populations were isolated, myeloid dendritic cells (mDC) and macrophages but not plasmacytoid dendritic cells (pDC) had suppressed capacity to stimulate CD4 T cell proliferation, with macrophage function declining as infection progressed. Macrophages but not pDC or mDC had suppressed capacity to induce IFN-γ release from CD4 T cells in acute infection, even after stimulation with virus-encoded TLR7/8 ligand. Changes in expression of costimulatory molecules did not explain loss of function after infection. Conversely, pDC and mDC had marked loss of IFN-α and IL-12 production, respectively, and macrophages lost production of both cytokines. In T cell co-cultures without TLR7/8 ligand macrophages were the primary source of IL-12 which was profoundly suppressed after infection and correlated with loss of IFN-γ release by T cells. TLR7/8-stimulated pDC, mDC and macrophages all produced IL-12 in T cell co-cultures which was suppressed in chronic infection. Supplementing IL-12 enhanced mDC-driven IFN-γ release from T cells, and IL-12 and IFN-α together restored function in TLR7/8-activated macrophages. These findings reveal loss of macrophage and mDC T cell-stimulating function in lymph nodes of SIV-infected rhesus macaques associated with diminished IL-12 and IFN-α production that may be a factor in AIDS immunopathogenesis.
Introduction
Mononuclear phagocytes including dendritic cells (DC) and macrophages are integral components of both innate and adaptive immunity. HIV and SIV infection leads to depletion of CD4 T cells and DC (1–5) and diminished Ag-specific T cell responses (6–8), but the relationship between mononuclear phagocyte function and the T cell response remains ill-defined. Many groups have examined the impact of HIV and SIV infection on production of pro-inflammatory cytokines by isolated DC and macrophages (3, 9–15) as well as the effect of HIV exposure in vitro on the IFN response (16). However, studies exploring the T-cell stimulating function of myeloid DC (mDC) and plasmacytoid DC (pDC) in HIV or SIV infection have been limited (11, 13, 14, 17), and virtually nothing is known about the APC function of macrophages in these infections.
The major site of virus replication and T-cell priming by mononuclear phagocytes is the lymph node and SIV-specific T cell responses in lymph nodes but not blood correlate with vaccine-induced protection from infection (18). SIV infection has profound effects on mononuclear phagocyte subsets in the lymph node. During acute infection there is increased recruitment and turnover of pDC, mDC and macrophages (15, 19–23), and pDC and macrophages from SIV-infected lymph nodes have reduced responsiveness to ex vivo stimulation (15). However, the capacity for lymph node DC and macrophages to serve as effective APC in HIV and SIV infection is understudied (24, 25). To address these gaps in knowledge, we performed a comprehensive study of DC and macrophage CD4 T-cell stimulating functions in lymph nodes of rhesus macaques with pathogenic SIV infection.
Materials and Methods
Animals, sample collection, and tissue processing
A total of 30 adult male Indian-origin rhesus macaques were used in this study. All protocols and experiments performed on macaques were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and were in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals. Five animals were infected by i.v. inoculation with SIVmac251 and sacrificed at acute infection (day 36) when inguinal and axillary lymph nodes were harvested, as previously reported (15). Pre-infection lymph node biopsies from these animals were also available for analysis. An additional 10 macaques were infected by i.v. inoculation with either SIVmac251 or SIVΔB670 and sacrificed at the chronic stage of infection (range = day 77 to 470, median = day 404) when inguinal and axillary lymph nodes were harvested, as previously described (26–28) (Table I). Inguinal and axillary lymph nodes from 15 healthy, SIV-naïve macaques were used as controls. Lymph nodes were digested and single cell suspensions generated using 1 mg/ml collagenase D (Sigma) and 20 ug/ml DNAse I (Roche) in RPMI 1640 with 2% FBS and 10 mM HEPES and cryopreserved for later experiments.
Table I.
Characteristics of animal cohort
| Animal ID | Virus | Time of sacrifice (day) | Plasma viral load (RNA copies/mL) | Reference |
|---|---|---|---|---|
| Acute infection | ||||
| M128-08 | SIVmac251 | 36 | 7,343 | (15) |
| M130-08 | SIVmac251 | 36 | 52,361 | (15) |
| M133-08 | SIVmac251 | 36 | 4,200 | (15) |
| M139-08 | SIVmac251 | 36 | 6,716 | (15) |
| M140-08 | SIVmac251 | 36 | 21,974 | (15) |
| Chronic infection | ||||
| R189 | SIVmac251 | 77 | 44,000,000 | (27) |
| R488 | SIVmac251 | 125 | 27,000,000 | (27) |
| M91-10 | SIVmac251 | 132 | 1,070 | (28) |
| R183 | SIVmac251 | 305 | 151,000 | (27) |
| M15001 | SIVΔB670 | 365 | 145,0001 | (26) |
| R481 | SIVmac251 | 442 | 46,7002 | (27) |
| R484 | SIVmac251 | 449 | 63,5002 | (27) |
| R489 | SIVmac251 | 456 | 178,0002 | (27) |
| M135-08 | SIVmac251 | 465 | 2,108 | (28) |
| R479 | SIVmac251 | 470 | 81,2002 | (27) |
Viral load measured at 336 days.
Viral load measured at 406 days.
Generation of the responder population of CD4 T lymphocytes
For responder cells we used allogeneic CD4 T cells isolated from an SIV-naïve rhesus macaque rather than autologous cells, as SIV infection affects CD4 T cell function and Ag specificity to varying degrees between animals (29) which would produce a confounding variable in our experiments. Given the extent of experiments we used isolated CD4 T cells from two different animals over the course of the study. Peliminary data indicated there was no discernable difference in CD4 T cell proliferation or IFN-γ production between the two donor animals (data not shown). PBMC were enriched for CD4 T lymphocytes by negative selection through magnetic-activated cell sorting with Ab against MHC-II, CD20, CD14, and CD8 according to the manufacturer’s protocol (Miltenyi). The resulting responder cells populations were >80% pure as measured through flow cytometric analysis, and were aliquoted and cryopreserved for later use.
Identification and selection of mononuclear phagocyte populations
mAb used in flow cytometry experiments were purchased from BD Biosciences (unless otherwise noted) and were specific for CD3 (clone SP34-2), CD20 (2H7, eBioscience), MHC-II (L243/G46-6), CD163 (GHI/61), CD11c (S-HCL-3), CD123 (7G3), and CD8 (RPA-T8). An enriched population of lymph node mononuclear phagocytes was generated by sorting CD3−CD20−CD8−MHC-II+ cells. To positively select subsets of mononuclear phagocytes from lymph nodes cells were sorted based on the following phenotype: pDC were CD3−CD20−MHC-II+CD163−CD123+CD11c−, mDC were CD3−CD20− MHC-II++CD163−CD123−CD11c+, and macrophages were CD3−CD20−MHC-II+CD163+ (15). All sorting was done using a biocontained FACSAria (BD Biosciences) and data were analyzed using FlowJo 8.8.7 (Tree Star).
Measurement of T lymphocyte proliferation through CFSE dilution
Twenty thousand enriched or positively selected mononuclear phagocytes were incubated in complete media (RPMI 1640 containing 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM glutamine, 10 mM HEPES, 0.1 mM nonessential amino acids, 0.1 mM sodium pyruvate) with or without toxic shock syndrome toxin-1 (TSST) at 1, 10 or 100ng/mL for 1 h at 37°C.
Cells were then washed and transferred to a sterile 96-well round bottom tissue-culture treated plate. Responder CD4 T cells isolated from one donor animal were labeled with CFSE according to the CellTrace CFSE cell proliferation kit protocol (Invitrogen). Two hundred thousand CFSE labeled responder cells were added to each well and the volume of all of the wells normalized to 200uL with complete media. The co-culture was allowed to incubate for 5 d. The proliferation of T lymphocytes was then measured through flow cytometric analysis by staining for CD3 and CD4 while recording CFSE dilution on the FITC channel. Dead cells were excluded through the use of a Live/Dead viability kit (Invitrogen). All terminal flow cytometric data were collected on an LSR-II flow cytometer with BD FACS Diva 6.0 and analyzed with FlowJo 8.8.7.
Detection of IFN-γ producing T lymphocytes by ELISPOT assay
Twenty thousand mononuclear phagocytes were placed directly into an anti-IFN-γ ELISPOT plate that had already been coated and blocked according to the manufacturer’s protocol (3420M-2A, Mabtech). The mononuclear phagocytes were either incubated in complete media or exposed to 1ug of the SIVmac251-derived ssRNA oligonucleotide Env976 that is a ligand for TLR7/8 and a potent activator of mononuclear phagocytes as previously shown (15). After a 2 h low-volume incubation 200,000 responder CD4 T cells isolated from one donor animal were added to each well and the volume of all of the wells normalized to 200uL. In assays measuring recovery of function, exogenous recombinant rhesus IFN-α2 (1000 IU/mL, PBL Interferon Source) and/or recombinant rhesus IL-12 (10ng/mL, R&D Systems) were added to each well. After 2 d of co-culturing, supernatants were harvested and stored at −80°C for IL-12 detection. The plates were then developed to quantify IFN-γ spot-forming units (SFU) according to the manufacturer’s protocol (3420M-2A, Mabtech).
Costimulatory molecule expression
Paired single cell suspensions of lymph nodes collected before and after infection were exposed to 10ug/mL of Env976 for 7 h at 37°C. The samples were then stained for viability, the surface markers mentioned above for cell identification, and CD40 (5C3), CD80 (L307.4), CD86 (2331 (FUN-1), and CD274 (PD-L1, 29E.2A3). Paired samples from the same animals were stained and analyzed together to control for potential daily differences in flow cytometer calibration.
Quantification of cytokine production through intracellular staining and ELISA
Lymph node cell suspensions were maintained in complete media with or without 10ug/mL of Env976 for 7 h at 37°C. The final 5 h of incubation were in the presence of 1ug/mL brefeldin A. The samples were then stained for viability, surface markers and the intracellular cytokines IFN-α (225.C) and IL-12 (C11.5) as previously described (15). Supernatants from sorted pDC, mDC, and macrophage co-cultures were collected after 48 h as mentioned above. The ELISA assay to detect the p40 subunit of IL-12 was performed according to the manufacturer’s protocol (CT719-10, U-Cytech).
Statistical analyses
Statistical analyses such as t-tests were performed using GraphPad Prism v6 software, while ANOVA and repeated measures analyses were done with R v3.1. P-values less than 0.05 were considered significant. Comparisons between treatment arms and/or study groups were tested by means of linear contrasts on the ANOVA parameters. The ANOVA assumptions of all models were graphically checked, and data were log-transformed when necessary. Tests used for each experiment are noted in figure legends.
Results
Enriched mononuclear phagocytes from acutely infected lymph nodes lack T-cell stimulating function
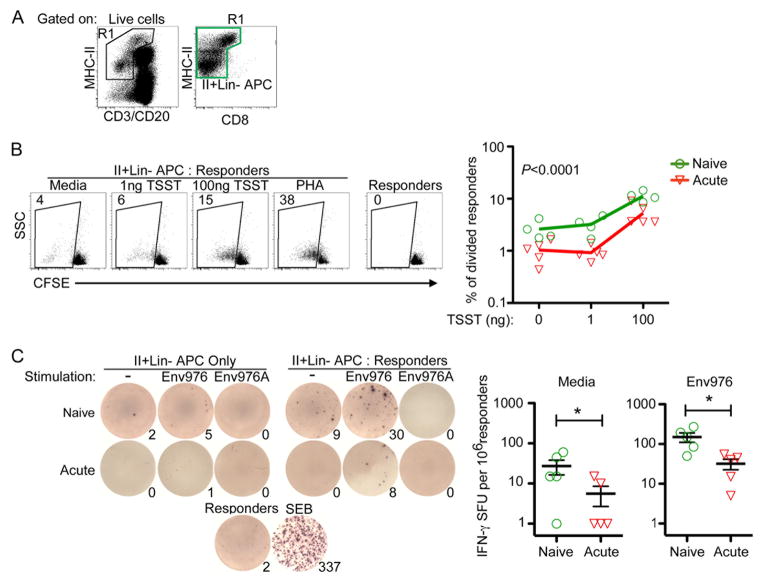
In order to determine the ability of lymph node mononuclear phagocytes to stimulate T cell proliferation, we cultured enriched mononuclear phagocytes isolated from lymph nodes of 5 acutely infected macaques (Table I) with CD4 T cells and measured T cell proliferation through CFSE dilution. Mononuclear phagocytes were enriched by sorting cells that expressed MHC-II but not CD3, CD8 or CD20 (Fig. 1A). The mononuclear phagocyte gate encompassed MHC-IIbright autofluorescent cells to include the majority of mDC in lymph nodes as we previously have shown (1). Allogeneic CD4 T cells isolated from one of two different SIV-naïve macaques were used as responders to avoid the potential for differences in autologous CD4 T cell function as a result of SIV infection. After infection enriched lymph node mononuclear phagocytes were less able to stimulate T cells to proliferate as CFSE dilution was significantly diminished, both in the presence and absence of the superantigen TSST (Fig. 1B). We next determined the ability of enriched mononuclear phagocytes to drive IFN-γ production from allogeneic CD4 T cells. Here we used the biologically relevant poly-uridine rich single-stranded RNA sequence Env976 encoded by SIVmac251 to stimulate pDC, mDC, and macrophages through TLR7/8 (15). Enriched mononuclear phagocytes from acutely infected lymph nodes were significantly less able to stimulate T lymphocytes to produce IFN-γ both in the absence and presence of TLR7/8-mediated activation as measured by ELISPOT assay (Fig. 1C).
Fig. 1. Enriched mononuclear phagocytes from SIV infected lymph nodes are impaired at stimulating allogeneic CD4 T cells.
(A) Gating strategy to generate enriched mononuclear phagocytes through live-cell sorting. The green gate represents the MHC-II+Lin− population that was added to each co-culture assay. The gate encompasses MHC-IIbright cells that have high autofluorescence. (B) (Left) MHC-II+Lin- mononuclear phagocytes from naïve or acutely-infected lymph nodes were incubated with CFSE-labeled allogeneic CD4 T cells with varying concentrations of TSST or PHA. Numbers represent the percentage of T cells that have diluted their CFSE intensity. (Right) Graphical representation of T cell division induced by enriched mononuclear phagocytes isolated from naïve or acutely infected lymph nodes. Each symbol represents an individual animal. An ANOVA was performed and subsequent multiple comparisons tests revealed a P-value of <0.0001. (C) (Left) MHC-II+Lin- cells from naïve or acutely-infected lymph nodes were either stimulated with Env976 or not and co-cultured with 200,000 allogeneic CD4 T cells directly in an anti-IFN-γ coated ELISPOT plate. Numbers represent IFN-γ spot-forming units (SFU) per 1 million responder CD4 T cells. (Right) Graphical representation of IFN-γ SFU by enriched mononuclear phagocytes isolated from naïve or acutely infected lymph nodes. Each symbol represents an individual animal. Repeated measures ANOVA and multiple comparisons tests were performed and each significant relationship is shown with capped bars. *P=0.03
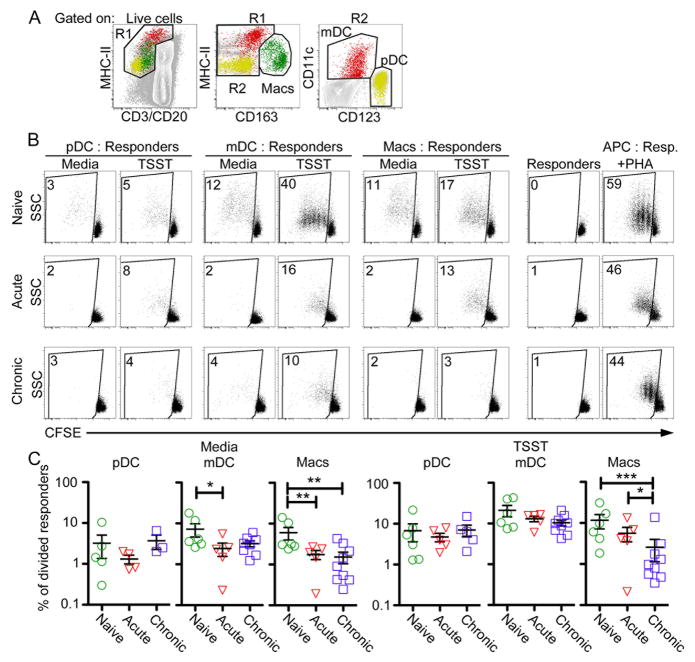
Suppressed mDC and macrophage capacity to stimulate CD4 T cell proliferation in SIV infection
To define which mononuclear phagocyte subset was responsible for this defect in T-cell stimulating function and determine if the defect persisted into chronic infection, we used flow cytometry to purify pDC, mDC, and macrophages from lymph nodes (Fig. 2A) of the acutely infected macaques along with lymph nodes from a group of 10 chronically infected macaques (Table I) and co-cultured them with CFSE-labeled CD4 T cells. Lymph node pDC were relatively weak at inducing CD4 T cell proliferation in naïve monkeys but did not lose this capacity as a result of acute or chronic SIV infection (Fig. 2B & C). Conversely, both mDC and macrophages induced strong CD4 T cell proliferation in the naïve state based on CFSE staining, and both responses were significantly impaired in acutely infected macaques. Moreover, macrophage induction of T cell proliferation was further reduced in chronic infection and was not corrected in the presence of TSST superantigen, in contrast to mDC from acute infection (Fig. 2B & C).
Fig. 2. mDC and macrophages have reduced capacity to stimulate allogeneic CD4 T cell proliferation in SIV infection.
(A) Gating strategy to isolate pDC (yellow), mDC (red), and macrophages (green) through live-cell sorting. (B) Isolated pDC, mDC, and macrophages were either pulsed with 100ng/mL TSST or not and co-cultured with CFSE-labeled T lymphocytes. Numbers represent the percentage of T cells that have diluted their CFSE signal intensity. (C) Graphical representation of T cell division induced by pDC, mDC, and macrophages isolated from naïve, acutely infected or chronically infected lymph nodes. Each symbol represents an individual animal. Repeated measures ANOVA and multiple comparisons tests were performed and each significant relationship is shown with capped bars. *P<0.05; **P<0.01; ***P<0.001.
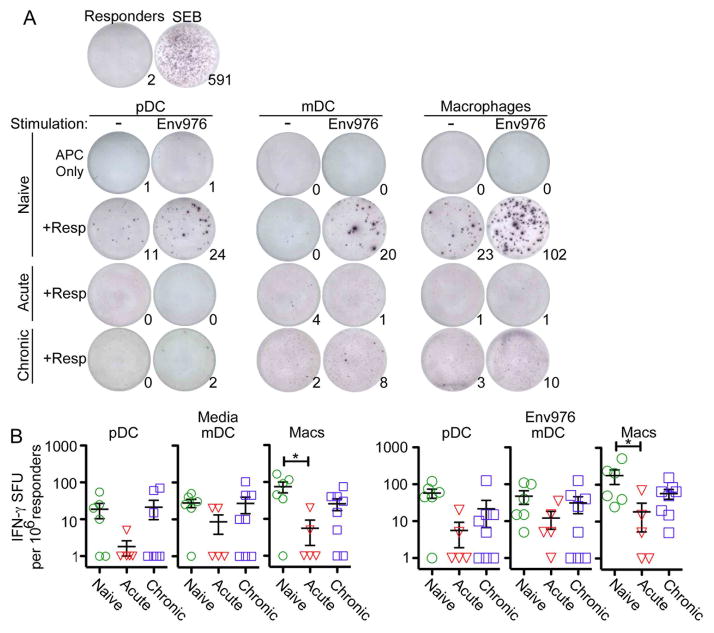
Macrophages lose capacity to stimulate IFN-γ production from CD4 T cells in acute SIV infection
In naïve lymph nodes pDC, mDC and macrophages all were capable of stimulating IFN-γ release from allogeneic CD4 T cells, with macrophages being the most potent inducer of IFN-γ release both with and without activation with Env976 (Fig. 3A, B). No IFN-γ production was detected by APC alone, in the absence of CD4 T cells (Fig. 3A). After acute SIV infection the capacity for macrophages to induce T-cell release of IFN-γ was significantly suppressed, even with TLR7/8 stimulation, but this function was largely restored in chronic infection. There was a trend towards loss of pDC- and mDC-mediated IFN-γ production by CD4 T cells in acute infection but this did not reach statistical significance (Fig. 3B).
Fig. 3. Macrophages lose capacity to induce IFN-γ production from allogeneic CD4 T cells in acute SIV infection.
(A) A representative ELISPOT assay depicting T cells producing IFN-γ when co-cultured with isolated pDC, mDC, or macrophages isolated from naïve, acutely infected or chronically infected lymph nodes. Each co-culture is shown with and without exposure to Env976. Numbers represent the number of IFN-γ spot-forming units (SFU). (B) Quantification of IFN-γ SFU is shown per 1 million responder cells. Each symbol represents an individual animal. Repeated measures ANOVA and multiple comparisons tests were performed and each significant relationship is shown with capped bars. *P<0.05.
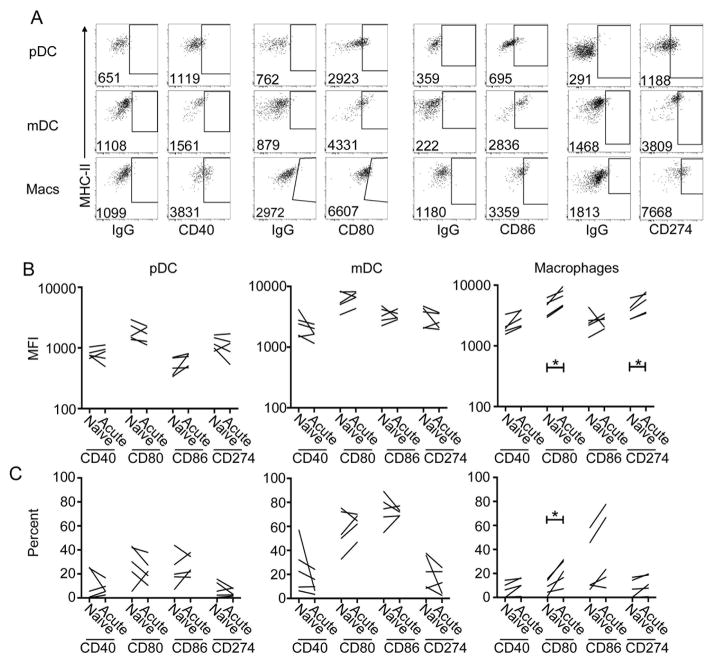
Loss of T cell-stimulating function is associated with minor changes in macrophage expression of costimulatory molecules in SIV infection
To begin determining the mechanism of defective T cell stimulation by DC and macrophages we first assayed for the expression of the costimulatory molecules CD40, CD80, and CD86 as well as CD274 (programmed death ligand-1) on paired samples of naïve and acutely infected lymph node suspensions stimulated with Env976 (Fig. 4A). There was no change in expression of any of these surface markers when comparing pre-infection and post-infection lymph node preparations from the same animals with the exception of macrophages, which had modest increases in expression of CD80 and CD274 after infection (Fig. 4B).
Fig. 4. Expression of markers of activation on stimulated lymph node pDC, mDC, and macrophages.
(A) Representative dot plots showing the expression of CD40, CD80, CD86, and CD274 on pDC, mDC, and macrophages from naïve lymph nodes after a 7-h exposure to Env976. The numbers represent the mean fluorescence intensity (MFI) of the whole cell population. The gates are based on isotype controls (IgG). (B) Quantification of the MFI of co-stimulatory molecule expression (top) and the percent of cells expressing the respective co-stimulatory molecules (bottom) of mononuclear phagocyte populations in paired naïve and acutely infected lymph nodes. Capped bars signify differences through paired t-test analysis. *P< 0.05.
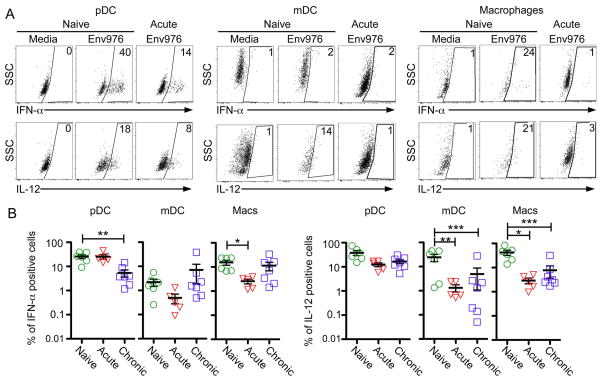
Mononuclear phagocytes from SIV-infected lymph nodes have diminished IFN-α and IL-12 production
We next focused on production of IFN-α and IL-12, two key cytokines released by activated mononuclear phagocytes that contribute to T cell responses. As expected, pDC in cell suspensions from SIV-naïve lymph nodes stimulated with the TLR7/8 ligand Env976 were the principal producer of IFN-α, with 40% of pDC producing this cytokine, but macrophages were also significant contributors to IFN-α production in the naïve state. mDC produced negligible amounts of IFN-α after stimulation with Env976 (Fig. 5A, B). SIV infection had variable effects on the capacity of lymph node cells to produce IFN-α, with significant loss of IFN-α production by pDC and macrophages in chronic and acute infection, respectively (Fig. 5B). In contrast to IFN-α, pDC, mDC and macrophages from naïve lymph nodes all were significant producers of IL-12 after stimulation with Env976. pDC production of IL-12 was unaffected by SIV infection, whereas the proportion of both mDC and macrophages that produced IL-12 after stimulation with Env976 was markedly suppressed in acute and chronic infection (Fig. 5B).
Fig. 5. Diminished IL-12 and IFN-α production by mononuclear phagocytes from SIV infected lymph nodes.
(A) Lymph node suspensions from naïve monkeys and monkeys with acute or chronic SIV infection were incubated with or without Env976 for 7 h with brefeldin A added in the final 5 h. Representative IFN-α and IL-12 production by pDC, mDC, and macrophages from SIV-naïve and acutely infected macaques are shown. (B) Quantification of the percentage of pDC, mDC, and macrophages producing IFN-α and IL-12 after stimulation with Env976 as determined by intracellular cytokine staining. Each symbol represents an individual animal. Capped bars signify differences found through repeated measures ANOVA and multiple comparisons tests. *P<0.05; **P< 0.01; ***P< 0.001
mDC and macrophages gain T-cell stimulating function with addition of IFN-α and Il-12
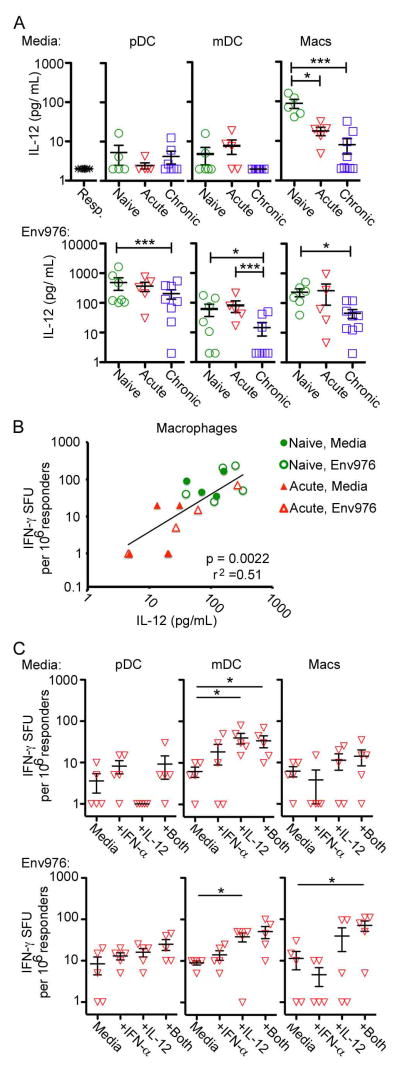
To explore if diminished IFN-γ production from T cells was directly related to suppressed cytokine production from mononuclear phagocytes, we measured the concentration of cytokine in supernatants from each CD4 T cell co-culture. We focused on IL-12 due to limited sample volume and evidence that IL-12 is more critical than IFN-α in driving T cell immune responses (30, 31). Importantly, IL-12 was never detected in any of the wells that contained CD4 T cell responders alone, in the absence of APC (Fig. 6A). Without TLR7/8 stimulation macrophages were the only mononuclear phagocyte population that produced significant amounts of IL-12 in co-culture with T cells, whereas after stimulation with Env976 pDC were the dominant producer of IL-12, followed by macrophages and to a lesser extent mDC (Fig. 6A). SIV infection had significant impact on the capacity for mononuclear phagocytes to secrete IL-12 in co-culture with allogeneic CD4 T cells. Unstimulated macrophages showed progressive loss of IL-12 production, which dropped 10-fold in chronic infection relative to naïve lymph nodes (Fig. 6A). In the presence of TLR7/8 stimulation pDC, mDC and macrophages from chronically infected lymph nodes had marked suppression of IL-12 production in co-cultures with CD4 T cells (Fig. 6A). There was a strong positive relationship between IL-12 production by macrophages isolated from naïve and acutely-infected lymph nodes and the number of IFN-γ producing CD4 T cells in the same cultures (Fig. 6B). This relationship was not significant for pDC (P=.18, r2=.10) and mDC (P=.45, r2=.03), consistent with the fact that loss of IL-12 from these cells in co-cultures was most evident during chronic infection (Fig. 6A), whereas loss of capacity to stimulate T cells to produce IFN-γ was most impacted during acute infection (Fig. 3B). We next performed stepwise supplementation of IFN-α, IL-12, or both cytokines to co-cultures of pDC, mDC or macrophages isolated from lymph nodes at acute infection and allogeneic CD4 T cells in ELIPSOT assays to determine if cytokine addition would restore or enhance T cell function. Again we focused on acutely infected samples as we observed the greatest stimulatory defect at this time point. Exogenous IL-12 with or without IFN-α enhanced T cell production of IFN-γ induced by mDC, in both unstimulated and Env976-stimulated conditions, whereas IFN-α and IL-12 together recovered the T cell stimulatory capacity of Env976-stimulate macrophages. Cytokine addition did not significantly impact the T-cell stimulating function of pDC isolated from lymph nodes during infection (Fig. 6C). Exogenous cytokines added to responder cells alone did not drive the production of IFN-γ (data not shown) revealing that mononuclear phagocytes were required for this response.
Fig. 6. Supplementing IFN-α and IL-12 enhances T cell stimulating function of mDC and macrophages in SIV infection.
(A) Quantification of IL-12 concentrations in supernatants of allogeneic CD4 T cells co-cultured with purified pDC, mDC, or macrophages from naïve, acutely infected or chronically infected lymph nodes with or without exposure to Env976, as determined by ELISA. Each symbol represents an individual animal. Capped bars signify differences found through repeated measures ANOVA and multiple comparisons tests. (B) Relationship between macrophage IL-12 concentration and the number of IFN-γ producing cells in T cell co-cultures. Data points for both unstimulated and Env976 stimulated SIV-naïve and acutely infected macaques are shown. The regression line was fit by a mixed effects regression model that accounted for multiple samples from each animal. The R2 value shown is similarly adjusted. The difference between the regression lines from the four measurement types was not significant. (C) pDC, mDC or macrophages isolated from naïve or acutely-infected lymph nodes with and without stimulation with Env976 were co-cultured with allogeneic CD4 T cells in IFN-γ ELISPOT assays with and without supplementation of IFN-α, IL-12 or both cytokines. Shown are IFN-γ SFU cells per 1 million responder cells. Each symbol represents an individual animal. Uncapped bars signify differences through paired T-test analysis. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Our study reveals that an enriched population of mononuclear phagocytes from lymph nodes of SIV-infected macaques has significant impairment in the ability to stimulate CD4 T cell proliferation and IFN-γ release that was not diminished by addition of superantigen or stimulation with virus-encoded TLR7/8 ligand. The loss of T cell-stimulating capacity was mediated primarily by dysfunction of macrophages and mDC, but not pDC, and was linked to deficient production of IFN-α and IL-12, two key cytokines in T cell priming (30, 31).
The most striking defect in lymph node mononuclear phagocyte function that has not previously been described was seen with macrophages, which lost capacity to both drive CD4 T cell proliferation and induce T cell release of IFN-γ in SIV-infected macaques. Macrophages accumulate in lymph nodes and gut mucosa in both SIV and HIV infection (15, 23, 32, 33), and the level of monocyte turnover is predictive of disease progression (21, 22). Our data suggest that loss of macrophage function in stimulating adaptive T cell responses may be a critical component in progressive HIV and SIV infection. Lymph node macrophages lost capacity to produce IFN-α, consistent with previous studies (15), although macrophages in gut mucosa of untreated HIV-infected patients produce proinflammatory molecules, suggesting there may be anatomical differences in macrophage function (32). Indeed macrophages in humans and rhesus macaques have considerable heterogeneity across tissues (33, 34), and it will be important in future studies to determine if T cell-stimulating function of the different macrophage subsets is uniformly or differentially affected by SIV infection and how this relates to disease progression.
Lymph node mDC in acute SIV infection failed to induce normal CD4 T cell proliferation and in both acute and chronic infection had markedly reduced production of IL-12 after stimulation with virus-encoded TLR7/8 ligand. While suppression of mDC-mediated stimulation of IFN-γ release from CD4 T cells was not statistically significant, addition of IL-12 strongly enhanced the capacity for mDC to drive cytokine production from T cells. Increased recruitment of mDC to lymphoid tissues is associated with disease progression in SIV-infected macaques (27), and in HIV infection, mDC from the blood of elite controllers but not individuals with chronic progressive infection stimulate CD4 T cell proliferation and IFN-γ release from HIV-specific CTL (17). Collectively these data are suggestive of a generalized defect in mDC T cell-stimulating function in HIV and SIV infection that reduces immune control of virus thereby promoting disease development. This may be associated with mDC-driven induction of regulatory T cells in lymph nodes, as demonstrated in both SIV and HIV infection (24, 25).
Our data show that mDC from virus-infected lymph nodes produce negligible amounts of IFN-α in response to stimulation with stimulatory virus RNA, consistent with the notion that HIV can escape intracellular recognition in mDC (16, 35, 36). However, recent studies suggest that mDC function in elite controllers is linked to robust production of type I IFN following exposure to virus (17). Together with the loss of IFN-α production by macrophages and the beneficial effect of IFN-α on macrophage function in our study, these data suggest that the IFN-α response is a critical component in mDC- and macrophage-mediated T cell responses in infection. We were not able to measure production of IFN-β in our study due to lack of cross-reactive reagents, and studies with HIV suggest that subversion of the IFN-β response in macrophages and DC is an important mechanism of immune evasion (16, 37). It is possible that SIV infection impacts IFN-β production by macrophages and DC in a manner that is independent from IFN-α, and analysis of this issue awaits further study.
pDC are known to have robust APC function (38, 39), and in our study lymph node pDC harvested prior to infection were strong inducers of IFN-γ release from T cells. Notably however, this function was maintained in SIV infection, despite loss of IFN-α and IL-12 production. These findings suggest that additional factors may be involved in driving pDC function distinct from macrophages and mDC.
Our findings are consistent with previous reports describing a loss of IL-12 production by PBMC from HIV-infected patients (40–43) and the beneficial effects of supplementation of IL-12 to CD4 and CD8 T cell responses in HIV-infected individuals (44). Factors in the plasma of HIV infected patients have also been shown to inhibit IL-12 production from monocyte-derived DC (45, 46). The findings suggest that direct administration of IL-12 may act to promote DC and macrophage function in stimulating antiviral T cell responses, and this approach has been shown to decrease virus load and prolong survival in SIV-infected macaques (47). However, IL-12 administration could also potentially be detrimental, as the broadly immunostimulatory effects of this cytokine (48) could create an increased pool of activated CD4 T cells that would serve as targets for infection.
We limited our analyses to using allogeneic CD4 T cells to control for differential autologous T cell responses in SIV-infected macaques, as have others in studying HIV-infected individuals (11, 13, 14, 24). Data from the rhesus macaque model suggest that lymph node DC stimulation of autologous T cells may also be dysfunctional in SIV infection (25). Our findings of marked mDC and macrophage dysfunction in acute infection and persistent macrophage dysfunction in chronic infection suggest that both the capacity to stimulate early virus-specific responses as well as responses to opportunistic pathogens in progressive infection may be impacted.
In summary, our findings identify previously unappreciated effects of pathogenic SIV infection on the T-cell stimulating function of macrophages and mDC in lymph nodes, indicating that immunosuppression during progressive infection may be due in part to mononuclear phagocyte dysfunction. Future studies aimed at assessing and reversing immunopathology in SIV and HIV infection should take into account the Ag presenting and T-cell stimulating function of these cells.
Acknowledgments
The authors thank Tim Sturgeon for technical expertise and Karen A. Norris for rhesus macaque blood samples.
Footnotes
This work was supported by NIH grants T32CA082084, R01AI071777 and P30CA047904.
References
- 1.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 2.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 3.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 4.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 5.Grassi F, Hosmalin A, McIlroy D, Calvez V, Debre P, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. Aids. 1999;13:759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shearer GM, Clerici M. Early T-helper cell defects in HIV infection. AIDS. 1991;5:245–253. doi: 10.1097/00002030-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Meyaard L, Schuitemaker H, Miedema F. T-cell dysfunction in HIV infection: anergy due to defective antigen-presenting cell function? Immunol Today. 1993;14:161–164. doi: 10.1016/0167-5699(93)90279-T. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 9.Yonkers NL, Rodriguez B, Asaad R, Lederman MM, Anthony DD. Systemic immune activation in HIV infection is associated with decreased MDC responsiveness to TLR ligand and inability to activate naive CD4 T-cells. PLoS One. 2011;6:e23884. doi: 10.1371/journal.pone.0023884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinson JA, Roman-Gonzalez A, Tenorio AR, Montoya CJ, Gichinga CN, Rugeles MT, Tomai M, Krieg AM, Ghanekar S, Baum LL, Landay AL. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 12.Chang JJ, Lacas A, Lindsay RJ, Doyle EH, Axten KL, Pereyra F, Rosenberg ES, Walker BD, Allen TM, Altfeld M. Differential regulation of toll-like receptor pathways in acute and chronic HIV-1 infection. Aids. 2012;26:533–541. doi: 10.1097/QAD.0b013e32834f3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabado RL, O’Brien M, Subedi A, Qin L, Hu N, Taylor E, Dibben O, Stacey A, Fellay J, Shianna KV, Siegal F, Shodell M, Shah K, Larsson M, Lifson J, Nadas A, Marmor M, Hutt R, Margolis D, Garmon D, Markowitz M, Valentine F, Borrow P, Bhardwaj N. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Yang Y, Al-Mozaini M, Burke PS, Beamon J, Carrington MF, Seiss K, Rychert J, Rosenberg ES, Lichterfeld M, Yu XG. Dendritic cell dysfunction during primary HIV-1 infection. J Infect Dis. 2011;204:1557–1562. doi: 10.1093/infdis/jir616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wonderlich ER, Wijewardana V, Liu X, Barratt-Boyes SM. Virus-encoded TLR ligands reveal divergent functional responses of mononuclear phagocytes in pathogenic simian immunodeficiency virus infection. J Immunol. 2013;190:2188–2198. doi: 10.4049/jimmunol.1201645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harman AN, Lai J, Turville S, Samarajiwa S, Gray L, Marsden V, Mercier SK, Jones K, Nasr N, Rustagi A, Cumming H, Donaghy H, Mak J, Gale M, Jr, Churchill M, Hertzog P, Cunningham AL. HIV infection of dendritic cells subverts the IFN induction pathway via IRF-1 and inhibits type 1 IFN production. Blood. 2011;118:298–308. doi: 10.1182/blood-2010-07-297721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Gayo E, Buzon MJ, Ouyang Z, Hickman T, Cronin J, Pimenova D, Walker BD, Lichterfeld M, Yu XG. Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers. PLoS Pathog. 2015;11:e1004930. doi: 10.1371/journal.ppat.1004930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, 3rd, Swanson T, Legasse AW, Sylwester A, Hansen SG, Smith AT, Stafova P, Shoemaker R, Li Y, Oswald K, Axthelm MK, McDermott A, Ferrari G, Montefiori DC, Edlefsen PT, Piatak M, Jr, Lifson JD, Sekaly RP, Picker LJ. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 21.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otani I, Mori K, Sata T, Terao K, Doi K, Akari H, Yoshikawa Y. Accumulation of MAC387+ macrophages in paracortical areas of lymph nodes in rhesus monkeys acutely infected with simian immunodeficiency virus. Microbes Infect. 1999;1:977–985. doi: 10.1016/s1286-4579(99)80515-2. [DOI] [PubMed] [Google Scholar]
- 24.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 25.Presicce P, Shaw JM, Miller CJ, Shacklett BL, Chougnet CA. Myeloid dendritic cells isolated from tissues of SIV-infected Rhesus macaques promote the induction of regulatory T cells. Aids. 2011;26:263–273. doi: 10.1097/QAD.0b013e32834ed8df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barratt-Boyes SM, Soloff AC, Gao W, Nwanegbo E, Liu X, Rajakumar PA, Brown KN, Robbins PD, Murphey-Corb M, Day RD, Gambotto A. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol. 2006;87:139–149. doi: 10.1099/vir.0.81445-0. [DOI] [PubMed] [Google Scholar]
- 27.Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6:e1001235. doi: 10.1371/journal.ppat.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kader M, Smith AP, Guiducci C, Wonderlich ER, Normolle D, Watkins SC, Barrat FJ, Barratt-Boyes SM. Blocking TLR7- and TLR9-mediated IFN-alpha production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog. 2013;9:e1003530. doi: 10.1371/journal.ppat.1003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen ZW, Shen Y, Kou Z, Ibegbu C, Zhou D, Shen L, Morrison P, Bogle C, McClure HM, Nahmias AJ, Sehgal PK, Letvin NL. Prolonged dominance of clonally restricted CD4(+) T cells in macaques infected with simian immunodeficiency viruses. J Virol. 2000;74:7442–7450. doi: 10.1128/jvi.74.16.7442-7450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 32.Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, Schinnerling K, Moos V, Schneider T. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis. 2014;209:739–748. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz AM, DiNapoli SR, Brenchley JM. Macrophages Are Phenotypically and Functionally Diverse across Tissues in Simian Immunodeficiency Virus-Infected and Uninfected Asian Macaques. J Virol. 2015;89:5883–5894. doi: 10.1128/JVI.00005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassol E, Cassetta L, Alfano M, Poli G. Macrophage polarization and HIV-1 infection. J Leukoc Biol. 2010;87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- 35.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasr N, Maddocks S, Turville SG, Harman AN, Woolger N, Helbig KJ, Wilkinson J, Bye CR, Wright TK, Rambukwelle D, Donaghy H, Beard MR, Cunningham AL. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood. 2012;120:778–788. doi: 10.1182/blood-2012-01-407395. [DOI] [PubMed] [Google Scholar]
- 38.Benitez-Ribas D, Adema GJ, Winkels G, Klasen IS, Punt CJ, Figdor CG, de Vries IJ. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. J Exp Med. 2006;203:1629–1635. doi: 10.1084/jem.20052364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 40.Chehimi J, Starr SE, Frank I, D’Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denis M, Ghadirian E. Dysregulation of interleukin 8, interleukin 10, and interleukin 12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 1994;10:1619–1627. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- 42.Chougnet C, Wynn TA, Clerici M, Landay AL, Kessler HA, Rusnak J, Melcher GP, Sher A, Shearer GM. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 43.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–1011. [PubMed] [Google Scholar]
- 44.Clerici M, Lucey DR, Berzofsky JA, Pinto LA, Wynn TA, Blatt SP, Dolan MJ, Hendrix CW, Wolf SF, Shearer GM. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 45.Buisson S, Benlahrech A, Gazzard B, Gotch F, Kelleher P, Patterson S. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J Infect Dis. 2009;199:1862–1871. doi: 10.1086/599122. [DOI] [PubMed] [Google Scholar]
- 46.Miller EA, Spadaccia MR, O’Brien MP, Rolnitzky L, Sabado R, Manches O, Frleta D, Bhardwaj N. Plasma factors during chronic HIV-1 infection impair IL-12 secretion by myeloid dendritic cells via a virus-independent pathway. J Acquir Immune Defic Syndr. 2012;61:535–544. doi: 10.1097/QAI.0b013e31826afbce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ansari AA, Mayne AE, Sundstrom JB, Bostik P, Grimm B, Altman JD, Villinger F. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J Virol. 2002;76:1731–1743. doi: 10.1128/JVI.76.4.1731-1743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]