Abstract
5-hydroxytryptamine (5-HT) was originally discovered as a vasoconstrictor. 5-HT lowers blood pressure when administered peripherally to both normotensive and hypertensive male rats. Because the serotonin transporter (SERT) can function bidirectionally, we must consider whether 5-HT can be transported from the bloodstream to the central nervous system (CNS) in facilitating the fall in blood pressure. The blood-brain barrier (BBB) is a highly selective barrier that restricts movement of substances from the bloodstream to the CNS and vice-versa, but the rat BBB has not been investigated in terms of SERT expression. This requires us to determine whether the BBB of the rat, the species in which we first observed a fall in blood pressure to infused 5-HT, expresses SERT. We hypothesized that SERT is present in the BBB of the male rat. To test this hypothesis, over 500 blood vessels were sampled from coronal slices of six male rat brains. Immunofluorescence of these coronal slices was used to determine if SERT and RecA-1 (an endothelial cell marker) colocalized to the BBB. Blood vessels were considered to be capillaries if they were between 1.5 and 23 μm (intraluminal diameter). SERT was identified in the largest pial vessels of the BBB (mean ± SEM= 228.70 ± 18.71 μm, N=9) and the smallest capillaries (mean ± SEM= 2.75 ± 0.12 μm, N=369). SERT was not identified in the endothelium of blood vessels ranging from 20 to 135 μm (N=45). The expression of SERT in the rat BBB means that 5-HT entry into the CNS must be considered a potential mechanism when investigating 5-HT-induced fall in blood pressure.
Keywords: 5-HT, blood brain barrier, rat, serotonin transporter
Introduction
5-HT (5-hydroxytryptamine, serotonin) is a neurotransmitter that is synthesized both centrally [in the raphe nuclei of the brain (Cooper et al, 2003)] and peripherally [in the enterochromaffin cells of the intestine (Mohammad-Zadeh et al, 2008)], and was initially discovered as a vasoconstrictor (Green 2006). As a vasoconstrictor, 5-HT has long been considered a substance that promotes development of and/or sustains high blood pressure (5-HT) because isolated arteries/vascular beds are hypersensitive to the contractile effects of 5-HT. Circulating levels of free 5-HT (platelet free) are elevated in both rodent models of hypertension and hypertensive vs normotensive humans (Watts et al, 2012). This idea inspired a study in which we infused 5-HT into conscious, freely moving normal and hypertensive rats over the course of a week. This was done over this time period given that hypertension is a chronic disease. We hypothesized that this infusion of 5-HT would cause an elevation of blood pressure through primarily a vascular mechanism, and a greater elevation in the hypertensive animals given vascular hypersensitivity to 5-HT. We did not observe a 5-HT-induced hypertension, but rather a 5-HT-induced hypotension (Diaz et al, 2008). We are committed to determining the mechanism(s) by which 5-HT causes this fall in blood pressure given that this could provide for a new therapy for hypertension. We have been unable to show that isolated arteries, largely responsible for controlling total peripheral resistance, relax directly to 5-HT (Davis et al, 2012) and thus other mechanisms have to be investigated.
5-HT is protonated at physiological pH, and thus does not readily pass through a cell membrane without assistance. 5-HT is transported into and out of cells by the serotonin transporter (SERT). SERT typically works against a 5-HT concentration gradient to bring 5-HT back into a cell once released. However, SERT can run in both directions (Blakely et al, 1994; Hilber et al, 2005). We have to consider that the infusion of 5-HT in the periphery could influence blood pressure by moving 5-HT from the blood to the brain given that interaction of 5-HT within the brain can cause profound changes in blood pressure (reviewed in Watts et al, 2012). Studies by Bulat and Supek (1967, 1968) support that 5-HT injected intravenously into rats caused a marked increase in 5-HT in the brain. On the other side, 5-HT has been shown to move from the brain to the blood (Nakatani et al, 2008). These studies provide evidence for the biological possibility of 5-HT from the periphery entering the brain, but do not provide a mechanism for this to occur. Discovery of SERT as a protein that can transport 5-HT came years after the studies by Bulat and Supek. No studies exist that support the presence of SERT on those structures in the rat brain thought to best regulate compound entry into the brain, the blood brain barrier (BBB).
The BBB is a highly selective barrier that is formed by the tight junctions between endothelial cells of blood vessels in the brain (Ballabh et al, 2004). It is debated whether the BBB lines all blood vessels in the brain, such as pial vessels, or just the capillaries (Brust et al, 2000; Roux and Couraud, 2005; Wakayama et al, 2002;). Pial vessels are intracranial vessels in the brain that branch into smaller vessels (Cipolla 2009), while capillaries are small vessels compromised of endothelial-cells that contain no smooth muscle (Cipolla 2009). Regardless of vessel definition, SERT has been localized to immortalized brain capillary endothelial cells of the rat [immortalized brain endothelial RBE4 cells (Brust et al, 2000)] and in mouse (both immortalized mouse brain capillary endothelial cells TM-BBB4 and mouse cerebral cortex; Wakayama et al, 2002). The missing piece for being able to state that entry of peripheral 5-HT to the brain is a potential mechanism that may support 5-HT-induced hypotension in the rat is evidence that the normal BBB of the rat expresses SERT.
We hypothesize that SERT is present in the endothelium of blood vessels (BBB) in the brain. RecA1 was used as an endothelial cell marker. Colocalization of the SERT and RecA-1 was determined via immunofluorescence of coronal rat brain cross-sections, and regions containing signal were be compared quantitatively. Because the size range of vessels that comprise the BBB is debated, we included vessels of all sizes from small vessels (1.5 to 23 μm) to larger vessels (24 to 317.571 μm) (Easton and Fraser, 1998; Sarker et al, 1998; Sokolova et al, 1985; Villringer et al 1994; Woneakin et al, 2012). This study examines the basic issue of whether SERT is expressed in normal rat brain BBB.
Materials and Methods
The Michigan State University Institutional Animal Care and Use Committee approved each of the following protocols.
Hematoxylin and eosin: determining the location of the brain sections
Fresh-frozen coronal brain sections (from male rats, 7–10 μm thickness, snap frozen in OCT embedding media, Zyagen, San Diego, CA, USA) were fixed in formaldehyde for 10 min, washed in PBS and incubated with hematoxylin (GHS 116, Gill No. 1, Sigma-Aldrich, MO, USA) for 5 min. Slides were washed in dH2O until the runoff was more blue than purple, and dipped for 1 s in ammonium hydroxide solution (1:5000, in H2O). Slides were washed again with dH2O and then incubated with eosin (Eosin Y, E4009, Sigma) for 5 min after which they were dipped in 70% EtOH for 10 min, twice in 100% EtOH for 10 min each, and CitriSolv clearing agent (1:1 in 100% EtOH, Thermo Scientific, MA, USA) for 5 min. Slides were next dipped in 100% CitriSolv three times, for 6 min each, mounted with Permount Mounting Medium (SP15-100, Thermo Fisher Scientific, Waltham, MA, USA) and coverslipped.
Immunofluorescence: Colocalization of RecA-1 and SERT
Fresh-frozen coronal brain sections from six male rats (from the same animals as described above) were used. Fresh-frozen vena cava (5–10 μm thickness, sectioned by the Investigative Histopathology laboratory at Michigan State University) were used as a positive control. Slides were thawed to room temperature and fixed in acetone for 10 min. Circles were made around the sections with an Immedge pen (H-4000, Vector Laboratories, Burlingame, CA, USA), slides were labeled, and sections were washed three times with Dulbecco’s Phosphate Buffered Saline (PBS, Product no. D-8537, Sigma-Aldrich, MO, USA) for 5 min per wash. All tissues were blocked with a mixture of species-specific blocking serums [5%, goat serum (S-1000, Vector) and rabbit serum (S-5000, Vector), in PBS] for 60 min. Four serial sections were used. Blocking serum was left on one section to act as the no primary control. Primary antibody RecA1 [anti-RecA1 (mouse monoclonal antibody, ab9774, 10 μg/mL, Abcam, MA, USA)] was added alone on one section to identify endothelial cells, and added in combination with either ST C-20 (goat polyclonal antibody, sc-1458, specific to amino acids 580–630 of SERT of human origin, 40 μg/mL, Santa Cruz Biotechnology, TX, USA) or ST N-14 (goat polyclonal antibody, sc-14514, specific to amino acids 1–50 of SERT of human origin, 40 μg/mL, Santa Cruz) on the remaining two sections for comparison of the SERT antibodies. Slides were incubated with either blocking serum or primary antibody at 4 C overnight in a humidified container. Slides were washed with PBS three times, 5 min per wash. Secondary antibodies [mixture of AlexaFluor 568 rabbit anti-goat (to identify C-20 and N-14) and AlexaFluor 488 goat anti-mouse (to identify RecA-1), 1:500 dilution in PBS, Jackson ImmunoResearch, PA, USA] was applied to all slides for 2 hours at room temperature in a humidified container. Slides were washed with PBS three times, 5 min per wash, then were coverslipped with Prolong Gold with DAPI (P36931, Invitrogen, CA, USA) and sealed with clear nail polish. Slides were imaged on a Nikon Eclipse inverted microscope with a Nikon Digital Sight DS-Qil camera (Nikon Group, Otowara, Japan). Fluorescent images were captured within Nikon NIS elements BR 3.00 software (Nikon Group), hematoxylin and eosin images were captured within MMI Cell Tools (MMI, Zurich, Switzerland, version 3.47).
Data Analysis
Images (4×, approximately 22 individual images per brain) were combined to show the entire coronal slice for each individual animal (Adobe Photoshop CC version 14.2.1, Adobe Systems, San Jose, CA, USA). Images were not altered except to universally adjust brightness and contrast. Exposure times for all images ranged from 100 ms to 1.6 s. A different exposure range was required per region and per magnification, due to variance in vessel size and morphology. This range was determined using the no primary control of each vessel to correct for autofluorescence. Blood vessels were considered to be capillaries if they were larger than 1.5 μm and smaller than 23 μm, as measured across their longest intraluminal distance (ILD). A μm to pixel conversion scale was established to measure ILD within Image J, as adapted from measurements within MMI Cell Tools. Pial vessels were defined as vessels larger than 23 μm. These vessels were separated into three categories for analysis by dividing the range into thirds: small pial vessels ranged from 30.999 to 126.523 μm (ILD), medium pial vessels ranged from 126.524 to 222.048 μm, and large pial vessels 222.049 to 317.571 μm. Pial vessels were studied first within all six brains, and then capillaries were sampled across the following eight regions: corpus callosum, right cortex, right caudate putamen, lateral septal complex complex, right nucleus accumbens, left nucleus accumbens, left caudate putamen, and left cortex. Every individual capillary could not be measured within this study due to their large quantity within the sections observed; thus, capillaries were sampled throughout each region as a representation of the full area. Each individual region was observed in the order listed above, through all brains, before the next region was observed, to prevent the section from being damaged by light exposure. A Grubbs Test (GraphPad Outlier Calculator, GraphPad Software) was used to identify outliers within regions, using a standard alpha of 0.05. A Mann-Whitney U Test was used to compare the size of vessels. A two-way ANOVA was used to compare hemispheres. Initial observation showed ST N-14 to be a redundant indicator relative to ST C-20, therefore sections stained with ST N-14 were not studied at the capillary level, and quantitative analysis of all blood vessels was completed using ST C-20.
Results
Location of coronal brain sections through hematoxylin and eosin stain
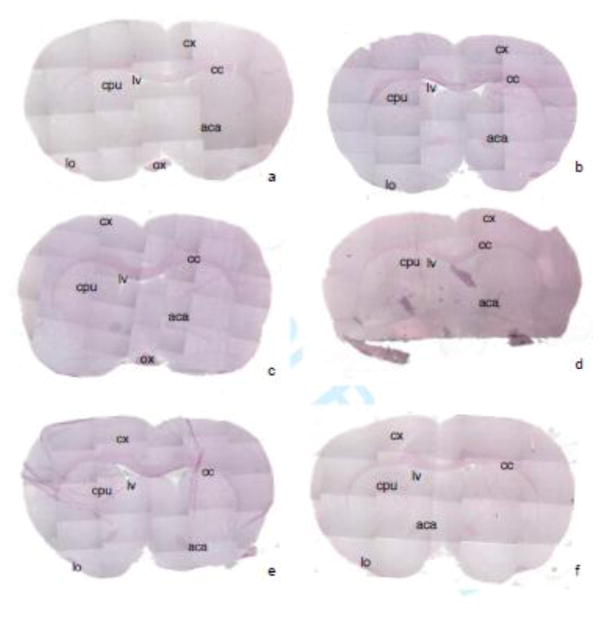
Figure 1 shows six male rat brains, coronally sliced, stained with hematoxylin (purple, nuclear marker) and eosin (pink, collagen and connective tissue marker). The formation of the optic chiasm (ox), corpus callosum (cc), lateral ventricles (lv), and anterior commissure, posterior (aca) (Paxinos 1986) were observed and used to determine the location of the brain slices. Also labeled are the cortex (cx), caudate putamen (cpu) and the lateral olfactory tract (lo) (Paxinos 1986). All six brains (figure 1 a–f) are between −0.26 and 1.60 mm (bregma) and 8.74 and 10.60 mm (interaural) (Paxinos 1986). The coronal brain sections from brains a-c are approximately from 9.20 mm (interaural) and 0.20 mm (bregma), the section from brain d is approximately from 10.60 mm (interaural) and 1.60 mm (bregma), and sections from brains e, and f are approximately from 8.74 mm (interaural) and −0.26 mm (bregma).
Figure 1. Location of coronal brain sections stained with hemotoxylin and eosin.
Coronal sections from six rat brains (Zyagen, a–f) are shown. Purple indicates hematoxylin, a nuclear marker. Pink indicates eosin, a collagen and connective tissue marker. All sections are between −0.26 and 1.60 mm (bregma) and 8.74 and 10.60 mm (interaural). The coronal sections from brains a–c are approximately from 9.20 mm (interaural) and 0.20 mm (bregma), the section from brain d is approximately from 10.60 mm (interaural) and 1.60 mm (bregma), and sections from brains e, and f are approximately from 8.74 mm (interaural) and −0.26 mm (bregma). Identified brain regions are: optic chiasm (ox), corpus callosum (cc), lateral ventricles (lv) and anterior commissure, posterior (aca), cortex (cx), caudate putamen (cpu) and the lateral olfactory tract (lo).
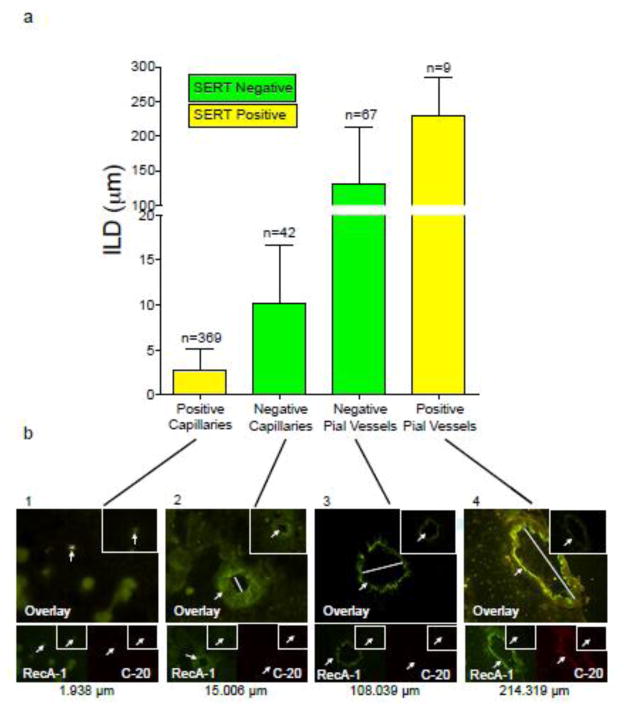
Immunofluorescence: 5-HT has the greatest potential to cross the BBB at the smallest capillaries and the largest pial vessels of the male rat brain
The term “blood vessel” will be used to reference both capillaries and pial vessels throughout this study. All blood vessels included in this study were positive for RecA-1, an endothelial cell marker. We identified RecA-1 positive vessels by observing a FITC signal stronger than that of any background signal, and the vessel was required to possess a clearly defined lumen. This positive signal was compared to that of the negative section, to ensure that non-specific staining was absent. Vessels observed were not regularly circular in shape, a result of the tissue processing. Blood vessels ranging from 1.50 to 317.57 μm (measured across their ILD) were observed in this study. Capillaries observed ranged from 1.50 to 22.49 μm and pial vessels ranged from 31.00 to 317.57 μm. Figure 2 shows SERT colocalization with RecA-1 and ILD of rat brain blood vessels. SERT-negative blood vessels are shown in green (N=109), while SERT-positive vessels are shown in yellow (N=378). Blood vessels negative for SERT were distributed across the ILD range observed, while blood vessels positive for SERT were exclusively at the smallest, and largest ILDs. SERT was not identified in blood vessels ranging from 20 to 135 μm (N=45).
Figure 2.
Figure 2a. SERT colocalization with RecA-1 and intraluminal diameter of rat brain blood vessels.
The graph shows SERT-positive and SERT-negative rat brain blood vessel sizes, with intraluminal diameter (ILD μm) on the y-axis and capillaries and pial vessels on the x-axis. Bars represent mean ILD ± SEM. Yellow indicates SERT-positive, colocalized vessels. Green indicates SERT-negative vessels. All vessels quantified have RecA-1 present. The number of vessels in each category and the range are noted above each bar.
Figure 2b. SERT colocalization with RecA-1 and intraluminal diameter of rat brain blood vessels.
Each of the four panels corresponds to a category of the graph in figure 2a, demonstrating the appearance of vessels from each category. The top panel is an overlay of the FITC and TRITC channels. In the bottom left of each panel RecA-1 (an endothelial cell marker) is shown on the FITC channel (green) while in the bottom right of each panel, ST C-20 (a SERT marker) is shown on the TRITC channel (red). In the upper right-hand corner of each image the negative control (without primary but with secondary present) is shown. Arrows indicate the vessel in each image and a bar is shown across the intraluminal diameter of the overlay images to indicate the size of each vessel. Magnification across the four panels is 100×, 100×, 40×, 40× from left to right.
The blood vessels shown in each panel of figure 2 (panels 1–4) are representative of all blood vessels within that subcategory, throughout all six male rat brains. Each panel shows an overlay of the RecA-1 (green, FITC channel) and ST C-20 (SERT, C-terminus specific, red, TRITC channel) antibodies at the top, with smaller images beneath the overlay showing isolated RecA-1 and ST C-20 staining. Areas of RecA-1 and SERT colocalization within the blood vessel (shown in panels 1 and 4), are observed as coalescent yellow. Panel 1 indicates a SERT-positive capillary (1.94 μm). Panel 2 indicates a SERT-negative capillary (15.00 μm). A SERT-negative pial vessel (108.04 μm) is shown in panel 3, and a SERT-positive pial vessel (214.32 μm) is shown in panel 4. Each image includes a negative control (insert in upper-right hand corner) with no primary antibody, which was exposed to the same secondary antibody as the ST C-20 blood vessels. Fresh-frozen vena cava was used as the positive control for the SERT antibody in this study (not shown) (Linder 2008).
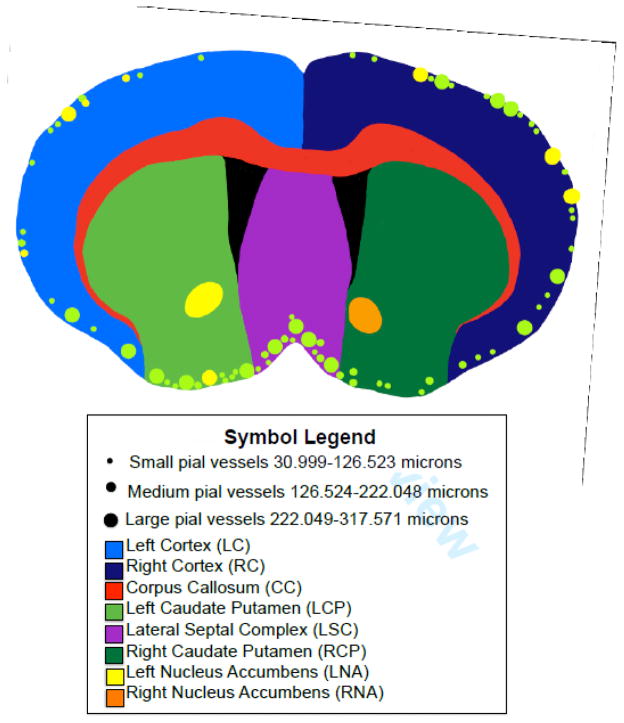
Figure 3 (adapted from a coronal brain image supplied by Zyagen with permission, section #4) is a summary of the locations of only the pial vessel (with approximate sizing) and general regions used for capillary sampling (figure 4 will cover this in a more quantitative manner). The smallest dots (approximately 4 px, Adobe Photoshop CC, Adobe Systems, as adapted within Noteability for iPad version 5.33, Ginger Labs, Inc.) indicate pial vessels ranging from 31.00 to 126.52 μm (ILD). The medium dots (approximately 6 px, Adobe Photoshop CC, Adobe Systems) indicate pial vessels ranging from 126.52 to 222.05 μm (ILD). The large dots (approximately 8 px, Adobe Photoshop CC, Adobe Systems) indicate pial vessels ranging from 222.05 to 317.57 μm (ILD). SERT-positive pial vessels are represented by yellow dots, and represent 9 distinct vessels throughout 4 of the 6 male rat brains in this study. These vessels were located around the edges of the right (4 vessels) and left (4 vessels) cortex, and one vessel was located in the left caudate putamen. SERT-negative pial vessels are indicated by green dots, and represent 67 distinct pial vessels throughout all 6 male rat brains. The 67 vessels were found along the periphery of all sections observed, aside from the left and right nucleus accumbens. A minority of pial vessels stained positive for SERT (9 out of 76 RECA positive, or 11.8%). Figure 3 also includes a color-coded diagram of each region of capillary sampling, as follows: corpus callosum (red), right cortex (dark blue), right caudate putamen (dark green), lateral septal complex (purple), right nucleus accumbens (orange), left nucleus accumbens (yellow), left caudate putamen (light green), and left cortex (light blue).
Figure 3. Summary: Colocalization and location of pial vessels and capillaries.
Different regions of the brain are shown, and locations of the pial vessels are identified. Green dots indicate where pial vessels without SERT are located, and yellow dots indicate vessels with SERT present. Small dots (4 px, Adobe Photoshop CC, Adobe Systems) represent vessels from 30.999 to 126.523 μm (ILD), medium dots (6 px, Adobe Photoshop CC, Adobe Systems) represent vessels from 126.524 to 222.048 μm (ILD), and large dots (8 px, Adobe Photoshop CC, Adobe Systems) represent vessels from 222.049 to 317.571 μm (ILD). Regions in which capillaries were sampled and grouped are shown, and are as follows: corpus callosum (CC, red), right cortex (RC, dark blue), right caudate putamen (RCP, dark green), lateral septal complex (LS, purple), right nucleus accumbens (RNA, orange), left nucleus accumbens (LNA, yellow), left caudate putamen (LCP, light green), and left cortex (LC, light blue).
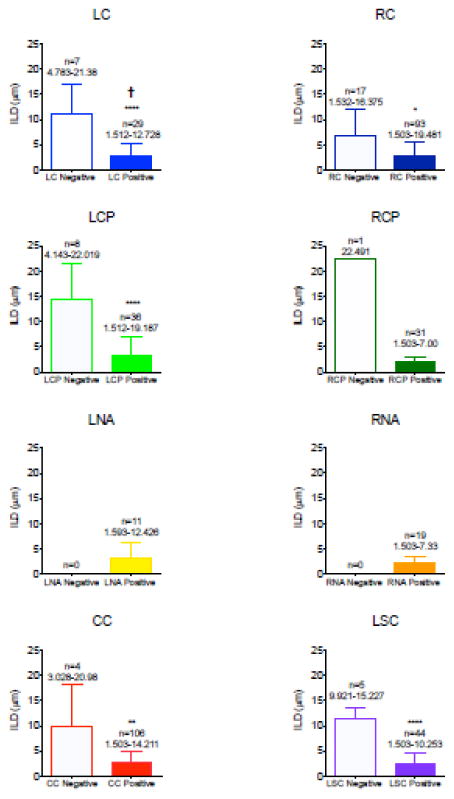
Figure 4. Quantification of SERT-positive capillaries per brain region.
Bar graphs represent the intraluminal diameter in micrometers (μm) of capillaries, specified per region. The regions identified are as follows (colors correspond to their representation in figure 3): left cortex (LC, light blue), right cortex (RC, dark blue), left caudate putamen (LCP, light green), right caudate putamen (RCP, dark green), left nucleus accumbens (LNA, yellow), right nucleus accumbens (RNA, orange), corpus callosum (CC, red), and lateral septal complex (LS, purple). Empty bars indicate the capillaries without SERT present, filled-in bars represent capillaries with SERT present. Above each bar is the number of capillaries sampled for that category and the ILD range observed. Statistical analysis comparing the ILD of vessels with and without SERT for each region was performed using an unpaired t test: * p-value of <0.05, ** p-value of <.01, **** p-value of <0.0001, and † indicates a significant difference between the hemispheres.
The capillaries of each individual region are further examined and quantified in figure 4 (maintaining the same color representations from figure 3). SERT-negative and SERT-positive capillary ILD is compared by region in figure 4. Within the corpus callosum, lateral septal complex, right and left cortex, and left caudate putamen, the SERT-positive capillaries were significantly smaller than the SERT-negative capillaries (p-values <0.05, Mann-Whitney U test, figure 4). Only one SERT-negative capillary was present within the right caudate putamen; as such, there were not enough vessels available for statistical comparison via the Mann-Whitney U test in this region. There were no SERT-negative capillaries present within the left and right nucleus accumbens (full areas could be observed due to the small size of the regions).
On average, 8.75 ± 0.22 capillaries were observed in each brain, per region. It is important to state whether any one brain contributed more capillaries to each region than another. Within the right cortex, left cortex, and lateral septal complex, both SERT-positive and SERT-negative capillaries were equally represented across all brains. In the right caudate putamen, all SERT-positive capillaries were equally represented across all brains. The only SERT-negative capillary in the right caudate putamen was found in brain d (figure 1), making this an outlier. Within the left caudate putamen, SERT-positive capillaries were equally distributed across all brains, but a greater quantity of SERT-negative capillaries was observed in brain f (figure 1) than the other five brains. Within the corpus callosum, SERT-positive capillaries were equally represented across all brains, but a disproportionate number of SERT-negative capillaries were found in brain c (figure 1) compared to the other brains. SERT was present in 90.3% of capillaries (1.503 – 23 μm, measured across their ILD) observed in this study.
Discussion
The findings presented are consistent with our hypothesis that SERT is expressed in the BBB of the male rat. A majority but not all of the vessels considered part of the BBB expressed SERT. Specifically, colocalization of SERT with RecA-1 was positive in the smallest capillaries (N=369, mean ILD ± SEM = 2.75 ± 0.12) and largest pial vessels investigated (N=9, mean ILD ± SEM = 228.7 ± 18.71). Vessels ranging from 20 to 135 μm (N=45) were negative for SERT in every instance within this study (~10% total of number vessels studied).
Impetus for the present study
One particular study provided the impetus for wanting to fill in the gap between 1) the in vitro studies supporting that immortalized cells express and transport 5-HT via SERT, and 2) the in vivo studies supporting movement of 5-HT between central and peripheral compartment. Linder et al (2011) demonstrated a 50% decrease in the absolute magnitude of blood pressure fall to chronically infused 5-HT in both male and female SERT-knockout compared to wild type rats. While a serotonergic system, including SERT, exists in peripheral vasculature (Linder et al, 1008; Ni et al, 2006), it is difficult to imagine a mechanism whereby loss of SERT in the vasculature would reduce the ability of chronically infused 5-HT to decrease blood pressure. If SERT moves 5-HT into the brain to affect BP, our findings fill in a gap to say this is possible. It remains to be seen if this is true, but because 5-HT receptors located centrally can change blood pressure (reviewed in Watts et al, 2012), we must consider this as a possible mechanism.
Differences in colocalization based on size
The greatest number of vessels that demonstrated positive colocalization of SERT and RecA-1 were the small capillaries. The average ILD of capillaries in the male rat brain is approximately 5.33 μm (Villringer et al, 1994), and the average ILD of capillaries within our study was 3.449 μm. We attribute this smaller mean to the large sampling size of our study. Definitions of appropriate capillary ILD range have evolved over time, as evidenced by previous studies: 1.4 to 5 μm rat brain, (Sokolova et al, 1985), 5 to 23 μm rat brain (Easton and Fraser, 1998), 8 to 14 μm rat brain (Sarker et al, 1998), and 1.5 to 19 μm rat femur (Woneakin et al, 2012). This full range, from 1.5 to 23 μm, was used to distinguish capillaries from pial vessels within this study, and this range was largely positive for SERT/Reca colocalization. Pial vessels were also positive, but the vessels sized between pial and capillaries were not. We have no good explanation for this non-conformity, but can suggest that our sampling size of these vessels (45) was considerably smaller than that of the capillaries (369). While possible, this does not explain why we were able to detect colocalization in the far fewer pial vessels (9).
Filling a gap for understanding the potential biological action of 5-HT
Regardless of how the BBB is defined, SERT was detected in both the smallest capillaries and largest pial vessels. The interaction of 5-HT with the BBB has been studied previously, but in different ways such that our understanding of the biological role of 5-HT in using the BBB to cross to and from the CNS, as well as cause changes in how other substances traverse the BBB, has been spotty.
Work on 5-HT and the BBB has been done in both immortalized culture lines and the whole animal. Studies nearly 50 years ago showed that 5-HT can move from brain to blood. The application of intravenous 5-HTP, 5-HT and 5-HIAA to the rat increased levels of these substances in the brain (Bulat and Supek, 1968). A decade later, Westergaard (1975, 1978) demonstrated 5-HT itself modified the plasma membrane of endothelial cells within arterioles, venuoles, and capillaries, enhancing the transfer of vesicles and protein within the brain. These studies were done before the discovery of monoamine transporters, and Brust and colleagues were the first to demonstrate mRNA and functional expression of SERT in the rat brain endothelium. Another step in our understanding came when Nakatani et al showed that 5-HT crosses the BBB from brain to periphery. They administered 5-HTP intravenously to male rats, and subsequently observed increased levels of 5-HT in the frontal cortex (Nakatani et al, 2008). In a second experiment, they surgically removed the gastrointestinal tract and kidneys and then inactivated the liver by destroying the portal vein and bile duct in male rats. This effectively eliminated all sources of 5-HT production within the periphery. Intravenously administered 5-HTP elevated brain levels of 5-HT two-fold from baseline, and elevations in peripheral 5-HT could be measured. This movement appears to require SERT given that administration of the SERT inhibitor fluoxetine in the surgically-modified rats prevented the significant increase of 5-HT levels in the brain. These data support that 5-HT can move from periphery to brain and brain to the periphery, and thus SERT, at least under the collective experimental conditions of the studies reported, can function in both directions. All of these studies lend support to our identification of SERT in the BBB of the male rat brain, but ours is the first in-depth study in which the location of SERT is thoroughly investigated. These studies are important because they challenge the commonly held idea that 5-HT cannot enter the CNS. This finding makes it such that we have to consider 5-HT entering the CNS from the periphery as one means by which 5-HT could influence blood pressure, just as we observed in the chronically infused rat (Diaz et al, 2008). This present study does not intend to answer the biological question of 5-HT crossing the BBB to change blood pressure, but rather raise the possibility.
Other possibilities
Once taken up by SERT, 5-HT may be degraded via MAO-A (which is present in capillaries [Yu 1984]), or it may be stored within a vesicle (Ni et al, 2006) and released within the lumen of the blood vessel. This means that 5-HT may not enter into the brain to exert biological function. Another mechanism of entry may be through other transporters, ones not examined in the present study. Specifically, plasma membrane monoamine transporter (PMAT), dopamine transporter (DAT), norepinephrine transporter (NET), and organic cation transporter (OCT3) are have all been identified in the brain. PMAT is highly expressed in the human brain and transports 5-HT (Daws 2009; Engel et al, 2004; Zhou et al, 2007). DAT and NET are both high affinity transporters of 5-HT (Daws 2009), and are expressed in both the rat (Ciliax et al, 1999; Daws 2009) and human brain (Daws 2009; Fan et al, 2014; Nyberg et al, 2013). OCT3 was identified as a 5-HT transporter in mice brains, and may mediate 5-HT signaling when SERT expression is diminished (Baganz et al, 2008). While all these transporters have been identified in the CNS, their specific expression in the BBB has not been validated, and the role of these transporters in allowing 5-HT to move between the brain and periphery is speculative. Finally, we have to consider that 5-HT may bypass the BBB altogether by entering through the area postrema, a medullary circumventrical organ exposed to circulating hormones (Hay, 2001).
Limitations
We recognize several limitations of the present study. Negative controls for capillaries could not be matched directly, but were selected representatively from each region. While the entire brain was viewed, section-by-section, quantification is of a sampling of about 70% of capillaries due to the high number of capillaries present. Images within this study were taken region by region to diminish the amount of photobleaching from brain to brain (all brains received the same amount of exposure to light per region). Other small SERT-positive structures that were observed could not be measured due to the inability to differentiate between imaging planes using our microscope. We chose sections that would provide a great number of vessels containing the BBB in this study, and thus did not observe specific brain regions that regulate blood pressure [such as the medulla oblongata (Guyenet 2006)]. This study did not undertake the physiological question of whether 5-HT administered peripherally enters the CNS.
Conclusion
Our experimental findings are consistent with our hypothesis that SERT and RecA-1 are colocalized in the male rat brain. Throughout six male rat brains, 90.3% of capillaries contained SERT. 5-HT has the highest potential to cross in the smallest (N=369, mean ILD ± SEM= 2.75 ± 0.12 μm) and largest blood vessels (N=9, mean ILD ± SEM= 228.70 ± 18.71 μm) that were observed in this study. This potential was not observed in vessels of medium size [20 μm to 135 μm (N=45)]. Putting our findings in the context of other studies, they suggest that we must consider the potential of 5-HT to cross the BBB from periphery to brain as one mechanism for reduction of blood pressure.
Acknowledgments
This research was supported by NIH RO1HL107495.
Footnotes
Conflict of Interest
There are no conflicts of interest.
References
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105(48):18976–81. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Brust P, Friedrich A, Krizbai IA, Bergmann R, Roux F, Ganapathy V, Johannsen B. Functional expression of the serotonin transporter in immortalized rat brain microvessel endothelial cells. J Neurochem. 2000;74(3):1241–8. doi: 10.1046/j.1471-4159.2000.741241.x. [DOI] [PubMed] [Google Scholar]
- Bulat M, Supek Z. The penetration of 5-hydroxytryptamine through the blood brain barrier. J Neurochem. 1967;14:265–271. doi: 10.1111/j.1471-4159.1967.tb09523.x. [DOI] [PubMed] [Google Scholar]
- Bulat M, Supek Z. Passage of 5-hydroxytryptamine through the blood-brain barrier, its metabolism in the brain and elimination of 5-hydroxyindoleacetic acid from the brain tissue. J Neurochem. 1968;15(5):383–9. doi: 10.1111/j.1471-4159.1968.tb11625.x. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409(1):38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cipolla M. The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2009. [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxf. University Press; New York: 2003. Serotonin (5-hydroxytryptamine) histamine and adenosine; pp. 271–320. [Google Scholar]
- Davis RP, Pattison J, Thompson JM, Tiniakov R, Scrogin KE, Watts SW. 5-hydroxytryptamine (5-HT) reduces total peripheral resistance during chronic infusion: direct arterial mesenteric relaxation is not involved. BMC Pharmacol. 2012 May 6;12:4. doi: 10.1186/1471-2210-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121(1):89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Ni W, Thompson J, King A, Fink GD, Watts SW. 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J Pharmacol Exp Ther. 2008:1031–8. doi: 10.1124/jpet.108.136226. [DOI] [PubMed] [Google Scholar]
- Easton AS, Fraser PA. Arachidonic acid increases cerebral microvascular permeability by free radicals in single pial microvessels of the anaesthetized rat. J Physiol. 1998;507(Pt 2):541–7. doi: 10.1111/j.1469-7793.1998.541bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279(48):50042–9. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- Fan Y, Chen P, Li Y, Ordway GA, Zhu MY. Effects of desipramine treatment on stress-induced up-regulation of norepinephrine transporter expression in rat brains. Psychopharmacology (Berl) 2015;232(2):379–90. doi: 10.1007/s00213-014-3674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol. 2006;147(Suppl 1):S145–52. doi: 10.1038/sj.bjp.0706427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hay M. Circumventricular organs: gateways to the brain. Subecellular mechanisms of area postrema activation. Clin Exp Pharmacol Physiol. 2001;28:551–557. doi: 10.1046/j.1440-1681.2001.03486.x. [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, Singer EA, Sitte HH. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacol. 2005;49:8110819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Linder AE, Ni W, Szasz T, Burnett R, Diaz J, Geddes TJ, Kuhn M, Watts SW. A serotonergic system in veins: serotonin transporter-independent uptake. J Pharmacol Exp Ther. 2008;325(3):714–22. doi: 10.1124/jpet.107.135699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder AE, Davis RP, Burnett R, Watts SW. Comparison of the function of the serotonin transporter in the vasculature of male and female rats. Clin Exp Pharmacol Physiol. 2011;38(5):314–22. doi: 10.1111/j.1440-1681.2011.05504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31(3):187–99. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Sato-Suzuki I, Tsujino N, Nakasato A, Seki Y, Fumoto M, Arita H. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur J Neurosci. 2008;27(9):2466–72. doi: 10.1111/j.1460-9568.2008.06201.x. [DOI] [PubMed] [Google Scholar]
- Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT) Clin Exp Pharmacol Physiol. 2006;33(7):575–83. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Jucaite A, Takano A, Kågedal M, Cselényi Z, Halldin C, Farde L. Norepinephrine transporter occupancy in the human brain after oral administration of quetiapine XR. Int J Neuropsychopharmacol. 2013;16(10):2235–44. doi: 10.1017/S1461145713000680. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Roux F, Couraud PO. Rat brain endothelial cell lines for the study of blood brain barrier permeability and transport functions. Cell Mol Neurobiol. 2005;25:41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker MH, Easton AS, Fraser PA. Regulation of cerebral microvascular permeability by histamine in the anaesthetized rat. J Physiol. 1998;507(Pt 3):909–18. doi: 10.1111/j.1469-7793.1998.909bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova IA, Manukhina EB, Blinkov SM, Koshelev VB, Pinelis VG, Rodionov IM. Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc Res. 1985;30(1):1–9. doi: 10.1016/0026-2862(85)90032-9. [DOI] [PubMed] [Google Scholar]
- Villringer A, Them A, Lindauer U, Einhäupl K, Dirnagl U. Capillary perfusion of the rat brain cortex An in vivo confocal microscopy study. Circ Res. 1994;75(1):55–62. doi: 10.1161/01.res.75.1.55. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev. 2012;64:359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama K, Ohtsuki S, Takanaga H, Hosoya K, Terasaki T. Localization of norepinephrine and serotonin transporter in mouse brain capillary endothelial cells. Neurosci Res. 2002;44(2):173–80. doi: 10.1016/s0168-0102(02)00120-7. [DOI] [PubMed] [Google Scholar]
- Westergaard E. Enhanced vesicular transport of exogenous peroxidase across cerebral vessels, induced by serotonin. Acta Neuropathol. 1975;32(1):27–42. doi: 10.1007/BF00686065. [DOI] [PubMed] [Google Scholar]
- Westergaard E. The effect of serotonin on the blood brain barrier to proteins. J Neural Transm Suppl. 1978;14:9–15. [PubMed] [Google Scholar]
- Woneakin N, Patumraj S, Niimi H. Capillary density changes in rat femur from youth to aging. Asian Biomedicine. 2012:285–289. [Google Scholar]
- Yu PH. Monoamine oxidase in young and adult rat brain capillaries. J Neural Transm. 1984;60(3–4):239–45. doi: 10.1007/BF01249096. [DOI] [PubMed] [Google Scholar]
- Zhou M, Engel K, Wang J. Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem Pharmacol. 2007;73(1):147–54. doi: 10.1016/j.bcp.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]