Abstract
Patterns of human locomotion are highly adaptive and flexible, and depend on the environmental context. Locomotor adaptation requires the use of multisensory information to perceive altered environmental dynamics and generate an appropriate movement pattern. In this study, we investigated the use of multisensory information during locomotor learning. Proprioceptive perturbations were induced by vibrating tactors, placed bilaterally over the plantar surfaces. Under these altered sensory conditions, participants were asked to perform a split-belt locomotor task representative of motor learning. Twenty healthy young participants were separated into two groups: no-tactors (NT) and tactors (TC). All participants performed an overground walking trial, followed by treadmill walking including 18 minutes of split-belt adaptation and an overground trial to determine transfer effects. Interlimb coordination was quantified by symmetry indices and analyzed using mixed repeated measures ANOVAs. Both groups adapted to the locomotor task, indicated by significant reductions in gait symmetry during the split-belt task. No significant group differences in spatiotemporal and kinetic parameters were observed on the treadmill. However, significant groups differences were observed overground. Step and swing time asymmetries learned on the split belt treadmill, were retained and decayed more slowly overground in the TC group whereas in NT, asymmetries were rapidly lost. These results suggest that tactile stimulation contributed to increased lower limb proprioceptive gain. High proprioceptive gain allows for more persistent overground after-effects, at the cost of reduced adaptability. Such persistence may be utilized in populations displaying pathologic asymmetric gait by retraining a more symmetric pattern.
Keywords: motor learning, biomechanics, touch, vibration, gait, sensation, perception
Introduction
Patterns of human locomotion are highly adaptive and flexible, depending on the environmental context. Changing environmental conditions require the system to adapt to meet the demands of the new environment (MacLellan and Patla 2006) and to ensure that postural stability is maintained during locomotion. Adaptive changes in locomotion can occur due to the system’s response to internal or external perturbations. External perturbations are perceived to result from particular environmental characteristics such as increased compliance of the support surface or altered gravitational conditions (Mulavara et al. 2010). Internal perturbations on the other hand are perceived to be the result of internal factors, such as changes in joint flexibility or reduced sensory acuity (Peters et al. 2011).
Split-belt walking, a task requiring the two lower limbs to move at different velocities, is often used as a paradigm to study locomotor adaptation in response to external perturbations (Reisman et al. 2005; Malone and Bastian 2010). The adaptive process involves complex interlimb coordination to achieve the phasing relationship required to walk on a split belt treadmill (Reisman et al. 2005; Mawase et al. 2013). Learning split-belt dynamics requires contributions from lower-limb somatosensory information, which uniquely conveys the altered locomotor dynamics through a mechanical connection at single and double support times. Knowledge of the movement of the belts and the resultant movement characteristics are captured in firing patterns of proprioceptors in lower limb musculature and plantar mechanoreceptors. Plantar cutaneous mechanoreceptors in particular provide critical information to the central nervous system about the subject’s interaction with the environment during locomotion (Kavounoudias et al. 1998). Electrical stimulation of the superficial peroneal nerve, innervating sensory and motor regions of the foot, result in coordinated bilateral reflex activity in lower limb muscles (Duysens et al. 1990; Zehr and Haridas 2003). Furthermore, direct plantar cutaneous electrical stimulation affects afferent feedback during locomotion, resulting in abnormal foot orientation and placement during locomotion (Zehr et al. 2014). These activation patterns have been observed to be phase dependent, suggesting cutaneous plantar perception is required to maintain complex interlimb phasing, or coordination, during locomotion (Zehr and Haridas 2003). Therefore, disrupting lower limb proprioception, which would constitute an internal perturbation, could potentially affect the way in which interlimb coordination is achieved on the split belt. Disrupting the function of plantar mechanoreceptors with plantar vibration may deprive the locomotor system of essential interlimb coordination information required to efficiently achieve a split belt locomotor pattern. This potentially drives the system to reorganize somatosensory perception and rely to a larger degree on alternative channels of information available to the somatosensory system, to attain the movement goals.
In this study, we investigated how the system performs a split-belt walking task, when interlimb coordination perception is perturbed. Plantar tactile vibration was applied to reduce the signal-to-noise ratio of peripheral somatosensory inputs, driving the system to reweight multisensory input. Injected supra-threshold noise inhibits the detection of both weak and strong signals and is counterproductive in static and dynamic balance tasks (Collins et al. 1997). We hypothesized that sensory reweighting driven by a vibratory proprioceptive perturbation would increase reliance on somatosensory perception. The increased effort required to perform this type of adaptation would lead to the formation of a more stable locomotor movement pattern, resulting in enhanced retention and transfer to a setting different from the training environment, i.e. overground walking conditions, in subjects who received plantar vibration.
Methods
Twenty participants (11 males, 9 females; age 26.0±5.4; mass 69.0±14.0 kg; height 169±9cm) were included in the study. All subjects had normal or corrected-to-normal vision and were free of any cognitive or musculoskeletal impairments which might affect gait or locomotor adaptation. Prior to the experiment, subjects were informed of the procedures and provided written consent. All procedures were approved by the institutional review board of our University’s Medical Center.
Participants were randomly assigned to one of two age-matched groups. While walking on the treadmill, half of the participants performed treadmill walking without an additional tactile stimulus (NT group) and the other half of the participants was exposed to an additional tactile stimulus (TC group), provided by vibrating force applicators. Six circular C2 tactors (Engineering Acoustics Inc., Casselberry, FL), each 7.6mm in diameter, were taped to the plantar surface of the bare feet. Two tactors were placed laterally on the ball of each foot, one placed over the 1st and one over the 5th metatarsal head, and one was placed over the lower surface of the calcaneus. Each tactor vibrated at a constant nominal sine frequency of 250Hz at an amplitude of 17.5 dB. There was no significant difference in the anthropometric parameters of the participants between the two groups (Table 1). Subjects were asked to walk overground and on a dual-belt instrumented treadmill. Each belt was individually driven, allowing the belt speed to be independently controlled (split-belt) or driven in unison (tied-belt). The instrumented treadmill (Bertec, Columbus OH) accommodated the recording of ground reaction forces (GRFs) at 300Hz through the dual force plates located underneath the belts. Kinematics were recorded using a 3D Investigator motion-tracking system (60Hz, Northern Digital Inc., Waterloo, Canada) to track smart marker clusters placed on the foot, shank, thigh, and sacrum. During treadmill locomotion, all participants wore a chest harness connected to a bodyweight support system (LiteGait; Mobility Research, Tempe, AZ) for safety. Overground walking parameters were quantified using force sensors, placed in the insoles of the participants. The force sensors were present in all conditions.
Table 1.
The anthropometric parameters of the participants in the no-tactors (NT) and tactors (TC) groups.
| No tactors (NT) | Tactors (TC) | p value | |
|---|---|---|---|
| Body mass (kg) | 74.7±9.46 | 63.2±15.9 | 0.13 |
| Height (m) | 1.72±0.07 | 1.65±0.09 | 0.07 |
| Age (yrs) | 24±5.3 | 28±5.1 | 0.13 |
| Preferable walking speed (m/s) | 0.99±0.18 | 0.91±0.11 | 0.18 |
Mean ± SD
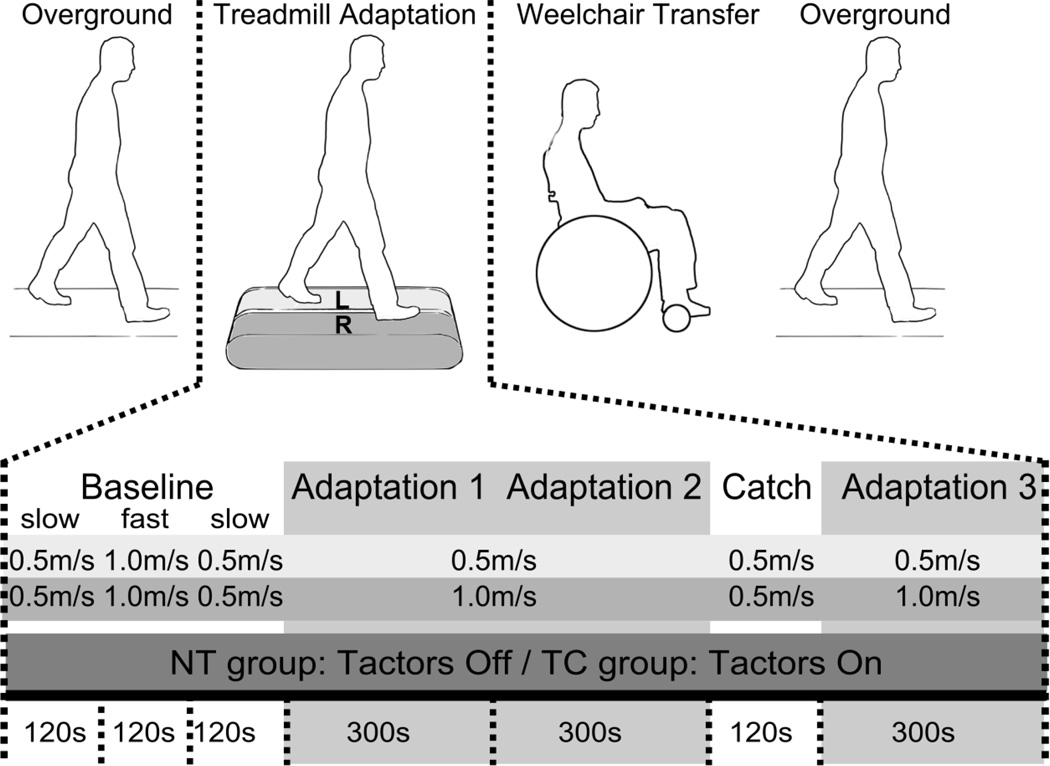
Participants performed overground and treadmill trials with tied and split-belt configurations. All participants performed an initial 120 s overground trial on an indoor jogging track, to determine baseline overground gait symmetry. Prior to the treadmill trials, participants performed a 300 s familiarization trial on the treadmill to get acquainted with locomotion while fitted with tactors, footswitches and suspension harness. Seven treadmill trials followed, separated by consistent 120 s rest periods (Figure 1). The treadmill belt velocities were the same in all participants and were either slow (0.5m/s) or fast (1.0m/s), depending on the particular condition (Reisman et al. 2009). Participants started with a slow walking trial (0.5 m/s) with the treadmill in a tied-belt configuration for 120 s. Following the initial slow trial, participants walked for 120s with the belts moving at the fast velocity. These two trials were not included in any statistical analyses and were only used to accommodate the participant to the two speeds of the split belt trials. The fast trial was followed by a second 120 s slow trial at 0.5 m/s. In the split-belt trials, all participants walked with the right belt at 1.0 m/s (fast leg) and the left belt at 0.5 m/s (slow leg). Two split-belt trials of 300 s each were performed in succession, separated by a rest period. Following the split-belt adaptation trials, participants performed a catch trial during which the belts were tied. Following the catch trial, participants performed a final 300 s split-belt trial. At the conclusion of the treadmill phase, participants were transported to the 200 m indoor jogging track via wheelchair while wearing opaque goggles to ensure transfer effects could be monitored in a controlled environment. The experiment was concluded with a 120 s overground walking trial on the indoor jogging track to determine learning transfer.
Figure 1.
The sequence of experimental procedures: the overground pre-adaptation phase, the treadmill adaptation phase, wheelchair transfer and the overground post-adaptation phase. Treadmill belt velocities were fixed as shown in all participants. In the TC group, tactors were on during the entire treadmill walking task, whereas the NT group received no tactile stimulation.
Spatiotemporal parameters were quantified during all treadmill walking trials. On the treadmill, Step length (SL) and step time (ST) were calculated based on the instance of heel-strike by using marker clusters placed on the feet. Gait characteristics during overground walking were calculated only in the temporal domain. Toe-off and touch-down events were quantified by footswitches located in the insoles of the participant and were used to compute stance time (STT) and swing time (SWT). In order to quantify interlimb coordination, the indices of step length symmetry (SLS) and step time symmetry (STS) were quantified on the treadmill (Eq. 1 and 2 respectively). Overground, Stance Time Symmetry (STTS) and Swing Time Symmetry (SWTS) were calculated (Eq. 3 and 4 respectively).
| (1) |
| (2) |
| (3) |
| (4) |
For the overground walking trials, an exponential decay function (Eq. 5) was used to fit the rate of change of STTS and SWTS (Smith et al. 2006; Mawase et al. 2014). In this equation, SI is the symmetry index of the specific gait parameter to which the exponential function is fitted; SI(1) indicates the starting value of the symmetry index, n refers to the step number after the beginning of the current treadmill walking condition, and b is the rate of change (Eq. 5). Using the right side of the equation, SI(n) is the symmetry index at gait cycle n that is estimated using a nonlinear least squares fitting method.
| (5) |
All treadmill trials other than baseline slow and fast trials prior to the adaptation trials were separated into an early and late component, each consisting of the first 3 gait cycles (starting from the first gait cycle) and last 3 gait cycles respectively (Vasudevan and Bastian 2010). As no change in movement patterns were expected in the baseline slow and fast trials, only the last 3 cycles of the respective trials were used. Each variable was analyzed using a 2 (group: NT, TC) × 5 (treadmill condition: slow baseline, early adaptation 1, late adaptation 2, early catch, early adaptation 3) mixed-model repeated measures ANOVA. The significant interactions were analyzed by corrected post-hoc t-tests. The post-hoc t-tests were corrected for multiple comparisons using the Bonferroni-Holm procedure. Overground performance in the pre-treadmill and post-treadmill trials was analyzed using a 2 (group: NT, TC) × 2 (condition: pre-treadmill, post-treadmill) mixed-model repeated measures ANOVA. Overground post-treadmill decay rates were analyzed using independent samples t-tests. For all statistical tests, alpha was set at 0.05. All values in the text represent the mean ± standard deviation of the respective variable.
Results
Spatiotemporal characteristics of split-belt treadmill adaptation
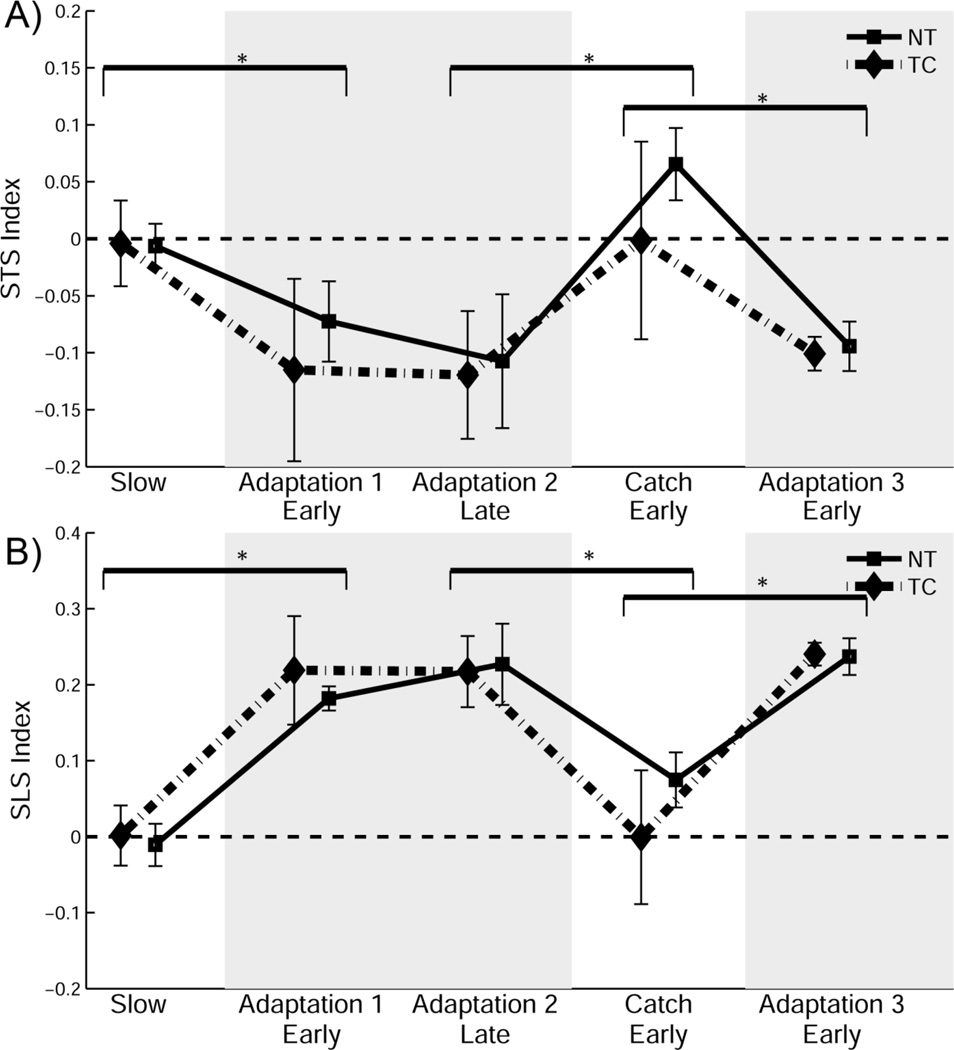
During treadmill locomotion, spatial symmetry was quantified as SLS and temporal symmetry was quantified as STS. As can be observed in figure 2a, statistical analyses revealed no significant group differences in STS during treadmill locomotion (F(1,18) < 0.001, p = 0.996). STS showed a significant effect of treadmill condition (F(4,72) = 75.656, p < 0.001). Between-condition analyses revealed STS was significantly decreased in early adaptation 1, compared to baseline (t(19) = −10.14, p < 0.001). In the early catch trial, STS values significantly increased, compared to late adaptation 2 (t(19) = 10.597, p < 0.001). By the end of the catch trial, STS returned to baseline values in both groups. STS decreased again in adaptation 3 early, compared to catch in both groups (t(19) = −9.974, p < 0.001). Figure 2b indicates the spatial characteristics of locomotor adaptation followed patterns similar to those observed in temporal adaptation. Analyses revealed no significant differences in SLS between groups during treadmill locomotion (F(1,18) = 1.313, p = 0.267). As can be observed in figure 2b, SLS did show a significant effect of condition (F(4,72) = 150.287, p < 0.001). SLS was significantly increased in early adaptation 1, compared to baseline (t(19) = −13.083, p < 0.001) and stabilized at a significantly higher index value in late adaptation 2 (t(19) = 9.982, p < 0.001). Following late adaptation 2, SLS returned to baseline values in early catch (t(19) = 12.071, p < 0.001). SLS increased again in early adaptation 3, compared to late catch (t(19) = −12.64, p < 0.001).
Figure 2.
The effect of plantar tactile vibration on the spatiotemporal symmetry indices during split-belt adaptation. (A) Mean step time symmetry index (STS) and (B) mean step length symmetry index (SLS) in the slow baseline, early adaptation, late adaptation, catch and final adaptation phases respectively. Means ± SD are shown. * p < 0.05.
Temporal characteristics of overground transfer
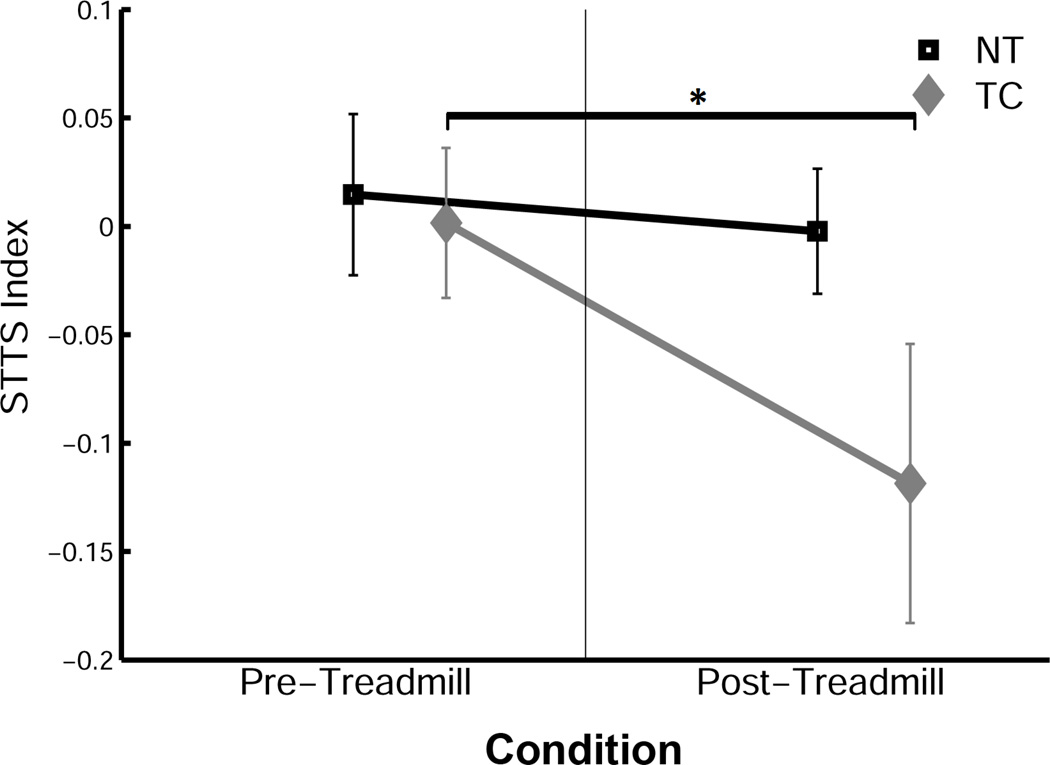
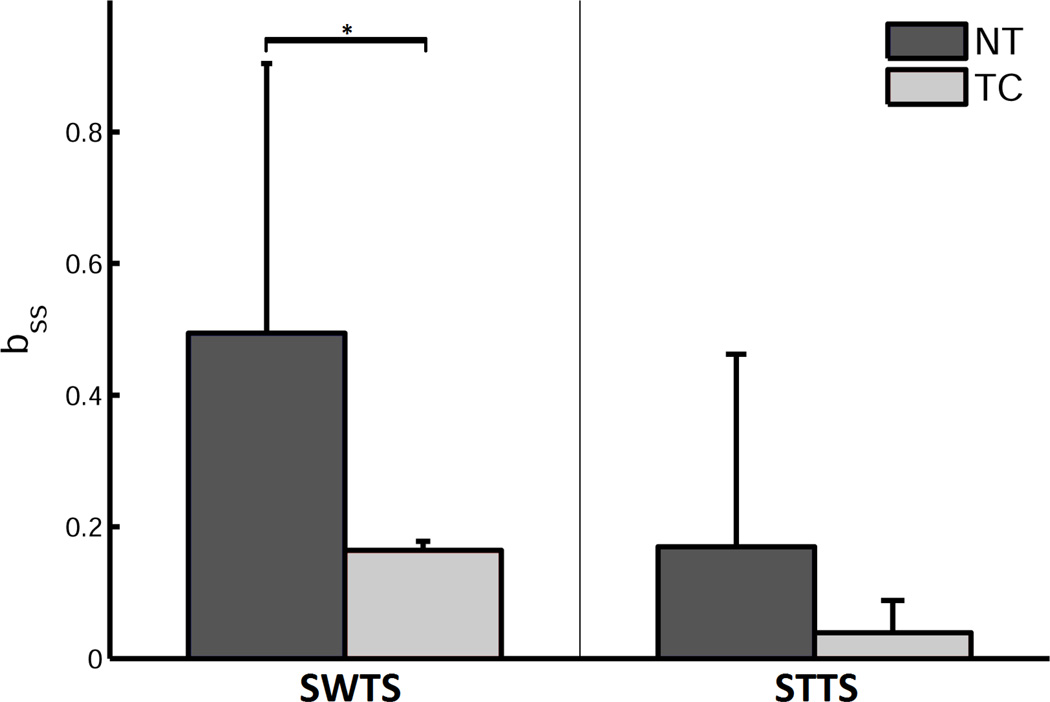
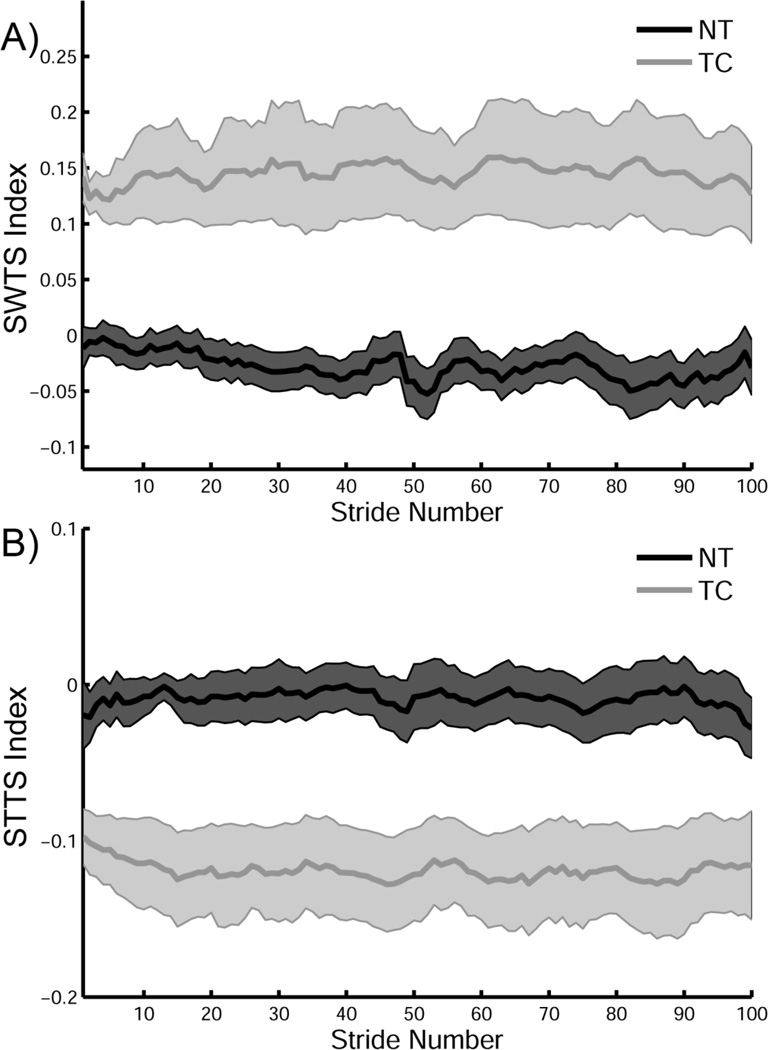
The transfer of the generated locomotor movement pattern during split-belt adaptation was quantified by the change in temporal parameters in a novel environment, i.e. overground walking without the plantar tactile perturbation. Specifically, decay rate parameter b was used to quantify the rate of de-learning. The TC group displayed overground gait patterns significantly different from those of the NT controls. During the overground walking after split-belt adaptation, the learning effect disappeared rapidly, within the initial few steps in the NT group. However, the TC group retained the generated locomotor movement pattern for a longer time, as indicated by a significant reduction in STTS during the overground trial (Figure 3; F(1,9) = 28.37,p < 0.05). An analysis of the decay rate of asymmetry was performed on STTS and SWTS, using a single decay-parameter exponential function. The decay rate of SWTS was only significantly lower in the TC group (Figure 4; t(13) = −1.984, p < 0.05). The aftereffect displays greater variability in TC (Figure 5a) than in NT for SWTS. The STTS was not significantly different, even though a similar trend was observed (Figure 5b).
Figure 3.
The effect of plantar tactile vibration on the step time symmetry index (STTS) during overground walking in the pre-adaptation and post-adaptation phases. Means ± SD are shown. * p < 0.05.
Figure 4.
The rate of change (bss) of the Swing Time Symmetry (SWTS) and Step Time Symmetry (STTS) indices for the two groups during the post-adaptation overground walking trials are shown. The smaller the bss value, i.e. value closer to zero, the smaller is the rate of change. Means ± SD are shown. * p < 0.05.
Figure 5.
The effect of plantar tactile vibration for all the subjects on A) Swing Time Symmetry (SWTS) and B) Step Time Symmetry (STTS) indices during post-adaptation overground walking for all the subjects. The SWTS and STTS mean (dark line) and standard deviations (shaded region) are provided for all the subjects for 100 strides for the no-tactors (NT) group (dark gray) and the tactors (TC) group (light gray). Note the greater variability in the TC group across 100 strides.
Discussion
In the present study we examined the effect of plantar tactile vibration on learning a split-belt locomotor adaptation task. The results indicate both spatial (step length: SLS) and temporal (step time: STS) parameters adapt to split-belt treadmill locomotion in both NT and TC. In both groups, adaptation effects observed during treadmill locomotion, primarily occurred in the initial three gait cycles. Adaptation during the split-belt walking conditions was reflected in a transient reduction in gait parameter asymmetries until a plateau was reached. SLS and STS retained a stable asymmetric value at the end of the split-belt trials. Furthermore, plantar tactile vibration did not change the rate of adaptation. Split-belt locomotor learning was also reflected in the aftereffect observed in step length and SLS and the temporal STS during the catch trial. The trends observed in the respective variables during the catch trial displayed the expected direction-reversal effect: asymmetry was initially high as a result of the system’s anticipation of a split-belt condition. When the tied belt was encountered instead, the locomotor pattern rapidly returned to normalcy. Consolidation of the split-belt dynamics was also reflected in the observation that all participants were able to rapidly adapt to the split-belt adaptation condition following the catch trial. Participants were able to rapidly attain stable locomotor performance. Overground, following split-belt locomotion, only the TC group temporarily retained the asymmetric walking pattern, indicated by a significantly slower rate of change of stance and swing time symmetries (STTS and SWTS respectively). However, inter-subject variability in the TC group was significantly larger than in the NT group, suggesting not all TC participants displayed the same rate of de-adaptation. In the NT group on the other hand, within-group de-adaptation was more uniform.
Sensory reweighting during locomotor adaptation
The various effects of plantar tactile vibration indicate peripheral somatosensory perception fulfills a significant role in locomotor learning. Plantar tactile vibration was applied to reduce the signal-to-noise ratio of peripheral somatosensory inputs, driving the system to reweight multisensory input. During locomotion, plantar pressure distributions in gait are not affected by systematic reductions in cutaneous sensitivity (Hohne et al. 2009; Zhang and Li 2013). Here, this is observed in the apparent absence of plantar tactile vibration driven effects on locomotor parameters. When tactile information becomes inaccurate through noise injection, the sensory reweighting that follows may increase the gain of lower limb proprioception while attenuating tactile input. The increased proprioceptive gain is maintained overground as a result of the asymmetrical properties of the sensory integration process. Although learning is not affected by plantar tactile vibration, overground transfer was significantly different between groups. Participants in the TC group retained the learned split-belt walking pattern overground, whereas participants in the NT group did not. Plantar tactile vibration may have driven learning through the reweighting of somatosensory information. Somatosensory perception contains contributions from intramuscular as well as cutaneous receptors. A reduction in cutaneous tactile acuity can lead to a reduction in the respective sensory weight, subsequently increasing the gain of intramuscular proprioception through attention-modulated intramodal reweighting (Pestilli et al. 2011). Increased attention-driven processing in higher-order sensory areas is reflected in more persistent aftereffects (Rosenkranz and Rothwell 2012).
Generalization of learning to different environments
Gradual changes in movement dynamics increase the probability of assigning variations to internal factors, for example resulting from reduced proprioceptive acuity. The resulting movement pattern does not depend on the specific training environment and is more likely to transfer to novel environments (Kluzik et al. 2008) such as different overground conditions. In that study, this process has been demonstrated only with perturbations of a single dimension. The perturbations the participants were exposed to in the current study operated at multiple proprioceptive levels. The split-belt treadmill affects locomotor kinematics by changing the dynamics of the lower limbs while they are interacting with the treadmill. Plantar tactile vibration, on the other hand, aimed at perturbing plantar load sensing and perceived interlimb phasing throughout the trials. Movement and perceptual characteristics experienced during split-belt locomotion, which are perceived to be environment specific, do not contribute to a transferable locomotor movement pattern (Berniker and Kording 2008; Torres-Oviedo and Bastian 2012). Similar large-scale external perturbations affecting generated locomotor movements have been shown to lead to rapid increases in variability in the interlimb phase relationship. Resulting changes in movement patterns are not maintained over time, nor do they display transfer to other locomotor tasks (Haudum et al. 2014). However, movement and perceptual characteristics which are perceived to originate from the body itself, are more likely to generalize. Overground transfer of a locomotor movement pattern of split-belt locomotion in healthy individuals is observed to be limited when the velocity of each belt remains constant during the entire session. When belt velocities are ramped up and down for the fast and slow belt respectively, overground transfer is observed for step symmetry parameters (Torres-Oviedo and Bastian 2012). In the Torres-Oviedo and Bastian study, the ramped belt velocities induced smaller movement variations, enhancing the transfer of the learned behavior. Larger variations on the treadmill resulted in an absence of transfer. Interlimb coordination of an inherently asymmetrical movement pattern, such as is required by split-belt locomotion, may be significantly more complex than stereotypical locomotion. The maintenance of the novel movement pattern in the current study suggests a change in the underlying neural organization has occurred as a result of plantar tactile vibration, corresponding with the predicted CNS's response to an internally perceived perturbation.
Implications for training and rehabilitation
The enhanced learning effects observed as a result of vibration driven sensory reweighting may have significant implications for varied motor learning paradigms, including rehabilitation of impaired locomotor behavior, particularly after stroke and in astronauts during and after long-duration spaceflight. Returning astronauts typically display reduced interlimb and postural control and coordination, and require significant training and rehabilitation to return to functional levels (Speers et al. 1998; Bloomberg and Mulavara 2003). Similarly, stroke survivors can develop a hemiparetic gait which significantly affects functional locomotor behavior and is primarily the result of control and coordination deficiencies. Hemiparetic stroke survivors generally possess intact motor learning mechanisms (van Vliet and Wulf 2006) Subsequently, training on a split-belt treadmill can lead to significant improvements in interlimb coordination and symmetry in overground walking. These transfer effects are typically more stable in individuals affected by hemiparetic gait patterns. The increased persistence of transfer is speculated to be the result of a reduction in functionality of higher-order cognitive systems in this population, impairing functional adaptability of the CNS (Reisman et al. 2009). The current results suggest an alternative explanation. Hemiparetic locomotion is the result of changes of internal factors. The reason why significant temporary aftereffects are observed in aforementioned populations may be because impairments and subsequent motor rehabilitation effects are perceived as coming from internal sources, leading to a greater likelihood of transfer of such movement patterns.
Limitations
This study employs a broader approach to motor learning by including adaptation and transfer aspects and sensory reweighting theory. However some methodological limitations need to be acknowledged. First of all, due to the nature of the overground walking trials and the setting in which these were performed, spatial kinematic characteristics and kinetics of the overground gait cycle could not be quantified. Second, selection of the vibrotactile stimulus properties, i.e. frequency and amplitude, was based on a preliminary study, which was not a split-belt adaptation study. The respective study was a regular treadmill locomotion experiment performed at this laboratory in which healthy young individuals were fitted with vibrating tactors at the same anatomical locations as in the current study. The results of the preliminary study are currently under review.
Conclusions
In summary, this study demonstrated that exposure to experimentally induced plantar vibrotactile perturbations affected the learning of a new locomotor task. The resulting sensory reweighting enhanced the ability to retain the generated locomotor movement pattern in a novel context while not affecting task learning itself. This phenomenon can potentially have a significant impact in rehabilitation and training, in which the ability to learn and retain the newly learned movement dynamics in different environmental contexts is of paramount importance.
Acknowledgements
This study was supported by funds from the NASA Experimental Program to Stimulate Competitive Research (EPSCoR) award number NNX11AM06A, the National Institute Of General Medical Sciences of the National Institutes of Health award number P20GM109090 and award number 1I01RX000604 from the Rehabilitation Research and Development Service of the VA Office of Research and Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NASA, NIH or the VA Office of Research and Development.
References
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci. 2008;11:1454–1461. doi: 10.1038/nn.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomberg JJ, Mulavara AP. Changes in walking strategies after spaceflight. IEEE Eng Med Biol Mag. 2003;22:58–62. doi: 10.1109/memb.2003.1195697. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Imhoff TT, Grigg P. Noise-mediated enhancements and decrements in human tactile sensation. Physical Review E. 1997;56:923–926. doi: [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res. 1990;82:351–358. doi: 10.1007/BF00231254. [DOI] [PubMed] [Google Scholar]
- Haudum A, Birklbauer J, Muller E. The effect of external perturbations on variability in joint coupling and single joint variability. Hum Mov Sci. 2014 doi: 10.1016/j.humov.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Hohne A, Stark C, Bruggemann GP. Plantar pressure distribution in gait is not affected by targeted reduced plantar cutaneous sensation. Clin Biomech (Bristol, Avon) 2009;24:308–313. doi: 10.1016/j.clinbiomech.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a 'dynamometric map' for human balance control. Neuroreport. 1998;9:3247–3252. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol. 2008;100:1455–1464. doi: 10.1152/jn.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan MJ, Patla AE. Adaptations of walking pattern on a compliant surface to regulate dynamic stability. Exp Brain Res. 2006;173:521–530. doi: 10.1007/s00221-006-0399-5. [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawase F, Haizler T, Bar-Haim S, Karniel A. Kinetic adaptation during locomotion on a split-belt treadmill. J Neurophysiol. 2013;109:2216–2227. doi: 10.1152/jn.00938.2012. [DOI] [PubMed] [Google Scholar]
- Mawase F, Shmuelof L, Bar-Haim S, Karniel A. Savings in locomotor adaptation explained by changes in learning parameters following initial adaptation. J Neurophysiol. 2014;111:1444–1454. doi: 10.1152/jn.00734.2013. [DOI] [PubMed] [Google Scholar]
- Mulavara AP, Feiveson AH, Fiedler J, et al. Locomotor function after long-duration space flight: effects and motor learning during recovery. Exp Brain Res. 2010;202:649–659. doi: 10.1007/s00221-010-2171-0. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M, Heeger DJ, Gardner JL. Attentional enhancement via selection and pooling of early sensory responses in human visual cortex. Neuron. 2011;72:832–846. doi: 10.1016/j.neuron.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BT, Miller CA, Brady RA, Richards JT, Mulavara AP, Bloomberg JJ. Dynamic visual acuity during walking after long-duration spaceflight. Aviat Space Environ Med. 2011;82:463–466. doi: 10.3357/asem.2928.2011. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci. 2012;32:9000–9006. doi: 10.1523/JNEUROSCI.0120-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers RA, Paloski WH, Kuo AD. Multivariate changes in coordination of postural control following spaceflight. J Biomech. 1998;31:883–889. doi: 10.1016/s0021-9290(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J Neurophysiol. 2012;107:346–356. doi: 10.1152/jn.00570.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet PM, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence? Disabil Rehabil. 2006;28:831–840. doi: 10.1080/09638280500534937. [DOI] [PubMed] [Google Scholar]
- Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103:183–191. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Haridas C. Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp Brain Res. 2003;149:260–266. doi: 10.1007/s00221-003-1377-9. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Nakajima T, Barss T, et al. Cutaneous stimulation of discrete regions of the sole during locomotion produces "sensory steering" of the foot. BMC Sports Sci Med Rehabil. 2014;6:33. doi: 10.1186/2052-1847-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li L. The differential effects of foot sole sensory on plantar pressure distribution between balance and gait. Gait Posture. 2013;37:532–535. doi: 10.1016/j.gaitpost.2012.09.012. [DOI] [PubMed] [Google Scholar]